I. INTRODUCTION
Diabetic neuropathies (DN) encompass a wide range of nerve abnormalities and are common, with prevalence rates reported between 5–100% depending on the diagnostic criteria(1–3). Diabetic neuropathies affect both peripheral and autonomic nervous systems and cause considerable morbidity and mortality in both Type 1 and Type 2 diabetic patients. Diabetic neuropathies are the most common forms of neuropathy, they account for more hospitalizations than all other diabetic complications combined, and are responsible for 50–75% of non-traumatic amputations(4,5). In older adults with diabetes, peripheral neuropathies are especially troublesome due to their detrimental effects on stability, sensorimotor function, gait, and activities of daily living(6–8). In the U.S. for 1999–2000, 28% of adults aged 70–79 years and 35% of adults aged ≥80 years had peripheral neuropathy based on a simple screen for reduced sensation at the foot.(9). In this review, we present and discuss the most recent approaches to the treatment of the common forms of diabetic neuropathy, including symmetric, focal and diffuse neuropathies (Box 1, Fig. 1). We will also provide the reader with algorithms for recognition and management of common pain and entrapment syndromes, and a global approach to recognition of syndromes requiring specialized treatments based upon our improved understanding of their etiopathogenesis. A comprehensive evaluation of autonomic neuropathy is beyond the scope of this review, but the reader is referred to two excellent reviews on this topic(10,11).
Box 1.
Classification of Diabetic Neuropathy
| Focal neuropathies | |
| • mononeuritis | |
| • entrapment syndromes | |
| Diffuse neuropathies | |
| • proximal motor (amyotrophy) | |
| ▪ co-existing chronic inflammatory demyelinating polyneuropathy (CIPD) | |
| ▪ monoclonal gammopathy of undetermined significance (MGUS) | |
| ▪ circulating GM1 antibodies and antibodies to neuronal cells | |
| ▪ inflammatory vasculitis | |
| Generalized symmetric polyneuropathies | |
| • acute sensory | |
| • autonomic | |
| • chronic sensorimotor distal polyneuropathy (DPN) | |
| ◦ large fiber | |
| ◦ small fiber |
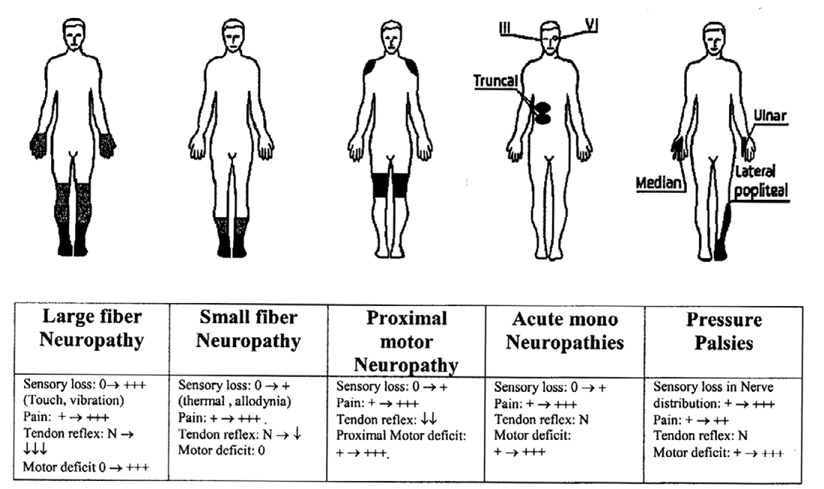
Fig. 1.
Schematic representation of different clinical presentations of diabetic neuropathy.
