Abstract
We have attempted to quantify the most up-to-date estimate of the association between cigarette smoking by the mother and preterm delivery. Studies were selected for inclusion in this review if they were prospective, reported data stratified across at least two levels of maternal smoking, and defined preterm delivery on the basis of gestational age. In a meta-analysis we combined results from multiple studies that reported on preterm delivery and maternal smoking during pregnancy. Pooled odds ratios were computed for various strata of smoking intensity with the Mantel-Haenszel fixed-effects model. Twenty studies met all inclusion criteria and were included in meta-analysis. The pooled point estimate from 20 prospective studies on any maternal smoking versus no maternal smoking was 1.27 (95% confidence interval, 1.21-1.33). Subgroup analyses stratifying maternal smoking on number of cigarettes per day suggest a dose-response relationship at low to moderate levels of smoking, which was not further increased at high levels of smoking. A nonsignificant level of publication bias appears to exist in the smoking-preterm delivery literature. Cigarette smoking is a preventable risk factor that is associated with preterm delivery. Consistent results across many study populations and research designs and evidence of a dose-response relationship support its causal role in preterm delivery.
Keywords: Preterm delivery, cigarette smoking, meta-analysis
Preterm delivery is the single most important contributor to infant mortality rates in technically developed countries,1 where rates of preterm delivery have increased over the last 20 years. In part, this observed trend may be attributed to increasing numbers of multiple births, more frequent use of ultrasonography to determine gestational age, and a growing tendency to register live births at very early gestational ages.2 However, maternal smoking is a potentially preventable cause for preterm delivery. Furthermore, a dose-response effect has been observed in single studies, in which mothers who smoke greater amounts during pregnancy have progressively higher rates of preterm deliveries. This study summarizes the currently available data on the association between smoking and preterm delivery and on a dose response of the association, by reporting pooled estimates for odds ratios in association with preterm delivery and smoking.
Material and methods
Studies and review articles relating preterm deliveries and maternal smoking were identified with MEDLINE (1966-1997) and by searches of references cited in identified articles. MEDLINE was searched with the subject headings premature labor and smoking, as well as the text words preterm delivery and preterm birth, which resulted in the identification of 104 citations. Any articles containing original data were retrieved in their entirety, with study type ascertained when the full text of the article was reviewed. Studies were limited to the English language. Although a few articles were identified in Eastern European literature by MEDLINE, they were mostly case reports or case-control studies and did not fulfill our inclusion criteria. Eight retrospective, case-control studies were also identified through this search and were not selected for analysis but are discussed in the Results section.
Studies selected for analysis had to meet the following requirements: (1) The study was prospective (ie, smoking exposure was ascertained before perinatal outcome); (2) the study reported data on rates of preterm delivery stratified across at least two levels of maternal smoking, or the study included a summary estimate (odds ratio or relative risk) with reported confidence intervals; (3) the study defined preterm delivery according to gestational age. The existence of publication bias was assessed among the selected studies by means of a funnel plot3 of individual study odds ratios plotted against study size.
The methods of Mantel and Haenszel4 were used to estimate effect size and confidence intervals. Calculations were conducted with Microsoft Excel5 software by methods described by Fleiss.6 Individual estimates from studies were pooled as odds ratios; odds ratio estimates were derived from the data for those studies that reported relative risks. The odds ratio is comparable to the risk ratio when the baseline risk is low (ie, when the event rate in the control group is <10%, as was the case in almost all of the identified studies).7 Whenever possible, adjusted odds ratios were used to provide more accurate estimates of the true underlying relation between smoking and preterm delivery. Most studies adjusted for maternal age, race, gravidity, parity, income, and other social and demographic factors.
Results
A total of 64 published articles were identified that reported original data on the association between maternal smoking and preterm delivery. Twenty studies in 21 reports8-28 met all the inclusion criteria and were included for analysis (see Table I). Four studies reported outcomes only in the form of mean gestational age changes for children born to smokers versus nonsmokers. Whereas these data support the association between maternal smoking and preterm delivery, in none of these reports were sufficient data presented to provide results that could be pooled with data from the other 20 studies. As a result, these 4 studies are not included in the analysis. A total of 43 studies were excluded from analysis, most because they were not prospective studies (references available from the authors on request).
Table I.
