Abstract
This study tests the hypothesis that perinatal taurine depletion produces autonomic nervous system dysregulation and increases arterial pressure in young male rats on a high sugar diet. Sprague-Dawley dams were taurine depleted (beta-alanine 3% in water) or untreated from conception to weaning. Their male offspring were fed normal rat chow with or without 5% glucose. At 7–8 weeks of age, male offspring were tested in a conscious, unrestrained or an anesthetic state. Body weight was slightly lower in the taurine-depleted rats with similar heart or kidney to body weight ratios. Plasma potassium, blood urea nitrogen, plasma creatinine, hematocrit, fasting blood glucose concentrations and glucose tolerance were all similar. In the taurine-depleted, high glucose group, mean arterial pressure and sympathetic nervous system activity were increased and baroreflex function was impaired. These findings suggest that perinatal taurine depletion causes autonomic nervous system dysfunction that may contribute to dietary high sugar-induced hypertension in this model.
Introduction
Perinatal environment can greatly influence adult function and disease development (Barker et al. 2002; Langley-Evans 2006). For instance, perinatal diets that are very low in nutritional values (e.g., proteins) can result in low birth weights in infants and subsequent insulin resistance, diabetes mellitus or arterial hypertension in adults (Barker et al. 2007; Eriksson et al. 2007). Mechanisms potentially underlying these changes include perinatal imbalances in circulating glucocorticoids, the renin-angiotensin system, oxidative stress and nephrogenesis. Substances that can inhibit or stimulate these factors either directly or indirectly (including perinatal angiotensin converting enzyme inhibitor treatment or taurine supplementation) could reduce these deleterious symptoms (Aerts and Van Assche 2002; Racasan et al. 2004; Wyss et al. 1994).
Taurine is a sulphur-containing beta-amino acid that plays many essential roles in prenatal and adult life (Sturman 1993), including intracellular volume regulation, cell membrane stabilization, neuromodulation, antioxidative stress, vasodilation, cardiac performance, learning and memory, renal growth and differentiation. Taurine is an essential amino acid during fetal life, due to its limited endogenous fetal biosynthesis (Aerts and Van Assche 2002). Maternal taurine supply to fetus and new born can be severely limited due to abnormal maternal protein intake or an imbalance in diet between protein and carbohydrate consumption (Barker et al. 2007; Forrester 2004; Langley-Evans 2006; Mendez et al. 2004). Though long-term effects of excess perinatal taurine exposure is not well established, perinatal taurine supplementation appears beneficial to the new born (Chesney et al. 1998).
Previously, we have demonstrated that prenatal or postnatal taurine depletion decreases renal blood flow and increases renal vascular resistance in adult, male rats (Roysommuti et al. 2004). In addition, both prenatal and postnatal taurine depletion significantly increases arterial pressure in the adult, male offspring; however, it does not significantly alter natriuretic and diuretic responses to acute intravenous saline load. Further, perinatal taurine depletion does not affect resting arterial pressure and heart rate. Many lines of evidence suggest that perinatal taurine depletion may increase an animal’s sensitivity to risk factors for adult hypertension (Dawson, Jr. et al. 1996; Schaffer et al. 2003).
High dietary carbohydrate intake is implicated in the pathogenesis of hypertension, and diets high in sugar increase arterial pressure in many animal models including spontaneously hypertensive rats (SHR) and in normotensive rats (Melancon et al. 2006; Reaven, 1990; Shimamoto and Ura 2006). The underlying mechanism may involve the renin-angiotensin system, insulin resistance, sympathetic nerve activation, and renal damage (Roysommuti et al. 2002). The present study tests the hypothesis that perinatal taurine depletion impairs autonomic nervous system control of arterial pressure in normotensive, young adult, male rats and this impairment is exacerbated by high dietary sugar.
