SUMMARY
High-density lipoproteins (HDL) are complexes of proteins (mainly apoA-I and apoA-II) and lipids that remove cholesterol and prevent atherosclerosis. Understanding distinct properties of the heterogeneous HDL population may help develop new diagnostic tools and therapies for atherosclerosis. Mature human HDL form two major subclasses differing in particle diameter and metabolic properties, HDL2 (large) and HDL3 (small). These subclasses are comprised of HDL(A-I) containing only apoA-I, and HDL(A-I/A-II) containing apoA-I and apoA-II. ApoA-I is strongly cardioprotective, yet the smaller, more hydrophobic apoA-II has unclear function. ApoA-II is thought to counteract the cardioprotective action of apoA-I by stabilizing HDL particles and inhibiting their remodeling. To test this notion, we performed the first kinetic stability study of human HDL subclasses. The results revealed that the stability of plasma spherical HDL decreases with increasing particle diameter; this may facilitate preferential cholesterol ester uptake from large lipid-loaded HDL2. Surprisingly, size-matched plasma HDL(A-I/A-II) showed comparable or slightly lower stability than HDL(A-I); this is consistent with the destabilization of model discoidal HDL observed upon increasing A-II/A-I ratio. These results clarify the roles of the particle size and protein composition in HDL remodeling and help reconcile conflicting reports regarding the role of apoA-II in this remodeling.
Keywords: Lipoprotein fusion, apolipoprotein dissociation, kinetic barriers, reverse cholesterol transport, atherosclerosis
INTRODUCTION
High-density lipoproteins (HDL) are macromolecular complexes differing in shape (nascent discoidal or mature spherical), diameter (7−12 nm), density (1.21−1.063 g/ml), protein and lipid composition, and function (recently reviewed in1-3). Plasma levels of HDL, HDL cholesterol (mainly in the form of cholesterol esters (CE) sequestered in the particle core), and the major HDL protein, apolipoprotein A-I (apoA-I), correlate inversely with the development of coronary artery disease, making HDL an attractive therapeutic target (4,5 and references therein). Cardioprotective action of HDL and apoA-I is attributed mainly to their central role in the reverse cholesterol transport (RCT), which is the sole pathway of cholesterol removal, along with their antioxidant, anti-inflammatory and anti-apoptotic action.6,7 Efforts to design new diagnostic tools and therapies for atherosclerosis to complement statins, low-density lipoprotein lowering drugs, have focused on raising HDL quantity and, most recently, quality.1-5 This necessitates understanding of the distinct functional properties of the heterogeneous HDL population. Our goal is to provide the physicochemical basis for understanding aspects of functional remodeling of human HDL differing in particle size and protein composition.
HDL heterogeneity reflects multiple stages of HDL remodeling in RCT.8 At an early stage, interaction of lipid-poor apoA-I with the plasma membrane, mediated by the ATP-binding cassette transporter A-1, leads to the formation of nascent discoidal HDL. These small particles are comprised of a cholesterol-containing phospholipid bilayer and two copies of apoA-I whose amphipathic α-helices wrap around the disk perimeter, conferring particle stability and solubility (reviewed in 9). Nascent HDL form preferred substrates for lecithin : cholesterol acyltransferase (LCAT) that is activated by apoA-I and, to a lesser extent, by other exchangeable (water-soluble) apolipoproteins. Apolar CE produced by LCAT move to the particle interior, converting disks to small spheroid HDL3 (d=7−9 nm, two copies of apoA-I per particle) (Fig. 1). Upon further action of LCAT and other plasma factors, such as CE and phospholipid transfer proteins, small HDL3 are converted to larger HDL2 particles (d=10−12 nm, three-to-four copies of apoA-I per particle). Conversion of HDL3 to HDL2 involves lipoprotein fusion and dissociation of lipid-poor apoA-I that re-enters RCT.8 After further remodeling, large HDL2 upload their cargo of CE to the liver via the selective uptake of apolar lipids mediated by the scavenger receptor SR-BI.11 At this final step, HDL disintegrate and the dissociated apolipoproteins re-enter RCT or are catabolized.
Figure 1.
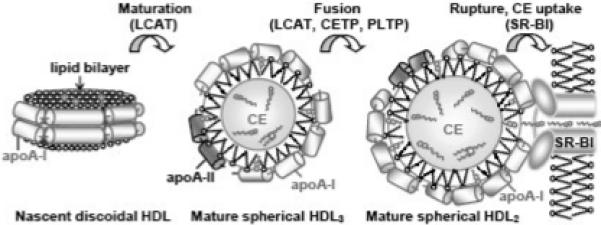
Cartoon representation of the major subclasses of plasma HDL differing in shape and size and their interconversions. Nascent discoidal HDL, mature spherical HDL3 (small, two copies of apoA-I per particle) and mature spherical HDL2 (large, three to four copies of apoA-I per particle) are shown. ApoA-II comprises ∼23% by weight of the total protein in HDL3 (i.e. one copy of apoA-II per particle), but only ∼10% in HDL2; CE content in HDL2 is nearly double of that in HDL3.10 LCAT reaction converts discoidal into small spherical HDL; further remodeling into large spherical HDL is mediated by LCAT and by cholesterol ester and phospholipid transfer proteins (CETP and PLTP).
In addition to apoA-I (243 a. a.) that comprises ∼70% of the total HDL protein mass, nearly 20% of the protein in human HDL is comprised of apoA-II (an S-S-linked dimer of two 77 a. a. molecules). These two major HDL proteins are distributed on two HDL subclasses: HDL(A-I) that contain apoA-I only, and HDL(A-I/A-II) that contain both apoA-I and apoA-II in an approximately 2:1 molar ratio.12 In contrast to apoA-I, whose cardioprotective action is well established, the function of apoA-II on HDL remains controversial.13-15 Mouse model studies yielded conflicting results that, depending on the model, suggested pro- or anti-atherogenic properties of apoA-II (16,17 and references therein). Until recently, the consensus was that apoA-II is a poor cardioprotector and may even be pro-atherogenic;13,14 however, recent epidemiologic studies report that apoA-II is cardioprotective.15,17,18 Furthermore, apoA-II has been proposed to counteract the atheroprotective effects of apoA-I via several mechanisms, including inhibition of various steps of HDL remodeling in RCT;14,19-22 however, not all reports claim that apoA-II inhibits HDL remodeling (reviewed in15). For example, some studies report that apoA-II prevents HDL remodeling and dissociation of lipid-poor apoA-I,19,21 which is the primary acceptor of cell cholesterol,22 while others report that apoA-II promotes such remodeling23 and may provide a reservoir of easily exchangeable apoA-I.24 The latter is consistent with the well-established ability of apoA-II to displace apoA-I from HDL.25 This and other conflicting evidence suggests that apoA-II plays a complex role in modulating metabolic remodeling of HDL, which may depend on the experimental system.15
We postulate that the effects of apoA-II on HDL remodeling and stability may be closely related. In fact, HDL remodeling in vivo by plasma factors and in vitro by thermal, chaotropic, detergent and other perturbations involves similar morphologic transitions, such as protein dissociation and lipoprotein fusion followed by rupture and release of apolar core lipids. Consequently, HDL remodeling in vivo and in vitro may be modulated by similar kinetic barriers.26-31 Therefore, analysis of the effects of apoA-II on HDL stability may help to better understand the role of this enigmatic protein in metabolic remodeling of HDL.
