Abstract
Modulation of glutamatergic neurotransmission by metabotropic glutamate2/3 (mGlu2/3) receptor agonists effectively treats seemingly diverse neuropsychiatric illness such as generalized anxiety disorder and schizophrenia. Activation of adenosine A1 heteroceptors, like mGlu2 autoreceptors, decreases glutamate release in the medial prefrontal cortex (mPFC) and other limbic brain regions. Previously, we have reported electrophysiological, neurochemical and behavioral evidence for interactions between the 5-hydroxytryptamine2A (5-HT2A) and mGlu2/3 receptors in the mPFC. The present studies were designed to investigate the effects in rats of adenosine A1 receptor activation/blockade on a behavior modulated by 5-HT2A receptor activation/blockade in the mPFC: head shakes induced in the rat by phenethylamine hallucinogens. An adenosine A1 receptor agonist, N6-cyclohexyladenosine (CHA) suppressed head shakes induced by activation of 5-HT2A receptors with the phenethylamine hallucinogen (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI). An adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), enhanced DOI-induced head shakes and blocked the suppressant action of an adenosine A1 receptor agonist on DOI-induced head shakes. Thus, the pattern of activity for an agonist and antagonist at the adenosine A1 receptor with respect to modulating DOI-induced head shakes is similar to the pattern observed with mGlu2/3 receptor agonists and antagonists. These novel observations with an adenosine A1 receptor agonist suggests that this pharmacological action could contribute to antipsychotic effects in addition to thymoleptic effects.
Keywords: head shakes, phenethylamine hallucinogens, DOI, adenosine, glutamate, medial prefrontal cortex
1. Introduction
Activation of metabotropic glutamate2/3 (mGlu2/3) receptors by orthosteric agonists recently has been shown to be an effective therapeutic approach for neuropsychiatric illness with suspected divergent etiology and pathophysiology such as generalized anxiety disorder and schizophrenia (Dunayevich et al., 2008; Patil et al., 2007). Blockade of neurotransmitter release, especially glutamate, via activation of mGlu2 autoreceptors is a prominent role played by mGlu2/3 receptor agonists (Cartmell et al., 2000b; Schoepp, 2001). Activation of mGlu2 receptors within the medial prefrontal cortex (mPFC) may decrease glutamate release induced by activation of cortical 5-HT2A receptors or the disinhibitory effects of NMDA receptor antagonists on local circuit interneurons in the hippocampus or mPFC (Jodo et al., 2004; Homayoun and Moghaddam, 2007). Thus, activation of mGlu2 autoreceptors appears to attenuate the electrophysiological, neurochemical and behavior effects of 5-HT2A receptor activation or NMDA receptor blockade (Benneyworth et al., 2007; Carli et al., 2004; Cartmell et al., 2000a; Galici et al., 2005; Gewirtz and Marek, 2000; Higgins et al., 2003; Homayoun et al., 2005; Marek et al., 2000; Moghaddam and Adams, 1998; Muschamp et al., 2004).
Activation of adenosine A1 receptors, like mGlu2 receptors, is known to decrease glutamate release in many limbic-related brain regions, as measured by electrophysiological recordings from layer V pyramidal cells of the medial prefrontal cortex (Brand et al., 2001; Marek et al., 2000; Stutzman et al., 2001). Previous preclinical in vivo testing with adenosine A1 receptor agonists has supported potential anxiolytic (Florio et al., 1998; Jain et al., 1995) and antipsychotic (Andine et al., 1999; Browne and Welch, 1982; Florio et al., 1998; Gotoh et al., 2002; Jain et al., 1995; Sills et al., 1999) action.
Therefore, the present studies were designed to investigate the effects of adenosine A1 receptor activation/blockade with respect to a behavior which may be mediated and/or modulated by increased glutamate release in the mPFC. Since head shakes induced by phenethylamine hallucinogens such as (1-(2,5,dimethoxy-4-iodophenyl))-2-aminopropane (DOI) appear to be mediated by activation of 5-HT2A receptors in the mPFC and are also suppressed by activation of mGlu2 autoreceptors, these DOI-induced head shakes were chosen as the first in vivo model system to test in the rat (Benneyworth et al., 2007; Gewirtz and Marek, 2000; Gonzalez-Maeso et al., 2007; Klodzinska et al., 2002; Willins and Meltzer, 1997). Another justification supporting DOI-induced head shakes as a model system is that in vivo microdialysis studies have suggested that systemic administration of phenethylamine hallucinogenic drugs is associated with increased extracellular glutamate in the mPFC and somatosensory cortex (Muschamp et al., 2004; Scruggs et al., 2003). Consistent with these studies in rodents, administration of phenethylamine hallucinogens have also been demonstrated to increase regional cerebral blood flow in the mPFC and other neocortical areas in healthy human volunteers (Vollenweider et al., 1997).