Materials and Methods
SD rats were bred at the animal unit of Faculty of Medicine, Khon Kaen University and maintained at constant humidity (60 ± 5%), temperature (24 ± 1°C), and light cycle (0600–1800 h). Female SD dams were either taurine depleted (beta-alanine 3% in tap water, TD) or untreated (control, C) from conception to weaning. The male offspring were fed normal rat chow with (TDG, CG) or without (TDW, CW) 5% glucose in their tap water throughout the experiment. All experimental procedures were preapproved by the Universities Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health guidelines.
At 7–8 weeks of age, under thiopental anesthesia, all male rats were implanted with femoral arterial and venous catheters. Three days later and after an overnight fast, arterial blood samples were obtained under a conscious condition for Na, K, BUN, creatinine, hematocrit, and fasting blood sugar determinations. Thereafter, glucose tolerance testing was initiated by intravenous injection of glucose (2 g/kg in saline), and blood glucose levels were measured at 0, 30, 60, and 120 minutes. Twenty-four hours later, non-fasting blood samples were collected and then arterial pressure pulses were continuously recorded (Biopac system, CA) in a conscious condition before and during infusion of phenylephrine (increased arterial pressure) or sodium nitroprusside (decreased arterial pressure).
Renal sympathetic nerve function was tested one day later under thiopental anesthesia by using stainless steel electrodes (12 MΩ, 0.01 Taper, A-M System, FL) connected to DAM80 amplifier (WPI, Sarasota, FL) and Biopac (Goleta, CA) system, respectively. Single unit recordings of renal nerve activity were conducted only on nerve units that responded to changes in arterial pressure following nitroprusside or phenylephrine infusion. Baroreflex sensitivity was measured as changes in heart rate and/or renal nerve activity per changes in mean arterial pressure.
Mean arterial pressure, heart rate, baroreflex sensitivity (BS) following phenylephrine or sodium nitroprusside, and power spectrum densities of arterial pressure pulse were determined offline by using Acknowledge software 3.8.1 (Biopac, Goleta, CA). Plasma Na, plasma K, BUN, and plasma creatinine concentrations were measured by an automatic analyzer, hematocrit by a standard technique and blood sugar by standard glucostrips and a glucometer (Accu-chek®, Germany). The autonomic nervous system control of arterial pressure was estimated from low frequency (0.3–0.5 Hz; sympathetic nerve activity) and high frequency (0.5–4.0 Hz; parasympathetic nerve activity) components of the power spectrum densities of baseline arterial pressure pulse using the Fourier analysis (Cerutti et al. 1991; Stauss and Kregel 1996).
All data were expressed as mean ± SEM. Statistical comparisons among groups (p < 0.05) were done by using one-way ANOVA and Duncan’Multi-Range (StatMost 3.6, Dataxiom Software, Los Angeles, CA).
Results
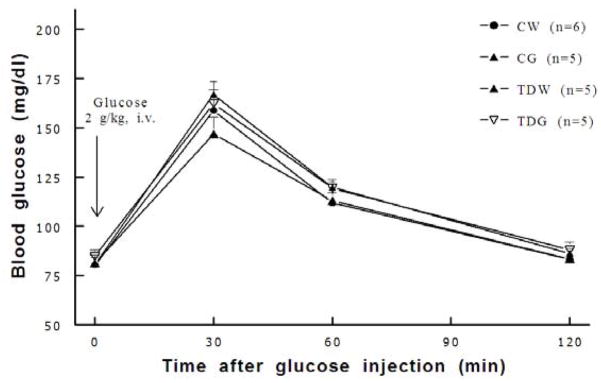
At 7–8 weeks of age, taurine depletion significantly lowered body weight by about 10%; however, kidney and heart weights (as absolute weights or as ratios to body weight) were not significantly different among groups (Table 1). Further, fasting and non-fasting plasma potassium levels were not significantly different among groups, and non-fasting plasma sodium levels were not significantly different among groups. In contrast, fasting plasma sodium concentrations were slightly and significantly lower in TDG compared to all other groups (Table 2). Blood urea nitrogen (BUN) and plasma creatinine were not affected by perinatal taurine depletion or dietary sugar supplementation (Table 3). Hematocrit, fasting blood glucose (Table 4), and glucose tolerance (Fig. 1) were also not significantly different among groups. In contrast, all glucose treated animals increased non-fasting blood sugar.