ApoA-II is widely believed to enhance HDL stability. Compared to apoA-I and other exchangeable apolipoproteins, apoA-II is thought to have higher lipid binding affinity and be practically non-exchangeable under normal physiologic conditions (reviewed in31), even though it readily dissociates from the particle surface in mice overexpressing human apoA-II16 or in reconstituted HDL (rHDL).32 Increased lipid binding affinity of apoA-II is attributed to the high hydrophobicity of its amphipathic α-helices that have ∼50% apolar surface, as compared to ∼30% in apoA-I and other exchangeable apolipoproteins.33 This may facilitate deeper penetration of the apoA-II helices into the phospholipid surface and their longer retention in this surface. In fact, earlier stability studies of mature plasma HDL showed that apoA-II dissociation starts at higher denaturant concentrations and higher temperatures than that of apoA-I.34,35 This lead to a widely accepted notion that apoA-II stabilizes HDL assembly and inhibits its remodeling; however, no comparative stability studies of plasma spherical HDL(A-I) and HDL(A-I/A-II) have been reported to substantiate this notion. Moreover, contrary to this notion, studies by Swaney and Palmieri suggested that A-II does not stabilize the HDL structure through interactions with A-I.36 Furthermore, the existing report on the stability of model spherical rHDL(A-I) and rHDL(A-I/A-II)37 should be treated with caution, since it relied on thermodynamic “end-point” measurements after incubation with denaturant, an approach that may not apply to lipoproteins whose stability is under kinetic control.26,27,30 Thus, to our knowledge, there is no strong experimental support for the stabilizing role of apoA-II on spherical HDL.
Moreover, several studies of discoidal rHDL clearly showed that the binary complexes containing model phospholipid dimyristoyl phosphatidylcholine (DMPC) and apoA-II are less stable than similar complexes containing apoA-I.32,38 The rank order of the disk stability, A-I > A-IIdimer > A-IImonomer ≥ C-I, correlated with the protein size rather than hydrophobicity, which was attributed to higher enthalpy of dissociation of larger proteins from the particle surface.32 This is consistent with a report that addition of apoA-II to discoidal rHDL(A-I) destabilizes apoA-I on the hybrid rHDL(A-I/A-II) particles.24 The results of these and other rHDL disk studies36 challenge the stabilizing role of apoA-II on HDL and suggest that the effect of apoA-II on the stability of spherical HDL should be re-evaluated.
Here, we report the first kinetic stability study of human plasma HDL(A-I) and HDL(A-I/AII), as well as of HDL2 and HDL3. Despite distinct differences in protein and lipid composition, function, and cardioprotective properties of the larger, more buoyant, lipid-rich HDL2 and smaller, denser, protein-rich HDL3,1,3,8,10,39-42 their stability studies have been limited to one work that used “end-point” measurements after incubation with Gdn HCl and reported higher stability of larger particles.43 Our results show that, contrary to the existing notions, i) the stability of plasma spherical HDL increases with decreasing particle size, and ii) compared to apoA-I, apoA-II has no detectable stabilizing effect on plasma spherical HDL and has a clear destabilizing effect on model discoidal rHDL, which increases with increasing A-II/A-I ratio. These results prompt us to re-evaluate the roles of particle size and protein composition in HDL stability, and help reconcile some of the conflicting reports on the role of apoA-II in HDL remodeling.
RESULTS
Effects of particle size on the stability of plasma spherical HDL
First, we analyzed thermal denaturation of human plasma HDL2 and HDL3 that were isolated by density centrifugation (Fig. 2A).44 In kinetic experiments, HDL denaturation was triggered by a rapid temperature increase (T-jump) from 25 °C to a higher constant value, and the time course of α-helical protein unfolding was monitored by circular dichroism (CD) spectroscopy at 222 nm. Thermal unfolding of HDL proteins has two-phase kinetics: the first (fast) phase corresponds to unfolding of HDL-anchored protein, and the second (slow) phase involves dissociation of a protein fraction and particle fusion followed by rupture.29 The results in Fig. 2B showed that, at any temperature explored, the second unfolding phase is faster in HDL2 than in HDL3, implying that HDL2 are remodeled faster and thus are less stable than HDL3 (Fig. 2B). This was confirmed by the melting data recorded during HDL heating at a constant rate by far-UV CD for protein unfolding, by 90° light scattering for changes in the particle size, and by differential scanning calorimetry (DSC) for the heat effects of HDL transitions (Fig. 2C, D). The heat capacity data in Fig. 2D (recorded from HDL in low-salt solution at 90 °C/h heating rate) showed two peaks. The first peak reflects apoA-I dissociation and HDL fusion into larger lipoprotein-like particles, and the second sharper peak reflects particle rupture, i.e. release of core lipids that coalesce into large droplets accompanied by dissociation of apoA-I and apoA-II (29,35 and references therein). In HDL2, these transitions occurred at lower temperatures compared to HDL3; for example, rupture was centered at 107 °C in HDL2 and 114 °C in HDL3 (Fig. 2D). Earlier, we showed that the temperatures of HDL fusion and rupture decrease upon i) reduction in the heating rate, which is characteristic of slow kinetically controlled reactions with high activation energy,26,29 and ii) increasing salt concentrations to near-physiologic range, and thereby shielding favorable electrostatic interactions on HDL.29 Here, to reduce the temperature of HDL transitions below 100 °C (i.e., to the range observable by spectroscopy), we reduced the heating rate to 6 °C/h and increased the salt concentration to 150 mM NaCl. The resulting CD (not shown) and 90° light scattering melting data (Fig. 2C) were consistent with the DSC data and showed lower transition temperatures for HDL2 as compared to HDL3. For example, a large increase in the light scattering corresponding to HDL2 rupture and lipid coalescence into large droplets was observed above 90 °C, whereas HDL3 rupture did not occur until above 100 °C under these conditions (Fig. 2C, grey and black lines). Taken together, the results in Fig. 2 clearly show that HDL2 are less stable than HDL3.
Figure 2.
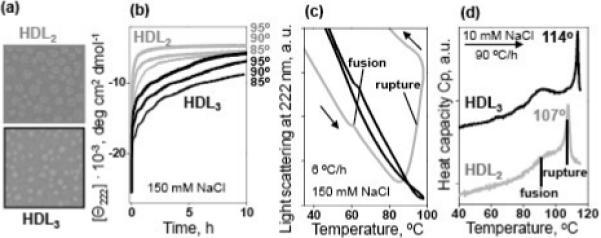
Comparison of thermal stability of human plasma HDL2 and HDL3.
(a) Negative staining electron micrographs of HDL2 and HDL3 used in our stability studies.
(b) Kinetic data of HDL2 (gray) and HDL3 (black) recorded in T-jumps from 25 to 85−95 °C (final temperatures are indicated on the lines); the time course of the protein unfolding was monitored by CD at 222 nm. Sample conditions are 33 μg/mL protein in buffer B (10 mM Na phosphate, pH 7.6, 1 mM EDTA, 0.05% NaN3, 150 mM NaCl).