I.A. Pathogenic Mechanisms
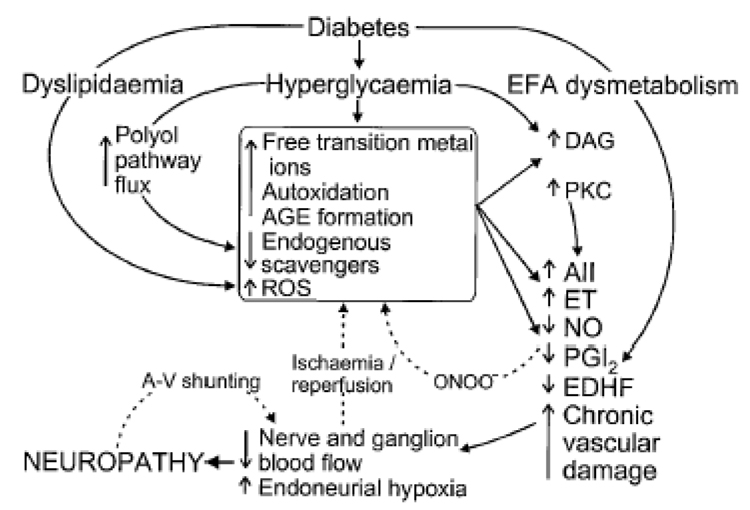
Figure 2 and figure 3 shows our current view on the pathogenesis of diabetes. The figure 2 depicts multiple etiologies, as discussed above, including metabolic, vascular, autoimmune, oxidative and nitrosative stress, and neurohormonal growth-factor deficiency. Inflammation is more clearly involved in the specific inflammatory neuropathies such as vasculitic and granulomatous disease than in diabetic neuropathy per se (12)though has not been studied in age-related neuropathies. P- and E-selectin, activated during the inflammatory process, predict the decline in peripheral nerve function among diabetic patients(13). Impaired blood flow and endoneurial microvasculopathy, mainly thickening of the blood vessel wall or occlusion, play a critical role in the pathogenesis of diabetic neuropathy. Metabolic disturbances in the presence of an underlying genetic predisposition, cause reduced nerve perfusion. Animal and human studies alike have shown major defects arising from chronic hyperglycemia and altered lipid metabolism(14). Oxidative stress-related mechanisms are also important in vascular dysfunction, and tend to increase vasoconstriction. These alterations in blood flow patterns appear to be important in the understanding of the arterio-venous shunting seen in vasa nervorum, which may occur in part due to autonomic nerve dysfunction. Sensory and local autonomic nerve function deficits appear to predominate in patients with critical limb ischemia(15).Improving blood flow to tissues may improve nerve conduction velocity in diabetic neuropathy(16). Oxidative and nitrosative stress and inflammation are implicated in several neurodegenerative disorders including Alzheimer’s disease and amyotrophic lateral sclerosis (ALS)(17). Oxidative stress is indicated as a contributor in diabetic neuropathy(18). It is greater in diabetic patients prior to development of peripheral neuropathy and particularly in those with peripheral neuropathy(19).Potentially, similar mechanisms play a role in the peripheral nerve with aging, as aging(20)and type 2 diabetes(21–25)are associated with an increased levels of subclinical systemic inflammatory markers, such as cytokines IL-6 and TNF-α, and acute phase proteins such as CRP.
Fig. 2.
Pathogenesis of diabetic neuropathy based upon oxidative/nitrosative stress and metabolic processes. AII, angiotensin II; AGE, advanced glycation end product; A-V, arteriovenous; DAG, diacylglycerol; EDHF, endothelium-derived hyperpolarizing factor; EFA, essential fatty acid; ET, endothelin-1; NO, nitric oxide; ONOO−, peroxynitrite; PGI2, prostacyclin; PKC, protein kinase C; ROS, reactive oxygen species.(106).
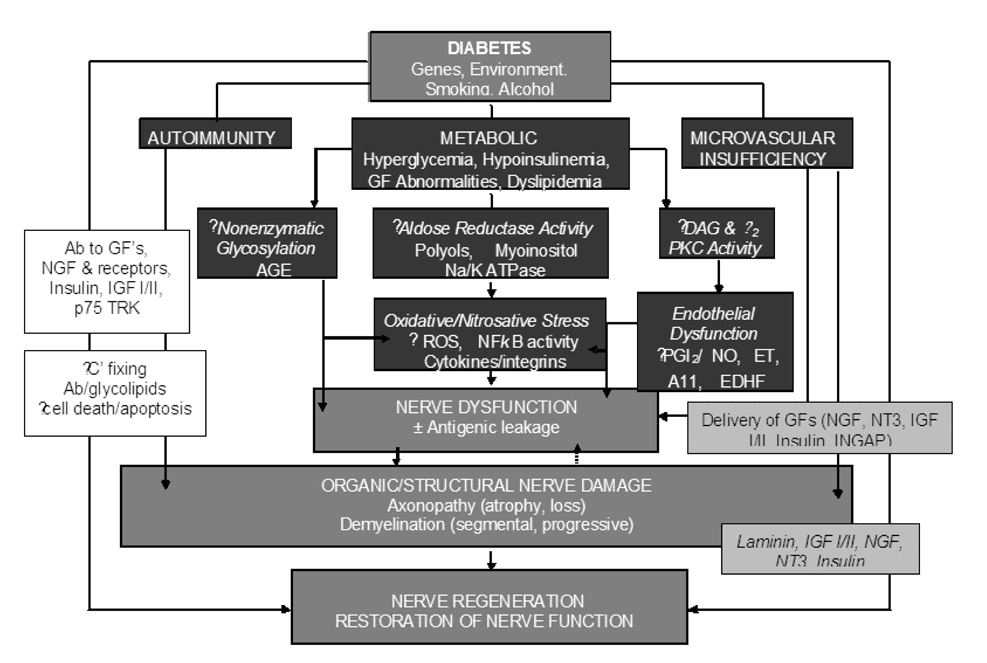
Fig.3.