Table 1.
Body (BW), heart (HW), and kidney (KW) weights among male offspring
| Treatment | BW (g) | KW (g) | HW (g) | KW/BW (%) | HW/BW (%) |
|---|---|---|---|---|---|
| CW (n=6) | 233±4 | 1.12±0.02 | 0.90±0.02 | 0.48±0.01 | 0.39±0.01 |
| CG (n=5) | 234±7 | 1.13±0.03 | 0.91±0.02 | 0.48±0.02 | 0.39±0.02 |
| TDW (n=5) | 210±5* | 1.07±0.04 | 0.85±0.02 | 0.51±0.03 | 0.41±0.01 |
| TDG (n=5) | 214±6 | 1.02±0.06 | 0.89±0.03 | 0.48±0.04 | 0.42±0.02 |
Data were mean±SEM.
P<0.05 when compared to CW. See text for abbreviations.
Table 2.
Fasting and non-fasting plasma sodium and potassium among male offspring
| Treatment | Plasma sodium (mEq/L) | Plasma potassium (mEq/L) | ||
|---|---|---|---|---|
| Fasting | Non-fasting | Fasting | Non-fasting | |
| CW (n=6) | 139.8±0.31 | 139.6±1.15 | 3.72±0.19 | 3.75±0.19 |
| CG (n=5) | 137.0±2.09 | 138.6±1.50 | 3.80±0.22 | 3.82±0.04 |
| DW(n=5) | 135.4±1.81 | 139.4±0.50 | 3.88±0.07 | 3.86±0.02 |
| DG (n=5) | 131.4±2.38* | 134.6±2.11 | 3.68±0.10 | 3.74±0.18 |
Data were mean±SEM.
P<0.05 when compared to CW. See text for abbreviations.
Table 3.
Fasting and non-fasting blood urea nitrogen and plasma creatinine
| Treatment | Blood urea nitrogen (mg/dl) | Plasma creatinine (mg/dl) | ||
|---|---|---|---|---|
| Fasting | Non-fasting | Fasting | Non-fasting | |
| CW (n=6) | 17.38±0.75 | 18.17±0.59 | 0.45±0.02 | 0.43±0.02 |
| CG (n=5) | 16.86±0.83 | 20.08±0.96 | 0.46±0.02 | 0.46±0.02 |
| TDW (n=5) | 16.72±0.62 | 18.84±1.02 | 0.46±0.02 | 0.42±0.02 |
| TDG (n=5) | 18.02±0.69 | 17.96±0.91 | 0.44±0.02 | 0.46±0.02 |
Data were mean±SEM. No significant differences among groups were observed. See text for abbreviations.
Table 4.
Fasting (FBG) and non-fasting (NFBG) blood glucose and hematocrit among male offspring
| Treatment | Blood glucose (mg/dl) | Hematocrit (%) | ||
|---|---|---|---|---|
| NFBG | FBG | Fasting | Non-fasting | |
| CW (n=6) | 83.5±2.9 | 80.3±3.7 | 42.7±0.7 | 42.2±0.8 |
| CG (n=5) | 105.6±5.3* | 80.8±1.6 | 43.2±0.6 | 42.2±1.0 |
| TDW (n=5) | 89.8±5.6 | 81.2±4.1 | 42.8±0.7 | 41.4±0.6 |
| TDG (n=5) | 107.0±4.9* | 84.6±3.4 | 43.4±0.6 | 42.2±0.8 |
Data were mean±SEM.