Prospective studies on smoking and preterm delivery
| Series | Year | Study |
PTD definition (weeks’ gestation) |
Exposure criteria | Unexposed criteria |
Exposed (PTD per total) |
Unexposed (PTD per total) |
Odds ratio |
95% Confidence interval |
Rate of preterm delivery (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin8 | 1991 | Sweden | <37 | Smoked before first prenatal check | None | 91/1291 | 86/2281 | 1.94 | 1.43-2.62 | 5 |
| Arbuckle and Sherman9 | 1989 | Nutrition Canada | <37 | Smoked during pregnancy | Never smoked | 31/308 | 28/373 | 1.38 | 0.81-2.35 | 9 |
| Donovan10 | 1977 | London, United Kingdom | <36 | Smoked at time of questionnaire | No smoking in prior year | 18/784 | 3/243 | 1.88 | 0.55-6.44 | 2 |
| Doucette and Bracken11 | 1993 | Yale University, New Haven, Conn | <37 | > 0 cigarettes/d in month 2 | 0 cigarettes/d in month 2 | 75/1076 | 122/2630 | 1.54 | 1.14-2.07 | 5 |
| Frazier et al12 | 1961 | Baltimore, Md | <38 | Smoked every day | Occasional and nonsmokers | 313/866 | 465/1491 | 1.25 | 1.05-1.49 | 33 |
| Henriksen et al13 | 1995 | Aarhus, Denmark | <37 | Smoked during pregnancy | Nonsmokers during pregnancy | 54/1180 | 72/2426 | 1.57 | 1.09-2.25 | 3 |
| Kramer et al14* | 1992 | Montreal, Quebec, Canada | <37 | Any smoking during pregnancy | None on average during pregnancy | — | — | 1.30 | 1.10-1.60 | — |
| Naeye15 | 1982 | CPP of NINCDS | <37 | Smokers | Nonsmokers | 127/635 | 116/635 | 1.12 | 0.85-1.48 | 19 |
| Nordentoft et al16* | 1996 | Copenhagen, Denmark | >25, <37 | 0-9 cigarettes/d at 20 wk | Nonsmoker at 20th wk | — | — | 1.30 | 0.88-1.93 | — |
| Obel17 | 1979 | Copenhagen, Denmark | <37 | Smoking at 28 wk | Not smoking at 28 wk | 87/853 | 84/1424 | 1.81 | 1.33-2.48 | 8 |
| Peacock et al18 | 1995 | London, United Kingdom | >32, <37 | Any cigarettes/d at booking, 28th wk | Nonsmoker | 28/409 | 26/309 | 0.80 | 0.46-1.39 | 8 |
| Rush and Kass19 | 1972 | Boston City Hospital | <37 | ≥1 cigarette/d | <1 cigarette/d | 113/531 | 104/441 | 0.88 | 0.65-1.19 | 22 |
| Russell et al20 | 1968 | Sheffield, United Kingdom | <38 | ≥5 cigarettes/d | None with <5 cigarettes/d | 126/639 | 189/1426 | 1.61 | 1.25-2.06 | 15 |
| Shiono et al21* | 1986 | California | >24, <37 | ≥1 pack/d during pregnancy | None during pregnancy | — | — | 1.20 | 1.10-1.40 | — |
| Siega-Riz et al22 | 1996 | West Los Angeles | <37 | Smoking | Not smoking | 28/295 | 483/7244 | 1.47 | 0.98-2.19 | 7 |
| Stein et al23 | 1987 | Oxford, United Kingdom | <37 | Smoking at 6 mo | Not smoking at 6 mo | 7/122 | 7/348 | 2.97 | 1.02-8.63 | 3 |
| van den Berg and Oechsli25 | 1984 | CHDS, Kaiser | <37 | Stopped because of pregnancy | Never smoked | 338/4547 | 195/4249 | 1.67 | 1.39-2.00 | 6 |
| Wen et al26 | 1990 | Alabama | <37 | Smoked at first prenatal visit | Not smoking | 671/5042 | 1477/12107 | 1.10 | 1.00-1.22 | 13 |
| Wisborg et al27 | 1996 | Aarhus Denmark | <37 | Smoked at 16 wk | Not smoking | 75/1404 | 103/2707 | 1.43 | 1.05-1.94 | 4 |
| Yerushalmy28 | 1964 | California | <37 | Any smoking during pregnancy | None | 218/2584 | 223/3010 | 1.15 | 0.95-1.40 | 8 |
| Combined† | — | — | — | — | — | — | — | 1.27 | 1.21-1.33 | — |
PTD, Preterm delivery; CPP, Collaborative Perinatal Project; NINCDS, National Institute of Neurological and Communicative Disorders and Stroke; CHDS, Child Health and Development Studies.
Individual cells not presented. Only odds ratios were given; hence preterm delivery rate was not calculable.
Mantel-Haenszel fixed-effects model.
The overall association between cigarette smoking and preterm delivery is reasonably well described by cohort studies. However, most did not identify the duration of exposure, with the authors choosing simply to dichotomize duration of cigarette use into any maternal smoking during pregnancy versus none. The level or intensity of smoking, however, was better characterized. Most cohorts were split into two or three levels of exposure (for example, groups in which mothers actively smoked 0-9, 10-19, or ≥20 cigarettes per day). Only 1 study8 looked at exposure to passive smoking, and so passive smoking exposure is not included in this review.
Only 2 of 20 studies showed a protective, but statistically nonsignificant, association between smoking and preterm delivery.18, 19 In the first,18 preterm delivery was defined as >32 weeks but <37 weeks of gestation; hence a large portion of the very early preterm deliveries are missing, where the observed association is likely to be strongest. For <32 weeks’ gestation, the same study found a relative risk of 1.95 (95% confidence interval, 1.30-2.93) for smoking and preterm delivery, pointing to the masking of smoking’s effect by narrowly defining preterm delivery. The data from the group with delivery at <32 weeks’ gestation were not reported in a manner that could be combined with the group with delivery at >32 weeks’ gestation; hence for this review the most conservative data (which showed a protective association) were used. The other article19 did not have a purely nonsmoking cohort for comparison; instead they used as controls women who on average smoked <1 cigarette per day (including nonsmokers). This study is similar to 2 other studies,12, 14 which, nevertheless, reported an increased odds of preterm delivery for mothers who smoked. Exposure misclassification of this sort would bring estimates toward the null (ie, decrease the association between smoking and preterm delivery).