Pathogenesis of diabetic neuropathies based upon Autoimmunity, Metabolic and Microvascular Insufficiency. Ab, antibody; AGE, advance glycation end products; C¢, complement; DAG, diacylglycerol; ET, endothelin; EDHF, endothelium-derived hyperpolarizing factor; GF, growth factor; IGF; insulin-like growth factor; NFkB, nuclear factor kB; NGF, nerve growth factor; NO, nitric oxide; NT3, neurotropin 3; PKC, protein kinase C; PGI2, prostaglandin I2; ROS, reactive oxygen species; TRK, tyrosine kinase.(101)
II. CLINICAL PRESENTATION AND DIAGNOSIS
II.A. Focal Neuropathies (Mononeuropathies and Entrapment Syndromes)
Mononeuropathies occur primarily in older adults. Their onset is generally acute, associated with pain, and they heal spontaneously, usually within 6–8 weeks. These neuropathies are caused by vascular obstruction, typically in the cranial nerves III, VI, and VII, ulnar, median, and peroneal. Mononeuropathies must be distinguished from entrapment syndromes which start slowly, progress and persist without intervention (Table 1).
Table 1.
Comparison of features of Mononeuropathies, Entrapment syndromes and Distal symmetrical polyneuropathy
| Feature | Mononeuropathy | Entrapment syndrome | Neuropathy |
|---|---|---|---|
| Onset | Sudden | Gradual | Gradual |
| Pattern | Single nerve but may be multiple | Single nerve exposed to trauma | Distal symmetrical poly neuropathy |
| Nerves involved | CN III, VI, VII, ulnar, median, peroneal | Median, ulnar, peroneal, medial and lateral plantar | Mixed, Motor, Sensory, Autonomic |
| Natural history | Resolves spontaneously | Progressive | Progressive |
| Treatment | Symptomatic | Rest, splints, local steroids, diuretics, surgery | Tight Glycemic control, Pregabalin, Duloxetine, Antioxidants, “Nutrinerve”, Research Drugs. |
| Distribution of Sensory loss | Area supplied by the nerve | Area supplied beyond the site of entrapment | Distal and symmetrical. “Glove and Stocking” distribution. |
Mononeuropathies, Entrapment syndromes and Distal symmetrical polyneuropathy; CN, cranial nerves.(101)
Common entrapment sites in diabetic patients involve the median, ulnar, peroneal, lateral cutaneous nerve of the thigh, and the tibial nerve in the tarsal canal. Their onset is gradual and is usually limited to a single nerve(26). Carpal tunnel syndrome is the most common entrapment syndrome, affecting one in three diabetic patients(27). It occurs three times more frequently in patients with diabetes compared with the normal healthy population (28)and may be related to diabetic cheiroarthropathy, repeated undetected trauma, metabolic changes, or an accumulation of fluid or edema within the confined space of the carpal tunnel(29). Surgical treatment of entrapment syndrome neuropathies are effective, but the decision to proceed with surgery should be based on severity of symptoms, appearance of motor weakness and failure of non-surgical treatment.
