Abstract
People with severe asthma account for 5% to 10% of all asthmatic patients; however, this small group uses the majority of health care resources. Novel methods are needed to cope with the burden that this minority of patients places on the health care system. A severe asthma clinic patient, who was monitored through the University of Alberta’s Virtual Asthma Clinic (Edmonton, Alberta) is presented. Despite optimization of his disease and individualized asthma education (provided by a certified asthma educator), the patient remained on oral glucocorticosteroids (OGS) to control his disease. Following optimization and stabilization, a further reduction in the dose of his OGS by the addition of the long-acting anticholinergic agent tiotropium bromide, was demonstrated. The role of tiotropium as a potential ‘steroid-sparing agent’ in severe refractory asthma is discussed, noting that if patients who are on OGS are not monitored for active inflammation, they may overuse the amount of prescribed systemic steroids, which can result in long-term steroid-related sequelae.
Keywords: Asthma, Internet monitoring, Severe asthma, Steroid-dependent asthma, Tiotropium
Abstract
Les personnes qui souffrent d’un asthme grave ne représentent que 5 % à 10 % de tous les patients asthmatiques. Ce sont pourtant ces malades qui utilisent la majeure partie des ressources en soins de santé. Il faut développer de nouvelles solutions pour faire face au fardeau qu’impose cette minorité de patients au système de soins de santé. On présente ici le cas d’un patient qui a été suivi par une clinique virtuelle de traitement de l’asthme de l’Université de l’Alberta (Edmonton, Alberta). Malgré l’amélioration de son état de santé et un enseignement individualisé sur l’asthme (prodigué par une éducatrice certifiée), ce patient continuait de prendre des corticostéroïdes par voie orale pour maîtriser sa maladie. Après optimisation et stabilisation du traitement, il a été possible de réduire sa dose de corticostéroïdes en ajoutant du bromure de tiotropium, un agent anticholinergique à longue action. Le présent article aborde le rôle du tiotropium à titre d’agent potentiel d’épargne des corticostéroïdes dans l’asthme grave réfractaire. Fait à noter, les patients risquent de faire une utilisation abusive de leurs corticostéroïdes par voie orale si l’on ne surveille pas la composante inflammatoire de leur maladie, ce qui peut, à longue échéance, être propice aux séquelles liées à l’emploi de corticostéroïdes.
Asthma is a chronic, inflammatory disorder of the airways associated with airway hyper-responsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing. These episodes are associated with variable airflow obstruction within the lung that is often reversible either spontaneously or with treatment (1). Asthma symptoms and airway inflammation can be controlled in the majority of individuals; however, approximately 5% to 10% of asthmatic patients are more difficult to control and appear to have an inherently different disease that we are only beginning to understand (2). A small percentage of asthmatic patients will remain dependent on systemic oral glucocorticosteroids (OGS) (3,4), resulting in a reduced quality of life, increased health care use and numerous steroid-induced side effects (5,6). This minority of patients contribute most significantly to the health care costs of asthma (7).
We describe the case of a patient with severe asthma who was followed in the severe asthma clinic and electronically monitored through the University of Alberta’s Virtual Asthma Clinic (VAC) (Edmonton, Alberta), an on-line tool previously described (8). Despite optimization with standard therapy and monitoring within the outpatient asthma clinic, and electronically via the VAC in between these periods, the patient remained dependent on OGS. We were able to significantly reduce his overall systemic steroid dose following the addition of the long-acting anticholinergic agent, tiotroprium bromide.
CASE PRESENTATION
A 43-year-old man with a history of adult-onset atopic asthma (positive allergen skin tests) that was diagnosed at 26 years of age, was referred to the severe asthma clinic at the University of Alberta. He had been using OGS to control his disease for three years before the initial assessment, with frequent emergency department visits and one previous hospitalization. He experienced increased respiratory symptoms primarily when outdoors, on awakening and with exercise. He had a reduced exercise tolerance (less than one flight of stairs). The patient was also an ex-smoker, with a total fifteen pack-year smoking history.
His asthma medications included prednisone 20 mg daily, fluticasone proprionate 500 μg plus salmeterol 50 mg (Advair diskus, GlaxoSmithKline Inc [GSK], USA) one puff twice daily, one to two puffs of salbutamol by pressurized metered dose inhaler with spacer two to three times per day as needed, and montelukast 10 mg daily.
The physical examination on his initial visit revealed normal vital signs, inflamed nasal mucosa with no visible nasal polyps and mild oropharyngeal thrush. The remainder of his examination, which included chest and cardiovascular investigations was normal.
Initial pulmonary function tests showed moderate to severe airflow obstruction with significant reversibility. His forced expiratory volume in 1 s (FEV1) improved from 1.83 L/min to 2.50 L/min postbronchodilator. Persistence of a component of fixed airflow obstruction was noted. Total lung capacity and diffusion capacity were normal. The patient’s initial peak expiratory flow rate (PEF) was 450 L/min.
