Summary
The ability of bacteria to sense and respond to both environmental and intracellular metal concentrations plays an important role in pathogenesis. The acquisition of manganese is vital for the virulence of several bacterial species. Although manganese uptake systems have been well studied in bacteria, no manganese efflux system has yet been identified. In this study we have identified a cation diffusion facilitator (CDF) protein (Sp1552) of unknown substrate specificity that functions as a manganese export system in Streptococcus pneumoniae. We designated the gene for this manganese efflux system mntE and found that the mutant strain was highly sensitive to manganese stress. Although the mutant was more resistant to oxidative stress and produced more H2O2 and pili, it had reduced virulence in a murine model of infection, indicating that manganese export plays a role in host pathogenesis. There was a distinct differential transcriptional response to extracellular and intracellular manganese accumulation. Our study indicates that manganese efflux is required for invasive disease and may provide a useful antimicrobial target to devise future therapeutics.
Introduction
Bacterial pathogens must acquire all the necessary cofactors for survival and replication from the host environment during the course of infection. One such limiting factor is the availability of various metals, including iron, zinc and manganese, which are required by both the host and the bacteria for essential cellular processes. Acquisition of metals is also essential for pathogenesis. Bacteria sense cation levels to respond to their environment via two-component regulators or transcriptional regulators that require a specific cation cofactor for promoter recognition. Hence, metal homeostasis is important not only for enzymatic function but also for appropriate transcriptional control of regulatory networks that govern gene expression under various environmental conditions.
Bacterial acquisition of manganese plays important roles in pathogenesis in a number of bacterial species. This is highlighted by the multiple strategies various species of bacteria have evolved to acquire manganese from the environment. Two main classes of manganese transporters have been identified in bacteria, Nramp H+-Mn2+ transporters and the ATP-binding cassette (ABC) Mn2+ permeases, with nearly all sequenced bacterial genomes containing transporters of one or both of these classes (Papp-Wallace and Maguire, 2006). Manganese transporters can also transport other ions including Fe2+, Zn2+, Cu2+, Co2+, Cd2+ and Ni2+ (Kehres et al., 2000; Low et al., 2003). Furthermore, some predicted metal importers have been shown to function for export, underscoring the need to experimentally confirm bioinformatics predictions (Brenot et al., 2007). No Nramp transporters have been characterized in pneumococcus, although an ABC transporter specific for manganese import, PsaABC, has been identified and found to play a role in many transcriptional pathways indicating that perturbation of manganese homeostasis by disrupting import elicits a specific cellular response (McAllister et al., 2004; McCluskey et al., 2004; Johnston et al., 2006). Manganese influx systems have also been characterized in many bacterial species (Kehres et al., 2000; Makui et al., 2000; McAllister et al., 2004). The converse experiment, disruption of a manganese export system, to ascertain the effect of intracellular manganese accumulation has not been performed as no export system whose primary substrate is Mn2+ has yet been identified in any bacteria to our knowledge.
Although many studies have focused on influx systems, efflux of cations is also a potential mechanism for regulating genes and maintaining homeostasis, particularly in an environment replete with certain cations. Bacterial efflux systems have been characterized for arsenic (ArsB), cadmium (CadA), zinc (CzcD), copper (CzcD and CopZA), cobalt (CzcD) and nickel (CzcD) (Moore and Helmann, 2005). One of the most ubiquitous classes of metal transporters is the cation diffusion facilitator (CDF) family of proteins, which transports various metals in both prokaryotes and eukaryotes (Haney et al., 2005). Members of this family have broad capabilities of metal transport; for example, FieF from Wautersia metallidurans is primarily an iron-detoxifying transporter but can also mediate resistance against other divalent cations such as Zn2+, Co2+, Cd2+ and Ni2+ (Munkelt et al., 2004). Studies on substrate specificity based on sequence homology or computation predictions need to be experimentally confirmed.
Bacteria tightly control the intracellular levels of cations such as zinc and manganese under dynamic environmental conditions (Chapuy-Regaud et al., 2001; Outten and O’Halloran, 2001). A selective efflux system is required to transport calcium out of the cell in order to maintain a 3-log difference between intra- and extracellular calcium concentrations (Rosch et al., 2008a). Zinc efflux systems have also been recently identified in Streptococcus pyogenes and Streptococcus pneumoniae (Brenot et al., 2007; Kloosterman et al., 2007). Elucidation of the structure of the CDF protein YiiP, which is responsible for efflux of both iron and zinc in Escherichia coli, has provided insights into the structure determinants of metal ion selectivity (Grass et al., 2005; Wei and Fu, 2005; Lu and Fu, 2007). Putative manganese exporters have been identified and characterized in eukaryotes, particularly plants (Delhaize et al., 2003; Hall and Williams, 2003). To our knowledge, evidence of bacterial manganese exporters has remained elusive, although there is some evidence of active efflux from bacterial cells (Fisher et al., 1973).
In this study, we describe a predicted CDF protein (Sp1552) of unknown substrate specificity that functions as a manganese efflux system in S. pneumoniae. We have designated the gene for this manganese efflux system mntE, and used the mutant strain (mntE–) that accumulated higher levels of intracellular manganese to study resistance to oxidative stress, production of hydrogen peroxide, pilus expression, host pathogenesis in a murine model, and differential transcriptional response to extracellular and intracellular manganese. Our results suggest that although the mutant is more resistant to oxidative stress and produces more H2O2 and pili, it has reduced pathogenicity in the host. The global transcriptome response indicates that S. pneumoniae has distinctly different responses to intracellular and extracellular manganese accumulation, providing a unique perspective on the bacterial response to environmental manganese.
Results
Identification of the manganese exporter
On the basis of sequence homology, Sp1552 has been predicted to be a CDF inorganic cation transport system of unknown substrate (Tettelin et al., 2001). We found the homologous gene to be conserved among several Gram-positive pathogens such as Staphylococcus aureus, Clostridium botulinum, Bacillus anthracis and S. pyogenes, and more distant homologues were identified in Gram-negative bacteria as well as Archaea (data not shown). The CDF family has been extensively studied in both eukaryotes and prokaryotes, and bacterial homologues have been implicated in zinc efflux (Grass et al., 2001). Members of this family can selectively export cadmium, cobalt, nickel, zinc, manganese or iron (Moore and Helmann, 2005). One hallmark of eukaryotic CDF manganese transporters is the presence of a DXXXD motif at transmembrane domain 5 (Montanini et al., 2007). This feature is conserved in Sp1552, indicating that Sp1552 may be functionally analogous to the CDF family of manganese transporters. The gene sp1552 is highly conserved in S. pneumoniae: comparative genomic hybridization (CGH) analysis has shown that it is part of the core pneumococcal genome and it is also present in all sequenced strains to date (Obert et al., 2006). Export systems for both zinc and calcium have been identified in S. pneumoniae; however, the substrate of the transporter encoded by sp1552 has not been experimentally determined (Kloosterman et al., 2007; Rosch et al., 2008a).
To identify the substrate and function of sp1552 in S. pneumoniae, we generated a deletion mutant of sp1552 by allelic replacement and confirmed it by sequencing. We could not achieve complementation of the deletion mutant on a plasmid, as the gene product appeared to be toxic to E. coli during cloning (data not shown) into various streptococcal vectors, including pIB163, pIB165, pIB167, pIB169 and pABG5 (Hanski et al., 1992; Biswas et al., 2008). The mutation was nonpolar, as evidenced by the normal transcription of both upstream and downstream genes (data not shown). A revertant strain was generated by crossing the parental locus into the sp1552– mutant and regenerating the parental TIGR4 genotype, as confirmed by PCR and sequencing. This mutant, sp1552–, and the TIGR4 and the wild-type revertant were exposed to various compounds to determine the substrate of the transporter. Additional substrate was expected to rescue an influx system mutant and inhibit an exporter mutant.