Therefore, in this study, we examined the effects of the adenosine A1 receptor agonist N6-cyclohexyladenosine (CHA) on DOI-induced head shakes to examine a behavior induced by activation of 5-HT2A receptors in the rat prefrontal cortex. The suppressant action of CHA on DOI-induced head shakes was tested for pharmacological specificity using the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX or PD116,948). The pharmacology of adenosine A1 receptor ligands (Bruns et al., 1987; Salvatore et al., 1993) with respect to DOI-induced head shakes is consistent with previous preclinical predictions that adenosine A1 agonists might demonstrate antipsychotic action.
2. Materials and Methods
2.1. Subjects
Male Sprague-Dawley rats (n=102) weighing between 150–300 g at the initial behavioral testing were used (Harlan, Indianapolis, IN). They were housed in suspended stainless wire cages(18 × 36 × 20 cm ) with two to four rats occupying each cage. The colony room was maintained at 20 °C and relative humidity (60%). The room was illuminated 12 hr/day (07:00–19:00). All rats had free access to laboratory chow (Teklad 4% Rat Diet) and water except during experimental sessions. All animals were treated in accord with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals. In addition, all protocols were approved by the Yale University Animal Care and Use Committee.
2.2. Behavioral observations
All experiments were performed between 9:00 and 16:00. The animals were transferred to a clear polycarbonate cage (43×21.5×20 cm) with a sawdust-covered floor. All the rats were habituated to the testing environment with a saline injection at least several days prior to the first DOI/vehicle, CHA/vehicle or DPCPX/vehicle injection. The animals were observed during consecutive 5 min periods for a total of 30 min following the DOI injection. In addition to counting each head shake response, forward locomotion (movement from one end to the other end of the cage was scored as one cross), and rearing (raising up on hind limbs) was also recorded.
2.3. Statistical analysis
A one-factor or two-factor ANOVA was carried out for measurement of head shake, rearing and horizontal locomotor activity. The Dunnett test or Neuman-Keuls test was used for the one-factor or two-factor ANOVAs, respectively. The effect of the adenosine A1 receptor agonist CHA was assessed using a between-subject design where a different group of rats were used for each dose (n=8). The effect of the adenosine A1 receptor antagonist DPCPX/vehicle with DOI/vehicle was tested using a with-in subject design (n=10) and a repeated measures ANOVA. The interaction of the adenosine A1 receptor antagonist DPCPX and the adenosine A1 receptor agonist CHA with respect to behavior induced by DOI was assessed using a between-subject design (n=10). The level of significance was set for p<0.05.
2.4. Drugs
Doses were calculated on the basis of the salt forms. The drugs were dissolved in saline, neutralized to a pH ~ 7.4, and injected IP in a volume of 1 ml/kg body weight. The adenosine A1 receptor agonist CHA (N6-cyclohexyladenosine) and the adenosine A1 receptor antagonist DPCPX (8-cyclopentyl-1,3-dimethylxanthine) were purchased from Sigma-Aldrich (St. Louis, MO) and Tocris (Ballwin, MO), respectively. The 5-HT2A/2B/2C receptor partial agonist DOI, (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride, was purchased from Research Biochemicals International (Natick, MA). At least a two week interval occurred between successive DOI injections to minimize tachyphylaxis of the DOI-induced head shakes. A dose of DOI (1.25 mg/kg, ip) producing a near-maximum of head shakes over a 30 min period was chosen for experiments testing suppression of head shakes by CHA (Gewirtz and Marek, 2000). DOI-induced head shakes increase in a monotonic dose-dependent manner through 9 mg/kg using Sprague-Dawley rats (Pranzatelli, 1990). DPCPX/vehicle, CHA/vehicle and DOI/vehicle were administered 30, 15 and 0 min, respectively, prior to beginning the 30 min observation period.
