Abstract
Using post-embedding immunogold electron microscopy, TAR DNA-binding protein of 43 kDa (TDP-43) was localized to neuronal cytoplasmic (NCI) and intranuclear (NII) inclusions, as well as unmyelinated neurites, in frontotemporal lobar degeneration with ubiquitinated inclusions (FTLD-U), amyotrophic lateral sclerosis (ALS), Alzheimer’s (AD), Pick’s disease (PiD) and Lewy body disease (LBD). The TDP-43 immunoreactive structures were morphologically heterogeneous. The most common was characterized by bundles of 10–20 nm diameter straight filaments with electron dense granular material within NCI, NII and neurites. This type of pathology was found in FTLD-U, ALS and some cases of AD. Less often inclusions in neuritic processes of FTLD-U and some cases of AD contained 10–17 nm diameter straight filaments without granular material. A final type of TDP-43 immunoreactivity was labeling of filaments and granular material associated tau filaments in neurofibrillary tangles of AD and Pick bodies of PiD or α-synuclein filaments in Lewy bodies of LBD. The results suggest that TDP-43 is the primary component of the granulofilamentous inclusions in FTLD-U and ALS. Similar inclusions sometimes accompany filamentous aggregates composed of other abnormal proteins in AD, PiD and LBD.
Keywords: Alzheimer's disease, amyotrophic lateral sclerosis, frontotemporal lobar degeneration with ubiquitinated inclusions, immunoelectron microscopy, Lewy body disease, Pick's disease, TAR DNA-binding protein of 43 kDa (TDP-43)
Introduction
TDP-43 immunoreactivity was first demonstrated in ubiquitin immunoreactive neuronal inclusions that are negative for tau and α-synuclein in frontotemporal lobar degeneration (FTLD-U) and amyotrophic lateral sclerosis (ALS) [2; 18]. These inclusions are commonly found in neuronal cell bodies (NCI), nuclei (NII) and neurites of the cerebral cortex, amygdala, hippocampus and striatum as well as motor neurons of the brain stem and spinal cord. They are also increasingly recognized in glia in some conditions [9]. The specificity of TDP-43 for FTLD-U was suggested in that other disorders, such as α-internexin-positive neuronal inclusions in neuronal intermediate filament inclusion disease, were not labeled by TDP-43 [18]. The first suggestion that TDP-43 may not be specific to FTLD-U and ALS was the report by Arai et al. that some cases of Pick’s disease (PiD) had TDP-43 immunoreactivity, which was later confirmed in other cases of PiD [2; 5]. Subsequently, TDP-43 immunoreactivity was demonstrated in neuronal inclusions of 20–30% of Alzheimer’s disease (AD) and more than 70% of cases with hippocampal sclerosis (HpScl) [1]. Later studies showed TDP-43 associated with Lewy body disease (LBD), particularly in cases with Parkinson’s disease and dementia [11; 17] and in both neurons and glia in parkinsonism-dementia complex and ALS of Guam [6; 9; 15].
Despite the growing list of disorders associated with TDP-43 immunoreactivity, there are relatively few immunoelectron microscopic studies of these inclusions [3; 12; 14; 19]. Early studies using ubiquitin antibodies [12; 14; 19] and pre-embedding immunolabeling methods demonstrated that NCI were composed of filaments of 10–15 nm [14] or 15–20 nm [12] diameter usually associated electron dense granular material. Ultrastructural localization of TDP-43 was recently reported in the hippocampus of a single case of FTLD-U using pre-embedding method and diaminobenzidine (DAB) as chromogen [3]. The authors showed TDP-43 immunolabeling of NCI in granular and membranous material with an occasional filament or microtubule as minor components [3]. In dystrophic neurites TDP-43 immunoreactivity was associated with filamentous material, but immuno-labeling was not demonstrated in inclusions composed largely of filaments or in NII. The authors suggested that TDP-43 was probably associated with non-filamentous material [3]. More recently, Hasegawa et al. have shown that TDP-43-immunoreactive inclusions in FTLD-U are filamentous [10]. The observations are in accord with our previous findings, in which we reported on TDP-43 immunoreactivity using immunogold electron microscopy and post-embedding staining methods in AD [1]. In that study, TDP-43 was associated with granule-coated filaments in NII and in cytoplasmic filaments distinct from tau filaments in neurons with neurofibrillary tangles. These studies suggested that TDP-43 may be a constituent of the filaments and that filamentous TDP-43 may sometimes associate with filaments composed of other disease-related proteins. In the present study, we extended these immunoelectron microscopic studies to include other neurodegenerative diseases, including FTLD-U, ALS, PiD and LBD.
Materials and Methods
Small pieces of tissue were collected from the hippocampus or parahippocampal gyrus of formalin-fixed brains from four pathologically confirmed cases of FTLD-U, one case of AD with HpScl, one case of PiD case and two cases of diffuse Lewy body disease (DLBD). The amygdala was sampled from one case of sporadic DLBD and one case of familial DLBD, a patient with A53T mutation in the gene for α-synuclein [20]. Samples were also taken from the anterior horns of the spinal cord of two ALS cases. All tissue was dehydrated in progressive ethanols and embedded in LR White as previously described [16]. Thin sections collected on Formvar-coated-nickel grids were floated with the section-side down on 2 ml of citrate buffer, pH 6, in a 100°C oven for 10 min, and cooled to room temperature prior to immunogold EM.
