Abstract
Peripheral ghrelin has been shown to act as a gut–brain peptide exerting a potent orexigenic effect on food intake. The dorsomedial nucleus of the hypothalamus (DMH) is innervated by projections from other brain areas being part of the network of nuclei controlling energy homeostasis, among others NPY/AgRP-positive fibers arising from the arcuate nucleus (ARC). The aim of the study was to determine if peripherally administered ghrelin affects neuronal activity in the DMH, as assessed by Fos expression. The number of Fos positive neurons was determined in the DMH, paraventricular nucleus of the hypothalamus (PVN), ARC, ventromedial hypothalamic nucleus (VMH), nucleus of the solitary tract (NTS) and in the area postrema(AP) in non-fasted Sprague–Dawley rats in response to intraperitoneally (ip) injected ghrelin (3 nmol/rat) or vehicle (0.15 M NaCl). Peripheral ghrelin induced a significant increase in the number of Fos-ir positive neurons/section compared with vehicle in the ARC (mean±SEM: 49±2 vs. 23±2 neurons/section, p=0.001), PVN (69±5 vs. 34±3, p=0.001), and DMH (142±5 vs. 83±5, p<0.001). Fos-ir positive neurons were mainly localized within the ventral part of the DMH. No change in Fos expression was observed in the VMH (53±8 vs. 48±6, p=0.581), NTS (42±2 vs.40±3, p=0.603), and in the AP (7±1 vs. 5±1, p=0.096). Additional double-labelling with anti-Fos and anti-AgRP revealed that Fos positive neurons in the DMH were encircled by a network of AgRP-ir positive fibers. These data indicate that peripheral ghrelin activates DMH neurons and that NPY-/AgRP-positive fibers may be involved in the response.
Keywords: Ghrelin, Dorsomedial hypothalamic nucleus, Fos, Brain, Rat, Food intake
1. Introduction
Various peptide hormones from the gastrointestinal (GI) tract are involved in the regulation of food intake by influencing the initiation and termination of meals (Woods et al., 1998). Ghrelin is a 28-amino acid peptide hormone which was primarily identified in the rat stomach as the first endogenous ligand of the growth hormone secretagogue (GHS) receptor (Kojima et al., 1999). Ghrelin is mainly synthesized in X/A-like endocrine cells of the gastric oxyntic mucosa (Dornonville et al., 2001) which contribute about 80% to the circulating levels of ghrelin (Kojima et al., 1999; Date et al., 2000). Peripheral ghrelin has been shown to act as a gut-brain peptide exerting a potent orexigenic effect on food intake in rats (Tschöp et al., 2000; Wren et al., 2000, 2001b; Nakazato et al., 2001; Rüter et al., 2003; Kobelt et al., 2005), mice (Asakawa et al., 2001; Wang et al., 2002), and humans (Wren et al., 2001a). Recent studies indicate that vagal afferents-dependent pathways may be involved in ghrelin-induced increase in food intake and Fos-immunoreactivity (Fos-ir) in the arcuate nucleus of the hypothalamus (ARC) after peripheral injection (Date et al., 2002). This was demonstrated by the attenuation of the spontaneous food intake induced by ghrelin after deafferentiation of the vagal nerve (Date et al., 2002), although other reports have contradicted these findings.
There is convincing evidence that peripheral ghrelin activates specific brain areas in the hypothalamus which are part of central networks involved in the regulation of feeding, metabolism, and the hypothalamic-pituitary adrenal (HPA) axis, as shown by the induction of Fos expression (Hewson and Dickson, 2000; Date et al., 2001; Lawrence et al., 2002; Traebert et al., 2002; Wang et al., 2002). Intranuclear Fos is the product of the immediate-early-gene c-fos, a well established marker of changes in neuronal activity in response to stimuli in rodents (Dragunow and Faull, 1989; Hoffman et al., 1993). We and others have previously demonstrated that peripheral ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus (PVN),mainly in the medial parvocellular part of the PVN (mpPVN) (Rüter et al., 2003; Chen et al., 2005; Hashimoto et al., 2007). In addition, peripheral ghrelin induces Fos expression in neuropeptide Y-/Agouti-related peptide (NPY-/AgRP)-positive neurons in the ARC (Hewson and Dickson, 2000; Wang et al., 2002). Chen et al. suggested that the effect of peripheral ghrelin on food intake is mediated via NPY-/AgRP-neurons in the ARC (Chen et al., 2004).
Other studies reported that the GHS receptor and NPY mRNA are co-expressed in ARC neurons (Willesen et al., 1999). 94% of these NPY neurons carry the GHS receptor (Willesen et al., 1999), and 90% of the NPY-ARC neurons are activated by peripheral ghrelin (Wang et al., 2002). Furthermore, it has been demonstrated that NPY is co-localized with AgRP in ARC neurons, and both neuropeptides are involved in orexigenic effects in rats (Clark et al., 1984; Hagan et al., 2000). Therefore, the above findings suggest an important role of NPY/AgRP-ARC-neurons on the regulation of food intake induced by peripherally injected ghrelin in rodents.
Neuroanatomical studies have shown that NPY-containing projections originating from the ARC innervate neurons located in the PVN and the dorsomedial hypothalamic nucleus (DMH) (Bai et al., 1985; Baker and Herkenham, 1995; Broberger et al., 1999). The DMH is another important hypothalamic nucleus which is involved in the regulation of feeding behavior in rodents (Bellinger and Bernardis, 2002).
Thus, the aim of this study was to elucidate changes in neuronal activity of the DMH in response to peripheral ghrelin by mapping Fos expression in the PVN, ARC, ventromedial hypothalamus (VMH), DMH, nucleus of the solitary tract (NTS), and the area postrema (AP) induced by peripheral injection of ghrelin in rats. Furthermore, we investigated the distribution of AgRP-positive fibers in the DMH in relation to Fos-ir using confocal microscopy in order to assess the hypothalamic circuits involved in mediation the effects of peripheral ghrelin on food intake.