Table I presents data on any maternal smoking during pregnancy versus no maternal smoking. When multiple data were present in the original report, efforts were made to dichotomize results into any maternal smoking versus no maternal smoking before inclusion in this analysis. The pooled estimate with 95% confidence interval for any smoking versus no smoking and preterm delivery is 1.27 (1.21-1.33). A sensitivity analysis that excluded the 2 studies with the largest and the 2 studies with the smallest point estimates resulted in no significant changes to the pooled estimate (data not shown).
Tables IIA and IIB present maternal smoking data stratified into light to moderate smoking (<20 cigarettes per day) and heavy smoking. When data were not available for 0 to 20 cigarettes per day, alternate levels (eg, 0-9, 1-14 cigarettes per day) were used. Similarly, the heavy exposure pooled estimate used ≥11, ≥15, or ≥16 cigarettes per day when other data were not available. It was thought that 20 cigarettes per day, representing 1 pack per day, was a sensible cut point for exposure. The pooled estimate with 95% confidence interval is 1.22 (1.13-1.32) for light to moderate smoking and 1.31 (1.20-1.42) for heavy smoking.
Table IIA.
Prospective studies on smoking and preterm delivery by level of smoking: Light to moderate smoking during pregnancy
| Series | Year | Exposure criteria | Unexposed criteria | Exposed (PTD/total) | Unexposed (PTD/total) | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin8 | 1991 | <20 cigarettes/d | No exposure | 80/1159 | 86/2281 | 1.89 | 1.38-2.59 |
| Doucette and Bracken11 | 1993 | <20 cigarettes/d in month 2 | 0 cigarettes/d in month 2 | 68/984 | 122/2630 | 1.53 | 1.12-2.07 |
| Kramer et al14 | 1992 | 1-10 cigarettes/d | 0 cigarettes/d on average during pregnancy | — | — | 1.18 | 1.03-1.36 |
| Nordentoft et al16 | 1996 | 0-9 cigarettes/d at 20 wk | Nonsmoker at 20 wk | — | — | 1.30 | 0.88-1.93 |
| Peacock et al18 | 1995 | 1-14 cigarettes/d at booking, 28th wk | Nonsmoker | 21/289 | 26/309 | 0.85 | 0.47-1.55 |
| Shiono et al21 | 1986 | <1 pack/d during pregnancy | None during pregnancy | — | — | 1.10 | 0.90-1.20 |
| van den Berg and Oechsli25 | 1977 | Smokes <15 cigarettes/d | Never smoked | 93/1600 | 195/4249 | 1.28 | 1.00-1.65 |
| Wisborg et al26 | 1996 | 1-10 cigarettes/d at 16 wk | Nonsmoker | 46/932 | 86/2339 | 1.36 | 0.94-1.96 |
| Yerushalmy28 | 1964 | 1-10 cigarettes/d at interview | None during pregnancy | 90/1059 | 223/3010 | 1.16 | 0.90-1.50 |
| Combined* | — | — | — | — | — | 1.22 | 1.13-1.32 |
PTD, Preterm delivery.
Mantel-Haenszel fixed-effects model.
Table IIB.
Prospective studies on smoking and preterm delivery by level of smoking: Heavy smoking during pregnancy
| Series | Year | Exposure criteria | Unexposed criteria | Exposed (PTD/total) | Unexposed (PTD/total) | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin8 | 1991 | Active; ≥20 cigarettes/d | No exposure | 11/132 | 86/2281 | 2.32 | 1.21-4.46 |
| Doucette and Bracken11 | 1993 | ≥21 cigarettes/d in month | 0 cigarettes/d in month 2 | 7/92 | 122/2630 | 1.69 | 0.77-3.74 |
| Kramer et al14 | 1992 | ≥11 cigarettes/d | 0 cigarettes/d on average during pregnancy | — | — | 1.27 | 1.08-1.50 |
| Nordentoft et al16 | 1996 | ≥16 cigarettes/d at 20 wk | Nonsmoker at 20 wk | — | — | 0.95 | 0.41-2.23 |
| Peacock et al18 | 1995 | ≥15 cigarettes/d at booking, 28th wk | Nonsmoker | 7/120 | 26/309 | 0.67 | 0.28-1.60 |
| Shiono et al21 | 1986 | ≥1 pack/d during pregnancy | None during pregnancy | — | — | 1.20 | 1.10-1.40 |
| van den Berg and Oechsli25 | 1977 | ≥15 cigarettes/d | Never smoked | 152/2077 | 195/4249 | 1.64 | 1.32-2.04 |
| Wisborg et al27 | 1996 | ≥11 cigarettes/d at 16 wk | Nonsmoker | 16/193 | 86/2339 | 2.37 | 1.36-4.13 |
| Yerushalmy28 | 1964 | ≥21 cigarettes/d at interview | None during pregnancy | 25/267 | 223/3010 | 1.29 | 0.84-1.99 |
| Combined* | — | — | — | — | — | 1.31 | 1.20-1.42 |
PTD, Preterm delivery.