The primary antibodies used were as follows: polyclonal antibody to TDP-43 (1:10 or 1:20; ProteinTech Group, Inc., Chicago, IL) and monoclonal antibody to TDP-43 (1:30, Abnova, Taipei, Taiwan); monoclonal antibody to phosphorylated neurofilaments (SMI 31, 1:20; Covance, Berkeley, CA); polyclonal antibody to ubiquitin (UBQ; 1:20) [4] and a monoclonal antibody to ubiquitin (Ubi-1; 1:20; Chemicon, Temecula, CA); a monoclonal antibody to phosphorylated tau (PHF-1; neat; Peter Davies, Albert Einstein College of Medicine); and a polyclonal to α-synuclein (NACP; 1:20) [8] and a monoclonal antibody to α-synuclein (LB509, 1:20, Zymed, South San Francisco, CA). To detect the location of the primary antibodies, we used 5-, 10- or 18 nm gold-conjugated secondary antibodies (1:20; Amersham/GE Healthcare, Piscataway, NJ; Jackson ImmunoResearch Laboratories, West grove, PA). Controls included incubating sections without primary antibodies.
At the dilutions used to optimally detect inclusions, TDP-43 showed only weak immunolabeling of nuclear chromatin, but did not bind to any other cellular component in neurons, glia or myelinated axons. Other structures, such as Hirano bodies, lipofuscin, neurofilaments, amyloid fibrils and astrocytic glial fibrils were all negative for TDP-43. In addition, no specific labeling was observed in samples when the primary antibody was omitted.
Results
FTLD-U
The NCI and NII in FTLD-U showed TDP-43 immunoreactivity in filamentous bundles often associated with electron dense granular material (Fig. 1). The filaments had a diameter of 10–17 nm, with thickness increasing to 20 nm or more in regions with heavy coating of electron dense granular material. The filament bundles tended to be more orderly and tightly packed in NII than in NCI. In some dystrophic neurites TDP-43 immunoreactivity was associated with tightly packed filamentous structures (Fig. 1E, F). Most filaments were readily recognized because granular