P<0.05 when compared to CW. See text for abbreviations.
Fig. 1.
All experimental groups displayed similar glucose tolerance (* P < 0.05 to CW). See text for abbreviations.
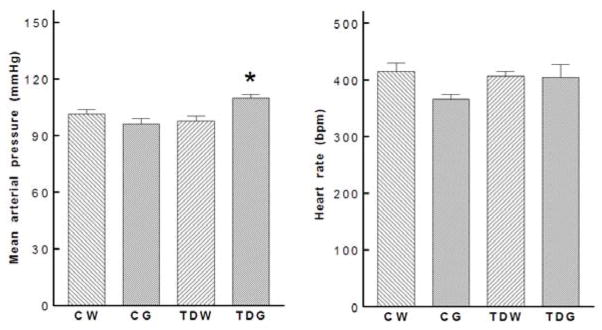
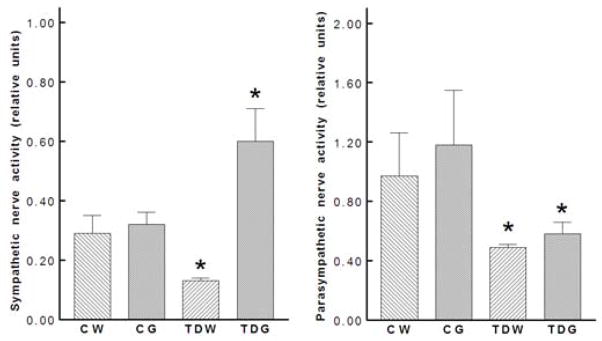
Neither dietary glucose supplementation in controls nor perinatal taurine depletion altered mean arterial pressures or heart rates. However, perinatal taurine depletion followed by high dietary glucose significantly increased mean arterial pressures (Fig. 2). Power spectrum analyses of arterial pressure indicated that taurine depletion blunted both sympathetic and parasympathetic components. In control rats glucose supplementation did not affect the either component, but in the taurine-depleted rats, glucose excess increased the sympathetic nerve component (Fig. 3). Further, in the taurine-depleted rats compared to all other groups, glucose decreased the parasympathetic nerve spectral component of arterial pressure. Thus, the ratio of sympathetic to parasympathetic activity was increased by taurine depletion.
Fig. 2.
High sugar intake increased mean arterial pressures in TDG while heart rates were not significantly different among groups (* P < 0.05 to CW). See text for abbreviations.
Fig. 3.
Perinatal taurine depletion decreased both sympathetic and parasympathetic nerve activities in adult, male rats and high sugar intake heightened the sympathetic nerve activity only in TDG (* P < 0.05 to CW). See text for abbreviations.
Baroreflex control of heart rate was significantly decreased in both taurine-depleted groups (Fig. 4), but the decrease was significantly greater in the taurine depleted rats on the high glucose diet. The high glucose diet did not affect baroreflex control in control rats. Baroreflex control of renal nerve activity (an indicator of autonomic nervous system control of renal vascular resistance) was also blunted by perinatal taurine depletion and was further blunted by high dietary glucose in the taurine depleted rats (Fig. 5). Further, baroreflex control of arterial pressure (within the normal range of arterial pressure, i.e., 80–120 mm Hg) was consistently blunted by perinatal taurine depletion
Fig. 4.
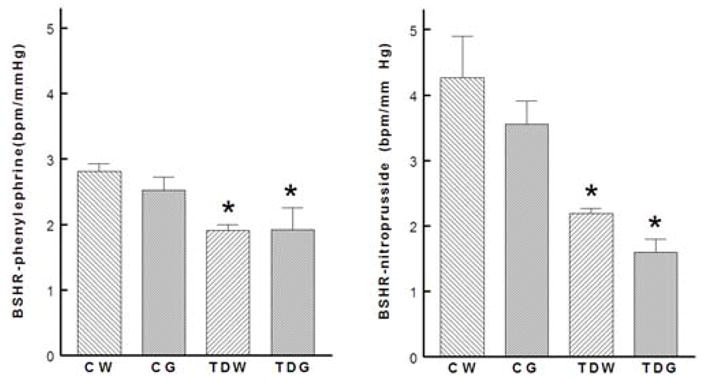
Perinatal taurine depletion decreased baroreflex sensitivity control of heart rate in adult, male rats (* P < 0.05 to CW). See text for abbreviations.