Mantel-Haenszel fixed-effects model.
Separating studies into two levels of smoking showed a trend toward a dose-response relationship between cigarette smoking during pregnancy and the incidence of preterm delivery. This trend was less apparent when studies were divided into 3 groups on the basis of predefined levels of smoking (see Tables IIIA, IIIB, and IIIC). For light smoking, defined as 0 to 10 cigarettes per day, the pooled estimate with 95% confidence interval was 1.25 (1.12-1.38). Moderate smoking, approximately 11 to 20 cigarettes per day, resulted in a pooled estimate of 1.38 (1.23-1.55). Heavy smoking, generally more than a pack per day, had a pooled estimate of 1.31 (1.19-1.45).
Table IIIA.
Prospective studies on smoking and preterm delivery by level of smoking: Light smoking during pregnancy
| Series | Year | Exposure criteria | Unexposed criteria | Exposed (PTD/total) | Unexposed (PTD/total) | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin8 | 1991 | 1-9 cigarettes/d | No exposure | 24/432 | 86/2281 | 1.50 | 0.94-2.39 |
| Doucette and Bracken11 | 1993 | 1-10 cigarettes/d in month 2 | 0 cigarettes/d in month 2 | 46/635 | 122/2630 | 1.61 | 1.13-2.28 |
| Kramer et al14 | 1992 | 1-10 cigarettes/d | 0 cigarettes/d on average during pregnancy | — | — | 1.18 | 1.03-1.36 |
| Nordentoft et al16 | 1996 | 0-9 cigarettes/day at 20 wk | Nonsmoker at 20 wk | — | — | 1.30 | 0.88-1.93 |
| Wisborg et al27 | 1996 | 1-10 cigarettes/d at 16 wk | Nonsmoker | 46/932 | 86/2339 | 1.36 | 0.94-1.96 |
| Yerushalmy28 | 1964 | 1-10 cigarettes/d at interview | None during pregnancy | 90/1059 | 223/3010 | 1.16 | 0.90-1.50 |
| Combined* | — | — | — | — | — | 1.25 | 1.12-1.38 |
PTD, Preterm delivery.
Mantel-Haenszel fixed-effects model.
Table IIIB.
Prospective studies on smoking and preterm delivery by level of smoking: Moderate smoking during pregnancy
| Series | Year | Exposure criteria | Unexposed criteria | Exposed (PTD/total) | Unexposed (PTD/total) | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin9 | 1991 | 10-19 cigarettes/d | No exposure | 56/727 | 86/2281 | 2.13 | 1.50-3.02 |
| Doucette and Bracken11 | 1993 | 11-20 cigarettes/d in month 2 | 0 cigarettes/d in month 2 | 22/349 | 122/2630 | 1.38 | 0.87-2.21 |
| Kramer et al14 | 1992 | ≥11 cigarettes/d | 0 cigarettes/d on average during pregnancy | — | — | 1.27 | 1.08-1.50 |
| Nordentoft et al16 | 1996 | 10-15 cigarettes/d at 20 wk | Nonsmoker at 20 wk | — | — | 1.79 | 1.12-2.85 |
| Wisborg et al27 | 1996 | ≥11 cigarettes/d at 16 wk | Nonsmoker | 16/193 | 86/2339 | 2.37 | 1.36-4.13 |
| Yerushalmy28 | 1964 | 11-20 cigarettes/d | None during pregnancy | 103/1258 | 223/3010 | 1.11 | 0.87-1.42 |
| Combined* | — | — | — | — | — | 1.38 | 1.23-1.55 |
PTD, Preterm delivery.
Mantel-Haenszel fixed-effects model.
Table IIIC.
Prospective studies on smoking and preterm delivery by level of smoking: Heavy smoking during pregnancy
| Series | Year | Exposure criteria | Unexposed criteria | Exposed (PTD/total) | Unexposed (PTD/total) | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Ahlborg and Bodin8 | 1991 | Active; ≥20 cigarettes/d | No exposure | 11/132 | 86/2281 | 2.32 | 1.21-4.46 |
| Doucette and Bracken11 | 1993 | >20 cigarettes/d in month 2 | 0 cigarettes/d in month 2 | 7/92 | 122/2630 | 1.69 | 0.77-3.74 |
| Shiono et al21 | 1986 | ≥1 pack/d during pregnancy | None during pregnancy | — | — | 1.20 | 1.10-1.40 |
| van den Berg and Oechsli25 | 1977 | Smokes ≥15 cigarettes/d | Never smoked | 152/2077 | 195/4249 | 1.64 | 1.32-2.04 |
| Yerushalmy28 | 1964 | ≥21 cigarettes/d at interview | None during pregnancy | 25/267 | 223/3010 | 1.29 | 0.84-1.99 |
| Combined* | — | — | — | — | — | 1.31 | 1.19-1.45 |
PTD, Preterm delivery.