3. Results
3.1. The adenosine A1 receptor agonist CHA suppresses DOI-induced head shakes
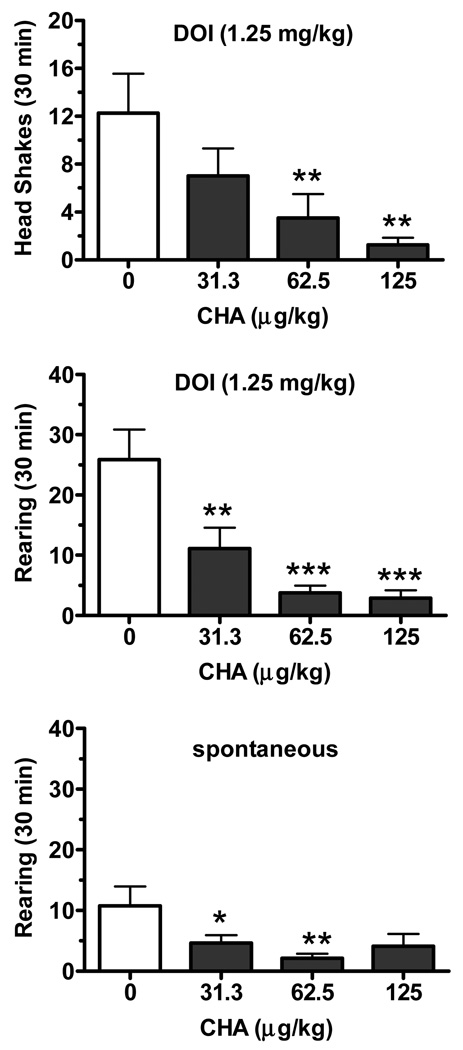
The adenosine A1 receptor agonist CHA (31.3–125 µg/kg, i.p.) suppressed DOI-induced head shakes in a dose-dependent manner (F(3,28)=4.44, p<0.05; Fig. 1). The 62.5 and the 125 µg/kg CHA dose conditions combined with DOI were significantly different from DOI (1.25 mg/kg) alone (p<0.05 and p<0.01, respectively, Newman-Keuls test). CHA (31.3–125 µg/kg, i.p.) also suppressed the frequency of rearing enhanced by DOI in a dose-dependent manner (F(3,28)=11.32, p<0.001; Fig. 1). The frequency of rearing was significantly decreased when rats were treated with each dose condition (p<0.01, Newman-Keuls test). A trend for CHA in reducing locomotion in DOI-treated rats was also found (F(3,28)=2.81, p=0.058, not shown). DOI tended to double locomotor activity, although only a trend was found when comparing DOI (1.25 mg/kg) vs vehicle (t(7)=2.095, p=0.074, not shown).
Fig. 1.
Dose-dependent suppression of DOI-induced head shakes and rearing by the adenosine A1 receptor agonist CHA (31.3–125 µg/kg, i.p.). The top panel displays the frequency of DOI (1.25 mg/kg, i.p.)-induced head shakes (mean ± SEM) 30 min following treatment with either vehicle or CHA (31.3, 62.5, 125 µg/kg; n=8/condition). In the absence of DOI, no head shakes were counted in rats similarly treated with vehicle or CHA (not shown) and observed for a 30 min period. The middle and lower panels display the frequency of rearing for the same rats under the same treatment conditions, except in the lower panel where DOI was not injected. * p<0.05; ** p<0.01; *** p<0.001 compared to the DOI/vehicle condition.
No spontaneous head shakes were observed in these rats over the 30 min observation periods used in the absence of DOI. However, CHA (31.3–125 µg/kg) did suppress basal levels of rearing behavior (F(3,28)=3.33, p<0.05; Fig. 1), with significant effects only at the 31.3 and 62.5 µg/kg dose levels (p<0.05 and p<0.01, respectively). CHA did not alter the basal horizontal locomotor activity (F(3,28)=1.75, p>0.1, not shown).