Fig. 5.
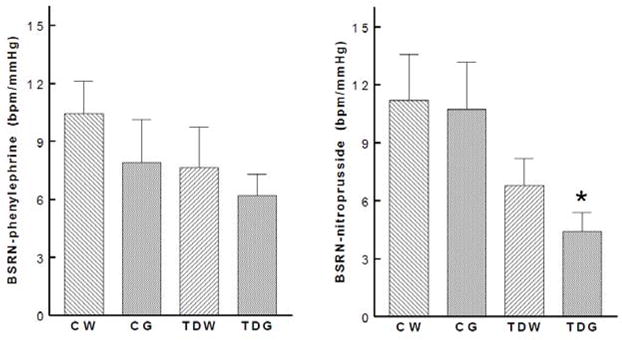
High sugar intake aggravated baroreflex sensitivity control of renal nerve activity in perinatal taurine depleted rats (* P < 0.05 to CW). See text for abbreviations.
Discussion
During perinatal life, large alterations in nutritional intake can lead to obesity, insulin resistance, hypertension, and other cardiovascular symptoms. These effects may be initiated during pre- or postnatal development (Barker et al. 2002; Harding, 2001; Langley-Evans 2006). The present study indicates that perinatal taurine depletion causes a dysregulation of the autonomic nervous system that is exacerbated by a high sugar diet. Further, these changes in autonomic nervous system regulation may contribute to increased arterial pressure in perinatal taurine-depleted rats on a high glucose diet. This provides another example of how the perinatal environment can alter function in adults and potentially contribute to disease (Barker et al. 2002; Harding 2001; Langley-Evans 2006).
Several lines of evidence indicate that imbalances in maternal protein to carbohydrate intake can result in low birth weight of offspring (Law et al. 2001; Shiell et al. 2001), which subsequently develop insulin resistance and a high risk of cardiovascular disease. Imbalance in glucocorticoids levels, renin-angiotensin system, and abnormal nephrogenesis during early life growth and development may underline these effects and modify the adaptive ability of the offspring at later life (Eriksson et al. 2007; Hanson et al. 2004; Mendez et al. 2004). The present study shows that perinatal taurine depletion in rats slightly decreases body weight, thus supporting the growth-promoting ability of taurine in the early life (Lourenco and Camilo 2002). While perinatal taurine depletion could have caused taurine concentrations to remain low throughout life, thus producing chronic taurine deficiency, this seems unlikely. After weaning, all animals were supplied with a normal rat chow that appears to “normalize” taurine content within 4–5 weeks in this animal model (Pacioretty et al. 2001). However, we did not independently measure taurine concentration in these rats. In addition, the animals in the present study displayed no signs of insulin resistance or diabetes mellitus. This suggests that insulin resistance may not be the key factor predisposing these animals to subsequent cardiovascular impairment (Reaven 1991). We have previously reported that in this model, high sugar diet can induce renal dysfunction without insulin resistance or hypertension (Roysommuti et al. 2002).
Taurine supplementation during perinatal or adult life reduces hypertension in animal models including SHR, cyclosporine A-induced hypertension and sugar-induced hypertension (Militante and Lombardini 2002). The present findings indicate that perinatal taurine depletion predisposed animals to dietary sugar-induced hypertension. Lack of taurine in the early life may underline the effect of perinatal protein malnutrition on adult hypertension (Aerts and Van Assche 2002). Interestingly, taurine, which is a sulphur-containing beta-amino acid that is found mainly in animal meat, is not present in most plant proteins and is very low in concentration in cow’s milk (Aerts and Van Assche, 2002). In contrast, it is high in concentration in human and rat milk. In humans, taurine concentrations are higher in non-vegetarians than vegetarians.