Mantel-Haenszel fixed-effects model.
Eight case-control studies on the relationship between preterm delivery and maternal smoking were identified.29-36 Unlike the prospective studies, however; they varied widely in their quality and procedures. For example, one study31 selected controls from deliveries >39 weeks of gestation, to avoid misclassification bias that might occur with incorrect gestational dates for preterm infants, but this artificially inflates the association of smoking with preterm delivery. Another study35 determined gestational age more carefully for cases than for controls, potentially introducing other biases.
Similarly, the definition of exposure to cigarette smoking varied widely from one case-control study to the next (see Table IV). Whereas some looked at smoking at a certain time point during pregnancy, others considered smoking status before pregnancy versus during pregnancy. One study37 looked at exposure to passive cigarette smoking. Only 2 studies stratified women according to the level of smoking29, 30; the others considered smoking a dichotomous variable. No case-control studies showed a protective effect from smoking on preterm delivery. Most studies reported odds ratios in the 2.0 to 3.0 range, with the overall range from 1.0 (no effect) to 4.20. Because of the great heterogeneity between studies (eg, in choice of control group and in exposure definition and the like), the data were not considered suitable for statistical pooling.
Table IV.
Case-control studies of smoking and preterm delivery
| Series | Year | Study | PTD definition (wk) | Exposure criteria | Unexposed criteria | Odds ratio | 95% Confidence interval |
|---|---|---|---|---|---|---|---|
| Berkowitz et al29* | 1982 | Yale | <37 | ≥10 cigarettes/d in 1st trimester | <10 cigarettes/d in 1st trimester | 2.10 | 1.40-3.10 |
| ≥10 cigarettes/d in 2nd trimester | <10 cigarettes/d in 2nd trimester | 2.40 | 1.60-3.60 | ||||
| ≥10 cigarettes/d in 3rd trimester | <10 cigarettes/d in 3rd trimester | 2.50 | 1.60-3.90 | ||||
| 1-9 cigarettes/d in 1st trimester | 0 cigarettes/d in 1st trimester | 1.20 | 0.70-2.20 | ||||
| 10-19 cigarettes/d in 1st trimester | 0 cigarettes/d in 1st trimester | 3.10 | 1.70-5.60 | ||||
| ≥20 cigarettes/d in 1st trimester | 0 cigarettes/d in 1st trimester | 1.60 | 0.90-2.70 | ||||
| 1-9 cigarettes/d in 2nd trimester | 0 cigarettes/d in 2nd trimester | 1.20 | 0.60-2.30 | ||||
| 10-19 cigarettes/d in 2nd trimester | 0 cigarettes/d in 2nd trimester | 2.80 | 1.50-5.10 | ||||
| ≥20 cigarettes/d in 2nd trimester | 0 cigarettes/d in 2nd trimester | 2.20 | 1.30-3.80 | ||||
| 1-9 cigarettes/d in 3rd trimester | 0 cigarettes/d in 3rd trimester | 1.00 | 0.50-1.90 | ||||
| 10-19 cigarettes/d in 3rd trimester | 0 cigarettes/d in 3rd trimester | 2.90 | 1.60-5.40 | ||||
| ≥20 cigarettes/d in 3rd trimester | 0 cigarettes/d in 3rd trimester | 2.20 | 1.10-4.70 | ||||
| de Haas et al30 | 1991 | Brigham and Womens | 20-37 | Yes vs no at 1st prenatal visit | — | 2.00 | — |
| <6 cigarettes/d at 1st prenatal visit vs none | — | 1.30 | — | ||||
| 6-10 cigarettes/d at 1st prenatal visit vs none | — | 2.20 | — | ||||
| ≥11 cigarettes/d at 1st prenatal visit vs none | — | 2.60 | — | ||||
| Ekwo et al31 | 1993 | With preterm rupture of membranes | <37 | Active; before and during pregnancy | — | 4.20 | 1.80-10.00 |
| Active plus passive | Control, ≥39 wk | 2.10 | 1.20-3.50 | ||||
| Active | Control, ≥39 wk | 1.70 | 0.70-4.30 | ||||
| Active plus passive | Control, ≥39 wk | 2.50 | 1.40-4.40 | ||||
| Ferraz et al32 | 1990 | Brazil | <2.5 kg, <37 | Smoking during pregnancy | — | 1.90 | 1.60-2.40 |
| Harger et al33 | 1990 | United States; preterm rupture of membranes cases | 20-36 | Smoked but stopped during pregnancy | Not smoking during pregnancy | 1.58 | 0.77-3.27 |
| 20-36 | Presently smoking during pregnancy | Not smoking during pregnancy | 2.08 | 1.37-3.13 | |||
| Hartikainen-Sorri and Sorri34 | 1989 | All births | <37 | Currently smoking | Nonsmoker | 2.40 | 1.30-4.50 |
| Spontaneous births | <37 | Currently smoking | Nonsmoker | 3.40 | 1.60-7.20 | ||
| Heffner et al35 | 1993 | Brigham and Womens | 20-36 | ≥1 cigarette/d during pregnancy | <500 cigarettes during lifetime and <1 cigarette/d | 1.90 | 1.30-2.70 |
| Williams et al36 | 1992 | Preterm rupture of membranes | <37 | Ever smoked during pregnancy | Never | 2.00 | 1.50-2.70 |
| Non-preterm rupture of membranes | <37 | Ever smoked during pregnancy | Never | 1.70 | 1.30-2.10 |
PTD, Preterm delivery.