2. Results
2.1. Effects of ghrelin administered ip on the number of Fos-ir positive neurons in four hypothalamic nuclei in fed rats
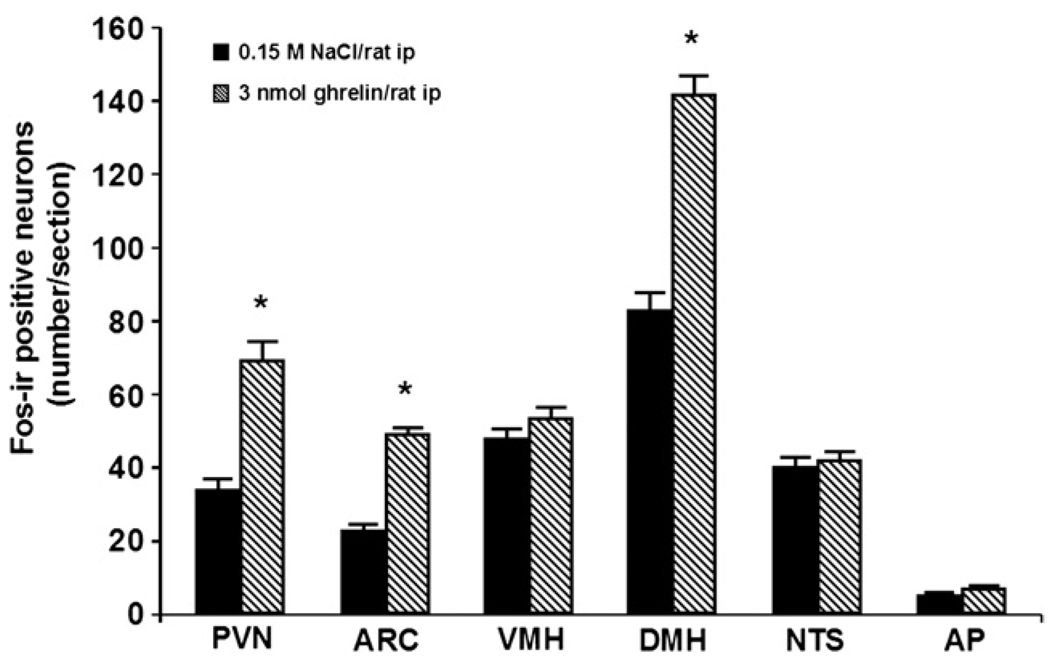
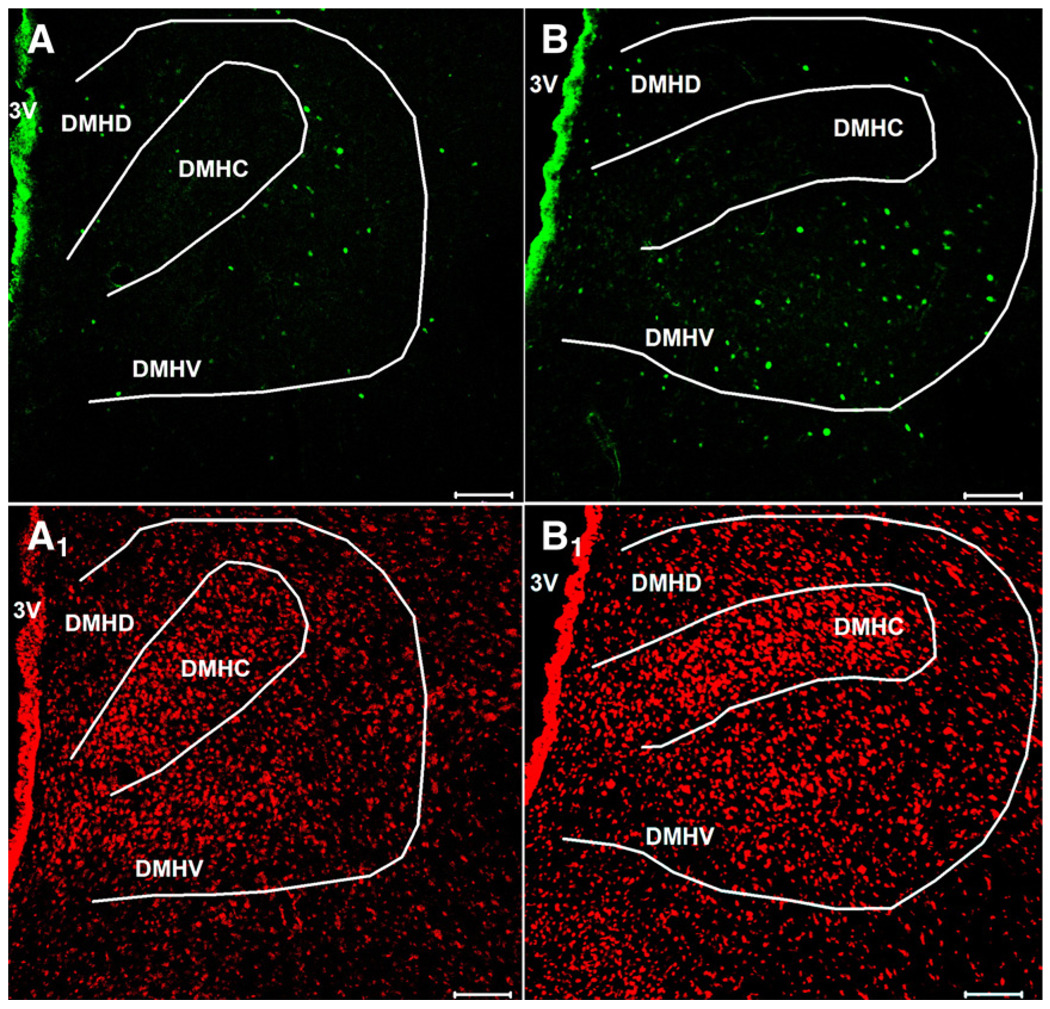
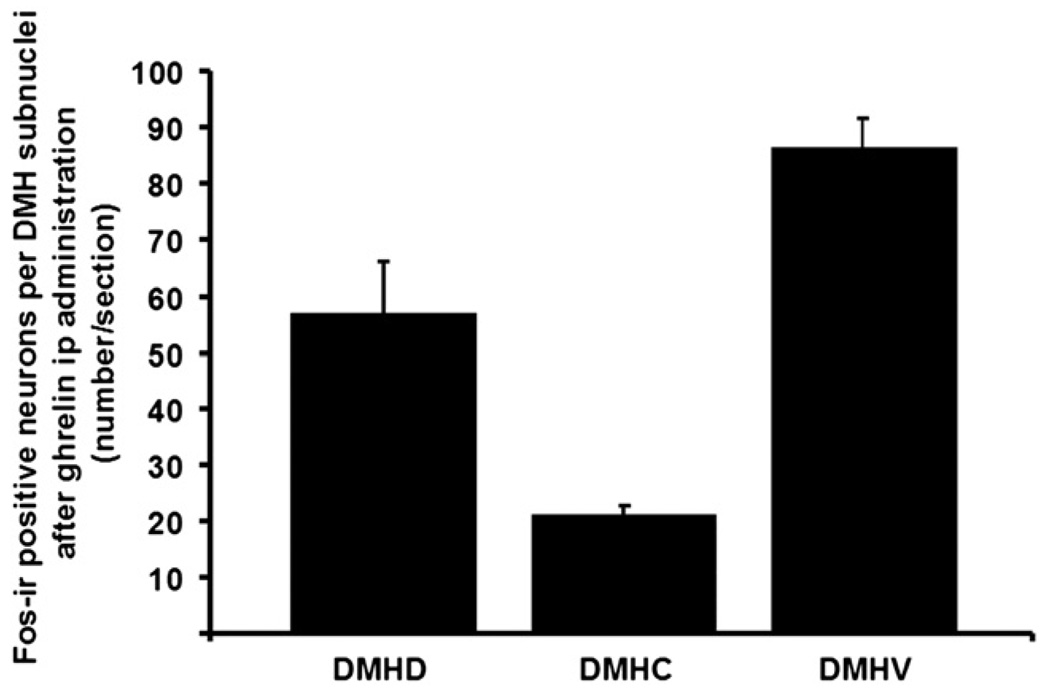
Ghrelin at a dose of 3 nmol/rat administered ip induced a robust 2-fold increase in the number of Fos-ir positive neurons in the ARC mainly localized in the medial part of this nucleus (mean±SEM: 49±2 vs. 23±2 neurons/section, p=0.001; Fig. 1 and Fig. 2) and by 1.9-fold in the PVN (69±5 vs. 34±3, p=0.001; Fig. 1 and Fig. 2) compared to vehicle-treated animals. Interestingly, ghrelin injected ip also induced a significant 1.7–fold increase in the number of Fos-ir positive neurons in the DMH (142±5 vs. 83±5, p<0.001; Fig. 1 and Fig. 3). The Fos-ir positive neurons induced by ip injection of 3 nmol ghrelin/rat were mainly localized within the ventral part of the DMH (mean± SEM: 87±6 neurons/section; Fig. 4).
Fig. 1.