(c) Melting data recorded by 90° light scattering to monitor changes in the particle size upon HDL heating and cooling at a constant rate of 6 °C/h. The directions of the temperature changes are shown by arrows; the data are shifted along the y-axis to avoid overlap. Sample conditions and color-coding are as in panel a. Negative slopes in the light scattering data result from an optical artifact of the CD instrument and from the temperature dependence of the refractive index.45 HDL fusion (that causes a small increase in the particle size) and rupture (that causes lipid coalescence into large droplets leading to a large increase in the light scattering29) are indicated.
(d) Excess heat capacity Cp(T) recorded by DSC upon HDL heating at a rate of 90 °C/h. Sample conditions are 2.5 mg/mL protein in buffer A (10 mM Na phosphate, pH 7.6, 1 mM EDTA, 0.05% NaN3) containing 10 mM NaCl. The data are shifted along the Y-axis to avoid overlap; the distance between the ticks is 1 kcal/mol·K. HDL fusion and rupture are indicated.
Next, the time course of protein dissociation and particle remodeling was analyzed by nondenaturing polyacrylamide gel electrophoresis (PAGE) (Fig. 3A). HDL2 and HDL3 were incubated at 85 °C and the aliquots taken after 1−60 min of incubation were analyzed. In HDL2, progressive protein dissociation was accompanied by an increase in the particle size due to fusion (Fig. 3A) and, eventually, rupture (observed by electron microscopy, data not shown). Interestingly, in HDL3 these transitions were preceded by an additional step that converted small HDL3 into larger HDL2-size particles (Fig. 3A). This additional step, which mimics aspects of HDL3 fusion into HDL2 during RCT,27 is probably responsible for the higher stability of HDL3 compared to HDL2 (Fig. 2).
Figure 3.
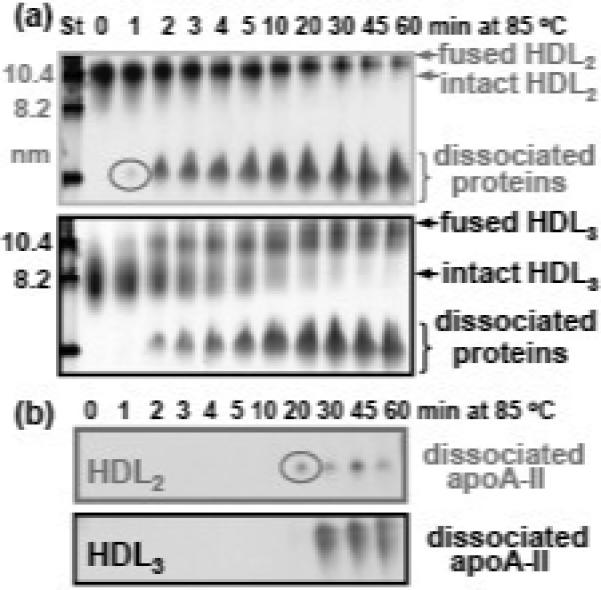
Time course of the heat-induced protein dissociation and particle fusion in HDL2 and HDL3. (a) HDL (1 mg/mL protein in buffer A) were incubated at 85 °C, and the aliquots taken after 1−60 min of incubation (as indicated on the lanes) were subjected to non-denaturing PAGE. Molecular size standards (St) are shown in nm. (b) Non-denaturing PAGE followed by Western blotting using antibodies against apoA-II. Circles indicate the first appearance of the total protein (a) and of apoA-II (b) dissociated from HDL2. Increased band intensity observed in HDL3 as compared to HDL2 reflects higher content of apoA-II in HDL3.10
Protein dissociation during incubation at 85 °C was further analyzed by Western blotting using antibodies against apoA-I (not shown) and apoA-II (Fig. 3B). Dissociated protein that was first observed by non-denaturing PAGE after 1 min incubation in HDL2 and after 2 min in HDL3 (Fig. 3A) was apoA-I, while dissociated apoA-II was not detected until 20 min of incubation in HDL2 and 30 min in HDL3 (Fig. 3B). Thus, the protein that dissociated from HDL during the first 10−15 min of incubation at 85 °C was a fraction of apoA-I, followed by further dissociation of apoA-I and apoA-II. This is consistent with the earlier studies showing two distinct stages in HDL denaturation corresponding to two calorimetric transitions (Fig. 2C). In the first transition, dissociation of a sub-population of apoA-I causes HDL fusion, and in the second transition, further dissociation of apoA-I and apoA-II causes HDL rupture and release of apolar lipids.29,35 Moreover, the results in Fig. 3 confirm that HDL3 are more stable than HDL2 and show that, despite relative enrichment of HDL3 in apoA-II,10 apoA-II dissociates from HDL3 slower than from HDL2 (Fig. 3B).
Does higher stability of HDL3 result from their smaller size or from their enrichment in apoA-II? To test the effects of particle size, HDL were separated by density centrifugation into multiple fractions from 1.08−1.21 g/mL (Fig. 4A). Higher-density lipoproteins are smaller, since proteins are denser than lipids, and the protein:lipid ratio increases with surface:volume ratio since proteins are located on the surface whereas lipids form the particle core. The thermal stability of each fraction was assessed in kinetic and melting experiments by far-UV CD, 90° light scattering and turbidity (Fig. 4). The melting data in Fig. 4B, C were recorded at a very slow heating rate (6 °C/h), facilitating the observation of HDL rupture below 100 °C. The results clearly show that the smaller the particle, the higher the rupture temperature (Fig. 4B, C) and the slower the protein unfolding (Fig. 4D). Therefore, smaller spherical HDL are more stable than their larger counterparts.
Figure 4.
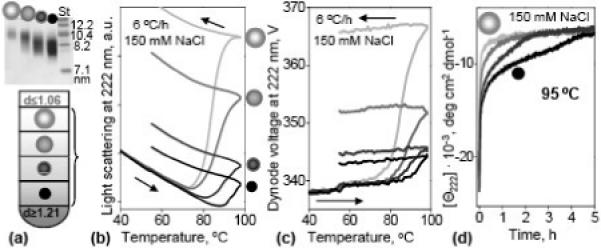
Effect of the particle size on the stability of plasma spherical HDL. (a) HDL were separated by density into four fractions, from small dense (dark) to large buoyant (light); the density of these fractions ranged from 1.068−1.089, 1.089−1.101, 1.101−1.125, and 1.125−1.21 g/ mL. The particle size was confirmed by non-denaturing PAGE (4−12% gradient, top panel). Each fraction was heated and cooled from 25 to 98 °C at a rate of 6 °C/h, and 90° light-scattering (b) and turbidity (c) melting data were recorded at 222 nm. Arrows show directions of the temperature changes. (d) Time course of the HDL protein unfolding in a T-jump from 25 to 95 °C monitored by CD at 222 nm. Sample conditions in (b-d) are 33 μg/mL protein in buffer B.