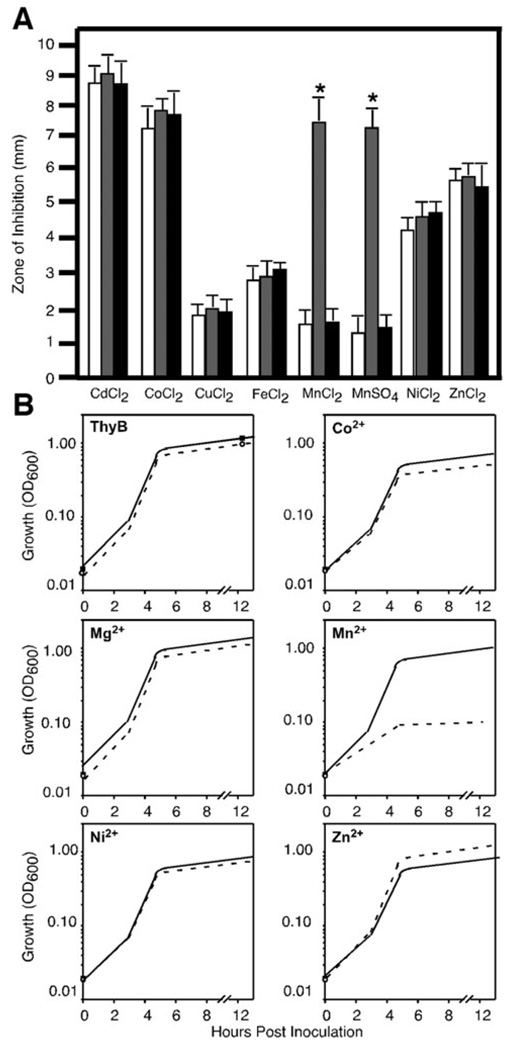
Cation sensitivity profiles of sp1552– revealed that it was selectively sensitive to manganese (P < 0.01), as evidenced by a significant increase in its zone of inhibition compared with the parental TIGR4 and wild-type revertant (Fig. 1A). Switching of the cognate anion indicated this sensitivity was selective for manganese. TIGR4 and sp1552– did not significantly differ in their sensitivities for any other cation tested, including cadmium and zinc. Sensitivities to cobalt and cadmium were also tested using 100 mM solutions infused into the disc, but no differences were observed between the strains (data not shown). Growth of the sp1552– mutant was severely restricted in media supplemented with manganese (Fig. 1B) with total growth inhibition observed at a manganese concentration of 1 mM. Growth of parental TIGR4 was not inhibited under all concentrations of manganese used in the study. We tested the ability of several metals, including cobalt, nickel, calcium and magnesium, to inhibit the growth of sp1552–, but none of them caused a significant growth defect (Fig. 1B) compared with that in TIGR4. These data indicate that the sp1552– strain has increased sensitivity for manganese, suggesting a possible role of this gene in manganese export.
Fig. 1. Cation sensitivity profiles of TIGR4, sp1552– and the WT revertant.
A. Bacterial lawns of TIGR4 (white bars), sp1552– mutant (grey bars) and WT revertant (black bars) were overlaid with filter discs saturated with 1 M solutions of the various cations tested. The zone of inhibition of growth was measured from the edge of the disc at 24 h. Data are representative of mean ± SD from four independent experiments. *P < 0.01.
B. Cultures of either TIGR4 (solid line) or the sp1552– mutant (dashed line) were grown in ThyB medium supplemented with cobalt, manganese, magnesium, nickel and zinc (concentrations given in Experimental procedures). Cultures were inoculated with 105 bacteria and growth was measured at OD600 over 12 h. Data are representative of one of three independent growth curves.
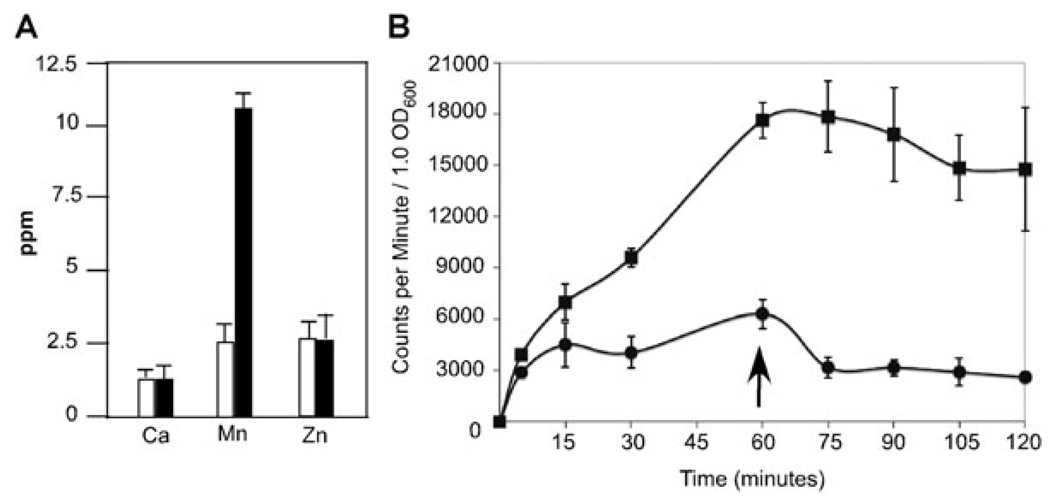
To confirm that the sp1552– mutant was deficient for manganese export, we measured the intracellular levels of manganese in both parental TIGR4 and sp1552– by inductively coupled plasma mass spectrometry (ICP-MS). The sp1552– strain accumulated five times more intracellular manganese than parental TIGR4 did (Fig. 2A). There was no difference between the TIGR4 and sp1552– strain with regard to intracellular concentrations of calcium or zinc (Fig. 2A) or those of magnesium [12.2 ± 0.6 p.p.m. for TIGR4 and 12.8 ± 0.3 p.p.m. for sp1552– per 107 colony-forming units (cfu)]. For cadmium, a slight decrease from 1.16 ± 0.04 p.p.m. for TIGR4 to 0.83 ± 0.06 p.p.m. for sp1552– per 109 cfu was observed, which might be the result of downregulation of manganese import because many manganese transporters can also transport cadmium (Kehres and Maguire, 2003).
Fig. 2. Intracellular concentrations of cations.
A. TIGR4 (white) and sp1552– (black) were grown in ThyC supplemented with calcium (1 mM), manganese (300 µM), magnesium (1 mM), zinc (300 µM) and cadmium (3 µM) to facilitate measurement of these specific cations. Cells were harvested and extracellular cations removed by washing in EDTA. Cation concentration was determined by ICP-MS in 108 bacteria (p.p.m., parts per million). Data represent mean ± standard deviation from at least two independent experiments. For all cations tested, an internal standard of known concentration was used to confirms measurements.
B. 54Mn2+ uptake was measured in both TIGR4 (circles) and sp1552– (squares) in log-phase cultures spiked with 500 µM MnCl2 and 1 µCi of 54Mn2+. Parallel cultures were measured to normalize accumulation to cellular growth. Counts per minute were determined and expressed as a function of OD600. After 1 h, cells were collected and re-suspended in media lacking isotope (arrow) to monitor efflux. Data represent mean ± standard deviation from three independent experiments.
We further confirmed the accumulation of manganese in the sp1552– strain by the 54Mn2+ uptake assay, wherein cultures were supplemented with radioactive manganese. The sp1552– mutant rapidly accumulated high levels of manganese, and continued to accumulate it over the course of 1 h (Fig. 2B). In contrast, the TIGR4 strain rapidly accumulated lower levels of manganese and subsequently maintained homeostasis. Re-suspending the bacteria in a medium not supplemented with 54Mn2+ (arrow in Fig. 2B) resulted in a gradual decrease of approximately 50% in intracellular radioactive manganese in the TIGR4 stain but not in the sp1552– strain. It should be noted that although the acidic media used in these experiments prevents most autolysis, we cannot eliminate the possibility that cell lysis mediates part of the manganese efflux. Data from the cation sensitivity and uptake assay indicate that Sp1552 functions as a manganese efflux system and that a mutant deficient in export accumulates significantly higher levels of intracellular manganese. In light of these data, we propose naming the gene mntE to be in accord with previously identified bacterial manganese import systems, mntA and mntH (Bartsevich and Pakrasi, 1995; Makui et al., 2000).