Number of Fos-ir positive neurons in selective hypothalamic nuclei induced by intraperitoneal injection of ghrelin in conscious fed rats. Fos-ir labelled neurons in the paraventricular nucleus of the hypothalamus (PVN), arcuate nucleus of the hypothalamus (ARC), ventromedial hypothalamus (VMH), and in the dorsomedial hypothalamic nucleus (DMH) were quantified 90 min after ip injection of ghrelin (3 nmol/rat) and vehicle. Data are means±SEM of 5 rats/group. *: p<0.05 vs. vehicle.
Fig. 2.
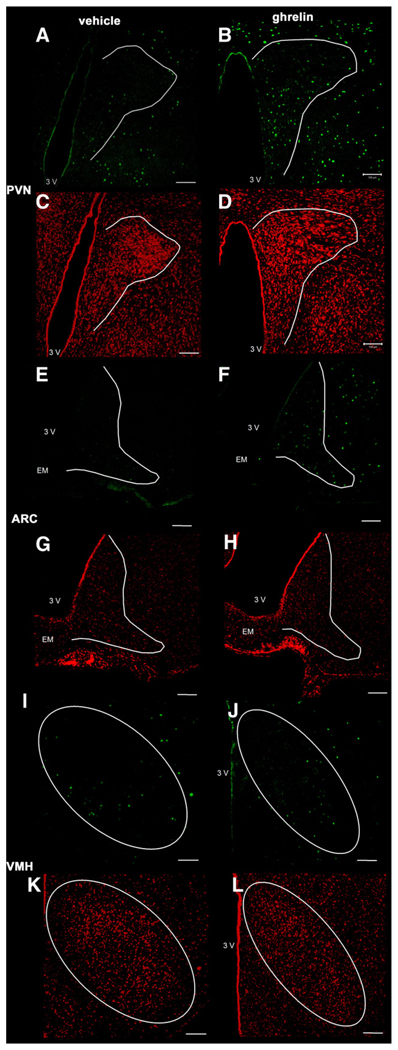
Representative images of the paraventricular nucleus of the hypothalamus (PVN), arcuate nucleus of the hypothalamus (ARC) and ventromedial hypothalamus (VMH) after ip administration of 3 nmol ghrelin or vehicle solution (0.15 M NaCl). Ghrelin induced an increase in the number of Fos-ir positive neurons (green staining) in the PVN (B) and in the ARC (F) but not in the VMH (J) compared to saline treated animals (A,E,I). Cell nuclei are stained red as a result of the counterstaining with propidium iodide in the same slice of animals treated with ghrelin- (B,F,J) or vehicle solution alone (A,E,I). The white outer line delineates the area of the PVN, ARC and VMH. The white scale bar represents 100 µm. 3V = third ventricle, EM = median eminence.