Dietary taurine supplementation appears to be directly related to taurine’s ability to decrease sympathetic nerve activity, likely at the level of the central nervous system (Mizushima et al. 1996; Sato et al. 1987). Taurine supplementation also reduces oxidative stress (Aerts and Van Assche 2002; Racasan et al. 2004). Interestingly, all perinatal taurine depleted rats on the normal glucose diet displayed resting autonomic nerve hypoactivity but sympathetic nerve activity was only selectively increased in the high sugar diet fed rats, suggesting that perinatal taurine depletion may not retard growth and development of the autonomic nervous system, but may dysregulate it. The selective action of high sugar intake on the sympathetic nervous system control suggests that taurine deficiency in early life may alter the adult central nervous system. Taurine injection into the brain has been shown to decrease the sympathetic outflow in the animals (Inoue et al., 1985); however, this effect does not appear be direct, but rather acts through other mechanism, e.g., adenosine or glutamate receptor systems (Albrecht and Schousboe 2005; Kohlenbach and Schlicker 1990). Hypothalamic sympathetic pathways play a very important role in pathogeneses of many models of hypertension (Carlson et al. 2001). Taurine depletion during development may modify the function of this brain area.
Baroreceptor reflex plays a crucial role for minute-to-minute regulation of arterial pressure, and recent evidence suggests that baroreceptor dysfunction may contribute to hypertension in some animal models, e.g., SHR (Carlson et al. 2001). In sustained hypertension, baroreflex sensitivity is usually blunted, either by arterial vascular resetting. In the present study, the baroreflex control of both heart rate and renal nerve activity was blunted in perinatal taurine-depleted rats, even though mean arterial pressure and heart rate were not significantly different in the taurine-depleted compared to control rats. In contrast, glucose supplementation led to both baroreflex blunting and increased arterial pressure in the taurine-depleted but not control rats. Together, this suggests that postprandial hyperglycemia and/or hyperinsulinemia may lead to sustained sympathetic overactivity and hypertension due to insufficient baroreceptor reflex adjustments. Renal nerve activity reflects sympathetic nervous system but not parasympathetic nervous system activity. Thus, the blunted baroreflex control of renal nerve activity by a high sugar diet in the perinatal taurine-depleted rats indicates that the changes in cardiovascular control in these rats are due primarily to the actions of sympathetic (versus parasympathetic) pathways. However, blunted renal excretory function is likely not the primary cause of hypertension in these animals, since the natriuretic and diuretic function is not different between groups (Roysommuti et al., 2004).
The renin-angiotensin system plays an important role in the pathogenesis of arterial hypertension including sugar-induced hypertension. Our previous study indicates that high sugar intake impairs renal function before the development of insulin resistance and hypertension (Roysommuti et al. 2002). This effect is abolished by treatment with an angiotensin converting enzyme inhibitor, captopril. The present experiment also further indicates that this high dietary glucose does not alter autonomic nervous system function in control rats. It is possible that a glucose-induced rise in angiotensin II may suppress the central baroreflex pathway (DiBona and Jones 2003; McMullan et al. 2007) and activate sympathetic nerve activity (Gao et al. 2005; Johns 2005) in the perinatal taurine depleted rats, which were more susceptibility to pressor agents than control. Over-expression of angiotensin II receptors has also been reported in perinatal protein restricted offspring (Pladys et al. 2004; Riviere et al. 2005), but not in the adult perinatal taurine depleted animals.