Role of manganese export in resistance to oxidative stress
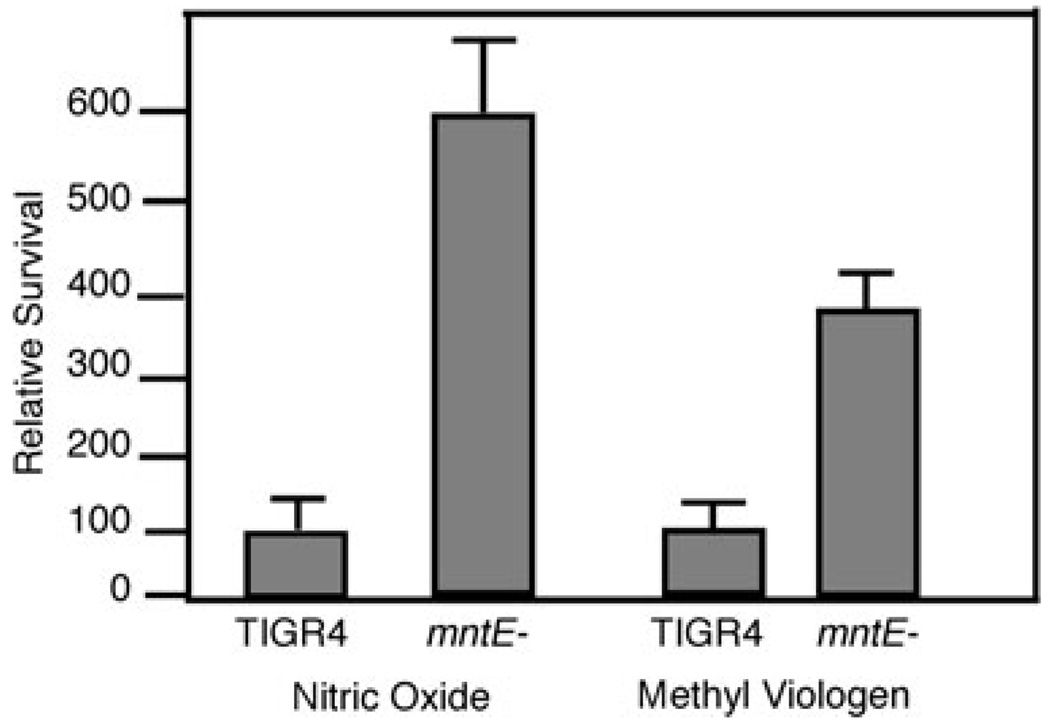
Manganese has long been known to play an important role in bacterial defence against reactive oxygen species (Inaika et al., 1999; Jakubovics et al., 2002; Papp-Wallace and Maguire, 2006). As the mntE– strain is deficient in manganese export, we hypothesized that it would be more efficient at handling reactive oxygen species because of an increase in available cellular manganese. Parental TIGR4 and mntE– strains were subjected to both superoxide and nitric oxide stress for 60 min and viability assessed by serial dilution and colony enumeration. Interestingly, the mntE– mutant showed a fourfold increase in survival over the parental TIGR4 in response to both oxidative and nitric oxide stresses (Fig. 3). One possible reason for this protection could be an increase in the manganese-dependent superoxide dismutase activity (Yesilkaya et al., 2000); however, there was no significant difference in superoxide dismutase activity between these two strains (data not shown). Manganese (II) bicarbonate complexes have been shown to reduce reactive oxygen species, providing an antioxidant defence independent of enzymatic factors (Archibald and Fridovich, 1981; Al-Maghredi et al., 2002; Daly et al., 2004). Our data also indicate that accumulation of high levels of intracellular manganese protects against reactive oxygen species.
Fig. 3. Oxidative killing of TIGR4 and mntE–
Strains were subjected to either superoxide (methyl viologen) or nitric oxide (GNSO) stresses. Bacterial cfu were enumerated immediately before adding oxidants and at 15 min intervals thereafter via serial dilution and direct counts. Data represent mean ± standard deviation of relative survival (% of TIGR4) from three independent replicates after a 60 min exposure.
Dysregulation of hydrogen peroxide production
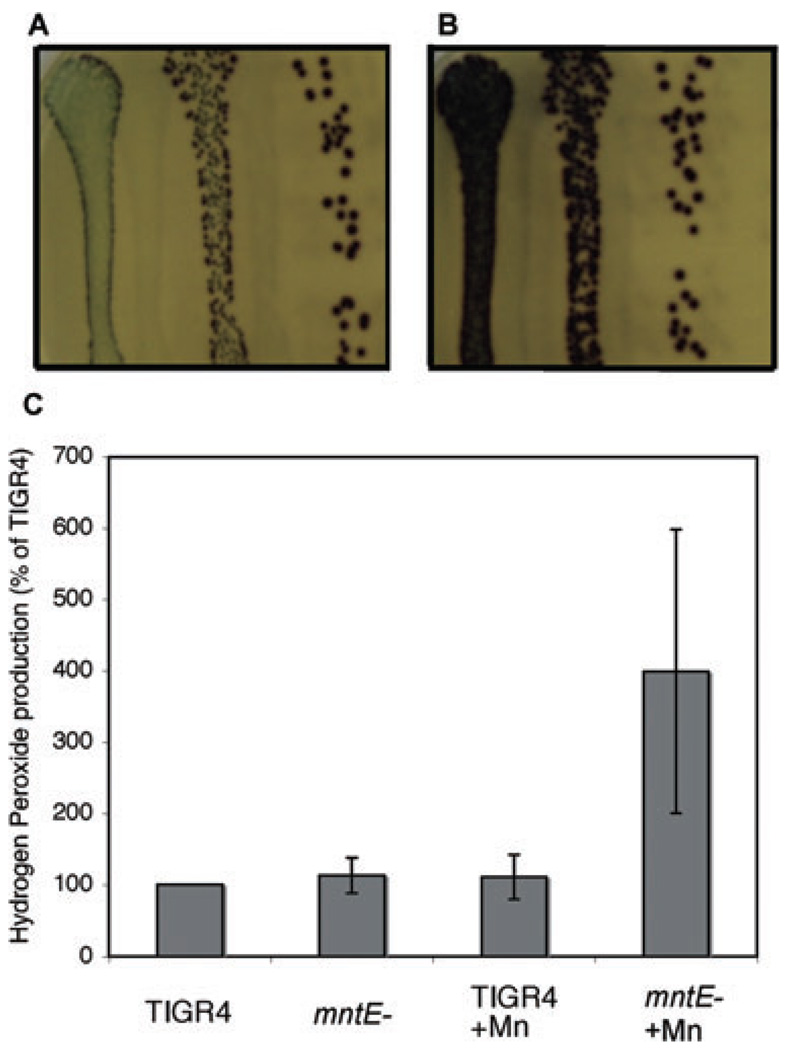
Species of streptococci produce prodigious amounts of H2O2, which plays important roles in processes such as competing with other bacterial species and as a potential quorum-sensing molecule in S. pneumoniae (Battig and Muhlemann, 2008; Kreth et al., 2008). We monitored the production of H2O2 by the mntE– mutant by an indicator plate assay (Brenot et al., 2005). Streptococci produce abundant H2O2 at low cell densities on solid media, but their H2O2 production is limited at high cell densities (Brenot et al., 2005). Both TIGR4 and mntE– produced equivalent amounts of H2O2 at low cell densities (Fig. 4A and B, far right). However, when cells were plated at high cell densities, H2O2 production by TIGR4 was dramatically reduced but that for the mntE– mutant continued to remain high (Fig. 5A and B, compare leftmost lanes). This phenotype is similar to that of an ahpC– mutant in S. pyogenes (Brenot et al., 2005). Although pneumococci do not encode a homologue of ahpC, it is interesting to note that a putative thiol peroxidase is immediately downstream of the psaABC manganese uptake system, indicating potential cross-talk between manganese signalling pathways and bacterial antioxidant defences (McAllister et al., 2004). TIGR4 and mntE– showed no difference in H2O2 production in liquid culture when cultured in ThyB media (Fig. 4C). However, under high manganese concentrations, the mntE– mutant showed a dramatic increase in H2O2 production (Fig. 4C). The high standard error of the mntE– mutant grown in manganese may be due in part to the measured absorbance approaching the upper limit of the linear range of the measurement assay at the dilution that all samples were measured for consistency. These data indicate that the availability of manganese may play a role in the ability of pneumococci to produce H2O2.
Fig. 4. Hydrogen peroxide production by TIGR4 and mntE–
A and B. TIGR4 (A) and the mntE– mutant (B) were serially diluted 1:10 (decreasing concentration from left to right) and streaked on to H2O2 indicator plates. Bacteria were grown under anaerobic conditions overnight on indicator plates, and images were taken after a 30 min exposure to atmospheric oxygen. Purple coloration indicates H2O2 production. Note that in the more dilute plating (far right of A and B) colonies produce equivalent amounts of H2O2, but in a plating that is 100 times more concentrated (far left of A and B) H2O2 production is restricted in TIGR4 but the mntE– mutant continues to produce prodigious amounts.
C. The amount of H2O2 produced by TIGR4 and mntE– mutant was measured after growth in ThyB and ThyB supplemented with manganese (100% = 10 µM H2O2 per 107 cfu).
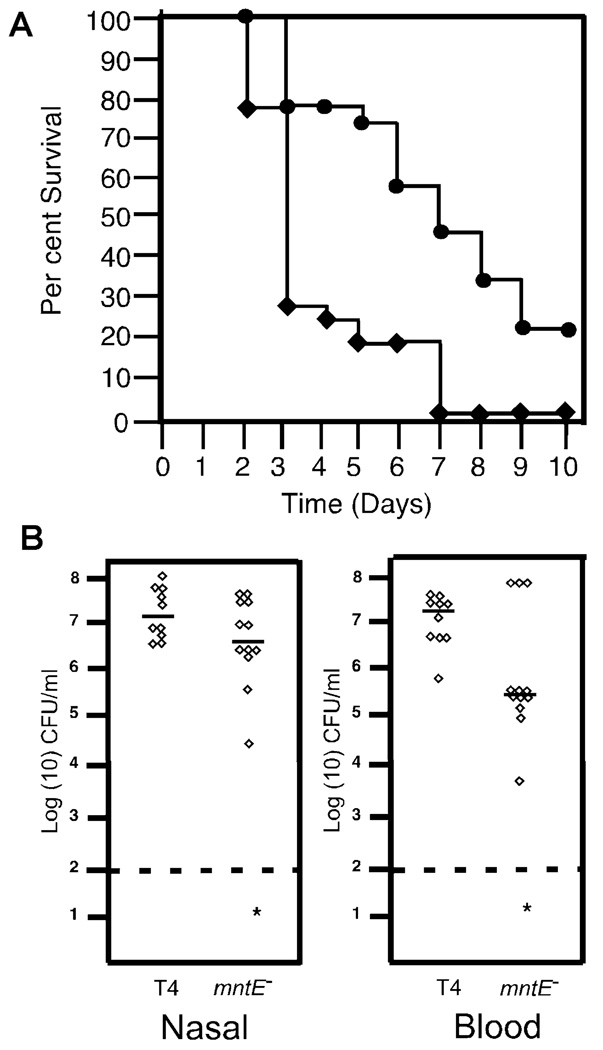
Fig. 5. Virulence of the manganese export mutant.