Following assessment and optimization of his treatment in accordance with current Canadian Asthma Consensus Guidelines, including review and monitoring in conjunction with a certified asthma educator, the patient was enrolled in the University of Alberta’s VAC. He was followed for a one-year period to monitor the progress and side effects of treatment.
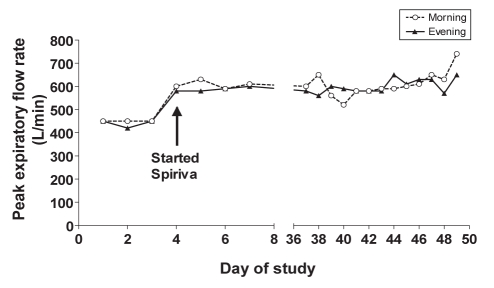
Shortly after the initial visit (during the optimization phase), his prednisone dose was maintained at 20 mg daily without worsening of his clinical status and baseline level of asthma symptoms. He continued to demonstrate a moderate degree of fixed airflow obstruction. At the three-month follow-up, the prednisone dose was decreased to a new baseline dose of 15 mg daily (PEF readings remained at 490 L/min). Induced sputum for cell counts was unsuccessfully attempted on three occasions. Based on the patient’s exercise limitation due to asthma and his ongoing frequent asthma symptoms (a few times per day), he was started on tiotropium bromide (18 μg), one capsule per day. Continued daily monitoring through the VAC showed significant improvement in his PEF values (600 L/min). Over the next six months his prednisone dose was decreased to 2 mg daily. Following the addition of tiotropium, the patient was followed in the VAC for a short period of time and was able to manage his disease without increasing the prednisone dose. He has improved his exercise capacity and quality of life, and maintained his PEFs in the range of 520 L/min to 740 L/min for the remainder of the monitoring period (Figure 1).
Figure 1).
Peak expiratory flow readings through on-line monitoring (Spiriva, Boehringer Ingelheim Pharmaceuticals Ltd, Canada)
DISCUSSION
Inhaled anticholinergic use as part of a medication regimen to manage asthma is not new; however, the steroid-sparing effect demonstrated in the present report is novel, and to our knowledge has not been previously published.
Our asthmatic patient is older (43 years of age), and older age has been demonstrated to cause a decline in beta-adrenergic responsiveness, possibly leading to a relative decrease in bronchodilator response to inhaled beta-agonist therapy, particularly in individuals older than 40 years of age (9). Our patient had been using salbutamol as his primary bronchodilator therapy for many years. This additional class of bronchodilation likely provided a mechanism for his improvement. Older studies (10) also suggest that anticholinergics with or without salbutamol may be preferable in patients older than 40 years of age.
Several recent review articles (3,11,12) highlight the importance of considering severe asthma a distinct phenotype, with a variety of mechanisms contributing to refractory disease. Our patient had demonstrable fixed airflow limitation along with a component of reversible disease, consistent with ‘complex airway disease’. There is significant physiological overlap – along with other similarities – between severe asthma and chronic obstructive pulmonary disease including fixed airflow obstruction, resistance to OGS therapy and when inflammation is characterized, a relative increased prevalence of predominantly neutrophilic (rather than eosinophilic) inflammatory response (13). The benefits of anticholinergics in chronic obstructive pulmonary disease are well documented (14,15) and it is possible that more asthmatic patients with a suboptimal response to beta-agonists may respond to a long-acting anticholinergic in much the same manner. This theory was explored by Fardona et al (16) in a recently published article, showing that in nonsmoking, moderately asthmatic patients, the addition of tiotropium and salmeterol in combination allowed for a 50% reduction in the dose of fluticasone required by patients, with better physiological parameters than the addition of salmeterol alone.
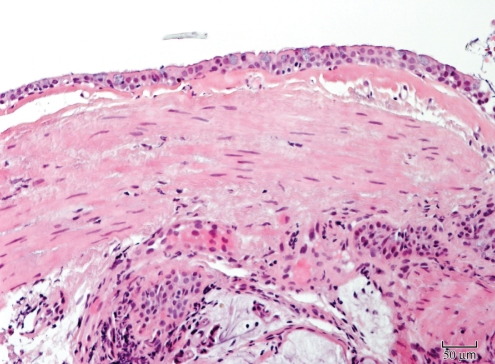
Animal studies support the theory that chronic stimulation of muscarinic receptors may be involved in airway remodelling via smooth muscle thickening. Gosens et al (17) used a guinea pig model to demonstrate that inhaled tiotropium considerably inhibited allergen-induced increases in airway smooth muscle mass, myosin expression and contractility. We are not aware of any human studies evaluating this hypothesis. Following the period of electronic monitoring within the present study, the patient underwent a clinical bronchoscopy for a better evaluation of his airways and to rule out significant upper or lower airway compromise. Marked smooth muscle and neuronal hypertrophy was noted on mucosal biopsies (Figure 2). The bronchoalveolar lavage did not show evidence of active airway inflammation. With gross visualization, there was a moderate degree of tracheobronchomalacia, most notable in his lower trachea, which was asymmetric – more so on the left within the trachea.