Fig. 3.
Ghrelin injected ip induces Fos-ir in the dorsomedial hypothalamic nucleus in fed rats. The dorsomedial hypothalamic nucleus showed Fos-ir (green staining) 90 min after the administration of ghrelin (3 nmol/rat) (B) while after ip saline injection only few Fos-ir positive neurons were observed (A). Cell nuclei are stained red as a result of the counterstaining with propidium iodide in the same slice of ghrelin- (B1) and saline treated (A1) rats. The white outer line marks the area of the dorsomedial hypothalamic nucleus. The white scale bar represents 100 µm. 3V = third ventricle, DMHD = dorsomedial hypothalamic nucleus, dorsal part; DMHC = dorsomedial hypothalamic nucleus, compact part; DMHV = dorsomedial hypothalamic nucleus, ventral part.
Fig. 4.
The Fos-ir positive neurons induced by ip administration of 3 nmol ghrelin/rat, were mainly localized within the ventral part of the DMH. DMHD = dorsomedial hypothalamic nucleus, dorsal part; DMHC = dorsomedial hypothalamic nucleus, compact part; DMHV = dorsomedial hypothalamic nucleus, ventral part. Data are means±SEM of 5 rats/group.
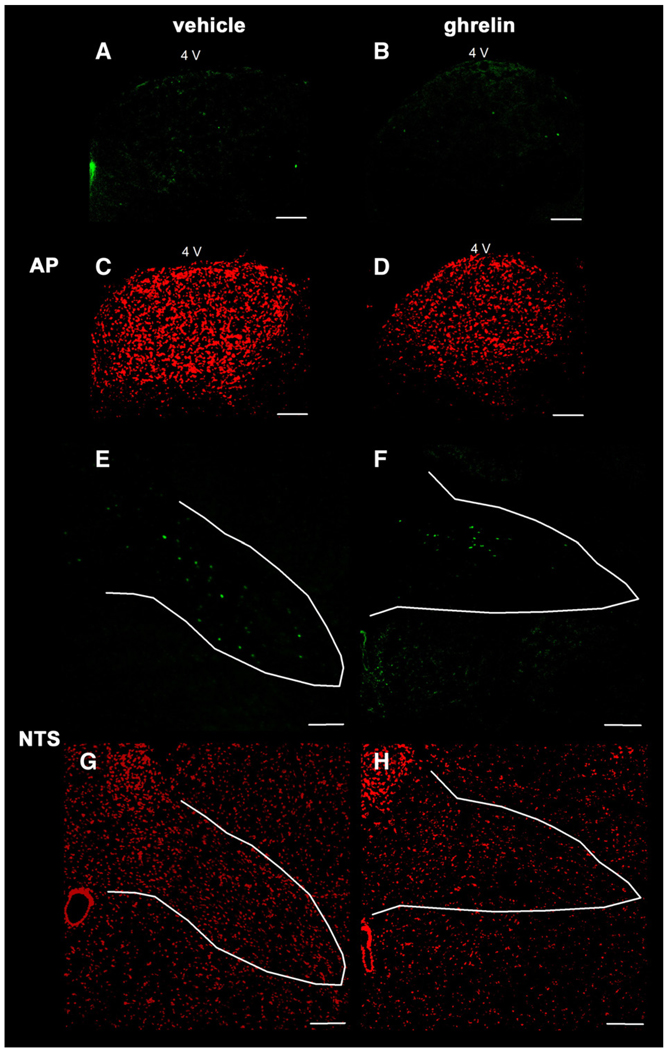
However, ghrelin at the dose of 3 nmol/rat had no effect on Fos expression in the VMH (53±3 vs. 48±3, p=0.177; Fig. 1 and Fig. 2), in the NTS (42±2 vs. 40±3, p=0.603; Fig. 1 and Fig. 5), and in the AP of the brainstem (7±1 vs. 5±1, p=0.096; Fig. 1 and Fig. 5) compared to vehicle treatment.
Fig. 5.
Representative images of the area postrem (AP) and the nucleus of the solitary tract (NTS) of the brainstem after ip injection of 3 nmol ghrelin/rat or vehicle solution (0.15 M NaCl). Ghrelin had no effect on the Fos expression in the AP (B) and in the NTS (F) compared to saline treated animals (A,E). Cell nuclei are stained red as a result of the counterstaining with propidium iodide in the same slice of animals treated with ghrelin- (B,F) or vehicle solution alone (A,E). The white outer line marks the area of the AP and the NTS. The white scale bar represents 100 µm. 4V = fourth ventricle.