A. Six-week-old female Balb/cJ mice were infected intranasally with 1 × 107 bacteria. Survival plot of mice infected intranasally with 1 × 107 TIGR4 (diamonds) or mntE– mutant (circles). Data are from 18 mice per group from two independent experiments.
B. Nasal and blood titres at 24 h are shown. Each symbol represents an individual mouse; five mice per experiment in duplicate are shown. The asterisk (*) indicates statistical significance of P < 0.05.
Role of manganese export in host pathogenesis
As the mntE– strain was significantly better at resisting reactive oxygen and nitrogen species normally encountered in the host, we expected it to be more virulent than the parental strain. The parental TIGR4 and mntE– mutant were assessed for their ability to sustain nasal colonization and establish invasive disease in an intranasal murine model of pathogenesis. The mntE– strain showed a significant defect in both nasal colonization (P = 0.042) and invasion into the blood (P = 0.017) (Fig. 5B). Overall survival data showed that mice infected with mntE– had a significant (P = 0.003) delay in time to death compared with the parental TIGR4 (Fig. 5A). These results indicate that although the mntE– mutant was more resistant to oxidative stress, loss of manganese export impaired pathogenesis in the host.
Disruption of manganese export influences pilus regulation
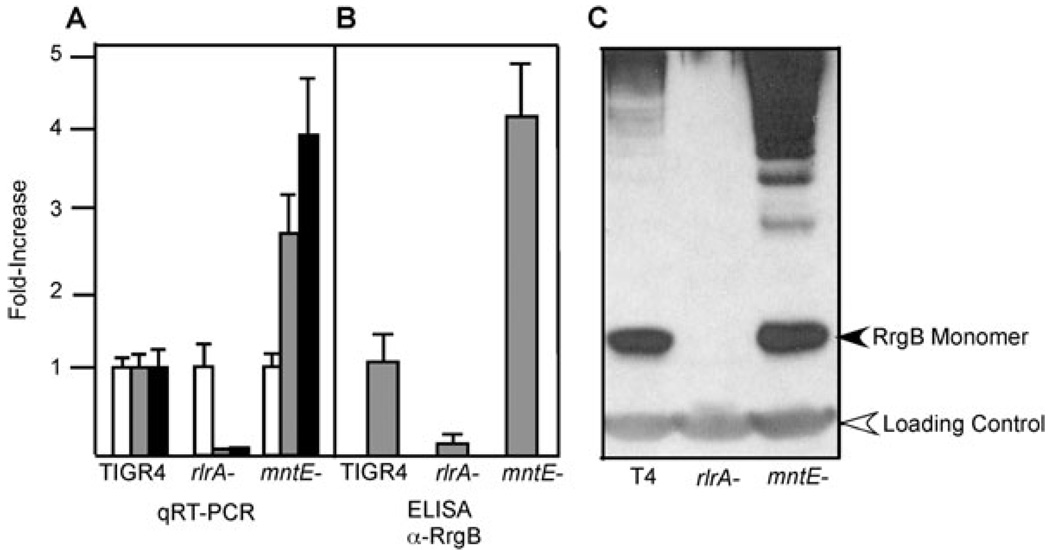
As the mntE– mutant was attenuated in a murine model of pathogenesis, we investigated whether this phenotype could be explained by differential regulation of important virulence determinants. Disruption of PsaR, which regulates the PsaABC manganese import system, has been shown to significantly upregulate the genes encoding the pilus subunits and the associated sortases (Lauer et al., 2005; Barocchi et al., 2006; Johnston et al., 2006). Hence, we expected the mntE– mutant to show decreased amounts of pilus expression and thereby reduced virulence because of accumulation of intracellular manganese. We used quantitative RT-PCR (qRT-PCR) to measure transcript abundance of the rlrA regulator, which is required for pilus transcription along with the major pilus subunit rrgB. There was an increase in both the rlrA and rrgB transcripts in the mntE– mutant as compared with parental TIGR4 (Fig. 6A), and this increase corresponded to an increase in the amount of pilus protein, as measured by both Western blot and ELISA using RrgB-specific antibodies (Fig. 6A and B). These results indicate that perturbation of intracellular manganese homeostasis may be a key cue for pilus expression. As manganese is highly abundant in the nasal and lung mucosa, differences in intracellular manganese levels may be an important cue for pathogenesis, particularly in the lung where pili play a role in invasive disease (Nelson et al., 2007; Rosch et al., 2008b). We expected that an increased amount of pili would increase the pathogenesis of the mutant, but the mntE– strain was not more virulent than parental TIGR4 in the murine model of infection. Additional factors may be responsible for this attenuation, necessitating a more global view of the transcriptional response due to intracellular manganese accumulation.
Fig. 6. Analysis of pilus expression.
Relative expressions of gyrA (white bars), rlrA (grey bars) and rrgB (black bars) were measured in the TIGR4, rlrA– and mntE– strains by qRT-PCR of RNA isolated from logarithmically growing cells (A). Expression levels were normalized to gyrA, which served as an internal control. The increased level of the rrgB transcript resulted in approximately a fourfold increase in RrgB protein expression, as measured by ELISA (B) as well as by Western blot using RrgB-specific antisera (C). The RrgB monomer and loading control cross-reactive band are indicated by arrows. Data for qRT-PCR and ELISA represents mean ± standard deviation from three independent experiments. Western blot is representative of three independent experiments.
Differential transcriptional response to extracellular versus intracellular manganese
Perturbation of manganese homeostasis has been shown to have an impact on the expression of many genes in S. pneumoniae, most notably the rlrA locus encoding the pilus and the psaABC locus encoding the manganese uptake system (Johnston et al., 2006). The global transcriptional response to environmental manganese is complex and includes the action of metalloregulatory proteins, perturbation of cellular iron availability, and differential activity of enzymes that require manganese as a cofactor (Guedon et al., 2003). We hypothesized that S. pneumoniae has separate transcriptional pathways to differentiate between high extracellular and intracellular manganese concentrations, which could help pinpoint the role of manganese in gene regulation. To elucidate this, global gene transcription in response to high (500 µM) manganese in parental TIGR4 as well as the transcription in response to intracellular manganese accumulation, as represented by the mntE– mutant, was examined.
We compared the transcriptome of S. pneumoniae grown in ThyB versus that grown in ThyB supplemented with 500 µM manganese. Table S1 lists all genes showing significant differences in transcript abundance and Table 1 indicates the number of genes in specific functional categories showing differential regulation. As expected, the operon downregulated the most was the PsaABC manganese uptake system, sp1647–sp1650, which has previously been shown to be repressed under conditions of high manganese (Johnston et al., 2006). Other loci that were significantly downregulated included sp0043, encoding the ComB protein involved in competence. The sp1869–1872 locus encoding an iron-compound ABC transporter was significantly upregulated in response to high levels of manganese, which agrees with previous results in Bacillus subtilis indicating that genes controlled by Fur (ferric uptake regulator) can be dysregulated by elevated concentrations of manganese (Guedon et al., 2003). Two predicted surface proteins, choline-binding protein D (sp2201) and a predicted cell wall-anchored protein (sp1992), were upregulated. We confirmed the expression patterns observed in the microarray by qRT-PCR, and found that both methods agreed (Table 2). These data indicate that S. pneumoniae elicits a specific transcriptional response to high levels of manganese in the environment, which includes repression of the manganese uptake system and upregulation of an iron import system.
Table 1.