material only coated segments of filaments, indicating that filaments were the dominant component of the inclusions. Inclusions within unmyelinated neurites also contained tightly packed filaments of 10–17 nm in diameter with or without associated dense granular material (Fig. 2). Of the neurites with TDP-43 immunoreactivity about 20% had minimal or no electron dense granular material. Filaments in these neuritic inclusions were randomly oriented. All filaments appeared to be straight without periodic constrictions. TDP-43 immunoreactivity was localized in both filaments and electron dense granular material associated with the filaments (Fig. 3A). The filaments were negative with a monoclonal antibody to neurofilament (Fig. 3B), which heavily labeled neurofilaments in axons within the same section (data not shown), demonstrating the specificity of the immunoelectron microscopy methods. Double labeling with antibodies to ubiquitin and TDP-43 showed co-localization of both proteins to the same inclusions (not shown).
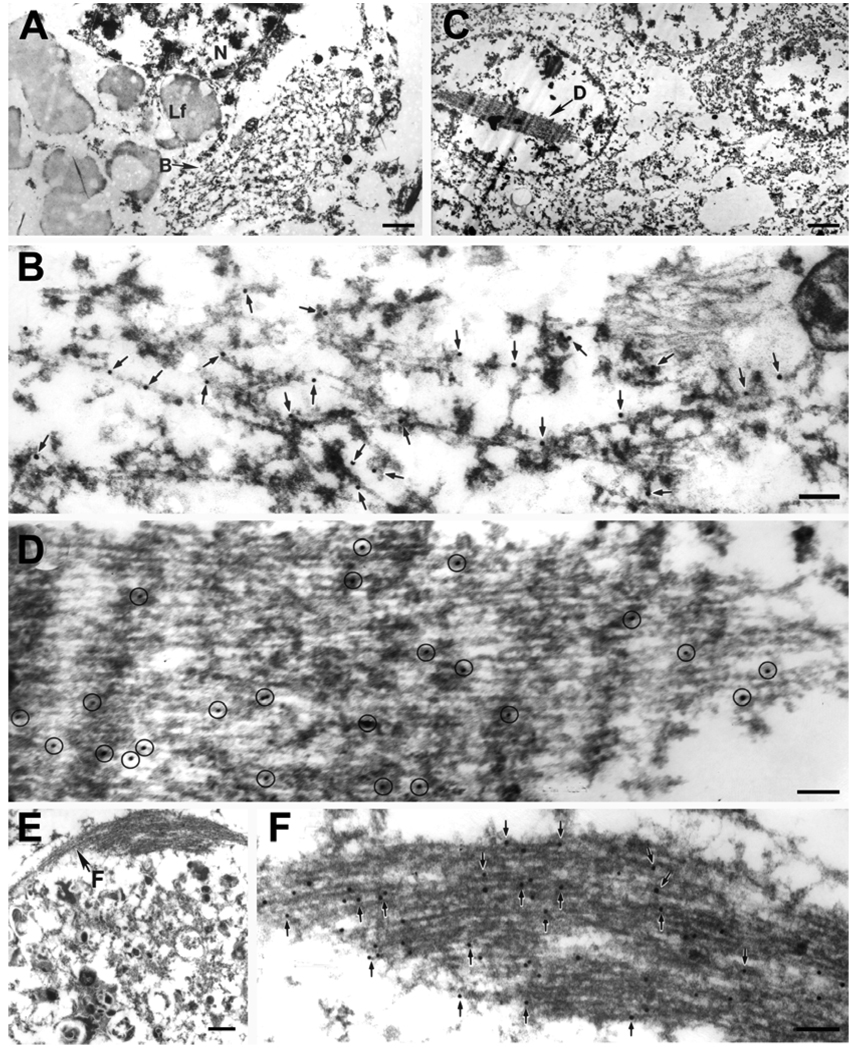
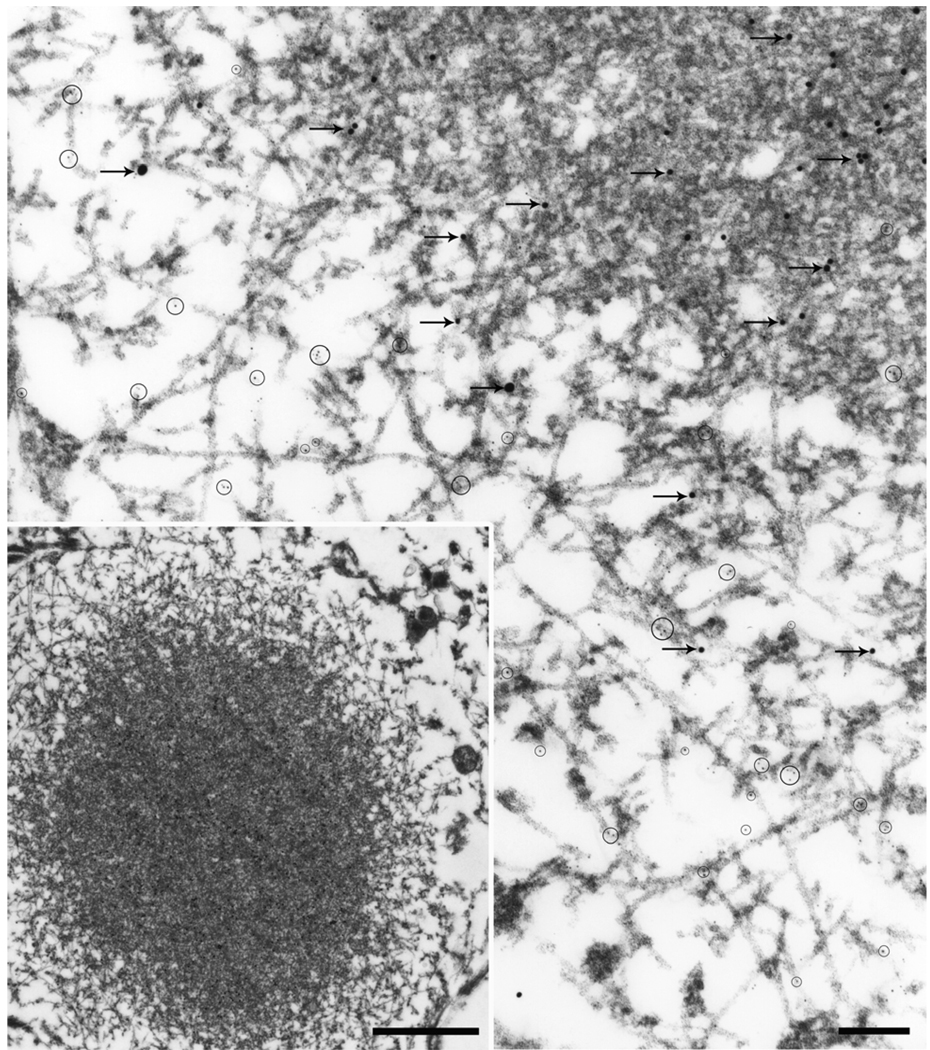
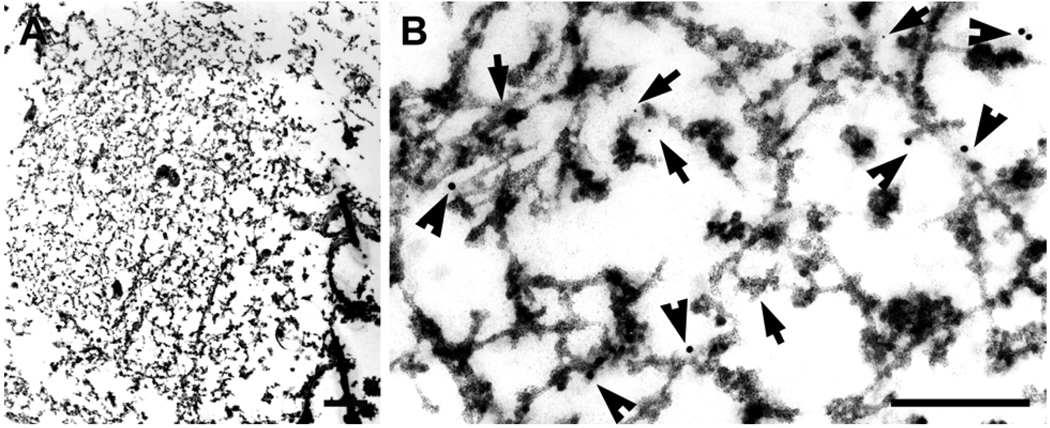
Fig. 1.
In FTLD-U a NCI (A, B), a lentiform NII in the dentate gyrus (C, D) and a compact inclusion in a dystrophic neurite (E, F) are illustrated. The NCI is a filamentous aggregate that displaces other cytoplasmic organelles. At higher magnification (B), TDP-43 immunogold particles decorate filaments and electron dense granular material (arrows). The NII (C) is composed of tightly packed filaments, with TDP-43 immunogold particles (circled) decorating the filaments at higher magnification (D). The dystrophic neurite (E) has degenerated organelles and dense granular material (bottom portion of image) and a bundle of filaments (F) that at higher magnification has TDP-43 immunogold particles (arrows). Lf, lipofuscin; N, nucleus. Bars, 1 µm in A; 1.5 µm in C; 0.5 µm in E; 0.15 µm in B, D, F.