Figure 2).
Mucosal biopsy showing marked muscular hyperplasia with no evident airway or mucosal inflammation (hematoxylin and eosin stain, original magnification ×200)
CONCLUSION
To our knowledge, the present case is the first published report of a steroid-dependent severe asthmatic patient to have a documented reduction in overall systemic steroid dose following initiation of a long-acting anticholinergic. We hypothesize that our case illustrates the need to treat severe asthma as a unique phenotype, not solely dependent on anti-inflammatory agents alone. It also documents a need for future clinical trials that explore the use of tiotropium as a potential ‘steroid-sparing agent’ in severe refractory asthma.
Acknowledgments
The authors thank Rene Dery, Irvin Mayers and Thomas Turner.
Footnotes
CONFLICTS OF INTEREST DECLARATION:
Drs Kapoor, Olsen and Puttagunta: none. Ms Cindy O’Hara has received honoraria for talks for continuing medical education from AstraZeneca, GSK, Novartis and Altana. Dr Vethanayagam has received industry support for research and program development over the past five years from AstraZeneca, GSK, Merck, Nycomed, Novartis, Topigen pharmaceuticals, the Research Institute of Fragrance Materials and CV Technologies. Industry support specific to tiotropium did not participate financially or otherwise in the written content of the present case report.
REFERENCES
- 1.Global Initiative for Asthma < http://www.ginasthma.com> (version current at September 15, 2007)
- 2.The ENFUMOSA Study Group The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 3.Holgate ST, Polosa R. The mechanisms, diagnosis and management of severe asthma in adults. Lancet. 2006;368:780–93. doi: 10.1016/S0140-6736(06)69288-X. [DOI] [PubMed] [Google Scholar]
- 4.Chanez P, Wenzel SE, Anderson GP, et al. Severe asthma in adults: What are the important questions? J Allergy Clin Immunol. 2007;119:1337–48. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 5.Saag KG, Furst DE. Major side effects of glucocorticoids. < www.uptodate.com> (version current at September 15, 2007)
- 6.O’Byrne PM, Vethanayagam D. Side effects of inhaled corticosteroids. In: Schleimer RP, O’Byrne PM, Szefler SJ, Brattsand R, editors. Inhaled Steroids in Asthma: Optimizing Effects in the Airways Lung Biology in Health and Disease Series. New York: Marcel Dekker Inc; 2001. pp. 49–60. [Google Scholar]
- 7.Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilization. Eur Respir J. 2004;23:723–9. doi: 10.1183/09031936.04.00004904. [DOI] [PubMed] [Google Scholar]
- 8.O’Hara C, Vethanayagam D, Majaesic C, Mayers I. Internet-based asthma education – a novel approach to compliance: A case report. Can Respir J. 2006;13:30–2. doi: 10.1155/2006/435028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullah MI, Newman GB, Saunders KB. Influence of age on response to ipratropium and salbutamol in asthma. Thorax. 1981;36:523–9. doi: 10.1136/thx.36.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis. 1987;70:171–9. [PubMed] [Google Scholar]
- 11.Green RH, Brightling CE, Bradding P. The reclassification of asthma based on subphenotypes. Curr Opin Allergy Clin Immunol. 2007;7:43–50. doi: 10.1097/ACI.0b013e3280118a32. [DOI] [PubMed] [Google Scholar]
- 12.Gaga M, Zerva E, Griva S, Castro M, Chanez P. Evaluation and management of severe asthma. Curr Med Chem. 2007;14:1049–59. doi: 10.2174/092986707780362961. [DOI] [PubMed] [Google Scholar]
- 13.Pavord ID, Birring SS, Berry M, Green RH, Brightling CE, Wardlaw AJ. Multiple inflammatory hits and the pathogenesis of severe airway disease. Eur Respir J. 2006;27:884–8. doi: 10.1183/09031936.06.00128105. [DOI] [PubMed] [Google Scholar]
- 14.van Noord JA, Bantje TA, Eland ME, et al. A randomized controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–94. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashkin D, Kesten S. Longterm treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest. 2003;123:1441–9. doi: 10.1378/chest.123.5.1441. [DOI] [PubMed] [Google Scholar]
- 16.Fardona T, Haggarta K, Leea DKC, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respir Med. 2007;101:1218–28. doi: 10.1016/j.rmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]