Global transcript analysis of the bacterial response to extracellular and intracellular manganese.
| Putative functional category | Number of genes responsive to high extracellular Mn | Number of genes responsive to high intracellular Mn |
|---|---|---|
| Transcriptional regulation | 2 | 10 |
| Carbohydrate transport | 1 | 3 |
| Amino acid and peptide transport | 0 | 8 |
| Carbohydrate metabolism | 9 | 39 |
| Protein metabolism | 4 | 6 |
| Metal transport/utilization | 8 | 9 |
| Other transport | 4 | 10 |
| Stress response and chaperones | 1 | 5 |
| Transcriptional regulators | 7 | 32 |
| Cell wall and surface proteins | 3 | 13 |
| Secretion | 0 | 2 |
| Competence | 1 | 3 |
| Electron transport, ATP synthesis | 3 | 0 |
| Hypothetical | 7 | 34 |
| Total genes | 52 | 172 |
Middle column (TIGR4 : TIGR4 + Mn) is a summary of the comparison of TIGR4 transcripts from bacteria grown in standard ThyB versus ThyB supplemented with 500 µM manganese to determine bacterial response to extracellular manganese. The far right column compares transcript abundance of TIGR4 to mntE– when both strains were cultured in ThyB supplemented with 500 µM manganese to induce intracellular manganese accumulation. Full data set in Tables S1 and S2.
Table 2.
qRT-PCR confirmation of gene expression patterns observed in microarrays.a
| Gene/condition | Fold change (microarray) | Fold change (qRT-PCR) |
|---|---|---|
| TIGR4 versus TIGR4 + Mn | ||
| SP0461 (rlrA) | NC | 1.5 |
| SP0463 (rrgB) | NC | 1.3 |
| SP1501 | NC | 1.2 |
| SP1650 | −30.3 | −36.1 |
| SP1992 | 2.0 | 3.4 |
| TIGR4 + Mn versus mntE– + Mn | ||
| SP0461 (rlrA) | 4.0 | 4.4 |
| SP0463 (rrgB) | 2.6 | 3.4 |
| SP1501 | 3.4 | 2.8 |
| SP1650 | −2.3 | −30.5 |
| SP1852 (galT-2) | −5.4 | −3.7 |
| Sp1992 | 3.6 | 4.3 |
| SP2148 (arcA) | −5.2 | −3.9 |
Data represent average from three experiments, with each gene measured in duplicate. NC, no significant change.
To differentiate between extracellular and intracellular manganese-induced signalling, we determined the global transcriptome response when manganese efflux was impaired (Table S2) by comparing transcripts of TIGR4 and mntE– when both strains were cultured under conditions of high manganese. Table 1 indicates the number of genes in specific functional categories showing differential regulation under these conditions. These conditions cause mntE– to accumulate approximately five times more intracellular manganese than TIGR4 does. Several loci were significantly upregulated, including the sp0461–sp0467, which encodes the pilus and associated sortases, confirming the qRT-PCR data. Several genes involved in central metabolic pathways were downregulated in response to manganese accumulation, including operons involved in lactose metabolism (sp1189–1193), glycosyl transferases (sp1764–1771) and sugar transporters (sp1895–1897). Manganese has been implicated to play a role in various steps of the sugar catabolism pathway in bacteria, and accumulation of intracellular manganese may therefore affect such pathways (Kehres and Maguire, 2003). It has been suggested that a high intracellular accumulation of manganese could effectively compete for magnesium, altering the activity of Mg2+-requiring enzymes involved in the carbon flux (Papp-Wallace and Maguire, 2006). The caxP gene (sp1551), recently identified to encode a calcium/manganese efflux protein, is also significantly upregulated when manganese accumulates intracellularly (Rosch et al., 2008a). The sp1857 gene, which has been recently demonstrated to be involved in zinc tolerance in S. pneumoniae, is also upregulated, indicating that various metal export systems may be co-regulated (Kloosterman et al., 2007). A brief comparison of various genes of interest showing differential regulation in response to high extracellular and intracellular manganese is detailed in Table 3. It should be noted that the transcriptional analysis was not exhaustive, as there was no detectable signal in a significant number of genes (16.8%), probably due to low expression or poor hybridization. These data indicate that S. pneumoniae elicits a specific response to the intracellular accumulation of manganese that is distinct from extracellular sensing pathways.
Table 3.
Representative transcripts of the bacterial response to extracellular and intracellular manganese.
| Gene ID | Annotation | Fold change in response to high extracellular Mn | Fold change in response to high intracellular Mn |
|---|---|---|---|
| SP0043 | Competence factor transport protein ComB | −2.3 | NC |
| SP0285 | Alcohol dehydrogenase, zinc containing | NC | −2.3 |
| SP0461 | Transcriptional regulator rlrA | NC | 4.0 |
| SP0463 | Pilus subunit rrgB | NC | 2.6 |
| SP0519 | dnaJ protein | −2.3 | NC |
| SP0641 | Serine protease, subtilase family | −14.7 | −2.6 |
| SP0877 | PTS system, fructose-specific IIABC components | −2.1 | 12.7 |
| SP1190 | Tagatose 1,6 diphosphate aldolase | NC | −2.5 |
| SP1649 | Manganese ABC transporter, permease protein | −61.9 | −3.8 |
| SP1759 | Preprotein translocase, SecA | NC | −2.8 |
| SP1869 | Iron-compound ABC transporter, permease protein | 3.7 | NC |
| SP1992 | Cell wall surface anchor protein | 2.0 | 3.6 |
| SP2157 | Alcohol dehydrogenase, iron containing | NC | −5.0 |
| SP2201 | Choline-binding protein D | 2.1 | 3.8 |
| SP2235 | Response regulator ComE | NC | 2.1 |
| SP2236 | Sensor histidine kinase ComD | NC | 2.9 |
| Total number of differentially expressed genes | 52 | 172 |
Middle column (TIGR4 : TIGR4 + Mn) is a representative sample of the comparison of TIGR4 transcripts from bacteria grown in standard ThyB versus ThyB supplemented with 500 µM manganese to determine bacterial response to extracellular manganese. The far right column is a representative sample of transcript abundance of TIGR4 and mntE– when both strains were cultured in ThyB supplemented with 500 µM manganese to induce intracellular manganese accumulation. NC, no change. Full data set in Tables S1 and S2.
Discussion
Manganese plays an important role in the virulence of many species of bacterial pathogens that have Nramp and ABC transporters for its uptake (Papp-Wallace and Maguire, 2006). Nramp orthologues have been identified in many bacterial species such as S. aureus, B. subtilis and Mycobacterium tuberculosis (Agranoff et al., 1999; Que and Helmann, 2000; Hornsburgh et al., 2002). The pneumococcus relies on the ABC-type transporter PsaABC to import manganese into the cytosol (McAllister et al., 2004). Numerous other streptococcal species (e.g. S. pyogenes, S. mutans, S. gordonii, S. parasanguis) also use a specialized ABC transporter for manganese uptake (Papp-Wallace and Maguire, 2006). However, no manganese efflux system yet has been identified in bacteria. Our data indicate that pneumococcus encodes a CDF protein that functions as a manganese efflux system, the gene for which we have designated mntE, which provides a unique insight into the role of intracellular manganese in transcriptional regulation and virulence. It is interesting that MntE does not appear to transport cadmium, a characteristic shared by many other manganese transporters (Kehres and Maguire, 2003). Future studies need to focus on elucidating the amino acids that determine metal ion specificity for this channel. This transporter is conserved among a number of Gram-positive bacteria, including the staphylococci, streptococci, bacilli and clostridia, and has more distant homologues in Gramnegative bacteria and Archaea, indicating a potential role for manganese efflux in many different biological systems.
Manganese is important in providing defence against oxidative stresses S. pneumoniae (McAllister et al., 2004). A well-understood example is the manganesedependent superoxide dismutase (sodA), a cytosolic protein found in many bacteria that reduces oxygen radicals to protect against oxidative stress (Yesilkaya et al., 2000). Manganese bicarbonate complexes themselves can reduce reactive oxygen species directly, providing an antioxidant defence independent of enzymatic factors (Daly et al., 2004). Manganese regulates oxidative stress tolerance in streptococci (Jakubovics et al., 2002) and manganese supplementation restores phenotypic defects in knockout strains of superoxide dismutase (Al-Maghredi et al., 2002). Our data indicate that intracellular accumulation of manganese not only increases tolerance to oxidative stresses but also dysregulates H2O2 production by the pneumococcus. The intracellular concentration of manganese may be the key factor controlling the ability of the pneumococcus to produce high amounts of H2O2 and survive in the absence of catalase.
An important aspect of bacterial gene regulation is the ability to sense and respond to environmental conditions. The availability of various elements is an important factor in bacterial pathogenesis as both bacteria and host compete for scarce resources. The bacterial response to various metal ion stresses is complex and involves cross-talk between metalloregulatory pathways and some unexpected patterns that include upregulation of amino acid biosynthesis systems in response to specific heavy metals (Moore et al., 2005). A recent study has found that the host immune system exploits this competition by secreting calprotectin, which chelates zinc and manganese, rendering them unavailable for bacterial growth (Corbin et al., 2008). The manganese-responsive regulator, PsaR, regulates not only manganese uptake but also the loci involved in pathogenesis, such as pili (Johnston et al., 2006). Other species of streptococci use manganese as a signal to control the expression of virulence genes (Arirachakaran et al., 2007). S. pneumoniae upregulates manganese uptake in response to acidic stress (Martin-Galiano et al., 2005) as well as in the lung and intraperitoneal cavity (Marra et al., 2002). The environment of the lung contains relatively high concentrations of calcium (5 mM), manganese (34 µM) and zinc (200 µM) (Chicharro et al., 1999; Vanthanouvong and Roomans, 2004). The signalling pathways involved in the uptake and sensing of divalent cations are likely to be complex and interconnected, as evidenced by recent studies indicating PsaR can responded to both manganese and zinc (Kloosterman et al., 2008).