3.2. The adenosine A1 receptor antagonist DPCPX enhances DOI-induced head shakes
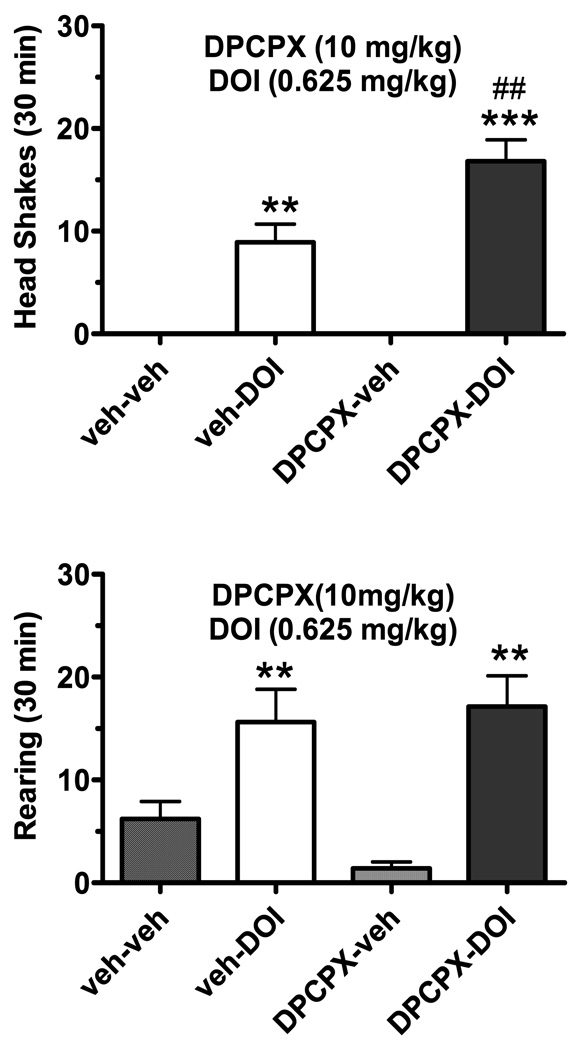
The adenosine A1 receptor antagonist DPCPX (10 mg/kg, i.p.) enhanced head shakes induced by a lower DOI dose (0.625 mg/kg, i.p.; Fig. 2) than the first experiment. A significant effect was observed for DPCPX (F(1,9)=6.93, p<0.05), DOI (F(1,9)=105.0, p<0.001), and the interaction between DPCPX and DOI (F(1,9)=6.93, p<0.05). DPCPX nearly doubled the frequency of DOI-induced head shakes (p<0.01, Newman-Keuls test). In contrast to this significant interaction, neither the adenosine A1 receptor antagonist factor nor the interaction factor between the A1 receptor antagonist and DOI were significant for either rearing behavior or forward locomotion (not shown).
Fig. 2.
The adenosine A1 receptor antagonist DCPCX enhances DOI-induced head shakes. The top panel displays the frequency of DOI (1.25 mg/kg, i.p.)-induced head shakes (mean ± SEM) in the presence and absence of the DCPCX (10 mg/kg, i.p.; n=10). The bottom shows the absence of an effect of this DCPCX dose on rearing associated with DOI.
3.3. DPCPX reverses the suppressant action of CHA on DOI-induced head shakes
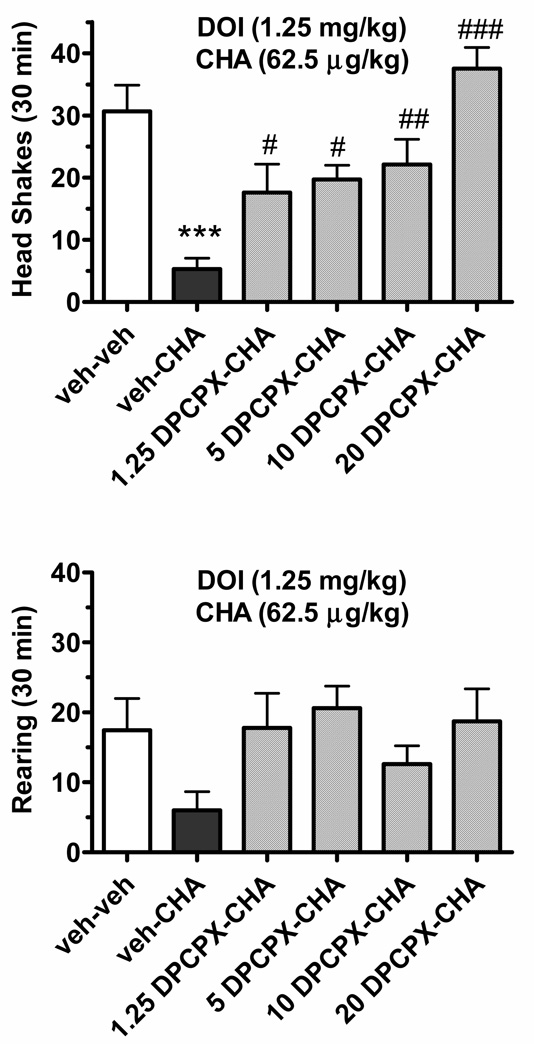
The adenosine A1 receptor antagonist DPCPX (1.25–20 mg/kg, i.p.) blocked the suppressant action of CHA (62.5 µg/kg, i.p.) on DOI(1.25 mg/kg, i.p.)-induced head shakes in a dose-dependent manner (F(5,53)=10.09, p<0.001, Fig. 3). CHA suppressed DOI-induced head shakes by 83% (p<0.001, Newman-Keuls test) while each DPCPX dose significantly reversed the suppressant action of CHA on DOI-induced head shakes (p<0.05 for the 1.25 and 5 mg/kg dose, p<0.01 for the 10 mg/kg dose, and p<0.001 for the 20 mg/kg dose). While CHA suppressed rearing that was increased by DOI, a dose-dependent reversal by DPCPX was not observed.