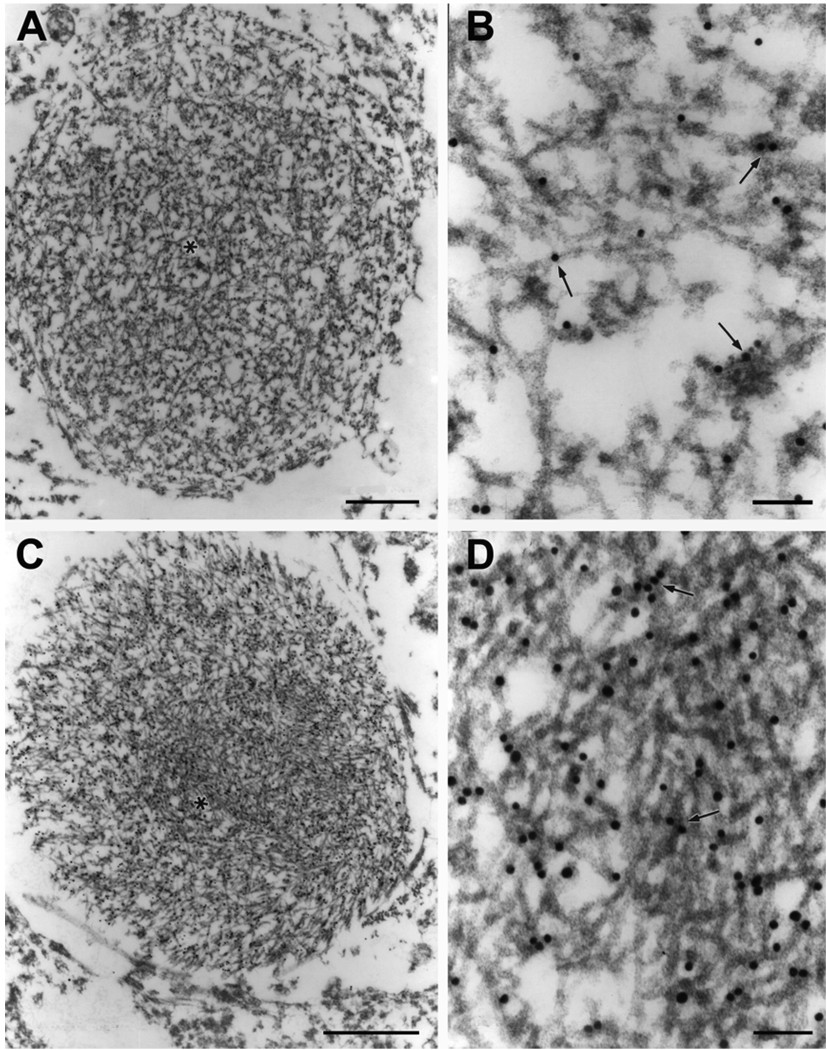
Fig. 2.
Round neuritic inclusions containing chaotic filaments with (A) or without (C) associated dense granular material; both are decorated with gold particles (arrows). Polyclonal antibody to TDP-43. *, enlarged areas in B and D. Bars, 1 µm in A and C; 0.1 µm in B and D.
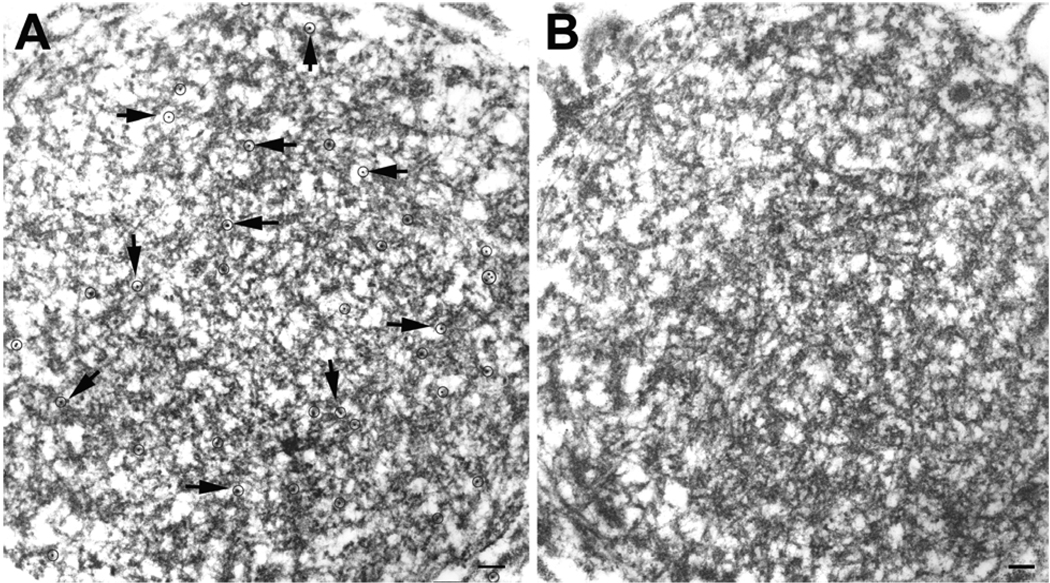
Fig. 3.
Semi-serial sections of a round neuritic inclusion are labeled by monoclonal antibody to TDP-43 (A), but not by an antibody to phosphorylated neurofilaments (B). Gold particles in (A) are circled and indicated with arrows. Bars, 0.15 µm.