Differences in gene regulation between conditions found in the respiratory mucosa and standard culture media can provide insight into the transcriptional pathways important for establishing disease in the human host. Using the mntE– mutant, we were able to show that pneumococcus elicits a distinct transcriptional response to intracellular accumulation of manganese, a response that is different from extracellular manganese-induced signalling pathways. Such differences allow the bacteria to fine-tune a transcriptional response in response to both dynamic intracellular and extracellular conditions. Our findings indicate that mntE– is more fit in terms of resistance to oxidative stress and production of more H2O2 and pili, but this fitness benefit is abrogated in the host as the mutation reduced the pathogenicity of the bacteria. It is possible that disruption of manganese export also has a downstream effect on iron uptake and signalling, as both our transcriptional analysis and previous studies suggest a significant degree of cross-talk between these pathways (Guedon et al., 2003; Hanks et al., 2006). Although we did not observe any significant difference in iron sensitivity in vitro, more subtle differences may be evident in vivo. Also, downregulation of the PsaABC locus to almost undetectable levels could play a role in attenuation in the host, as this locus has been implicated in virulence (Marra et al., 2002). Downregulation of PsaABC is not sufficient to mediate protection against high levels of manganese in the environment, indicating that while downregulation of uptake does play an important role, an active efflux system is also required to maintain homeostasis. This indicates that manganese efflux is required at some stage of invasive disease, and provides a unique antimicrobial target for the development future therapeutics.
Experimental procedures
Media and growth conditions
Streptococcus pneumoniae TIGR4 and TIGR4R was grown on tryptic soy agar (EMD Chemicals, Gibbstown, NJ) supplemented with 3% sheep blood or in defined semi-synthetic casein liquid media supplemented with 0.5% yeast extract (C+Y) (Lacks and Hotchkiss, 1960; Rosch et al., 2008a). Erythromycin (1 µg ml−1) and kanamycin (400 µg ml−1) were added when appropriate. Todd Hewitt Broth (ThyB) and ThyB depleted of cations (ThyC) using Chelex resin (Bio-Rad, Hercules, CA) were formulated as previously described (Hanks et al., 2006). When appropriate, pH was adjusted to 6.5 using concentrated hydrochloric acid and subsequently sterile filtered. Cultures of S. pneumoniae were innoculated from frozen stock and incubated at 37°C in 5% CO2.
Primers/mutant construction
Mutants in TIGR4 and TIGR4R were made by PCR-based overlap extension (Wurch et al., 1998). Briefly, regions flanking upstream and downstream of the target gene were amplified by PCR and spliced to an antibiotic cassette. The final PCR product was transformed into the pneumococcus by conventional methods, replacing the targeted gene with the antibiotic cassette. To confirm transformation, primers outside the transformed region were used for PCR and subsequent region sequencing. To construct sp1552–, the gene was replaced with an erythromycin resistance cassette. To generate the wild-type revertant strain of sp1552–, primers JR50 and JR53 of the list were used to amplify sp1552 along with flanking sequence from TIGR4 chromosomal DNA. The PCR product was subsequently purified and transformed into sp1552– and grown on tryptic soy agar supplemented with 3% sheep blood and 1 mM MnCl2 which is toxic to sp1552–. Of the approximately 105 colonies obtained, there were no spontaneous colonies in control transformations. PCR and sequencing were used to confirm reversion of sp1552– to the parental genotype. Oligos used and strains generated are listed in Table 4.
Table 4.
Primers and strains used in this study.
| Strains/primers | Characteristics/sequence |
|---|---|
| Strains | |
| TIGR4 | Pathogenic strain |
| mntE– | sp1552 replaced by Erm cassette |
| WT revertant | mntE– crossed back to TIGR4 sequence |
| Primers | |
| ErmF | GGAAATAAGACTTAGAAGCAAAC |
| ErmR | CCAAATTTACAAAAGCGACTC |
| JR50 | CAAGGGCAATGACCAAGATAGCATAGG |
| JR51 | GTTTGCTTCTAAGTCTTATTTCCCTTGAGATTTGAGATAGATTGCTTCAT |
| JR52 | GAGTCGCTTTTGTAAATTTGGCTTTCATCAAGAAACCAAAAAAGAATAG |
| JR53 | CCAGTCGGATTGGGAAGAAAGTCATCG |
| Sp0461-RT1 | GACTGCACAATCATATGTGTGACCC |
| Sp0461-RT2 | CCATCGCAACAGGCTACCG |
| Sp0463-RT1 | ATGCTGCTGTGATTGCTGCC |
| Sp0463-RT2 | TCAATGCCTTGTCCAGTCGC |
| GyrA-RT1 | TGTGACCCCAGACAAACCCC |
| GyrA-RT2 | CTTGCTCATACGTGCCTCGG |
| Sp1501-RT1 | CAATCATCATAGCACGCGCC |
| Sp1501-RT2 | GCAGAAGCGCAATCTACTGGG |
| Sp1650-RT1 | GTTCCGATTGGGCAAGACCC |
| Sp1650-RT2 | TAGATAACATCAACGCCGTCGC |
| Sp1852-RT1 | CACGCGCAAAGATAGCACCC |
| Sp1852-RT2 | ATTGAGGTCATGGGCTTGGC |
| Sp1992-RT1 | TGTTCCTGCAGTTGCTTGCG |
| Sp1992-RT2 | ATGAAATCAAAGGCGCACCG |
| Sp2148-RT1 | TTATGTTGCACCGTCCAGGC |
| Sp2148-RT2 | TGACGATCACGGATGTTGGC |
Cation sensitivity
To determine cation sensitivity, 1 M solutions of calcium chloride, calcium nitrate, cobalt chloride magnesium chloride, manganese chloride, manganese sulphate, iron chloride, nickel chloride and zinc chloride (Sigma-Aldrich, St Louis, MO) were prepared in Milli-Q water and sterile filtered. A freshly plated bacterial lawn was overlaid with a sterile, 5 mm filter disc with 10 µl of the cation solutions. Plates were incubated overnight, and the zone of inhibition was measured for each disc. Statistical significance was determined by Friedman test. For growth curves, the pH of standard ThyB was adjusted to 6.3 to facilitate solubility of the various cations. Cations were added to final concentrations of 1 mM for magnesium, manganese, calcium and nickel. Cobalt was added at to a final concentration of 100 µM and zinc to a final concentration of 500 µM. Higher concentrations of cobalt and zinc were found to have detrimental effects on the growth of TIGR4.
Elemental analysis
Intracellular elemental composition was measured by ICP-MS as previously described (Outten and O’Halloran, 2001). Cells were grown in chelexed ThyC supplemented with 1 mM calcium chloride, 300 µM manganese chloride, 1 mM magnesium chloride and 300 µM zinc chloride. To measure intracellular cadmium, this medium was further supplemented with 3 µM cadmium chloride. Bacteria were collected by centrifugation and the pellet washed five times in PBS + 10 mM EDTA. Pellet was dried overnight and analysed by the Bodycote Testing Group (Santa Fe Springs, CA). Bacterial cfu and dry weight were enumerated before sample analysis.
54Mn uptake assay
Manganese uptake in live cells was measured as previously described (Maguire, 2007). Strains were grown in ThyB, pH 6.5 in a water bath at 37°C to OD600 = 0.4, after which 1 µCi of 54Mn2+ (Perkin Elmer, Wellesley, MA) per millilitre of media along with 500 µM MnCl2 was added. Aliquots were taken at indicated 15 min intervals, centrifuged to collect cells, and the pellet was washed twice in PBS + 10 mM EDTA to remove extracellular cations. After 1 h, bacteria were collected by centrifugation and re-suspended in ThyB medium lacking isotope to monitor efflux. Counts per minute were measured in the samples by a Perkin Elmer 1480 Wizard 3 Automatic Gamma Counter. A caesium standard and background control was used for all measurements. Data were normalized to OD600 to compensate for cellular growth.
RNA isolation and microarray analysis
To measure the global transcriptional response to high manganese, bacteria were cultured in ThyB supplemented with 500 µM manganese chloride. To measure transcriptional response to high intracellular manganese, both TIGR4 and the sp1552– mutant were cultured in ThyB supplemented with 500 µM manganese chloride. Bacterial RNA was harvested from mid-log-phase cultures by the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA). Microarray experiments were performed as described previously (Orihuela et al., 2004). Wholegenome S. pneumoniae oligonucleotide microarrays (version 6) obtained from the PFGRC at the J. Craig Venter Institute (http://pfgrc.jcvi.org/) were used for microarray experiments (lot numbers 6QSP11062007A and 6QSP11012007A). The array consisted of 70-mer oligonucleotide probes representing 2060 open reading frames from S. pneumoniae strain TIGR4 and 457 from strains G54 and R6, and each probe sequence was spotted at least five times on the array. Data have been submitted to NCBI under accession GSM358422, GSM358242, GSM358241, GSM358231, GSM358229 and GSM358101.