Fig. 3.
Dose-dependent attenuation by the adenosine A1 receptor antagonist DCPCX of the suppressant action of the adenosine A1 agonist CHA on DOI-induced head shakes. The top panel shows the frequency of DOI (1.25 mg/kg, ip)-induced head shakes in rats also treated with vehicle or CHA (62.5 µg/kg, i.p.) and either vehicle or DCPCX (1.25–20 mg/kg; n=10/condition). The bottom panel shows the frequency of rearing under similar treatment conditions where again, all rats received DOI treatment immediately prior to the observation period. * p<0.05, compared to the vehicle-vehicle condition; # p<0.05, ## p<0.01, ### p<0.001 compared to the CHA-vehicle condition, respectively.
4. Discussion
This is the first known demonstration that activation of adenosine A1 receptors, potentially by suppressing glutamate release in the prefrontal cortex and associated limbic regions, blocks the behavioral effect of a serotonergic hallucinogen used as a preclinical psychosis screen. The demonstration that acute administration of phenethylamine hallucinogens and other 5-HT2A receptor agonists into the prelimbic region of the rodent medial prefrontal cortex induces head shakes (Willins and Meltzer, 1997) is particularly important for a number of reasons. First, the DOI-induced head shake response is suppressed or enhanced by drugs which decrease or increase glutamate release, respectively, by acting on mGlu2 receptor autoreceptors (Benneyworth et al., 2007; Gewirtz and Marek, 2000; Klodzinska et al., 2002). Second, two different selective 5-HT2A receptor antagonists were found to possess modest antipsychotic effects in large multi-centered studies intermediate between placebo and haloperidol (Marder, 1999; Meltzer et al., 2004). This raises the possibility that the prefrontal cortex may be an important component of the circuitry mediating the antipsychotic responses observed with the mGlu2/3 receptor agonist prodrug LY2140023 and the 5-HT2A receptor antagonists. In addition to mGlu2/3 receptor agonists and adenosine A1 receptor agonists both suppressing DOI-induced head shakes, activation of both adenosine A1 receptors and mGlu2 receptors is known to suppress excitatory synaptic currents induced by 5-HT2A receptor activation in the rat or mouse medial prefrontal cortex using slice preparations (Benneyworth et al., 2007; Klodzinska et al., 2002; Marek et al., 2000; Stutzman et al., 2001; Zhai et al., 2003).
These results extend previous preclinical in vivo research suggesting antipsychotic properties for adenosine A1 receptor agonists (Andine et al., 1999; Browne and Welch, 1982; Florio et al., 1998; Gotoh et al., 2002; Jain et al., 1995; Sills et al., 1999). Serotonergic hallucinogens as a pharmacological model for psychosis actually predated the use of non-competitive NMDA receptor antagonists or amphetamine/methamphetamine (Shaw and Woolley, 1956; Woolley and Shaw, 1954). Head shakes/head twitches induced by activation of 5-HT2A receptors in rodents has been one of the most commonly used preclinical behavioral models to back translate the psychotomimetic effects of LSD, mescaline and psilocybin analogues. Adenosine A1 receptor agonists, like mGlu2/3 receptor agonists are capable of suppressing the presumed psychotomimetic effects in rodents of all three major classes of challenge agents, non-competitive NMDA receptor antagonists, amphetamine and serotonergic hallucinogens (Heffner et al., 1989; Andine et al., 1999; Gewirtz and Marek, 2000; Rorick-Kehn et al., 2007). This sharing of psychopharmacological effects between adenosine A1 receptor agonists and mGlu2/3 receptor agonists is especially poignant given the clinical observations that a prodrug for a mGlu2/3 receptor agonist improved both the positive and negative symptoms of schizophrenia in a double-blind, placebo-controlled study (Patil et al., 2007).