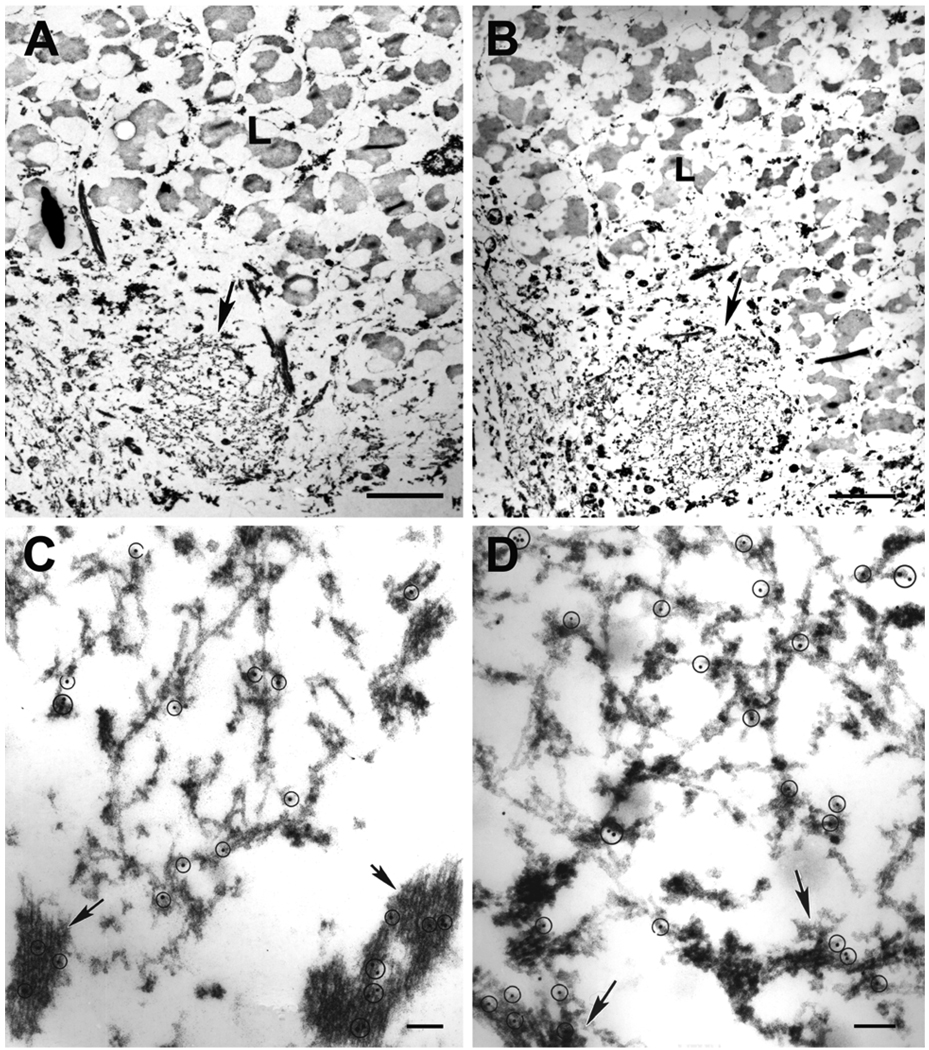
The findings were similar to those reported previously in studies using ubiquitin for immunoelectron microscopy [14]; however, several novel findings were also made. First, some neuritic inclusions had an electron dense amorphous core and a rim with filaments that were more heavily labeled for TDP-43 (Fig. 4). In some cases the rim appeared to have two zones of loose filaments. Those adjacent to the core were free of associated material, while those in the outermost rim were coated with granular material. The same filament could sometimes be traced from the region without electron dense coating to the periphery where most of the filaments had electron dense granular coating. Double labeling with antibodies to ubiquitin and TDP-43 showed that ubiquitin was localized mainly to the dense amorphous core, while TDP-43 was present in the core and the filamentous rim (Fig. 5).
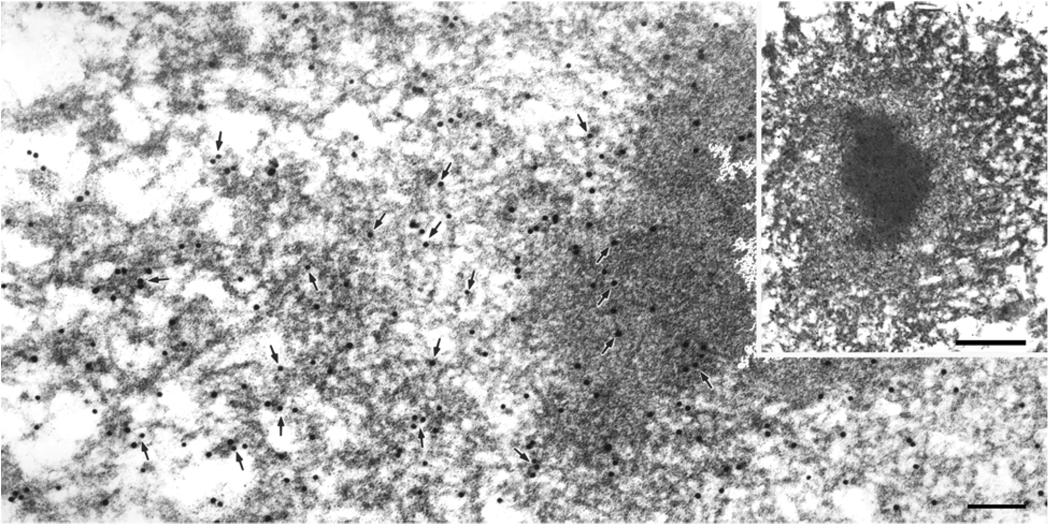
Fig. 4.
A neuritic inclusion with a dense core and pale rim (inset) has filaments that are heavily labeled with polyclonal antibody to TDP-43. Arrows point to gold particles. The rim is made of disorganized loose filaments. Note filaments in the zone adjacent to the core appear to be free of associated material and are contiguous with filaments in the core and with filaments in the outermost zone, where they become coated with granular material. Bars, 0.15 µm; 1 µm in inset.
Fig. 5.
Double immunostaining for ubiquitin (20-nm gold; arrows) and TDP-43 (5-nm gold; circles) of a Lewy-like inclusion in FTLD-U (inset) at higher magnification shows dense central region with heavy ubiquitin immunolabeling and sparse TDP-43 surrounded by a loose filamentous zone with more TDP-43 than ubiquitin. Bars, 0.3 µm; inset 1.0 µm
ALS
NCI in spinal cord motor neurons showed similar ultrastructure and immunolabeling features to the TDP-43-immunoreactive NCI in FTLD-U (Fig. 6). The inclusions contained coated filaments in loose or densely packed arrangements. The inclusions were not labeled by antibodies to phosphorylated neurofilaments or α-synuclein (data not shown).