To analyse the microarray data, a series of filtration algorithms was applied to eliminate spots with poor-quality data. Spots flagged (as bad, absent or not found) by the image analysis software GenePix 6.0 (Axon Corp., Union City, CA) and spots having a signal-to-noise ratio less than 1.5 or a background-corrected signal reading less than 20 in both Cy3 and Cy5 channels were excluded from analysis. To correct the intensity bias, loess normalization was applied to each microarray. Of at least five replicate spots on each array, only genes whose corresponding spots passed the filtering criterion at least 50% of the times were reported in further analysis. Of the 11 960 spots dedicated to TIGR4, approximately 16.8% were eliminated because of poor quality or low signal intensity. These were typically gene specific, with all five spots failing to give adequate signal intensity, indicative of either poor hybridization to the oligonucleotide probe or low abundance of the specific transcript. For each experiment, results were summarized from three independent microarray replicates, which included one dye flip experiment for adjusting the dye bias. The average of the fold changes from at least two replicated experiments for each gene was calculated, and those with a minimum twofold change were listed in respective tables. All genes included in the table had a P-value less than 0.05, using a t-distribution test.
Quantitative RT-PCR
Purified mRNA was quantified via NanoDrop with appropriate standards. cDNA synthesis and quantitative PCR were performed using the Superscript III Platinum SYBR Green Two-Step qRT-PCR kit (Invitrogen, San Diego, CA) on a ABI PRISM 7300. Relative transcript abundance of genes was normalized to gyrA, which served as an internal control. Primers used to confirm gene expression are listed in Table 4. The qRT-PCR experiments were performed in triplicate from independently isolated RNA samples.
Western blot and ELISA analysis of pilus expression
Logarithmically growing cells in C+Y media (OD620 = 0.5) were collected by centrifugation and lysed in 0.1% Triton X-100. To ensure equal loading, protein concentration was determined for each lysate via A-280 and loaded accordingly. Duplicate gels stained with Coomassie were used to confirm measured protein concentrations to confirm equivalent loading. Lysates were run on 4–12% NuPAGE Bis-Tris gels (Invitrogen) for 5–6 h to resolve higher-molecular-weight pilus polymers. Proteins were subsequently transferred to PVDF membranes by Western blot. Pilus proteins were detected using rabbit anti-RrgB (1:5000) in PBS + 0.1% Tween-20 + 5% non-fat dry milk.
ELISA analysis using equivalent numbers of bacteria, as determined by cfu, was used to confirm the Western analysis. Strains were grown in C+Y to an OD600 of 0.5, diluted in 0.1 M carbonate buffer (pH 9.6) and transferred in serial dilutions to 96-well ELISA plate. The plates were spun at 2000 g for 10 min, and the supernatant was removed. The plates were dried under a vent hood for 1 h before blocking in 10% FBS for 2 h. Rabbit polyclonal antiserum against Sp0463 (RrgB) was diluted 1:1000 in 10% FBS. The plates were washed five times with wash buffer (1% Tween 20, 1 mM Tris, 154 mM NaCl) and incubated with primary antibody for 1 h. The plates were washed five times and incubated with AP-anti-Rabbit IgG (Southern Biotech, Birmingham, AL) (1:2000) for 1 h. The plates were washed five times, incubated for 20 min in AP-yellow substrate (Sigma), and read at OD405 in a Spectramax 340 plate reader (Molecular Devices, Sunnyvale, CA).
Oxidant stress challenge
S-nitrosoglutathione (GSNO) killing was performed as previously described (Stoeher et al., 2007). For superoxide killing, 60 mM methyl viologen (Sigma) was used as previously described (Tseng et al., 2002). Briefly, triplicate samples of bacterial cultures were grown to an OD600 of 0.50 in C+Y media. Cells were centrifuged, re-suspended in PBS, and cfu were enumerated to quantify input. GNSO or superoxide was then added to the cultures at the desired concentrations and aliquots were taken at 15 min intervals for serial dilution and plating to enumerate bacteria.
Hydrogen peroxide measurement
Hydrogen peroxide (H2O2) indicator plates were made using standard ThyB plates supplemented with ABTS (Sigma, A1888) at a final concentration of 3 mg ml−1 in plates and horseradish peroxidase (Sigma, P8250) at a final concentration of 0.2 mg ml−1 to serve as a colorimetric indicator of H2O2 production. Plates were then dried for 24 h. Serial dilutions (1:10) of bacterial cultures were added to the plates and incubated anaerobically in GasPack anaerobic pouches at 37°C overnight. Plates were then exposed to room air and colour development on the indicator plates was monitored. Images were taken 30 min after exposure to atmospheric oxygen. For measurement of H2O2 production in liquid media, bacteria were cultured in C+Y media to an OD of 0.8 and subsequently back-diluted 1:20 into fresh ThyB or ThyB supplemented with 1 mM MnCl2. Bacteria were allowed to grow for 2 h at 37°C/5% CO2, and then 1 ml aliquots were taken, bacteria collected via centrifugation, and re-suspended in sterile PBS. Bacteria were then incubated at room temperature for 20 min to allow H2O2 production. H2O2 production was measured with a H2O2 assay kit from National Diagnostics (Atlanta, GA). A PBS blank and serial dilutions of a H2O2 standard solution were used as a background measurement and standard curve respectively. Bacteria were enumerated by serial dilution on TSA + 3% blood agar plates and respective values for H2O2 production were normalized for cfu.
Mouse challenge
Virulence studies were performed as previously described (Orihuela et al., 2004). Exponential cultures were centrifuged, washed in sterile PBS, and re-suspended at the appropriate concentration in PBS as confirmed by serial dilution and plating on blood agar plates. Six-week-old female BALB/cJ mice (Jackson Laboratory, Bar Harbor, ME) were maintained in BSL2 facilities. All experiments were performed under inhaled isoflurane (2.5%). Bacteria were introduced by intranasal administration of 107 cfu in 25 µl of PBS. Mice were monitored daily for signs of infection. Nasal passages were lavaged and blood was extracted from the tail vein 24 and 72 h post infection, diluted, and plated on blood agar to ascertain bacterial colonization and bacteraemia. Statistical analysis was performed using Kaplan–Meier survival estimates for survival and Student’s t-test for bacterial titres.
Acknowledgements
We thank Caroline Obert for assistance with the statistical analysis and The Institute for Genomic Research (TIGR) for supplying the microarrays. This work was support in part by NIH R01AI27913, U54HL070590 and the American Lebanese Syrian Associated Charities.