Involvement of prefrontal cortical 5-HT2A receptors in these effects is supported by previous studies demonstrating that (1) activation of 5-HT2A receptors in the rat PFC alone is sufficient to induce head shakes and (2) a rescue of forebrain (prefrontal cortex, neocortex and claustrum) 5-HT2A receptors is sufficient to support hallucinogen-induced head twitches in a mouse strain where hallucinogen-induced head twitches had been lost following constitutive disruption of 5-HT2A receptors (Gonzalez-Maeso et al., 2007; Willins and Meltzer, 1997). Furthermore, both electrophysiological and in vivo dialysis experiments have suggested that activation of mGlu2 receptors within the mPFC suppresses glutamate release induced by 5-HT2A receptor activation (Benneyworth et al., 2007; Marek et al., 2001; Marek et al., 2000; Muschamp et al., 2004).
At a more fundamental level, the constellation of shared electrophysiological, biochemical and behavioral effects between adenosine A1 receptor agonists and mGlu2/3 receptor agonists or mGlu2 potentiators emphasizes similar biological roles played by mGlu2 heteroceptors and adenosine A1 heteroceptors (Fredholm and Dunwiddie, 1988; Goodman et al., 1983; Schoepp, 2001). For example, activation of mGlu2 receptors and adenosine A1 receptors suppresses glutamate release from thalamocortical pathways (Fontanez and Porter, 2006; Marek et al., 2001). The plethora of behavioral effects predicting antipsychotic action for the activation of adenosine A1 heteroceptors and mGlu2 receptors may also involve a wider limbic-related distribution of these receptors where they may play similar roles.
Electrophysiological studies in the mPFC are consistent with the hypothesis that adenosine A1 receptors suppress glutamate release induced by 5-HT2A receptors in a fashion similar to mGlu2 receptor activation. Given (1) the similarity between the effects of mGlu2 receptor activation and adenosine A1 receptor activation in the PFC; (2) the similar laminar distribution of rodent 5-HT2A receptors, mGlu2 receptors and adenosine A1 receptors (Fastbom et al., 1987; Lopez-Gimenez et al., 1997; Marek et al., 2001; Marek et al., 2008; Marek et al., 2000), and (3) the suggestion that 5-HT2A and mGlu2 receptors interact via a molecular complex (Gonzalez-Maeso et al., 2008), then the presence or absence of a molecular complex between 5-HT2A and adenosine A1 receptors may be important for evaluating this new theory postulated to explain physiological interactions between 5-HT2A and mGlu2 receptors of relevance for psychosis and antipsychotic drug effects. Conversely, activation of a number of Gq-linked GPCRs acts similar to 5-HT2A receptors at inducing spontaneous 5-HT-induced EPSCs (e.g., α1-adrenergic, orexin-2, neurokinin-3, and mGlu5 receptors). These Gq-linked GPCRs might be expected to similarly interact with glutamatergic autoreceptors (mGlu2, mGlu4, mGlu8) and class A GPCR heteroceptors (adenosine A1 heteroceptors and u-opioid heteroceptors) similar to the proposed interaction of 5-HT2A and mGlu2 receptors. It is also conceivable that the effects of adenosine A1 receptor agonists might be directly acting on other receptor that also oppose the effects of 5-HT2A receptors such as 5-HT1A receptors (Zgombick et al., 1989). However, this type of relationship would not appear as parsimonious as the alternative hypothesis that a number of Gi/Go-coupled GPCRs similarly oppose the effects of 5-HT2A receptor activation by virtue of sharing aspects of post-receptor transduction pathways.
Other brain regions may mediate the effects of systemic administration of adenosine A1 receptor agonists and antagonists on DOI-induced head shakes. Adenosine A1 receptor activation may suppress neurotransmitter release in a number of regions associated with neuropsychiatric illness ranging from the PFC, hippocampus, the striatum, thalamus, and brainstem nuclei associated with attention and arousal via a number of mechanisms (Fredholm and Dunwiddie, 1988; Ochiishi et al., 1999; Rivkees et al., 1995). The distribution of mGlu2 receptor mRNA and protein in limbic-related regions is similar to the pattern observed for adenosine A1 receptor mRNA and protein.
The present results may be relevant to understanding additional domains of behavior which might be impacted by modulation of adenosine A1 receptors. Previously, drugs which block adenosine A1 receptors such as caffeine and PD 116,600 have been found to impair performance of rats on a differential-reinforcement-of-low rate (DRL) 72-s operant schedule consistent with an enhancement of impulsivity (Marek et al., 1993). Activation of 5-HT2A receptors has been found to increase motoric impulsivity while blockade of 5-HT2A receptors appears to enhance the ability of animals to wait before making a response when performing on DRL 72-s schedules (Ardayfio et al., 2008; Carli et al., 2004; Winstanley et al., 2004). Given the growing literature supporting these findings, determining the effects of adenosine A1 receptor agonists and mGlu2 receptor potentiators or mGlu2/3 receptor agonists in tasks assessing dysfunction of the prefrontal cortex is of great heuristic and potential therapeutic interest.