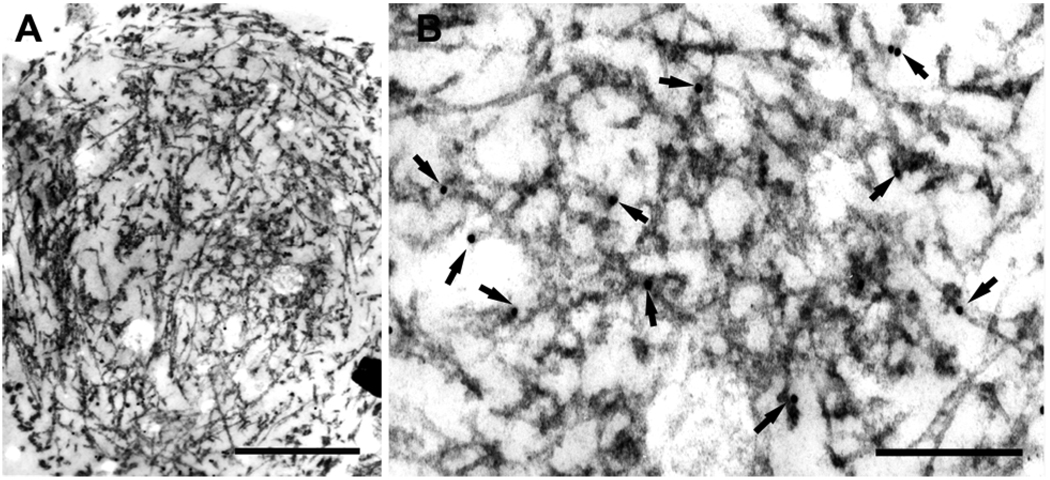
Fig. 6.
Semi-serial sections of a spinal cord motor neuron in ALS, immunostained for ubiquitin (A, C) and TDP-43 (B, D). Upper panels show the same cytoplasmic filamentous inclusion (arrows). L, lipofuscin. Bars, 3 µm. Each inclusion is shown at higher magnification in C and D. Note similar localization of ubiquitin and TDP-43 to granule-coated filaments, either loosely arranged or in tightly packed bundles (arrows). Gold particles in circles. Bars, 0.15 µm.
AD
In a previous study, we showed that TDP-43 immunoreactive inclusions in AD included both NCI and NII [1]. The filaments in both NII and NCI were 10–17 nm diameter straight filaments with coating by electron dense granular material. In some cases the granule-coated filaments were present in neurons that also had NFTs that contained tau filaments with distinct immunoreactivity and fine structural profiles. In the present study we show that in AD TDP-43 immunoreactivity can also be detected in unmyelinated neurites as aggregates of uncoated filaments (Fig. 7).
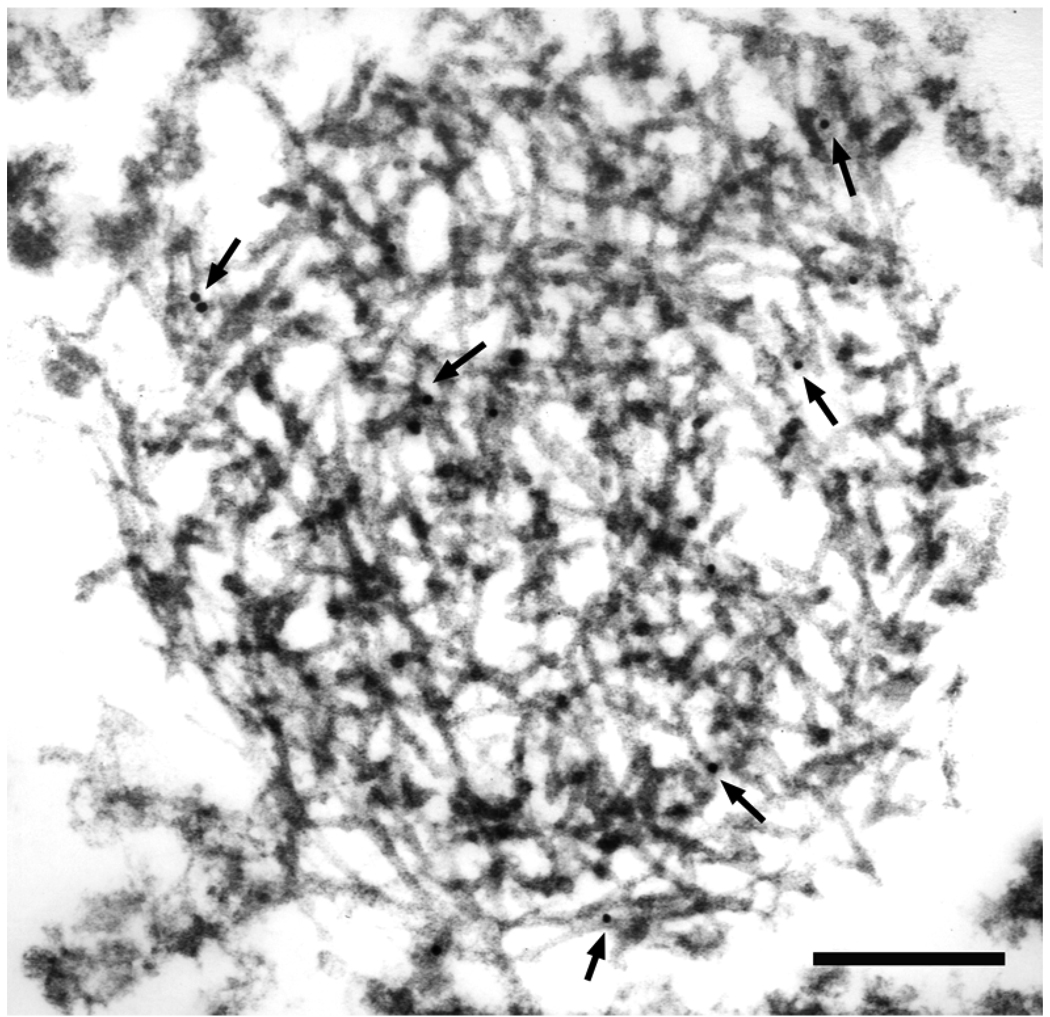
Fig. 7.