Effects of A-II on the stability of plasma spherical HDL
To differentiate between the effects of the particle diameter and apoA-II content, HDL2 and HDL3 were isolated by density centrifugation, followed by immunoaffinity chromatography to separate HDL3(A-I/A-II) from HDL3(A-I).12 Size homogeneity of the final preparations was confirmed by non-denaturing PAGE and by negative staining electron microscopy (EM); protein composition was confirmed by sodium dodecyl sulfate (SDS) and by non-denaturing PAGE followed by Western blotting using antibodies for apoA-I or apoA-II (Fig. 5A). Analysis by CD, light scattering and turbidity in melting and kinetic experiments showed that the stability of HDL3(A-I/A-II) was comparable or slightly lower that that of HDL3(A-I) (Fig. 5B-D). Similarly, the stability of HDL2(A-I/A-II) was comparable to that of HDL2(A-I); a similar effect was observed in total HDL (data not shown). We conclude that, contrary to the accepted notion, apoA-II has no detectable stabilizing effect on plasma spherical HDL(A-I/A-II) as compared to size-matched HDL(A-I). Therefore, higher stability of HDL3 may not result from their enrichment in apoA-II as compared to HDL2.
Figure 5.
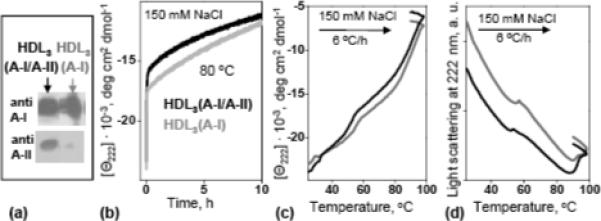
Comparison of thermal stability of HDL(A-I/A-II) and HDL(A-I). HDL3(A-I/A-II) (black) and HDL3(A-I) (grey) were isolated by immunoaffinity,12 and their protein composition was assessed by non-denaturing PAGE followed by Western blotting using antibodies for apoA-I and apoA-II (a). Time course of HDL protein unfolding monitored by CD in a T-jump from 25−80 °C (b). Melting data recorded at 222 nm by CD (c) and 90° light-scattering (d) during HDL heating at a constant rate of 6 °C/h. Sample conditions in (b-d) are 33 μg/mL proteins in buffer B.
Effects of A-II on the stability of reconstituted discoidal HDL
The absence of a stabilizing effect of apoA-II on plasma spherical HDL is consistent with earlier studies of discoidal rHDL reconstituted from model phospholipids such as DMPC (14:0, 14:0) and apoA-I and/or apoA-II, which provide simple structural and functional models for nascent HDL.24,32,36,38 Thermal denaturation of the apoA-I:DMPC and apoA-II:DMPC binary discoidal complexes showed that the former are more stable,32,38 a result that is independent of the disk diameter.46 To test whether this result extends to ternary complexes of DMPC with apoA-I and apoA-II, we prepared such hybrid complexes varying in A-II:A-I molar ratio.24 EM analysis of these hybrid particles confirmed that the disk size and morphology remained invariant upon addition of apoA-II; protein composition of these hybrid disks was confirmed by Western blotting (not shown). Melting data of these rHDL(A-I/A-II) disks, taken together with similar data of rHDL(A-I) and rHDL(A-II) disks, as well as their kinetic T-jump data showed a clear trend: the higher the apoA-II content the lower the disk stability (Fig. 6).
Figure 6.
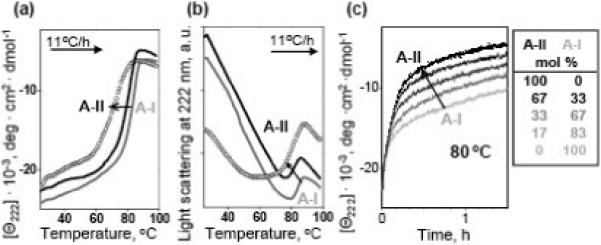
Effects of apoA-II on thermal stability of discoidal rHDL. Sample conditions are 20 μg/ mL protein in buffer A. DMPC complexes containing apoA-I alone (grey line), apoA-II alone (circle), or both apoA-I and apoA-II (black line) were heated at a rate of 11 °C/h, and the melting data were recorded at 222 nm by CD (a) and 90° light scattering (b). Black lines correspond to AII:A-I molar ratio of 1:2; the data at other ratios (not shown to avoid overlap) demonstrated a similar trend. (c) Time course of rHDL protein unfolding monitored by CD in a T-jump from 25−80 °C; arrow indicates increase in the apoA-II:apoA-I ratio (molar fractions are listed in the table). Increase in apoA-II:apoA-I ratio reduces the apparent melting temperature (a, b) and accelerates the denaturation of rHDL (c), indicating reduced stability.
This result is consistent with stability studies by Durbin and Jonas who used discoidal rHDL with lipid composition ((16:0,18:1)-PC and cholesterol) that was representative of plasma HDL; incorporation of increasing amounts of apoA-II into these apoA-I-containing rHDL reportedly destabilized the apoA-I molecule and eventually displaced it from the rHDL surface.24 In summary, our results using discoidal rHDL showed that the disk stability gradually decreases with increasing apoA-II/apoA-I ratio, a result that is consistent with the earlier studies.24,32,38 Taken together, our stability studies of plasma spherical and model discoidal HDL (Fig. 5, 6) refute the widely accepted notion of specific stabilizing interactions formed by A-II on HDL.
DISCUSSION
Large lipid-loaded HDL are less stable: Physical origins and functional implications
Our studies of human HDL have revealed that smaller spherical particles are more stable than their larger, lipid-loaded counterparts. This is evident from far-UV CD, 90° light scattering, turbidity, DSC and non-denaturing PAGE data showing slower denaturation kinetics and higher apparent temperatures of fusion and rupture in smaller particles (Fig. 2, 4). Higher stability of smaller particles is consistent with an additional denaturation step observed by non-denaturing PAGE in HDL3, i.e. fusion of HDL3 into HDL2-size particles (Fig. 3). The kinetic barrier associated with this step probably decelerates HDL3 disintegration and thereby increases HDL3 stability as compared to HDL2. Increased stability of HDL3 does not result from their enrichment in apoA-II, since our melting and kinetic data clearly show that the stability of HDL3(A-I/A-II) is comparable or lower than that of HDL3(A-I) (Fig. 5). Therefore, higher stability of smaller HDL must result from other factors; these may include higher surface-to-core and protein-to-lipid ratios in smaller particles, as well as their distinct lipid composition. Thus, compared to large HDL, small HDL have reduced content of sphingomyelin and increased content of free fatty acids,41, 47 which may affect particle fusion and rupture.
Increased stability of smaller particles reported here is consistent with recent study by Pownall and colleagues of HDL disruption by streptococcal serum opacity factor that causes HDL fusion and dissociation of an fraction of apoA-I; the authors use kinetic approach to show that smaller HDL undergo slower disruption and fusion than their larger counterparts.48 This suggests that increased stability of smaller particles is not limited to thermal denaturation but extends to HDL perturbation by other factors.
The rank order of particle stability reported here, HDL3>HDL2, is consistent with distinct functional properties of these HDL subclasses (1,8,41 and references therein). Small protein-rich HDL play dominant roles at early stages of RCT as preferential acceptors of cell cholesterol and substrates for LCAT, while large CE-loaded HDL2 bind with higher affinity to SR-BI receptor at the final stage of RCT; this high-affinity binding was proposed to promote selective CE uptake from lipid-rich over lipid-poor HDL.49,50 We propose that receptor-mediated selective uptake of apolar lipids resembles aspects of HDL rupture upon denaturation, since both these reactions involve HDL disintegration, release of apolar lipids and dissociation of apoA-I and apoA-II.51 Thus, reduced stability of HDL2 (i.e., increased propensity to fuse and rupture), together with their increased affinity for HDL receptor,49 may contribute to preferential lipid uptake from these large CE-loaded particles over their smaller protein-rich counterparts.