5. Conclusions
The present study provides a third psychotomimetic animal model predicting antipsychotic action for adenosine A1 receptor agonists. Adenosine A1 receptor agonists have a primary role in suppressing glutamate release from heteroceptors from axons throughout the forebrain, including the prefrontal cortex . These results are consistent with the hypothesis that drugs which suppress glutamate release from dysfunctional limbic circuits, such as mGlu2 receptor agonists or potentiators, will be useful antipsychotic drugs.
Acknowledgements
These experiments were performed in the Department of Psychiatry at the Yale University School of Medicine and the Ribicoff Research Facilities of the Connecticut Mental Health Center. Support from the National Institutes of Health Grants RO1 MH62186 and K08 MH01551 and the state of Connecticut is gratefully acknowledged. I also thank Allyson Abo, Sara Heron, Angela Keene, and Kim Reiss for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andine P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J. Pharmacol. Exp. Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. The 5-hydroxytryptamine2A receptor antagonist R-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and enhancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J. Pharmacol. Exp. Ther. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A1 and A3 receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacol. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- Browne RG, Welch WM. Stereoselective antagonism of phencyclidine's discriminative properties by adenosine receptor agonists. Science. 1982;217:1157–1159. doi: 10.1126/science.6287578. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LAH, J D, Hays SJ, Huang CC. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-diproprylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch. Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2Areceptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacol. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparision with the atypical antipsychotic, clozapine. Psychopharmacol. 2000a;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Perry KW, Salhoff CR, Monn JA, Schoepp DD. The potent, selective mGlu2/3 receptor agonist LY379268 increases extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindole3-acetic acid in the medial prefrontal cortex of the freely moving rat. J. Neurochem. 2000b doi: 10.1046/j.1471-4159.2000.0751147.x. in press. [DOI] [PubMed] [Google Scholar]
- Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacol. 2008;33:1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- Fastbom J, Pazos A, Palacios JM. The distribution of adenosine A1 receptors and 5'-nucleotidase in the brain of some commonly used experimental animals. Neurosci. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- Florio C, Prezioso A, Papaioannou A, Vertua R. Adenosine A1 receptors modulate anxiety in CD1 mice. Psychopharmacol. 1998;136:311–319. doi: 10.1007/s002130050572. [DOI] [PubMed] [Google Scholar]
- Fontanez DE, Porter JT. Adenosine A1 receptors decrease thalamic excitation of inhibitory and excitatory neurons in the barrel cortex. Neurosci. 2006;137:1177–1184. doi: 10.1016/j.neuroscience.2005.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release. Trends Pharmacol Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriquez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J. Pharmacol. Exp. Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacol. 2000;23:569–576. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signalling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Goodman RR, Kuhar MJ, Hester L, Snyder SH. Adenosine receptors: Autoradiographic evidence for their location on axon terminals of excitatory neurons. Science. 1983;220:967–969. doi: 10.1126/science.6302841. [DOI] [PubMed] [Google Scholar]
- Gotoh L, Kawanami N, Nakahara T, Hondo H, Motomura K, Ohta E, Kanchiku I, Kuroki T, Hirano M, Uchimura H. Effects of the adenosine A1 receptor agonist N6-cyclopentyladenosine on phencyclidine-induced behavior and expression of the immediate early genes in the discrete brain regions of rats. Mol. Brain Res. 2002;100:1–12. doi: 10.1016/s0169-328x(02)00136-5. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacol. 1989;98:31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and 'impulsive-type' behaviors produced by NMDA receptor antagonism. Psychopharmacol. 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J. Neurophysiol. 2005;93 doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Kemp N, Adeyemo O, Buchanan P, Stone TW. Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 1995;116:2127–2133. doi: 10.1111/j.1476-5381.1995.tb16421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Suzuki Y, Katayama T, Hoshino H-Y, Takeuchi S, Niwa S-I, Kayama Y. Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampal-prefrontal pathway. Cerebral Cortex. 2004;15:663–669. doi: 10.1093/cercor/bhh168. [DOI] [PubMed] [Google Scholar]