2.2. Results of double-staining with anti-Fos, anti-AgRP and anti-NPY in the DMH
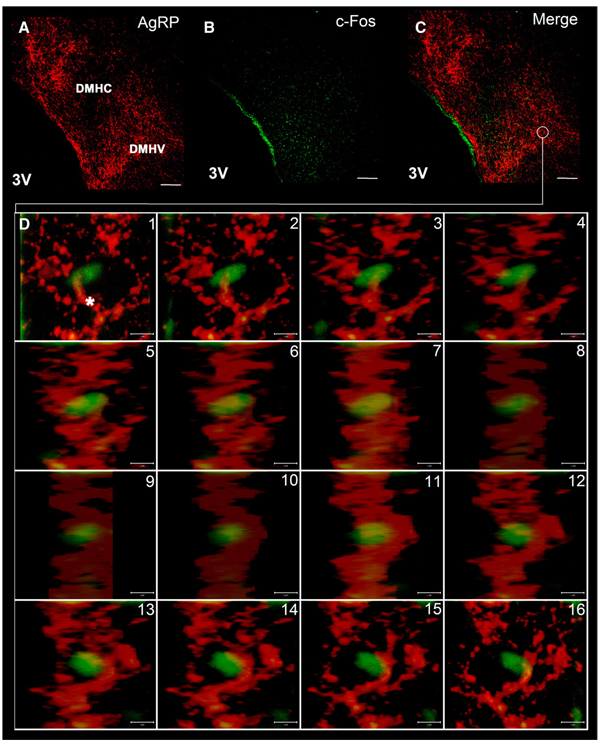
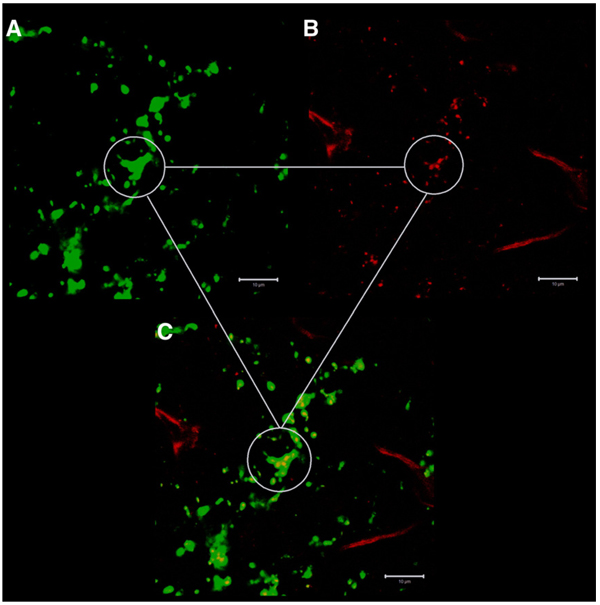
Double-labelling immunohistochemistry investigations with anti-Fos and anti-AgRP revealed that a vast majority of the ghrelin-induced Fos positive neurons in the DMH are encircled in a network of AgRP-ir positive fibers (Fig. 6). Confocal analysis by laser scanning microscope at high magnification (y-projection of a z-stack) revealed that Fos positive neurons in the DMH were associated with a dense network of AgRP-ir positive fibers in the ventral part of this nuclei (Fig. 6). Additional staining with anti- AgRP and anti-NPY revealed a strong co-localization of AgRP and NPY in nerve fibers in the DMH (Fig. 7).
Fig. 6.
Double-staining of Fos positive neurons with anti-AgRP in the DMH. Overview of double-staining with anti-AgRP (A) and anti-Fos (B) in the DMH. Merge image of double-staining showed that Fos-ir positive neurons (green staining) were encircled in a network of AgRP-ir positive fibers (red staining) in the DMH (C). A y-projection of a z-stack analysed by confocal laser scanning microscope demonstrated that Fos positive neurons were associated by AgRP-ir positive fibers (asterisk) (D). The white scale bar represents 100 µmin A, B and C, and 5 µmin D. DMHC = dorsomedial hypothalamic nucleus, compact part, DMHV = dorsomedial hypothalamic nucleus, ventral part, 3V = third ventricle.
Fig. 7.
Double-staining with anti-AgRP and anti-NPY in the DMH. At high magnification additional labelling with anti-AgRP (green staining in A) and anti-NPY (red staining in B) revealed a strong co-localization of AgRP and NPY in nerve fibers in the DMH (C). The white scale bar represents 10 µm.
3. Discussion
This study shows for the first time that peripheral ghrelin affects neuronal activity in the DMH. We also found that peripheral administration of ghrelin significantly increased Fos positive neurons in the ARC in non-fasted rats as reported before in mice or rats (Wang et al., 2002; Hewson et al., 2002; Takayama et al., 2007). In addition, ghrelin administered peripherally increased the neuronal activity in the PVN which is consistent with our previous observations (Rüter et al., 2003).However, no effects on the Fos expression pattern was observed in the VMH, NTS and in the AP of the brainstem.
The present study also showed that the Fos positive cells in the DMH were surrounded by a network of Agouti-related peptide (AgRP)-positive fibers. The distribution of NPY/AgRP nerve fibers and terminals is in line with previous morphological reports showing dense NPY-ir in the DMH in rodents (Bai et al., 1985; Broberger et al., 1998). Some studies established that AgRPir nerve fibers in the DMH emanate from projections of NPY/AgRP-synthesizing neurons located in the ARC (Broberger et al., 1998) including all anatomical levels of the ARC (Thompson and Swanson, 1998).However, some studies suggested that the DMH receives NPY-positive projections from both the ARC and the brainstem(Sahu et al., 1988; Broberger et al., 1998, 1999). The use of double labelling with anti-NPY and anti-AgRP in our study revealed the co-localization of both AgRP and NPY in nerve fibers localized in the DMH. As the ARC is the only brain site of AgRP synthesis (Broberger et al., 1998), the present data indicate that the induction of Fos expression in the DMH by peripheral injected ghrelin, is mediated by NPY/AgRP fibers projecting from the ARC to the DMH. As peripheral ghrelin activates NPY/AgRP positive neurons in the ARC (Wang et al., 2002), the present data together with the above mentioned results suggest that neuronal NPY/AgRP-projections from the ARC to the DMH mediate the activation of DMH neurons. Furthermore, there are studies showing that AgRP and NPY directly injected into the PVN and DMH induce food intake in rodents (Kim et al., 2000). In NPY-deficient mice, an increase in AgRP synthesis was reported without inhibition of ghrelin-induced food intake (Tschöp et al., 2002). However, there are findings of a considerably decrease in the ghrelin-induced food intake in AgRP-receptor deficient mice (Shaw et al., 2005). Moreover, treating animals with antibodies against NPY and/or AgRP, can block the ghrelin-induced food intake (Nakazato et al., 2001). These findings, as well as the lack of ghrelin-induced food intake in NPY-/AgRP-deficient mice, demonstrate the important role of these peptides in the transduction of ghrelin signals.