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agranoff DD, Monahan IM, Mangan JA, Butcher PD, Krishna S. Mycobacterium tuberculosis expresses a novel pH-dependent divalent cation transporter belonging to the Nramp family. J Exp Med. 1999;190:717–724. doi: 10.1084/jem.190.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Maghredi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch Biochem Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arirachakaran P, Benjavongkulchai E, Leungpailin S, Ajdic D, Banas JA. Manganese affects Streptococcus mutans virulence gene expression. Caries Res. 2007;41:503–511. doi: 10.1159/000110883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barocchi MA, Ries J, Zogaj X, Hemsley C, Albiger B, Kanth A, et al. A pneumococcal pilus influences virulence and host inflammatory responses. Proc Natl Acad Sci USA. 2006;10:2857–2862. doi: 10.1073/pnas.0511017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsevich VV, Pakrasi HB. Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in pho-tosynthetic oxygen evolution process. EMBO J. 1995;14:1845–1853. doi: 10.1002/j.1460-2075.1995.tb07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battig P, Muhlemann K. Influence of the spxB gene on competence in Streptococcus pneumoniae. J Bacteriol. 2008;190:1184–1189. doi: 10.1128/JB.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I, Jha JK, Fromm N. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology. 2008;154:2275–2282. doi: 10.1099/mic.0.2008/019265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenot A, King KY, Caparon MG. The PerR regulon in peroxide resistance and virulence in Streptococcus pyogenes. Mol Microbiol. 2005;55:221–234. doi: 10.1111/j.1365-2958.2004.04370.x. [DOI] [PubMed] [Google Scholar]
- Brenot A, Weston BF, Caparon MG. A PerR-regulated metal transporter (PmtA) is an interface between oxidative stress and metal homeostasis in Streptococcus pyogenes. Mol Microbiol. 2007;63:1185–1196. doi: 10.1111/j.1365-2958.2006.05577.x. [DOI] [PubMed] [Google Scholar]
- Chapuy-Regaud S, Jones HE, Campbell AK, Trombe MC. Validation of the use of aequorin for cytoplasmic free calcium determination by chemiluminescence in Streptococcus pneumoniae. J Soc Biol. 2001;195:271–276. [PubMed] [Google Scholar]
- Chicharro JL, Serrano V, Urena R, Guiterrez AM, Carvajal A, Fernandez-Hernando P, Lucia A. Trace elements and electrolytes in human resting mixed saliva after exercise. Br J Sports Med. 1999;33:204–207. doi: 10.1136/bjsm.33.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gammaradiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Kataoka T, Hebb DM, White RG, Ryan PR. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell. 2003;15:1131–1142. doi: 10.1105/tpc.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Buxbaum L, Toth K, Eisenstadt E, Silver S. Regulation of manganese accumulation and exchange in Bacillus subtilis W23. J Bacteriol. 1973;113:1373–1380. doi: 10.1128/jb.113.3.1373-1380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Fan B, Rosen BP, Franke S, Nies DH, Rensing C. ZitB (YbgR), a member of the cation diffusion facilitator family, is an additional zinc transporter in Escherichia coli. J Bacteriol. 2001;183:4664–4667. doi: 10.1128/JB.183.15.4664-4667.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Micobiol. 2005;183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- Guedon E, Moore CM, Que Q, Wang T, Ye RW, Helmann JD. The global transcriptional response of Bacillus subtilis to manganese involved the MntR, Fur, TnrA, and sigmaB regulons. Mol Microbiol. 2003;49:1477–1491. doi: 10.1046/j.1365-2958.2003.03648.x. [DOI] [PubMed] [Google Scholar]
- Hall JL, Williams LE. Transition metal transporters in plants. J Exp Bot. 2003;54:2601–2613. doi: 10.1093/jxb/erg303. [DOI] [PubMed] [Google Scholar]
- Haney CJ, Grass G, Franke S, Rensing C. New developments in the understanding of the cation diffusion facilitator family. J Ind Microbiol Biotechnol. 2005;32:215–226. doi: 10.1007/s10295-005-0224-3. [DOI] [PubMed] [Google Scholar]
- Hanks TS, Liu M, McClure MJ, Fukumura M, Duffy A, Lei B. Differential regulation of iron and manganese specific MtsABC and heme-specific HtsABC transporters by the metaloregulator MtsR of Group A Streptocococus. Infect Immun. 2006;74:5132–5139. doi: 10.1128/IAI.00176-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanski E, Horwitz PA, Caparon MG. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–1289. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- Inaika T, Matsumura Y, Tsuchido T. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J Bacteriol. 1999;181:1939–1943. doi: 10.1128/jb.181.6.1939-1943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Smith AW, Jenkinson HF. Oxidative stress tolerance is manganese regulated in Streptococcus gordonii. Microbiology. 2002;148:3255–3263. doi: 10.1099/00221287-148-10-3255. [DOI] [PubMed] [Google Scholar]
- Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun. 2006;74:1171–1180. doi: 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry, and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJE, Kuipers OP. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+ resistance gene czcD in Streptococcus pneumoniae. Mol Microbiol. 2007;65:1049–1063. doi: 10.1111/j.1365-2958.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- Kloosterman T, Witwicki RM, vander Kooi-Pol MM, Bijlsma JJ, Kuipers OP. Opposite effects of Mn2+ and Zn2+ on the PsaR-mediated expression of the virulence genes pcpA, prtA and psaBCA of Streptococcus pneumoniae. J Bacteriol. 2008;190:5382–5393. doi: 10.1128/JB.00307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Zhang Y, Herzberg MC. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J Bacteriol. 2008;190:4632–4640. doi: 10.1128/JB.00276-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S, Hotchkiss RD. A study of the genetic material determining enzyme in the pneumococcus. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Lauer P, Rinaudo CD, Soriani M, Margarit I, Maione D, Rosini R, et al. Genome analysis reveals pili in Group B Streptococcus. Science. 2005;309:105. doi: 10.1126/science.1111563. [DOI] [PubMed] [Google Scholar]
- Low YL, Jakubovics NS, Flatman JC, Jenkinson HF, Smith AW. Manganese-dependent regulation of the endocarditis-associated virulence factor EfaA of Entereococcus faecalis. J Med Microbiol. 2003;52:113–119. doi: 10.1099/jmm.0.05039-0. [DOI] [PubMed] [Google Scholar]
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- McAllister LJ, Tseng H, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53:889–901. doi: 10.1111/j.1365-2958.2004.04164.x. [DOI] [PubMed] [Google Scholar]
- McCluskey J, Hinds J, Husain S, Witney A, Mitchell TJ. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol Microbiol. 2004;51:1661–1675. doi: 10.1111/j.1365-2958.2003.03917.x. [DOI] [PubMed] [Google Scholar]
- Maguire ME. Magnesium, manganese, and divalent cation transport assays in intact cells. Methods Mol Biol. 2007;394:289–305. doi: 10.1007/978-1-59745-512-1_14. [DOI] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P, Cellier MFM. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol. 2000;35:1065–1078. doi: 10.1046/j.1365-2958.2000.01774.x. [DOI] [PubMed] [Google Scholar]
- Marra A, Lawson S, Asundi JS, Brigham D, Hromockyj AE. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology. 2002;148:1483–1491. doi: 10.1099/00221287-148-5-1483. [DOI] [PubMed] [Google Scholar]
- Martin-Galiano AJ, Overweg K, Ferrandiz MJ, Reuter M, Wells JM, de la Campa AG. Transcriptional analysis of the acid tolerance response in Streptococcus pneumoniae. Microbiology. 2005;151:3935–3946. doi: 10.1099/mic.0.28238-0. [DOI] [PubMed] [Google Scholar]
- Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family; improved signature and prediction of substrate specificity. BMC Genomics. 2007;8:107–133. doi: 10.1186/1471-2164-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CM, Helmann JD. Metal ion homeostasis in Bacillus subtilis. Curr Opin Microbiol. 2005;8:188–195. doi: 10.1016/j.mib.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Moore CM, Gaballa A, Hui M, Ye RW, Helmann JD. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol Microbiol. 2005;57:27–40. doi: 10.1111/j.1365-2958.2005.04642.x. [DOI] [PubMed] [Google Scholar]
- Munkelt D, Grass G, Nies DH. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J Bacteriol. 2004;186:8036–8043. doi: 10.1128/JB.186.23.8036-8043.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AL, Ries J, Bagnoli F, Dahlberg S, Rounioja S, Tschop J, et al. RrgA is a pilus-associated adhesin and virulence factor in S. pneumoniae. Mol Microbiol. 2007;66:329–340. doi: 10.1111/j.1365-2958.2007.05908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obert C, Sublett J, Kaushal D, Hinojosa E, Barton T, Tuomanen EI, Orihuela CJ. Identification of a Candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect Immun. 2006;74:4766–4777. doi: 10.1128/IAI.00316-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela CJ, Radin JN, Sublett JE, Gao G, Kaushal D, Tuomanen EI. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun. 2004;72:5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- Que Q, Helmann JD. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diptheria toxin repressor family of proteins. Mol Microbiol. 2000;35:1454–1468. doi: 10.1046/j.1365-2958.2000.01811.x. [DOI] [PubMed] [Google Scholar]
- Rosch JW, Sublett J, Gao G, Wang Y, Tuomanen EI. Calcium efflux is essential for bacterial survival in the eukaryotic host. Mol Microbiol. 2008a;70:435–444. doi: 10.1111/j.1365-2958.2008.06425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch JW, Mann B, Thornton J, Sublett J, Tuomanen E. Convergence of regulatory networks on the pilus locus on Streptococcus pneumoniae. Infect Immun. 2008b;76:3187–3196. doi: 10.1128/IAI.00054-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeher UH, Kidd SP, Staffor SL, Jennings MP, Paton JC, McEwan AG. A pneumococcal MerR-like regulator and S-nitroglutathione reductase are required for systemic virulence. J Infect Dis. 2007;196:1820–1826. doi: 10.1086/523107. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Tseng HJ, McEwan AG, Paton JC, Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanthanouvong V, Roomans GM. Methods for determining the composition of nasal fluid by X-ray microanalysis. Microsc Res Tech. 2004;63:122–128. doi: 10.1002/jemt.20020. [DOI] [PubMed] [Google Scholar]
- Wei Y, Fu D. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP (FieF) J Biolog Chem. 2005;280:33716–33724. doi: 10.1074/jbc.M506107200. [DOI] [PubMed] [Google Scholar]
- Wurch T, Lestienne F, Pauwels PJ. A modified overlap extension PCR method to create chimeric genes in the absence of restriction enzymes. Biotechnol Tech. 1998;12:653–657. [Google Scholar]
- Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ, Andrew PW. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun. 2000;68 doi: 10.1128/iai.68.5.2819-2826.2000. 2819–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]