This argument may not apply to discoidal particles containing only a few molecules of CE. Such rHDL were used to assess the influence of particle size on SR-BI-mediated selective uptake of CE.50 The results showed comparable efficiency of CE uptake from large and small particles, which is consistent with comparable stability of rHDL disks of different diameters reported in our work (46 and references therein). This suggests that the size of spherical, but not necessarily discoidal, HDL (i.e., the size of the HDL core) is an important determinant of the particle stability and the efficiency of CE uptake.
Recently, we proposed an inverse correlation between HDL stability and function: less stable HDL and their precursors tend to undergo faster metabolic remodeling at early stages of RCT, which may accelerate cholesterol removal and benefit cardioprotection.46,51,52 The results reported here support this notion and extend it to the final stage of RCT. In fact, according to epidemiological studies, HDL2 are more cardioprotective than HDL3 (1,39,40,42 and references therein), which may result, in part, from the enhanced ability of HDL2 to mediate cell cholesterol efflux and CE uptake via the SR-BI receptor.42,49 We propose that reduced stability of HDL2 revealed in this work (Fig. 2-4) may accelerate these and other functional reactions in RCT and thereby contribute to the strong cardioprotective action of HDL2.
ApoA-I stabilizes HDL at least as well as apoA-II
Our studies of plasma spherical HDL clearly show that HDL(A-I/A-II) have comparable or slightly lower stability than the size-matched HDL(A-I) (Fig. 5). Although this result challenges the widely accepted notion of the stabilizing role of apoA-II on HDL, it is consistent with the earlier studies of discoidal rHDL24,32,36,38 and with our current data showing that the higher the AII/A-I ratio, the lower the disk stability (Fig. 6). Taken together, these results refute supposed specific stabilizing effects of apoA-II on HDL disks or spheres, such as the formation of salt bridges between apoA-I and apoA-II, which were proposed to stabilize HDL assembly and inhibit its remodeling.21 Thus, the inhibitory effects of apoA-II on HDL remodeling by plasma factors, such as LCAT and lipid transfer proteins,14,19-21 may not result from the direct stabilizing action of apoA-II on HDL. Possible reasons for these effects may include conformational changes in apoA-I induced by apoA-II, including partial or complete displacement of apoA-I from the HDL surface, which may hinder LCAT activity,23,24 competition between apoA-II and apoA-I for binding to LCAT and other plasma factors, etc.
In this and other studies showing destabilization of discoidal rHDL upon increasing apoA-II/apoA-I ratio, this ratio varied from 100% apoA-II to 100% apoA-I (Fig. 6). In comparison, in human spherical HDL(A-I/A-II) that contain approximately 2 mol apoA-I per 1 mol apoA-II,12 apoA-II comprises only about 24% of the total protein mass. This may partially explain why the observed difference in stability of size-matched human HDL(A-I) and HDL(A-I/A-II) is very small (Fig. 5). Artificial enrichment of human HDL(A-I/A-II) with A-II may increase this difference and help directly compare the effects of high apoA-II content in HDL disks and spheres. However, the results in Fig. 5 clearly show that at physiologic A-II/A-I ratios, apoA-I on human HDL is at least as stabilizing as apoA-II.
How can one reconcile the lack of apoA-II-induced HDL stabilization with the high hydrophobicity of this protein? Although the fraction of the apolar α-helical surface (thought to form the lipid binding site in the exchangeable apolipoproteins) is larger in apoA-II (about 1/2 as compared to 1/3 in apoA-I), the total helical length is smaller in this smaller protein (18 kD as compared to 28 kD for apoA-I). As a result, the total apolar helical area is comparable in apoA-II and apoA-I, which is consistent with comparable stability of plasma HDL(A-I/A-II) and HDL(AI) (Fig. 5).
How can one reconcile the lack of stabilizing effects of apoA-II on HDL(A-I/A-II) with the observation that apoA-I starts dissociating from these particles at lower temperatures and lower denaturant concentrations as compared to apoA-II?34,35 ApoA-I forms two distinct populations on plasma HDL that manifest themselves in two consecutive transitions observed by DSC, CD, light scattering and EM (Fig. 2B, C, Fig. 5B, C): a labile population that dissociates concomitantly with HDL fusion, and a tightly bound population that dissociates upon HDL disintegration, rupture and release of apolar core (reviewed by Pownall and Ehnholm31). Even though apoA-II does not dissociate from HDL until particle rupture,29,34,35 our results show that the presence of apoA-II on human HDL has no large effect on either the fusion or the rupture transition (Fig. 5). Therefore, HDL fusion and rupture are apparently driven by apoA-I dissociation, while apoA-II plays the role of a “passive bystander” rather than an important stabilizing factor in these transitions. This conclusion is consistent with the notion that apoA-I is a key determinant of the structural and functional properties of HDL subclasses, whereas apoA-II is probably a modulator of RCT rather than its strong determinant.15,31
Roles of the particle size and apoA-II content in HDL stability
In summary, our results show, for the first time, that the stability of human plasma HDL i) decreases with increasing particle diameter, and ii) is comparable for size-matched HDL(A-I) and HDL(A-I/A-II). This shifts our understanding of the key determinants for the stability and remodeling of human HDL and suggests that, in mature human HDL of normal composition, the particle diameter but not the apoA-II content importantly influences lipoprotein stability. Therefore, the existing controversy regarding the effects of apoA-II on HDL remodeling may stem, in part, from the indirect effects of apoA-II on the particle stability, e.g. through changes in the particle size. Such changes depend on the availability of other proteins and lipids, which is different in different experimental systems. For example, in mice overexpressing human apoA-II, a reduction in HDL size was observed in direct proportion to the level of apoA-II expression,16 which may explain the increased particle stability upon increase in apoA-II levels. Another example is in vitro remodeling of rHDL by CE transfer protein leading to formation of the large and small particles from HDL(A-I) but only small particles from HDL(A-I/A-II).21 ApoA-I dissociation was observed from the former but not the latter particles, which may reflect higher stability of the smaller particles (rather than the putative stabilizing salt bridges between apoA-I and apoA-II proposed by the authors21).
Although many earlier studies recognized the importance of HDL size and used homogeneous-size particle preparations in their functional and stability studies, they usually conducted spectroscopic measurements after HDL incubation with Gdn HCl to determine HDL stability (37,43,53,54 and references therein). However, HDL denaturation is a slow kinetically controlled reaction, hence such “end-point” data depend strongly on the length of incubation, and their interpretation is often inconclusive if not implausible; thus, such data suggested near-zero stability of human HDL27 or destabilization of apoA-II upon transfer from aqueous solution to DMPC disks.37 We propose that using a quantitative kinetic approach in denaturant- or T-jump experiments,27,29 together with the melting experiments in qualitative stability studies (Figs. 2-4), yields a much more accurate assessment of lipoprotein stability. This approach helps resolve some of the existing controversies regarding lipoprotein stability, such as the effects of particle size and apoA-II content reported here.