Point estimates are for the same women followed from the first trimester through the third trimester; these are not independent observations.
A funnel plot to assess potential publication bias in the prospective studies is presented in Fig 1, which was created with the use of data presented in Table I. Publication bias occurs most often when small studies with “negative” results are less likely to be published. A biased plot would be asymmetric around the pooled estimate, spreading out unevenly to both sides. Among prospective studies of smoking and preterm delivery, there is a right-skewed distribution. This suggests that some studies with point estimates <1.27 (the pooled estimate) may have been conducted but were not published.
Fig 1.
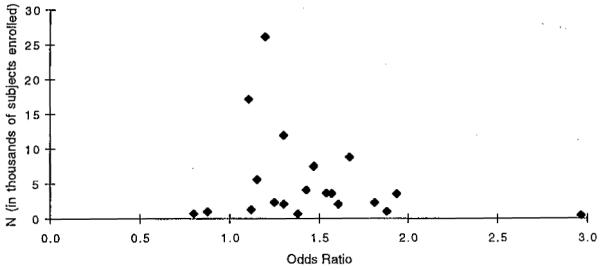
Funnel plot to evaluate publication bias—all prospective studies of any smoking versus none and preterm delivery. This figure was created with data presented in Table I.
Comment
A truncated dose-response trend was observed when studies were separated into 3 groups of low, medium, and heavy smoking. It is possible that heterogeneity in design and execution of studies masked a dose-response relationship that, whereas it was apparent within individual studies, was less evident in the pooled groups. The heaviest smokers had an association between smoking and preterm delivery that was essentially the same as that for moderate smokers. An alternate explanation lies in the nature of the exposure; there are fewer people who smoke large amounts (≥40 cigarettes per day) relative to the low and medium smoking groups. As a result the heavy smoking group may be increasingly undifferentiated from the medium smoking group to create a clear dose-response contrast between medium and heavy smokers.
When a random-effects model was used to generate pooled odds ratios, results were essentially unchanged (data not shown). This type of model may better incorporate heterogeneity between studies than other models, and because its results were comparable to those found by the fixed-effects methods of Mantel and Haenszel, we may infer sufficient homogeneity between studies for this report.
There are several limitations to the data. Publication bias may affect the results of this study, but we believe its contribution is minimal. The overall pooled estimate of 1.27 closely approximates the point estimate of the largest study, with an odds ratio of 1.20. This suggests that, despite the possible existence of some publication bias, the pooled estimate is likely a valid estimate of the true underlying effect.
Another potentially limiting factor is that various methods were used to estimate gestational age, ranging from extrapolating the self-reported last menstrual period to estimates that were based on a combination of last menstrual period, ultrasonography, and clinical examination. Furthermore, there was some heterogeneity of exposure criteria, with cigarette smoking ascertained at different points in pregnancy and smoking categorization differing from one study to the next. Heterogeneity also existed in the rates of preterm delivery from one study to the next, ranging from 2% to 33%. This might be attributable to geographic differences between sites, different target populations and recruitment, and time when the study was conducted (earlier studies tended to have higher rates; studies published before 1983 had among them the four highest rates of preterm delivery). Finally, there are multiple known causes of preterm labor, which may have been differentially controlled among studies. Adjusted odds ratios were used whenever available to better control for possible sources of confounding, but the adjustments differed from one study to the next.
Despite these potential limitations, this analysis provides the most up-to-date and validated summary of the effect of maternal cigarette smoking on the incidence of preterm delivery. A prior meta-analysis conducted in 1984 found pooled odds ratios of 1.32 to 1.56 when preterm delivery was variously defined as before 34, 35, 36, 37, or 38 weeks of gestation.39 The estimate for preterm delivery based on a gestational age <37 weeks, which was the cut point used for this meta-analysis, found a pooled odds ratio of 1.40 (95% confidence interval, 1.30-1.51). This estimate was based on 42,000 subjects pooled from 5 studies, whereas the current meta-analysis pooled 20 studies and >100,000 subjects to get a pooled odds ratio of 1.27 (95% confidence interval, 1.21-1.33). The current meta-analysis used adjusted odds ratios whenever possible, whereas the 1984 meta-analysis used strictly unadjusted values. This report was limited to prospective studies to avoid any potential bias that might overestimate the effect of smoking in retrospective data collection.
A benefit of meta-analysis used to summarize data is that a broad range of study sites and populations are represented in the pooled estimate. This expands the generalizability of results, providing greater credibility and wider clinical and public health significance to the finding. Furthermore, there are a large number of observations in the overall analysis, which is oftentimes prohibitively difficult in any single study.
Maternal smoking has been shown to be associated with many other problems for the newborn in addition to preterm labor. There are strong associations with low birth weight,40 spontaneous abortion,41 abruptio placentae,42 ectopic pregnancy,43 impaired respiratory function in newborns,44 and psychiatric adjustment of the child in later life,45 to name just a few.