A filamentous inclusion in parahippocampal gyrus of AD is labeled with monoclonal antibody to TDP-43 (arrows point to gold particles). The majority of filaments are uncoated, with a diameter of 13–14 nm. Bar, 0.3 µm.
PiD
Many Pick bodies were present in the cytoplasm of granule cells in dentate fascia of the hippocampus. They were composed of circumscribed aggregates of tau filaments with admixture of cytoplasmic organelles and electron dense granule material. Both polyclonal and monoclonal antibodies to TDP-43 showed immunoreactivity with filaments, some that were coated with granular material, in Pick bodies (Fig. 8).
Fig. 8.
Pick body in a hippocampal dentate granule neuron (A) (higher magnification in B) is doubly labeled with TDP-43 (small gold particles, arrows) and tau (large gold particles, arrowheads). TDP-43 appears to be associated with filaments. Bars, 1 µm in A, 0.3 µm in B.
LBD
At the light microscopic level TDP-43 immunoreactivity was present in NCI and neurites (data not shown). Immunoelectron microscopy with double immunolabeling for TDP-43 and α-synuclein showed their colocalization in neuronal cytoplasm (Fig. 9) and unmyelinated neurites (Fig. 10). Both antibodies were localized to filaments and associated dense material.
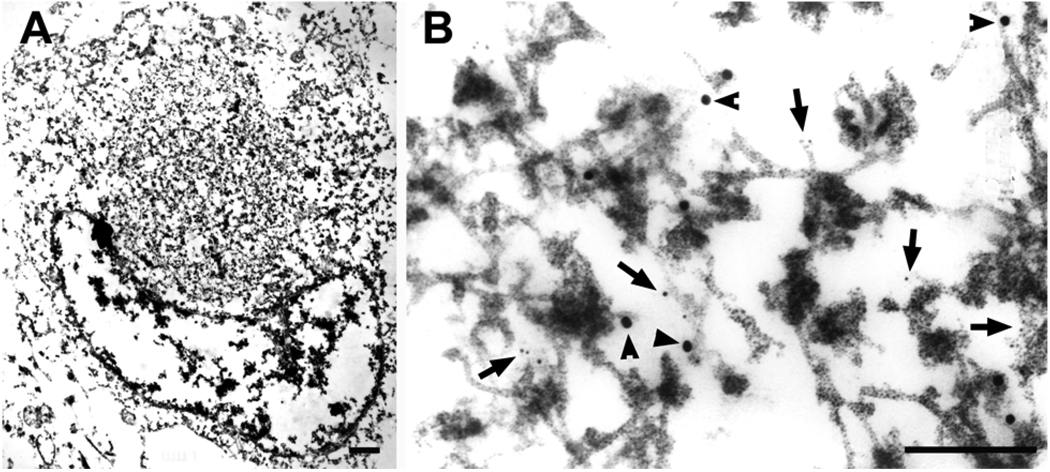
Fig. 9.
A Lewy body in the amygdala (A) (higher magnification in B) is doubly labeled with TDP-43 (large gold particles, arrowheads) and α-synuclein (LB509, small gold particles, arrows). Both antibodies are localized to filaments and dense granular material. Bars: left panel, 1 µm; right panel, 0.3 µm.
Fig. 10.
An unmyelinated neurite in parahippocampal gyrus of LBD (A) (higher magnification in B) shows labeling of TDP-43 (arrows point to gold particles) on filaments and associated dense material. Bar: left panel, 0.6 µm; right panel, 0.3 µm.
Discussion
The high resolution provided by post-embedding immunogold electron microscopy provided evidence that TDP-43 immunoreactive structures in neuronal inclusions in a range of neurodegenerative disorders are filamentous. The TDP-43 immunoreactive structures fell into three major types. The most common type was characterized by filaments with associated granular material. Less often filaments were detected without granular material. Finally, in the setting of other neurodegenerative diseases, TDP-43 immunoreactivity was present in granular material associated with other distinct types of filament, such as tau or α-synuclein filaments. The NII in FTLD-U and AD and the NCI in FTLD-U and ALS were most often composed of granule-coated filaments. In general, NII tended to have more densely packed filaments than NCI. The TDP-43-immunoreactive neurites in FTLD-U and AD were most often composed of granule-coated filaments, but uncoated filaments were occasionally found in unmyelinated neurites. TDP-43 immunoreactivity was associated with granular material and not clearly associated with filaments other than those recognized by tau antibodies in neurofibrillary tangles of AD and Pick bodies of PiD, and as α-synuclein filaments in Lewy bodies and neurites of LBD.
The morphology of the filaments in NCI in FTLD-U and ALS were similar to those reported previously using ubiquitin Immunoelectron microscopy in these disorders [12; 14]. These studies showed ubiquitin immunoreactivity in filamentous inclusions. Since there is no evidence that ubiquitin can form filaments, it is most likely that ubiquitin is conjugated to the protein that makes up the filaments. The majority of inclusions evaluated in the present study in nuclei (NII) and neuronal cytoplasm (NCI) were filamentous structures, and some had associated electron dense granular material. Inclusions composed exclusively of amorphous granular material were not detected. These results contrast with a recent Immunoelectron microscopy study of a single case of FTLD-U, which reported that granular material was the major constituent of TDP-43 immunoreactive inclusions [3]. The discrepancy between the present findings and that report may be technical. The previous study used a peroxidase-based pre-embedding immunoelectron microscopy with diaminobenzidine as the chromogen. This method produces an electron dense deposit that can be difficult to differentiate from the granular material that is present in the inclusions both within the cytoplasm and coating the filaments. It is possible that chromogen might have obscured the fine structure of the filaments, especially for the granule-coated filaments. It is noteworthy that in a previous study using similar methods, Kinoshita and coworkers [14] mentioned that they could not precisely characterize the nature of ubiquitin immunoreactive structures because they were obscured by electron dense deposits of chromogen. In support of this notion is the fact that a recent immunogold electron microscopic study also showed TDP-43 immunoreactivity in filaments in a case of FTLD-U [10].