In summary, while perinatal taurine-depletion blunts baroreceptor reflexes and suppresses autonomic nervous system activity, excess dietary glucose increases sympathetic nerve activity. The baroreceptor reflex was also further blunted by high sugar intake. These data further support an important role for maternal dietary taurine during the perinatal period.
Acknowledgments
This work was supported by grants from the Faculty of Medicine, Khon Kaen University and National Institutes of Health grants, AT 00477 (JMW) from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements.
Abbbreviation
- SHR
spontaneously hypertensive rat
- CG
control with glucose
- CW
control without glucose
- TDW
taurine depletion without glucose
- TDG
taurine depletion with glucose
Contributor Information
Sanya Roysommuti, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Atchariya Suwanich, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
Dusit Jirakulsomchok, Department of Physiology, Khon Kaen University, Faculty of Medicine, Khon Kaen 40002, Thailand.
J. Michael Wyss, Department of Cell Biology, School of Medicine, University of Alabama at Birmingham, Birmingham, AL 35294.
References
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–6. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–21. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Maternal and social origins of hypertension. Hypertension. 2007;50:565–71. doi: 10.1161/HYPERTENSIONAHA.107.091512. [DOI] [PubMed] [Google Scholar]
- Carlson SH, Roysomutti S, Peng N, Wyss JM. The role of the central nervous system in NaCl-sensitive hypertension in spontaneously hypertensive rats. Am J Hypertens. 2001;14:155S–62S. doi: 10.1016/s0895-7061(01)02083-0. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol. 1991;261:H1292–9. doi: 10.1152/ajpheart.1991.261.4.H1292. [DOI] [PubMed] [Google Scholar]
- Chesney RW, Helms RA, Christensen M, Budreau AM, Han X, Sturman JA. The role of taurine in infant nutrition. Adv Exp Med Biol. 1998;442:463–76. doi: 10.1007/978-1-4899-0117-0_56. [DOI] [PubMed] [Google Scholar]
- Dawson R, Jr, Eppler B, Patterson TA, Shih D, Liu S. The effects of taurine in a rodent model of aging. Adv Exp Med Biol. 1996;403:37–50. doi: 10.1007/978-1-4899-0182-8_4. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY. Endogenous angiotensin affects responses to stimulation of baroreceptor afferent nerves. J Hypertens. 2003;21:1539–46. doi: 10.1097/00004872-200308000-00019. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–21. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- Forrester T. Historic and early life origins of hypertension in Africans. J Nutr. 2004;134:211–6. doi: 10.1093/jn/134.1.211. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–9. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Hanson M, Gluckman P, Bier D, Challis J, Fleming T, Forrester T, Godfrey K, Nestel P, Yajnik C. Report on the 2nd World Congress on Fetal Origins of Adult Disease, Brighton, U.K., June 7–10, 2003. Pediatr Res. 2004;55:894–7. doi: 10.1203/01.PDR.0000115682.23617.03. [DOI] [PubMed] [Google Scholar]
- Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi H, Lee LC, Iyoda I, Sasaki S, Okajima H, Takeda K, Yoshimura M, Nakagawa M, Ijichi H. Centrally induced vasodepressor and sympathetic nerve responses to taurine. Jpn Circ J. 1985;49:1180–4. doi: 10.1253/jcj.49.1180. [DOI] [PubMed] [Google Scholar]
- Johns EJ. Angiotensin II in the brain and the autonomic control of the kidney. Exp Physiol. 2005;90:163–8. doi: 10.1113/expphysiol.2004.029025. [DOI] [PubMed] [Google Scholar]
- Kohlenbach A, Schlicker E. GABAB receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Br J Pharmacol. 1990;100:365–9. doi: 10.1111/j.1476-5381.1990.tb15810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CM, Egger P, Dada O, Delgado H, Kylberg E, Lavin P, Tang GH, von Hertzen H, Shiell AW, Barker DJ. Body size at birth and blood pressure among children in developing countries. Int J Epidemiol. 2001;30:52–7. doi: 10.1093/ije/30.1.52. [DOI] [PubMed] [Google Scholar]