MATERIALS AND METHODS
Isolation of plasma HDL subclasses
Human HDL from six healthy volunteers were used. Single-donor HDL were isolated from fresh EDTA-treated plasma by density gradient ultracentrifugation in the density range 1.063−1.21 g/mL.44 HDL migrated as a single band on agarose gel. HDL stock solution (8−10 mg/mL protein measured by a modified Lowry assay) was dialyzed against buffer A (10 mM Na phosphate, pH 7.6, 1 mM EDTA, 0.05% NaN3) and stored in the dark at 4 °C. The stock solution was used within 8 weeks during which no protein degradation was detected by SDS PAGE and no changes in the protein secondary structure or lipoprotein stability were observed by CD spectroscopy and DSC.
HDL2 and HDL3 were separated from human HDL by density gradient ultracentrifugation.44 Briefly, HDL solutions were adjusted to 1.125 g/mL density by KBr and were ultracentrifuged in a Beckman Ti50.2 rotor at 4 °C for over 55 h. The density fractions from 1.063−1.125 g/mL (HDL2) and 1.125−1.210 g/mL (HDL3) were collected and used for further studies. The particle size was confirmed by negative staining EM (Fig. 2A) and by 10% non-denaturing PAGE (Fig. 3A, 0 lanes).
HDL(A-I) and HDL (A-I/A-II) were separated by immunoaffinity chromatography following published protocols.12 Briefly, monoclonal antibodies against apoA-II (Biodesign) were covalently attached to cyanogen bromide-activated Sepharose 4B beads (GE Healthcare). HDL were incubated with the beads at 4 °C overnight. HDL(A-I), which did not bind to the beads, were collected and dialyzed against buffer A. Column beads were washed with solution containing 0.01 M Tris, pH 8.0, 150 mM NaCl until no eluted protein was detected by UV absorption. HDL(A-I/A-II), which were eluted by a solution of 0.1 M acetic acid, pH 2.88, were immediately neutralized in 1 M Tris, pH 8.0 and dialyzed against buffer A. Protein composition in HDL(A-I) and HDL(A-I/A-II) was confirmed by SDS PAGE (not shown) and by nondenaturing PAGE followed by Western blotting using antibodies for apoA-I or apoA-II (Fig. 5A).
Reconstitution of model discoidal rHDL
Discoidal rHDL were reconstituted from DMPC (95+% purity, Avanti Polar Lipids) and apoA-I and/or apoA-II. Single-donor human apoA-I and apoA-II were isolated from delipidated plasma HDL as described.32 Protein purity assessed by SDS PAGE was >95%. Binary complexes of DMPC with apoA-I or apoA-II, rHDL(A-I) and rHDL(A-II), were obtained by spontaneous reconstitution after overnight co-incubation of protein and lipid (1:4 mg/mg ratio) at 24 °C, followed by centrifugation at 13,000 rpm to remove excess lipid. Discoidal rHDL(A-I/A-II) of varying A-I/A-II content were obtained following established protocols24 by incubating rHDL(A-I) disks with apoA-II using various A-II:A-I molar ratios from 1:5 to 1:2; under these conditions, apoA-II reportedly binds to discoidal rHDL without changing particle size or displacing apoAI.24 In addition, hybrid rHDL(A-I/A-II) were prepared by incubating lipid with a mixture of both proteins37 (1:4 protein:lipid weight ratio) using a broad range of apoA-I/ apoA-II molar ratios listed in Fig. 6C; non-denaturing PAGE showed that all protein was incorporated in these rHDL. Non-denaturing PAGE followed by Western blotting using antibodies against apoA-I or apoA-II was used to confirm the presence of both proteins in hybrid rHDL. rHDL were visualized by negative staining EM to confirm their discoidal morphology.
Gel electrophoresis and electron microscopy
Plasma HDL from various subclasses (intact or at various stages of thermal denaturation) were analyzed by non-denaturing PAGE (8% or 10%) and by SDS PAGE using a homogeneous system (12%) or a gradient (5−12%). The gels were run at 80 V for 20 min and at 120 V for 2 h and were stained with Coomassie blue. For Western blots, the proteins were transferred to a PVDF membrane for 1 h at 100 V. The membranes were blocked in 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20, 5% non-fat milk buffer, probed with antibodies for apoA-I or apoA-II in the blocking buffer, and visualized using ECL system (NEN Life Science Products).
Plasma and reconstituted HDL were visualized at 22 °C by negative staining EM using a CM12 transmission electron microscope (Philips Electron Optics) as described.27 Particle size analysis was carried out in Photoshop computer graphics using 200−300 particles per image.
Circular dichroism, light scattering and turbidity
CD, light scattering and turbidity data were recorded using AVIV 400 or AVIV 62DS spectropolarimeters with thermoelectric temperature control. Briefly, far-UV CD spectra (185−250 nm), kinetic data, and melting data were recorded from HDL solutions of about 33 μg/mL protein concentration in buffer B (10 mM Na phosphate, pH 7.6, 1 mM EDTA, 0.05% NaN3, 150 mM NaCl). Near-physiologic salt concentration was used to destabilize HDL and facilitate spectroscopic observation of their thermal disruption below 100 °C.29 In the melting experiments, CD data were recorded at 222 nm during sample heating and cooling with 1 °C increment and 300−600 s accumulation time per data point, which corresponds to a scan rate of 11−6 °C/h; 90° light scattering at 222 nm was monitored simultaneously with the CD signal by using fluorescence accessory in the AVIV 400 instrument.45 Heat-induced turbidity changes were monitored by measuring dynode voltage in CD experiments.45 In T-jump experiments, HDL denaturation was triggered by a rapid temperature increase from 25 °C to higher constant values and the time course of the α-helical unfolding was monitored at 222 nm. The dead time in these experiments (<60 sec) is much smaller than the typical time scale of protein unfolding, dissociation and lipoprotein fusion (minutes to hours). For protein unfolding that is coupled to protein dissociation and lipoprotein fusion, the unfolding rate k(T) measured in a T-jump to temperature T is related to kinetic lipoprotein stability ΔG*(T) at this temperature as ΔG* = -RT ln k(T). The CD data were normalized to protein concentration and expressed as molar residue ellipticity, [Θ].
Differential scanning calorimetry
Excess heat capacity, Cp(T), was measured using an upgraded Microcal MC-2 differential scanning microcalorimeter from degassed HDL solutions of 2.5 mg/mL protein concentration in buffer A containing 10 mM NaCl. The data, which were recorded during sample heating from 5 to 115 °C at a rate of 90 °C/h, were corrected for buffer baseline and normalized to protein concentration. ORIGIN software was used for the analysis and display of CD and DSC data.
All chemicals used in this work were of highest purity analytical grade. All experiments were repeated three to six times to ensure reproducibility.
ACKNOWLEDGEMENTS
We thank Cheryl England and Michael Gigliotti for help with lipoprotein and apolipoprotein isolation, Donald L. Gantz for expert help with electron microscopy, Dr. Sissel Lund-Katz, Dr. Sanna Makela, and Sunitha Chari for generous advice and help with separation of HDL subclasses; and David Plotkin for help with particle size analysis and manuscript editing. We are indebted to Dr. Henry Pownall for his generous gift of human plasma apoA-II that was used at the final stages of this project. This work was supported by the National Institutes of Health grants GM067260 and HL026355.