DMH neuronal activation by peripheral ghrelin may have implications in brain circuitry through which ghrelin promotes food intake. The DMH neurons send projections to the PVN, a primary center for energy regulation which was also found to be activated by peripheral injection of ghrelin (Hashimoto et al., 2007). The DMH is also involved in the regulation of energy homoeostasis in rodents. In this context, we recently demonstrated that an injection of the satiety inducing peptide CCK-8S activates CRF-positive neurons in the DMH (Kobelt et al., 2006). Furthermore, it has been substantiated that peripheral administration of leptin leads to an increased Fos expression within the DMH (Elmquist et al., 1997). Accordingly, the present and previous findings indicate that DMH neurons participate in the regulation of feeding behavior.
Besides, the DMH is also involved in a neuronal network mediating the stress response (DiMicco et al., 2002). Therefore, an increased Fos expression in DMH neurons after peripheral ghrelin injection could also reflect an activation of the hippocampal–amygdalic–hypothalamic pathway involved in the regulation of the stress response of the organism. It should be mentioned that the experimental condition of the present study might have induced stress. The animals receive an ip injection of ghrelin without having the opportunity to eat food afterwards, even though ghrelin would normally induce feeding behavior in animals even fed ad libitum. The induction of hunger by ghrelin and a simultaneous deprivation of food as performed in our experiments might provoke stress in the animals and result in an increased Fos expression in the PVN and DMH, especially since those nuclei are known to be involved in the stress response (DiMicco et al., 2002). However, in a different study design allowing the animals a food intake ad libitum after ghrelin ip administration, the release of gastrointestinal agents signaling satiety might distort the expression patterns of Fos (Mönnikes et al., 1997; Kobelt et al., 2006).
In contrast to this and earlier studies (Wang et al., 2002; Rüter et al., 2003), there were reports about increased Fos expression in the NTS and AP after ghrelin injection (Hashimoto et al., 2007; Takayama et al., 2007). The different patterns of Fos expression in the brainstem nuclei might depend on the varying means of administration. Hashimoto et al. and Takayama et al. administered ghrelin intravenously (Hashimoto et al., 2007; Takayama et al., 2007). In this study as well as in previous studies, ghrelin was given intraperitoneally (Wang et al., 2002; Rüter et al., 2003). After ip injection, as opposed to iv injection, the peptide first passes the liver and might in part be degraded.
Earlier studies about changes in neuronal activation patterns of different nuclei after peripheral administration of ghrelin reported no increase in Fos expression in the DMH (Wang et al., 2002; Takayama et al., 2007). It is unclear what caused these differences. An important feature of the present study compared to previous studies is the use of confocal laser scanning microscopy. This technique allows to suppress unspecific background signals (Kobelt et al., 2004).This way, even relatively light signals can be detected at a low microscopic magnification in 25 µm brain sections (Kobelt et al., 2004). Especially the high contrast and specific excitations of fluorochromes in confocal laser scanning microscopy might explain those differences. Also, the use of Fos-antibodies of different manufacturers as well as varying concentrations of different lots of antibodies might explain differing results.
In conclusion, the present findings show that peripheral ghrelin induces Fos expression in DMH neurons and provide neuro-anatomical support for the concept that an activation of a NPY/AgRP-ARC–DMH pathway may be part of the neuronal circuitry through which peripheral ghrelin influences feeding behavior.
3.1. Experimental procedures
3.1.1. Animals
Male Sprague–Dawley rats (Harlan-Winkelmann Co., Borchen, Germany) weighing ~250 g were housed under conditions of controlled illumination (12:12 h light/dark cycle), humidity, and temperature (22+2 °C) for at least 21 days prior to the experiments. Animals were fed with a standard rat diet (Altromin ®, Lage, Germany) and tap water ad libitum. All animals were accustomed to the experimental conditions for a period of 14 days by handling them daily and putting them in the position to mimic the procedure of intraperitoneal (ip) injection and placing them in individual cages for 2 h per day.
Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the state authority for animal research conduct.
3.1.2. Peptide preparation
Rat ghrelin (Tocris, Ellisville, USA) was dissolved in distilled water (1 mg/ml) and stored at −20 °C. Immediately before the experiments, the peptide was diluted in vehicle solution consisting of sterile 0.15 M NaCl solution (Braun, Melsungen, Germany) to reach the final concentration of 3nmol/0.5 ml (~10 µg/0,5ml). The peptide solution was kept on ice for the duration of the experiments. Dose of ghrelin was based on previous reports (Hewson and Dickson, 2000).