The presence of TDP-43 immunoreactivity associated with uncoated filaments, either throughout their length or in short segments, in a subset of inclusions in FTLD-U, AD and LBD argues for TDP-43 being a major constituent of the filaments. The variability of granule coating of TDP-43 immunoreactive filaments as they pass from the central dense region to the periphery of a subset of the inclusions in both FTLD-U and LBD is further support for the notion that TDP-43 is a component of the filaments. Moreover, double immunostaining and single immunostaining of adjacent sections failed to localize tau, α-synuclein or neurofilaments in these inclusions.
The first in vitro studies demonstrating the ability of TDP-43 to form filaments recently has been reported by Hasegawa et al. with phosphorylated recombinant TDP-43[10]. In support of these in vitro studies and our immunoelectron microscopic findings in human brain disorders, is the fact that solubility changes in TDP-43 in FTLD-U are analogous to changes in solubility of other proteins that form pathologic filaments [18]. Accumulation of abnormal forms of proteins into filamentous lesions is a feature of neurodegenerative disorders. For example, abnormal forms of tau protein form filaments in neurofibrillary tangles and dystrophic neurites of AD and in Pick bodies of PiD, while α-synuclein forms filaments in LBD. In some disorders (e.g., AD and LBD), inclusions may contain a mixture of abnormal filaments [13]. More studies are needed to determine the cause and mechanism of TDP-43 fibril formation in the nucleus and cytoplasm. A recent in vitro study suggests that proteolytic cleavage of TDP-43 in FTLD-U may be mediated by caspases [22]. It remains to be determined whether filamentous aggregates are composed of full-length or fragments or a mixture of full-length and fragments of TDP-43 and the importance of phosphorylation.
In this study all the inclusions detected in neuronal cell processes, regardless of the disorder, were not myelinated. Although it is not possible to exclude that these are distal axons, it is more likely that they are dendritic. In none of the cases studied was TDP-43 immunoreactivity ever detected in myelinated axons and our unpublished observations of FTLD using double labeling for phosphorylated neurofilaments and ubiquitin did not show ubiquitin labeling in the axoplasm of myelinated axons [14]. The TDP-43 immunoreactive structures were unlikely to be in astrocytic processes since they were not positive for glial fibrillary acidic protein (not shown). A dendritic localization of TDP-43 is of interest given recent findings of Wang and coworkers [21] who showed that in rat hippocampal slices TDP-43 was localized in dendritic processing bodies (P-bodies) and that repetitive stimuli increased its co-localization with two RNA-binding proteins known to regulate mRNA transport and local translation in neurons, suggesting that TDP-43 may have normal functions in the cytoplasm of dendritic processes. If cleavage of TDP-43 is involved in transformation to a form that more readily aggregates, evidence for local activation of caspases in this compartment may be relevant. [7]
In summary, using post-embedding immunogold EM we show that TDP-43 immunoreactivity is present in filamentous aggregates often, but not always, associated with electron dense granular material. TDP-43 can be found in isolation in FTLD-U and ALS, but also associated with distinctly different types of abnormal filaments composed of tau in AD and PiD or α-synuclein in LBD. The mechanism of TDP-43 filament formation and its relation to dendritic pathology remains to be studied.
Acknowledgments
Supported by NIH grants P50-AG25711, P50-AG16574, P50-NS40256, P01-AG17216 and P01-AG03949
REFERENCES
- 1.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 3.Cairns NJ, Newmann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White ICL, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM-Y, Mackenzie IRA. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickson DW, Wertkin A, Mattiace LA, Fier E, Kress Y, Davies P, Yen SH. Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer's disease. Acta Neuropathol (Berl) 1990;79:486–493. doi: 10.1007/BF00296107. [DOI] [PubMed] [Google Scholar]
- 5.Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP. TDP-DNA binding protein 43 in Pick Disease. J Neuropathol Exp Neurol. 2008;67:62–67. doi: 10.1097/nen.0b013e3181609361. [DOI] [PubMed] [Google Scholar]
- 6.Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VM-Y, Trojanowski JQ. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol (Berl) 2008;115:133–145. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- 7.Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growthcone motility? Neuromolecular Med. 2002;2:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- 8.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol (Berl) 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008 doi: 10.1002/ana.21425. 10.1002/ana.21425 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of TDP-43, tau and α-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 12.Iseki E, Li F, Odawara T, Hino H, Suzuki K, Kosaka K, Akiyama H, Ikeda K, Kato M. Ubiquitin-immunohistochemical investigation of atypical Pick’s disease without Pick bodies. J Neurol Sci. 1998;159:194–201. doi: 10.1016/s0022-510x(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 13.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita A, Tomimoto H, Suenaga T, Akiguchi I, Kimura J. Ubiquitin-related cytoskeletal abnormality in frontotemporal dementia: immunohistochemical and immunoelectron microscope studies. Acta Neuropathol (Berl) 1997;94:67–72. doi: 10.1007/s004010050673. [DOI] [PubMed] [Google Scholar]
- 15.Kuzuhara S. TDP-43 accumulation in ALS/parkinsonism-dementia complex (ALS/PDC) of the Kii peninsula of Japan [abstr] Neuropathology. 2007;27:61. [Google Scholar]
- 16.Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW. Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol. 2003;162:213–218. doi: 10.1016/S0002-9440(10)63812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig HI, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM-Y, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related disease. Acta Neuropathol (Berl) 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 18.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S. Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol. 1992;239:426–430. doi: 10.1007/BF00856806. [DOI] [PubMed] [Google Scholar]
- 20.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 21.Wang I-F, Wu L-S, Chang H-Y, Shen C-K. TDP-43, the signature protein of FTLD-U is a neuronal activity-responsive factor. J Neurochem. 2008;105:797–806. doi: 10.1111/j.1471-4159.2007.05190.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y-J, Xu Y-F, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson DW, Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]