ABBREVIATIONS
- HDL
high-density lipoprotein
- rHDL
reconstituted high-density lipoprotein
- apo
apolipoprotein
- HDL(A-I)
HDL containing apolipoprotein A-I only
- HDL(A-I/A-II)
HDL containing both apopolipoprotein A-I and A-II
- RCT
reverse cholesterol transport
- CE
cholesterol ester
- LCAT
lecithin:cholesterol acyltransferase
- SR-BI
scavenger receptor class B type I
- DMPC
dimyristoyl phosphatidylcholine
- CD
circular dichroism
- DSC
differential scanning calorimetry
- EM
electron microscopy
- T-jump
temperature-jump
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- EDTA
ethylenediamine tetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Joy T, Hegele RA. Is raising HDL a futile strategy for atheroprotection? Nat. Rev. Drug Discov. 2008;7(2):143–155. doi: 10.1038/nrd2489. [DOI] [PubMed] [Google Scholar]
- 2.Scanu AM, Edelstein C. HDL: bridging past and present with a look at the future. FASEB J. 2008;22(12):4044–4054. doi: 10.1096/fj.08-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Movva R, Rader DJ. Laboratory assessment of HDL heterogeneity and function. Clin. Chem. 2008;54(5):788–800. doi: 10.1373/clinchem.2007.101923. [DOI] [PubMed] [Google Scholar]
- 4.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 2006;116(12):3090–3100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls SJ, Nissen SE. New targets of high-density lipoprotein therapy. Curr. Opin. Lipidol. 2007;18(4):421–426. doi: 10.1097/MOL.0b013e32821f603b. [DOI] [PubMed] [Google Scholar]
- 6.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995;36:211–228. [PubMed] [Google Scholar]
- 7.von Eckardstein A, Nofer JR, Assman G. High density lipoproteins and arteriosclerosis. Role of cholesterol efflux and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2001;21:13–27. doi: 10.1161/01.atv.21.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Rye KA, Clay MA, Barter PJ. Remodelling of high density lipoproteins by plasma factors. Atherosclerosis. 1999;145(2):227–238. doi: 10.1016/s0021-9150(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 9.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 2007;282(31):22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 10.Malle E, Marsche G, Panzenboeck U, Sattler W. Myeloperoxidase-mediated oxidation of high-density lipoprotein: Fingerprints of newly recognized potential proatherogenic lipoproteins. Arch. Biochem. Biophys. 2006;445:245–255. doi: 10.1016/j.abb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 12.Cheung MC, Albers JJ. Characterization of lipoprotein particles isolated by immunoaffinity chromatography. Particles containing A-I and A-II and particles containing A-I but no A-II. J. Biol. Chem. 1984;259(19):12201–122219. [PubMed] [Google Scholar]
- 13.Hedrick CC, Lusis AJ. Apolipoprotein A-II: a protein in search of a function. Can. J. Cardiol. 1994;10(4):453–459. [PubMed] [Google Scholar]
- 14.Blanco-Vaca F, Escolà-Gil JC, Martín-Campos JM, Julve J. Role of apoA-II in lipid metabolism and atherosclerosis: Advances in the study of an enigmatic protein. J. Lipid Res. 2001;42(11):1727–1739. [PubMed] [Google Scholar]
- 15.Tailleux A, Duriez P, Fruchart JC, Clavey V. Apolipoprotein A-II, HDL metabolism and atherosclerosis. Atherosclerosis. 2002;164(1):1–13. doi: 10.1016/s0021-9150(01)00751-1. [DOI] [PubMed] [Google Scholar]
- 16.Pastier D, Dugué S, Boisfer E, Atger V, Tran NQ, van Tol A, Chapman MJ, Chambaz J, Laplaud PM, Kalopissis AD. Apolipoprotein A-II/A-I ratio is a key determinant in vivo of HDL concentration and formation of pre-beta HDL containing apolipoprotein A-II. Biochemistry. 2001;40(41):12243–12253. doi: 10.1021/bi010348m. [DOI] [PubMed] [Google Scholar]
- 17.Birjmohun RS, Dallinga-Thie GM, Kuivenhoven JA, Stroes ES, Otvos JD, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 2007;116:2029–2035. doi: 10.1161/CIRCULATIONAHA.107.704031. [DOI] [PubMed] [Google Scholar]
- 18.Winkler K, Hoffmann MM, Seelhorst U, Wellnitz B, Boehm BO, Winkelmann BR, März W, Scharnagl H. Apolipoprotein A-II is a negative risk indicator for cardiovascular and total mortality: findings from the Ludwigshafen Risk and Cardiovascular Health Study. Clin. Chem. 2008;54(8):1405–1406. doi: 10.1373/clinchem.2008.103929. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi L, Lucchini A, Vecchio G, Sirtori CR, Franceschini G. Human apolipoprotein A-II inhibits the formation of pre-beta high density lipoproteins. Biochim. Biophys. Acta. 1996;1304(1):32–42. doi: 10.1016/s0005-2760(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 20.Pussinen PJ, Jauhiainen M, Ehnholm C. ApoA-II/apoA-I molar ratio in the HDL particle influences phospholipid transfer protein-mediated HDL interconversion. J. Lipid Res. 1997;38:12–21. [PubMed] [Google Scholar]
- 21.Rye KA, Wee K, Curtiss LK, Bonnet DJ, Barter PJ. Apolipoprotein A-II inhibits high density lipoprotein remodeling and lipid-poor apolipoprotein A-I formation. J. Biol. Chem. 2003;278(25):22530–22536. doi: 10.1074/jbc.M213250200. [DOI] [PubMed] [Google Scholar]
- 22.Rye KA, Barter PJ. Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 2004;24(3):421–428. doi: 10.1161/01.ATV.0000104029.74961.f5. [DOI] [PubMed] [Google Scholar]
- 23.Labeur C, Lambert G, Van Cauteren T, Duverger N, Vanloo B, Chambaz J, Vandekerckhove J, Castro G, Rosseneu M. Displacement of apo A-I from HDL by apo A-II or its C-terminal helix promotes the formation of pre-beta1 migrating particles and decreases LCAT activation. Atherosclerosis. 1998;139:351–362. doi: 10.1016/s0021-9150(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 24.Durbin DM, Jonas A. The effect of apolipoprotein A-II on the structure and function of apolipoprotein A-I in a homogeneous reconstituted high density lipoprotein particle. J. Biol. CheAm. 1997;272(50):31333–31339. doi: 10.1074/jbc.272.50.31333. [DOI] [PubMed] [Google Scholar]
- 25.Lagocki PA, Scanu AM. In vitro modulation of the apolipoprotein composition of high density lipoprotein. Displacement of apolipoprotein A-I from high density lipoprotein by apolipoprotein A-II. J. Biol. Chem. 1980;255(8):3701–3706. [PubMed] [Google Scholar]
- 26.Gursky O, Ranjana, Gantz DL. Complex of human apolipoprotein C-1 with phospholipid: Thermodynamic or kinetic stability? Biochemistry. 2002;41:7373–7384. doi: 10.1021/bi025588w. [DOI] [PubMed] [Google Scholar]