- Lourenco R, Camilo ME. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp. 2002;17:262–70. [PubMed] [Google Scholar]
- McMullan S, Goodchild AK, Pilowsky PM. Circulating angiotensin II attenuates the sympathetic baroreflex by reducing the barosensitivity of medullary cardiovascular neurones in the rat. J Physiol. 2007;582:711–22. doi: 10.1113/jphysiol.2007.128983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon S, Bachelard H, Badeau M, Bourgoin F, Pitre M, Lariviere R, Nadeau A. Effects of high-sucrose feeding on insulin resistance and hemodynamic responses to insulin in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2006;290:H2571–81. doi: 10.1152/ajpheart.01002.2005. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Wynter S, Wilks R, Forrester T. Under- and over-reporting of energy is related to obesity, lifestyle factors and food group intakes in Jamaican adults. Public Health Nutr. 2004;7:9–19. doi: 10.1079/phn2003508. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–93. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- Pacioretty L, Hickman MA, Morris JG, Rogers QR. Kinetics of taurine depletion and repletion in plasma, serum, whole blood and skeletal muscle in cats. Amino Acids. 2001;21:417–27. doi: 10.1007/s007260170006. [DOI] [PubMed] [Google Scholar]
- Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–9. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–8. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Insulin and hypertension. Clin Exp Hypertens A. 1990;12:803–16. doi: 10.3109/10641969009073501. [DOI] [PubMed] [Google Scholar]
- Reaven GM. Insulin resistance, hyperinsulinemia, and hypertriglyceridemia in the etiology and clinical course of hypertension. Am J Med. 1991;90:7S–12S. doi: 10.1016/0002-9343(91)90028-v. [DOI] [PubMed] [Google Scholar]
- Riviere G, Michaud A, Breton C, VanCamp G, Laborie C, Enache M, Lesage J, Deloof S, Corvol P, Vieau D. Angiotensin-converting enzyme 2 (ACE2) and ACE activities display tissue-specific sensitivity to undernutrition-programmed hypertension in the adult rat. Hypertension. 2005;46:1169–74. doi: 10.1161/01.HYP.0000185148.27901.fe. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Khongnakha T, Jirakulsomchok D, Wyss JM. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am J Hypertens. 2002;15:773–9. doi: 10.1016/s0895-7061(02)02974-6. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Jirakulsomchok D, Jirakulsomchok S, Wyss JM. Perinatal taurine status influences renal hemodynamics in adult conscious rats. FASEB J. 2004;18 (4 Part I):A292–3. [Google Scholar]
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension. 1987;9:81–7. doi: 10.1161/01.hyp.9.1.81. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Azuma J, Takahashi K, Mozaffari M. Why is taurine cytoprotective? Adv Exp Med Biol. 2003;526:307–21. doi: 10.1007/978-1-4615-0077-3_39. [DOI] [PubMed] [Google Scholar]
- Shiell AW, Campbell-Brown M, Haselden S, Robinson S, Godfrey KM, Barker DJ. High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspring. Hypertension. 2001;38:1282–8. doi: 10.1161/hy1101.095332. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Ura N. Mechanisms of insulin resistance in hypertensive rats. Clin Exp Hypertens. 2006;28:543–52. doi: 10.1080/10641960600851900. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Kregel KC. Frequency response characteristic of sympathetic-mediated vasomotor waves in conscious rats. Am J Physiol. 1996;271:H1416–22. doi: 10.1152/ajpheart.1996.271.4.H1416. [DOI] [PubMed] [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–47. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Roysommuti S, King K, Kadisha I, Regan CP, Berecek KH. Salt-induced hypertension in normotensive spontaneously hypertensive rats. Hypertension. 1994;23:791–6. doi: 10.1161/01.hyp.23.6.791. [DOI] [PubMed] [Google Scholar]