3.2. Experimental design
Non-fasted rats received an ip injection (final volume: 0.5 ml) of ghrelin (3 nmol/rat, n=5) or vehicle solution (0.15MNaCl, n=5). Immediately after the ip injection, animals were deprived of food to avoid an influence of increased food intake on Fos expression in the brain, but had ad libitum access to water (Wang et al., 2002). 90 min after the injection, animals were deeply anesthetized with 100 mg/kg ketamine (Ketanest®, Curamed, Karlsruhe, Germany) and 10 mg/kg xylazine (Rompun ® 2%, Bayer, Leverkusen, Germany) and heparinized with 2500 U heparin ip (Liquemin®, Hoffmann-La Roche, Grenzach-Whylen, Germany). Transcardial perfusion was performed as described before (Geisler et al., 2002). It was started with a 10-s flush of a plasma substitute (Longasteril®70; Fresenius, Bad Homburg, Germany), followed for 20 min by a mixture of 4%w/v paraformaldehyde, 0.05% v/v glutaraldehyde, and 0.2% (v/v) picric acid in 0.1M phosphate buffer, pH 7.4, and finished with a 5% w/v sucrose solution for 5 min. After dissection, brains were kept in a 5% w/v sucrose solution overnight and then cut into 1.0 to 4.5mmcoronal blocks. For cryoprotection, the blocks were moved through a graded series of sucrose (15% and 27.3%), then shock-frozen in hexane at −70 °C, and stored at −80 °C until further processing.
3.2.1. Immunohistochemistry
3.2.1.1. Staining for Fos-immunoreactivity (Fos-ir)
First, 25 µm free-floating brain sections were pre-treated with 1% w/v sodium borohydride in phosphate buffered saline (PBS) for 15 min. Subsequently, sections were incubated in a solution containing 5% w/v bovine serum albumin (BSA) and 0.3% v/v Triton X-100 in PBS for 60 min for blockade of unspecific antibody binding. Thereafter, the diluted primary antibody solution (rabbit antirat c-Fos, Oncogene Research Products, Boston, USA; 1:4000 in a solution of 5% w/v BSA, 0.3% v/v Triton X-100, and 0.1% w/v sodium azide in PBS) was applied for 42 h at room temperature.
After rinsing sections in PBS three times and incubation in a solution containing 5% w/v BSA and 0.3% v/v Triton X-100 in PBS for 60 min, FITC-labelled goat-anti-rabbit IgG (Sigma, St. Louis, USA) was applied for 12 h at room temperature in an appropriate dilution (1:600 in 5% w/v BSA in PBS). Sections were rinsed in PBS three times and stained with propidium iodide (2.5 µg/ml in PBS) for 15 min to counterstain cell chromatin. Tissue sections were finally embedded in 15 µl anti-fading solution (100 mg/ml 1,4-Diazabicyclo [2.2.2] octane (Sigma, St. Louis, USA) in 90% v/v glycerine, 10% v/v PBS, pH 7.4), and analyzed using a confocal laser scanning microscope (cLSM 510, Carl Zeiss, Germany).
3.2.1.2. Double-staining for Fos- and AgRP-immunoreactivity
(AgRP-ir). Free-floating brain sections (25 µm) were pretreated with a 1% w/v sodium borohydride solution for 15 min. Subsequently, sections were incubated in a solution containing 5% w/v normal donkey serum (Jackson ImmunoResearch Laboratories Inc., Pennsylvania, USA) and 0.3% v/v Triton X-100 in PBS for 60 min for blockade of unspecific antibody binding. Afterwards, the diluted primary antibody solution (rabbit anti-c-Fos; 1:2000 and goat anti-AgRP; Neuromics, Minnesota, USA; 1:500 in a solution of 5%w/v donkey normal serum, and 0.1%v/v sodium azide in PBS) was applied for 42 h at room temperature. After rinsing the sections in PBS three times and incubation in a solution containing 5% w/v donkey serum and 0.1% v/v sodium azide in PBS for 2 h, sections were incubated with TRITC-labelled donkey-anti-goat IgG (Jackson ImmunoResearch Laboratories Inc) for 12 h at room temperature (1:200 in 5% w/v donkey in PBS). Sections were rinsed again three times in PBS and incubated in a solution containing 3% w/v goat normal serum (Jackson ImmunoResearch Laboratories Inc) and 0.1% v/v sodium azide in PBS for 2 h. Then, the secondary antibody goat biotin-SP-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc) was applied for 12 h at room temperature (1:1000 in 3% w/v goat normal serum, and 0.1% v/v sodium azide in PBS). Sections were rinsed in PBS three times and incubated for 1 h in Soerensen buffer (pH 8.0). Thereafter, sections were incubated in Soerensen buffer (pH 8.0) containing 20 µg/ml avidin D conjugated with fluorescein (Vector Laboratories, Burlingame, USA) for 5 h at room temperature. Then, sections were rinsed in Soerensen buffer (pH 8.0) three times and stained with DAPI (2 µg 4′-6-Diamidino-2-phenylindole/ ml in PBS; Sigma) for 10 min to counterstain cell chromatin. Sections were rinsed in PBS three times again, then embedded in anti-fading solution and analyzed using a confocal laser scanning microscope.
3.2.1.3. Double-staining for NPY- and AgRP-immunoreactivity (NPY-ir and AgRP-ir)
Free-floating brain sections (25 µm) were pre-treated with a 1% w/v sodium borohydride solution for 15min. Subsequently, sections were incubated in a solution containing 5% w/v donkey serum and 0.3% v/v Triton X-100 in PBS for 60 min for blockade of unspecific antibody binding. Afterwards, the diluted primary antibody solution (anti-NPY guinea pig, Abcam, Germany; 1:500 and goat anti-AgRP 1:500 in a solution of 5% w/v donkey serum, and 0.1% v/v sodium azide in PBS) was applied for 42 h at room temperature. After rinsing the sections in PBS three times and incubation in a solution containing 5% w/v donkey serum and 0.1% v/v sodium azide in PBS for 2 h, FITC-labelled donkey-anti-goat IgG and TRITC-labelled donkey-anti-guinea pig (Jackson ImmunoResearch Laboratories Inc) were applied for 12 h at room temperature (both dilutes 1:200 in 5% w/v donkey serum in PBS). Sections were rinsed in PBS three times and stained with DAPI (2 µg/ml in PBS) for 15 min to counterstain cell chromatin. Sections were rinsed in PBS three times again, then embedded in anti-fading solution and analyzed using a confocal laser scanning microscope.