- 27.Mehta R, Gantz DL, Gursky O. Human plasma high-density lipoproteins are stabilized by kinetic factors. J. Mol. Biol. 2003;328(1):183–192. doi: 10.1016/s0022-2836(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 28.Pownall HJ. Remodeling of human plasma lipoproteins by detergent perturbation. Biochemistry. 2005;44(28):9714–9722. doi: 10.1021/bi050729q. [DOI] [PubMed] [Google Scholar]
- 29.Jayaraman S, Gantz DL, Gursky O. Effects of salt on thermal stability of human plasma high-density lipoproteins. Biochemistry. 2006;45:4620–4628. doi: 10.1021/bi0524565. [DOI] [PubMed] [Google Scholar]
- 30.Gursky O. Apolipoprotein structure and dynamics. Curr. Opin. Lipidol. 2005;16(3):287–294. doi: 10.1097/01.mol.0000169348.61191.ac. [DOI] [PubMed] [Google Scholar]
- 31.Pownall HJ, Ehnholm C. The unique role of apolipoprotein A-I in HDL remodeling and metabolism. Curr. Opin. Lipidol. 2006;17(3):209–213. doi: 10.1097/01.mol.0000226110.66942.e8. [DOI] [PubMed] [Google Scholar]
- 32.Jayaraman S, Gantz DL, Gursky O. Kinetic stabilization and fusion of apolipoprotein A-2:DMPC disks: comparison with apoA-1 and apoC-1. Biophys. J. 2005;88(4):2907–2918. doi: 10.1529/biophysj.104.055921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J. Lipid Res. 1992;33(2):141–166. [PubMed] [Google Scholar]
- 34.Nichols AV, Gong EL, Blanche PJ, Forte TM, Anderson DW. Effects of guanidine hydrochloride on human plasma high density lipoproteins. Biochim. Biophys. Acta. 1976;446:226–239. doi: 10.1016/0005-2795(76)90113-6. [DOI] [PubMed] [Google Scholar]
- 35.Tall AR, Deckelbaum RJ, Small DM, Shipley GG. Thermal behavior of human plasma high density lipoprotein. Biochim. Biophys. Acta. 1977;487(1):145–153. doi: 10.1016/0005-2760(77)90051-0. [DOI] [PubMed] [Google Scholar]
- 36.Swaney JB, Palmieri E. Hybrid association between human apolipoproteins A-I and A-II in aqueous solution and in phospholipid recombinants. Biochim. Biophys. Acta. 1984;792(2):164–171. doi: 10.1016/0005-2760(84)90218-2. [DOI] [PubMed] [Google Scholar]
- 37.Boucher J, Ramsamy TA, Braschi S, Sahoo D, Neville TA, Sparks DL. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J. Lipid Res. 2004;45:849–858. doi: 10.1194/jlr.M300431-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Reijngoud DJ, Phillips MC. Mechanism of dissociation of human apolipoproteins A-I, A-II, and C from complexes with dimyristoylphosphatidylcholine as studied by thermal denaturation. Biochemistry. 1984;23(4):726–734. doi: 10.1021/bi00299a022. [DOI] [PubMed] [Google Scholar]
- 39.Patsch JR, Karlin JB, Scott LW, Smith LC, Gotto AM., Jr. Inverse relationship between blood levels of high density lipoprotein subfraction 2 and magnitude of postprandial lipemia. Proc. Natl. Acad. Sci. USA. 1983;80(5):1449–1453. doi: 10.1073/pnas.80.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller NE. Associations of high-density lipoprotein subclasses and apolipoproteins with ischemic heart disease and coronary atherosclerosis. Am. Heart J. 1987;113(2):589–597. doi: 10.1016/0002-8703(87)90638-7. [DOI] [PubMed] [Google Scholar]
- 41.Skipski VP, Barclay M, Barclay RK, Fetzer VA, Good JJ, Archibald FM. Lipid composition of human serum lipoproteins. Biochem. J. 1967;104(2):340–352. doi: 10.1042/bj1040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kontush A, Therond P, Zerrad A, Couturier M, Négre-Salvayre A, de Souza JA, Chantepie S, Chapman MJ. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 2007;27(8):1843–1849. doi: 10.1161/ATVBAHA.107.145672. [DOI] [PubMed] [Google Scholar]
- 43.Catalano G, Duchene E, Julia Z, Le Goff W, Bruckert E, Chapman MJ, Guerin M. Cellular SR-BI and ABCA1-mediated cholesterol efflux are gender-specific in healthy subjects. J. Lipid Res. 2008;49(3):635–643. doi: 10.1194/jlr.M700510-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Pownall HJ, Hosken BD, Gillard BK, Higgins CL, Lin HY, Massey JB. Speciation of human plasma high-density lipoprotein (HDL): HDL stability and apolipoprotein A-I partitioning. Biochemistry. 2007;46(25):7449–7459. doi: 10.1021/bi700496w. [DOI] [PubMed] [Google Scholar]
- 45.Schumaker VN, Puppione DL. Sequential flotation ultracentrifugation. Methods Enzymol. 1986;128:151–170. doi: 10.1016/0076-6879(86)28066-0. [DOI] [PubMed] [Google Scholar]
- 46.Benjwal S, Verma S, Rohm KH, Gursky O. Monitoring protein aggregation during thermal unfolding in circular dichroism experiments. Protein Sci. 2006;15:635–639. doi: 10.1110/ps.051917406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skipski VP, Barclay M, Barclay RK, Fetzer VA, Good JJ, Archibald FM. Lipid composition of human serum lipoproteins. Biochem. J. 1967;104(2):340–352. doi: 10.1042/bj1040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han. M, Gillard BK, Courtney HS, Ward K, Rosales C, Khant H, Ludtke SJ, Pownall HJ. Disruption of human plasma high-density lipoproteins by streptococcal serum opacity factor requires labile apolipoprotein A-I. Biochemistry. 2009 doi: 10.1021/bi802287q. PMID: 19191587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guha M, Gantz DL, Gursky O. Effects of acyl chain length, unsaturation and pH on thermal stability of model discoidal high-density lipoproteins. J. Lipid Res. 2008;49(8):1752–17461. doi: 10.1194/jlr.M800106-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Beer MC, Durbin DM, Cai L, Jonas A, de Beer FC, van der Westhuyzen DR. Apolipoprotein A-I conformation markedly influences HDL interaction with scavenger receptor BI. J. Lipid Res. 2001;42(2):309–313. [PubMed] [Google Scholar]
- 51.Thuahnai ST, Lund-Katz S, Williams DL, Phillips MC. Scavenger receptor class B, type I-mediated uptake of various lipids into cells. Influence of the nature of the donor particle interaction with the receptor. J. Biol. Chem. 2001;276:43801–43808. doi: 10.1074/jbc.M106695200. [DOI] [PubMed] [Google Scholar]
- 52.Guha M, Gao X, Jayaraman S, Gursky O. Correlation of structural stability with functional remodeling of high-density lipoproteins: The importance of being disordered. Biochemistry. 2008;47(44):11393–11397. doi: 10.1021/bi8014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao X, Jayaraman S, Gursky O. Mild oxidation promotes and advanced oxidation prevents protein dissociation and remodeling of human plasma high-density lipoprotein in vitro. J. Mol. Biol. 2008;376(4):997–1007. doi: 10.1016/j.jmb.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leroy A, Toohill KL, Fruchart JC, Jonas A. Structural properties of high density lipoprotein subclasses homogeneous in protein composition and size. J. Biol. Chem. 1993;268(7):4798–4805. [PubMed] [Google Scholar]