In his presidential address before the Royal Society of Medicine in 1965, Bradford Hill noted the following 9 conditions that help strengthen causal inference for an observed association: strength of the association, consistency, specificity, temporality, dose response, plausibility, coherence, experiment, and analogy. With all the data that are now available on the association between cigarette smoking and preterm delivery, these criteria have been met and any controversy regarding maternal smoking and preterm delivery appears to have been sufficiently addressed.
REFERENCES
- 1.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–43. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 2.Joseph KS, Kramer MS, Marcoux S, Ohlsson A, Wen SW, Allen A, et al. Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med. 1998;339:1434–9. doi: 10.1056/NEJM199811123392004. [DOI] [PubMed] [Google Scholar]
- 3.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 5.Microsoft Excell 98 [computer program] Microsoft Corp; Redmond (WA): 1998. [Google Scholar]
- 6.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–45. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. J Clin Epidemiol. 1994;47:881–9. doi: 10.1016/0895-4356(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 8.Ahlborg G, Jr, Bodin L. Tobacco smoke exposure and pregnancy outcome among working women: a prospective study at prenatal care centers in Orebro County, Sweden. Am J Epidemiol. 1991;133:338–47. doi: 10.1093/oxfordjournals.aje.a115886. [DOI] [PubMed] [Google Scholar]
- 9.Arbuckle TE, Sherman GJ. Comparison of the risk factors for pre-term delivery and intrauterine growth retardation. Paediatr Perinat Epidemiol. 1989;3:115–29. doi: 10.1111/j.1365-3016.1989.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 10.Donovan JW. Randomised controlled trial of anti-smoking advice in pregnancy. Br J Prev Soc Med. 1977;31:6–12. doi: 10.1136/jech.31.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucette JT, Bracken MB. possible role of asthma in the risk of preterm labor and delivery. Epidemiology. 1993;4:143–50. doi: 10.1097/00001648-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Frazier TM, Davis GH, Goldstein H, Goldberg ID. Cigarette smoking and prematurity: a prospective study. Am J Obstet Gynecol. 1961;81:988–96. doi: 10.1016/s0002-9378(15)33448-7. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen TB, Wilcox AJ, Hedegaard M, Secher NJ. Bias in studies of preterm and postterm delivery due to ultrasound assessment of gestational age. Epidemiology. 1995;6:533–7. doi: 10.1097/00001648-199509000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, McLean FH, Eason EL, Usher RH. Maternal nutrition and spontaneous preterm birth. Am J Epidemiol. 1992;136:574–83. doi: 10.1093/oxfordjournals.aje.a116535. [DOI] [PubMed] [Google Scholar]
- 15.Naeye RL. Factors that predispose to premature rupture of the fetal membranes. Obstet Gynecol. 1982;60:93–8. [PubMed] [Google Scholar]
- 16.Nordentoft M, Lou HC, Hansen D, Nim J, Pryds O, Rubin P, et al. Intrauterine growth retardation and premature delivery: the influence of maternal smoking and psychosocial factors. Am J Public Health. 1996;86:347–54. doi: 10.2105/ajph.86.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obel EB. Pregnancy complications following legally induced abortion: an analysis of the population with special reference to prematurity. Dan Med Bull. 1979;26:192–9. [PubMed] [Google Scholar]
- 18.Peacock JL, Bland JM, Anderson HR. Preterm delivery: effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ. 1995;311:531–5. doi: 10.1136/bmj.311.7004.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rush D, Kass EH. Maternal smoking: a reassessment of the association with perinatal mortality. Am J Epidemiol. 1972;96:183–96. doi: 10.1093/oxfordjournals.aje.a121447. [DOI] [PubMed] [Google Scholar]
- 20.Russell CS, Taylor R, Law CE. Smoking in pregnancy, maternal blood pressure, pregnancy outcome, baby weight and growth, and other related factors. A prospective study. Br J Prev Soc Med. 1968;22:119–26. doi: 10.1136/jech.22.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiono PH, Klebanoff MA, Rhoads GG. Smoking and drinking during pregnancy. Their effects on preterm birth. JAMA. 1986;255:82–4. [PubMed] [Google Scholar]
- 22.Siega-Riz AM, Adair LS, Hobel CJ. Maternal underweight status and inadequate rate of weight gain during the third trimester of pregnancy increases the risk of preterm delivery. J Nutr. 1996;126:146–53. doi: 10.1093/jn/126.1.146. [DOI] [PubMed] [Google Scholar]