3.2.1.4. Data and statistical analysis
Semi-quantitative assessment of Fos-immunoreactivity (Fos-ir) was achieved by counting the number of Fos-ir positive cells. Cells with green nuclear staining were considered Fos-ir-positive. Every third of all consecutive coronal 25 µm sections was counted for Fos-ir positive staining bilaterally in the PVN (10 sections/rat; Bregma−1.30 to −2.12 mm), bilaterally in the ARC (20 sections/rat; Bregma −2.12 to −3.60 mm), bilaterally in the VMH (20 sections/ rat; Bregma −2.12 to −3.60 mm), bilaterally in the DMH (10 sections/rat, bregma −2.56 to −3.60 mm), bilaterally in the NTS (15 sections/rat; Bregma −13.24 to −14.30mm), and unilateral in the AP (6 sections/rat; Bregma −14.08 to −13.68 mm). Anatomic correlations were made according to landmarks given in Paxinos and Watson's stereotaxic atlas (Paxinos and Watson, 1997). The investigator counting the number of Fos-ir positive cells was blinded to treatments received by the animals. The average number of Fos-ir positive cells per section for the brain nuclei mentioned above was calculated for five rats per experimental group. All data are expressed as means±SEM and analyzed by normality and equal variance test. Differences between groups were evaluated by the Student t-test, with p<0.05 considered as significant.
Acknowledgments
We are grateful to Christa Josties for her excellent technical support. This work was supported by a grant from the German Research Foundation (DFG) to H.M. (DFG: Mö 458/4–3), and grants to H.M. (Charité: UFF 2006-521), and Y.T. (Research Career Scientist Award, Department of Veterans Affairs and NIHDK R01 33061).
REFERENCES
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Baker RA, Herkenham M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus: neuropeptidergic identity and consequences of adrenalectomy on mRNA levels in the rat. J. Comp. Neurol. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol. Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology. 1999;70:295–305. doi: 10.1159/000054490. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem. Biophys. Res. Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dornonville dlC, Bjorkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Hakanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul. Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB. Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology. 1997;138:839–842. doi: 10.1210/endo.138.2.5033. [DOI] [PubMed] [Google Scholar]
- Geisler S, Heilmann H, Veh RW. An optimized method for simultaneous demonstration of neurons and myelinated fiber tracts for delineation of individual trunco- and palliothalamic nuclei in the mammalian brain. Histochem. Cell Biol. 2002;117:69–79. doi: 10.1007/s00418-001-0357-z. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2000;279:R47–R52. doi: 10.1152/ajpregu.2000.279.1.R47. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Fujihara H, Kawasaki M, Saito T, Shibata M, Otsubo H, Takei Y, Ueta Y. Centrally and peripherally administered ghrelin potently inhibits water intake in rats. Endocrinology. 2007;148:1638–1647. doi: 10.1210/en.2006-0993. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J. Neuroendocrinol. 2000;12:1047–1049. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front. Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- Kim MS, Rossi M, Abusnana S, Sunter D, Morgan DG, Small CJ, Edwards CM, Heath MM, Stanley SA, Seal LJ, Bhatti JR, Smith DM, Ghatei MA, Bloom SR. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49:177–182. doi: 10.2337/diabetes.49.2.177. [DOI] [PubMed] [Google Scholar]
- Kobelt P, Tebbe JJ, Tjandra I, Bae HG, Rüter J, Klapp BF, Wiedenmann B, Mönnikes H. Two immunocytochemical protocols for immunofluorescent detection of c-Fos positive neurons in the rat brain. Brain Res. Brain Res. Protoc. 2004;13:45–52. doi: 10.1016/j.brainresprot.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, van der Voort I, Veh RW, Werner CR, Klapp BF, Wiedenmann B, Wang L, Taché Y, Mönnikes H. CCK inhibits the orexigenic effect of peripheral ghrelin. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2005;288:R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- Kobelt P, Paulitsch S, Goebel M, Stengel A, Schmidtmann M, van der Voort I, Tebbe JJ, Veh RW, Klapp BF, Wiedenmann B, Taché Y, Mönnikes H. Peripheral injection of CCK-8S induces Fos expression in the dorsomedial hypothalamic nucleus in rats. Brain Res. 2006;1117(1):109–117. doi: 10.1016/j.brainres.2006.08.092. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- Mönnikes H, Lauer G, Bauer C, Tebbe J, Zittel TT, Arnold R. Pathways of Fos expression in locus ceruleus, dorsal vagal complex, and PVN in response to intestinal lipid. Am. J. Physiol. 1997;273:R2059–R2071. doi: 10.1152/ajpregu.1997.273.6.R2059. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Rüter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Taché Y, Mönnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Sahu A, Kalra SP, Crowley WR, Kalra PS. Evidence that NPY-containing neurons in the brainstem project into selected hypothalamic nuclei: implication in feeding behavior. Brain Res. 1988;457:376–378. doi: 10.1016/0006-8993(88)90710-x. [DOI] [PubMed] [Google Scholar]
- Shaw AM, Irani BG, Moore MC, Haskell-Luevano C, Millard WJ. Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice. Peptides. 2005;26(10):1720–1727. doi: 10.1016/j.peptides.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Takayama K, Johno Y, Hayashi K, Yakabi K, Tanaka T, Ro S. Expression of c-Fos protein in the brain after intravenous injection of ghrelin in rats. Neurosci. Lett. 2007;417:292–296. doi: 10.1016/j.neulet.2007.02.089. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J. Neuroendocrinol. 2002;14:580–586. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Statnick MA, Suter TM, Heiman ML. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology. 2002;143:558–568. doi: 10.1210/endo.143.2.8633. [DOI] [PubMed] [Google Scholar]
- Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metab. 2001a;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001b;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]