- Klodzinska A, Bijak M, Tokarski K, Pilc A. Group II mGlu receptor agonists inhibit behavioral and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Behavior. 2002;73:327–332. doi: 10.1016/s0091-3057(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- Marder SR. Limitations of dopamine-D2 antagonists and the search for novel antipsychotic strategies. Neuropsychopharmacol. 1999;21(S6):S117–S121. [Google Scholar]
- Marek GJ, Heffner TG, Richards JB, Shaughnessy RA, Li AA, Seiden LS. Effects of caffeine and PD 116,600 on the differential-reinforcement-of-low rate 72-s (DRL 72-s) schedule of reinforcement. Pharmacol. Biochem. Behav. 1993;45:987–990. doi: 10.1016/0091-3057(93)90153-k. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neurosci. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Wright RA, Johnson BG, Fell M, Svensson K, Benvenga M, Chaney S, Schoepp DD, Monn J. Activation of mGlu2 receptors mediates the "antipsychotic-like" activity of mGlu2/3 receptor agonists in three psychotomimetic drug models and regional distribution of mGlu2 vs. mGlu3 receptors. Neuropharmacol. 2008;55:608–609. [Google Scholar]
- Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine2A and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 2000;292:76–87. [PubMed] [Google Scholar]
- Meltzer HY, Arvanitis L, Bauer D, Rein W, Group M-TS. Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorders. Am. J. Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonists in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Regina MJ, Hull EM, Winter JC, Rabin RA. Lysergic acid diethylamide and (−)-2,5-dimethoxy-4-methylamphetamine increase extracellular glutamate in rat prefrontal cortex. Brain Res. 2004;1023:134–140. doi: 10.1016/j.brainres.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Ochiishi T, Chen L, Yukawa A, Saitoh Y, Sekino Y, Arai T, Nakata H, Miyamoto H. Cellular localization of adenosine A1 receptors in rat forebrain: Immunohistochemical analysis using adenosine A1 receptor-specific monoclonal antibody. J. Comp. Neurol. 1999;411:301–316. [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nature Medicine. 2007;13:1103–1108. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pranzatelli MR. Evidence for involvement of 5-HT2 and 5-HT1C receptors in the behavioral effects of the 5-HT agonist 1-(2,5-dimethoxy-4-iodophenyl aminopropane)-2 (DOI) Neurosci. Lett. 1990;115:74–80. doi: 10.1016/0304-3940(90)90520-j. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Price SL, Zhou FC. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- Rorick-Kehn LM, Johnson BG, Knitowski KM, Salhoff CR, Witkin JM, Perry KW, Griffey KI, Tizzano JP, Monn JA, McKinzie DL, Schoepp DD. In vivo pharmacological characterization of the structurally novel, potent, selective mGlu2/3 receptor agonist LY404039 in animal models of psychiatric disorders. Psychopharmacol. 2007;193:121–136. doi: 10.1007/s00213-007-0758-3. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc. Natl. Acad. Sci. USA. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Scruggs JL, Schmidt D, Deutch AY. The hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (DOI) increases cortical extracellular glutamate levels in rats. Neurosci. Lett. 2003;346:137–140. doi: 10.1016/s0304-3940(03)00547-0. [DOI] [PubMed] [Google Scholar]
- Shaw E, Woolley DW. Some serotoninlike activities of lysergic acid diethylamide. Science. 1956;124:121–122. doi: 10.1126/science.124.3212.121. [DOI] [PubMed] [Google Scholar]
- Sills TL, Azampanah A, Fletcher PJ. The adenosine A1 receptor agonist N6-cyclopentyladenosine blocks the disruptive effect of phencyclidine on prepulse inhibition of the acoustic startle response in the rat. Eur. J. Pharmacol. 1999;369:325–329. doi: 10.1016/s0014-2999(99)00088-6. [DOI] [PubMed] [Google Scholar]
- Stutzman GE, Marek GJ, Aghajanian GK. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neurosci. 2001;105:55–69. doi: 10.1016/s0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacol. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J. Pharmacol. Exp. Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacol. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Woolley DW, Shaw E. A biochemical and pharmacological suggestion about certain mental disorders. Proc. Natl. Acad. Sci., USA. 1954;40:228–231. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgombick JM, Beck SG, Mahle CD, Craddock-Royal B, Maayani S. Pertussis toxin-sensitive guanine nucleotide-binding protein(s) couple adenosine A1 and 5-hydroxytrytamine1A receptors to the same effector systems in rat hippocampus: Biochemical and electrophysiological studies. Mol. Pharmacol. 1989;35:484–494. [PubMed] [Google Scholar]
- Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacol. 2003;28:45–52. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]