- 23.Stein A, Campbell EA, Day A, McPherson K, Cooper PJ. Social adversity, low birth weight, and preterm delivery. BMJ. 1987;295:291–3. doi: 10.1136/bmj.295.6593.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berg BJ. Epidemiologic observations of prematurity: effects of tobacco, coffee and alcohol. In: Reed DM, Stanley FJ, editors. The epidemiology of prematurity. Urban und Schwarzenberg; Baltimore: 1977. [Google Scholar]
- 25.van den Berg BJ, Oechsli FW. Prematurity. In: Bracken MB, editor. Perinatal epidemiology. Oxford University Press; New York: 1984. [Google Scholar]
- 26.Wen SW, Goldenberg RL, Cutter GR, Hoffman HJ, Cliver SP. Intrauterine growth retardation and preterm delivery: prenatal risk factors in an indigent population. Am J Obstet Gynecol. 1990;162:213–8. doi: 10.1016/0002-9378(90)90853-y. [DOI] [PubMed] [Google Scholar]
- 27.Wisborg K, Henriksen TB, Hedegaard M, Secher NJ. Smoking during pregnancy and preterm birth. Br J Obstet Gynaecol. 1996;103:800–5. doi: 10.1111/j.1471-0528.1996.tb09877.x. [DOI] [PubMed] [Google Scholar]
- 28.Yerushalmy J. Mother’s cigarette smoking and survival of the infant. Am J Obstet Gynecol. 1964;88:505–18. doi: 10.1016/0002-9378(64)90509-5. [DOI] [PubMed] [Google Scholar]
- 29.Berkowitz GS, Holford TR, Berkowitz RL. Effects of cigarette smoking, alcohol, coffee and tea consumption on preterm delivery. Early Hum Dev. 1982;7:239–50. doi: 10.1016/0378-3782(82)90086-x. [DOI] [PubMed] [Google Scholar]
- 30.de Haas I, Harlow BL, Cramer DW, Frigoletto FD., Jr. Spontaneous preterm birth: a case-control study. Am J Obstet Gynecol. 1991;165:1290–6. [PubMed] [Google Scholar]
- 31.Ekwo EE, Gosselink CA, Moawad A. Previous pregnancy outcomes and subsequent risk of preterm rupture of amniotic sac membranes. Br J Obstet Gynaecol. 1993;100:536–41. doi: 10.1111/j.1471-0528.1993.tb15304.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferraz EM, Gray RH, Cunha TM. Determinants of preterm delivery and intrauterine growth retardation in north-east Brazil. Int J Epidemiol. 1990;19:101–8. doi: 10.1093/ije/19.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Harger JH, Hsing AW, Tuomala RE, Gibbs RS, Mead PD, Eschenbach DA, et al. Risk factors for preterm premature rupture of fetal membranes: a multicenter case-control study. Am J Obstet Gynecol. 1990;163:130–7. doi: 10.1016/s0002-9378(11)90686-3. [DOI] [PubMed] [Google Scholar]
- 34.Hartikainen-Sorri AL, Sorri M. Occupational and socio-medical factors in preterm birth. Obstet Gynecol. 1989;74:13–6. [PubMed] [Google Scholar]
- 35.Heffner LJ, Sherman CB, Speizer FE, Weiss ST. Clinical and environmental predictors of preterm labor. Obstet Gynecol. 1993;81:750–7. [PubMed] [Google Scholar]
- 36.Williams MA, Mittendorf R, Stubblefield PG, Lieberman E, Schoenbaum SC, Monson RR. Cigarettes, coffee, and preterm premature rupture of the membranes. Am J Epidemiol. 1992;135:895–903. doi: 10.1093/oxfordjournals.aje.a116385. [DOI] [PubMed] [Google Scholar]
- 37.Ekwo EE, Gosselink CA, Woolson R, Moawad A. Risks for premature rupture of amniotic membranes. Int J Epidemiol. 1993;22:495–503. doi: 10.1093/ije/22.3.495. [DOI] [PubMed] [Google Scholar]
- 38.Colditz GA, Burdick E, Mosteller F. Heterogeneity in meta-analysis of data from epidemiologic studies: a commentary. Am J Epidemiol. 1995;142:371–82. doi: 10.1093/oxfordjournals.aje.a117644. [DOI] [PubMed] [Google Scholar]
- 39.McIntosh ID. Smoking and pregnancy. II. Offspring risks. Public Health Rev. 1984;12:29–63. [PubMed] [Google Scholar]
- 40.Secker-Walker RH, Vacek PM, Flynn BS, Mead PB. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet Gynecol. 1997;89:648–53. doi: 10.1016/s0029-7844(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 41.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340:333–9. doi: 10.1056/NEJM199902043400501. [DOI] [PubMed] [Google Scholar]
- 42.Ananth CV, Smulian JC, Vintzileos AM. Incidence of placental abruption in relation to cigarette smoking and hypertensive disorders during pregnancy: a meta-analysis of observational studies. Obstet Gynecol. 1999;93:622–8. doi: 10.1016/s0029-7844(98)00408-6. [DOI] [PubMed] [Google Scholar]
- 43.Saraiya M, Berg CJ, Kendrick JS, Strauss LT, Atrash HK, Ahn YW. Cigarette smoking as a risk factor for ectopic pregnancy. Am J Obstet Gynecol. 1998;178:493–8. doi: 10.1016/s0002-9378(98)70427-2. [DOI] [PubMed] [Google Scholar]
- 44.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mothers smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–5. doi: 10.1164/ajrccm.158.3.9711057. [DOI] [PubMed] [Google Scholar]
- 45.Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry. 1998;55:721–7. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]