II.B. Diffuse Neuropathies (proximal motor neuropathies)
Proximal motor neuropathy can be clinically identified based on proximal muscle weakness and muscle wasting. It may be symmetric or asymmetric in distribution, and is sometimes associated with pain in the lateral aspect of the thigh. Patients usually present with weakness of the iliopsoas, obturator and adductor muscles, together with relative preservation of the gluteus maximus and minimus, and hamstrings(30,31). Those affected have great difficulty rising out of chair unaided, although heel or toe standing is surprisingly good. In the classic form of diabetic proximal motor neuropathy, axonal loss is the predominant process and the condition coexists with distal symmetric polyneuropathy (DPN)(32). Electrophysiologic evaluation reveals lumbosacral plexopathy(33). Common features include:
Primarily affects the elderly
Onset may be gradual or acute
Begins with pain in the thighs and hips or buttocks
Pain followed by significant weakness of the proximal muscles of the lower limbs with inability to rise from the sitting position (positive Gower’s maneuver)
Begins unilaterally and spreads bilaterally
Coexists with DPN
Spontaneous muscle fasciculation, or provoked by percussion
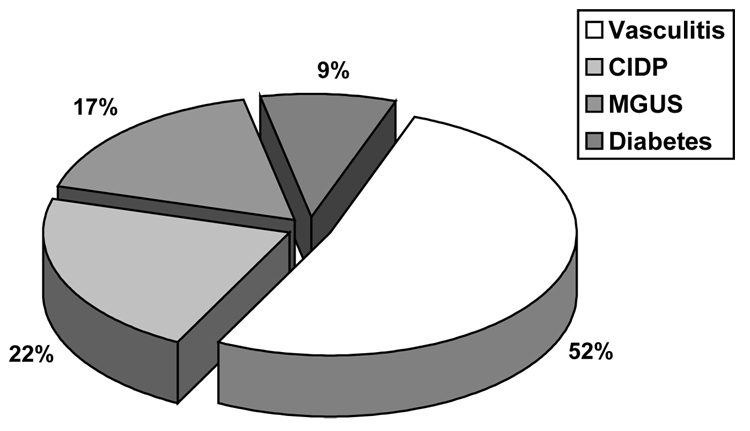
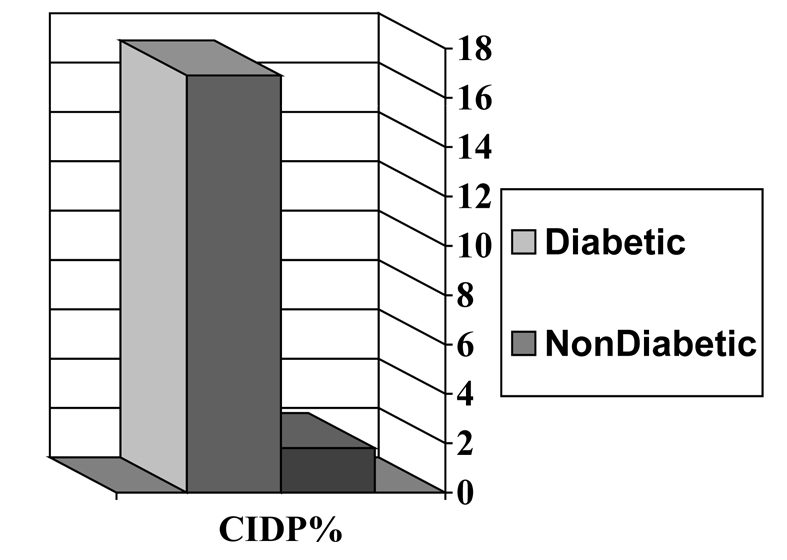
Proximal motor neuropathy is now recognized as being secondary to a variety of causes unrelated to diabetes, but which occur more frequently in patients with diabetes than in the general population. It includes patients with chronic inflammatory demyelinating polyneuropathy (CIDP), monoclonal gammopathy of undetermined significance (MGUS), circulating GM1 antibodies and antibodies to neuronal cells, and inflammatory vasculitis(34,35). Vinik et al (36) (Fig. 4) found that almost half of patients with proximal neuropathies have a vasculitis and all but 9% have CIDP, MGUS, or a ganglioside antibody syndrome(36,37). Sharma examined over 1000 patients with neurologic disorders and found that CIDP was 11x more frequent among diabetic than non-diabetic patients (Fig. 5)(38).
Fig. 4.
Disabling Peripheral Neuropathies in Older Adults (38)
Fig. 5.
Frequency of chronic inflammatory demyelinating polyneuropathy (CIPD)(38). There is an 11-fold greater frequency of CIPD in patients with diabetes.
In contrast, if demyelination predominates and the motor deficit affects proximal and distal muscle groups, the diagnosis of CIDP should be considered. It is important to divide proximal syndromes into these two subcategories since the CIDP variant responds dramatically to intervention(36,38,39), with IVIG, plasmaphereis, steroids and immunosuppresive agents (36) whereas proximal motor neuropathy runs its own course over months to years (Box 2). Until more evidence is available, we consider them as separate syndromes.
Box 2.
Decline in neurologic function between 20–80 years
| Function | Percent Dysfunction |
|---|---|
| Vibratory sensation | 97 |
| Stability (Rombergism) | 32 |
| Handwriting speed | 30 |
| Handgrip strength | 22 |
| Ankle jerk | 9 |
| Ataxia (finger nose test) | 8 |
| Pain perception | 0 |
These conditions should be distinguished from spinal stenosis syndromes common in older individuals, which occur due to: 1) encroachment on nerve roots as they emerge from the spinal cord, 2) osteophytes which narrow joint space and cause compression, 3) hypertrophy of the ligamentum flavum due to aging, 4) disk dehydration due to aging, and 5) arachnoiditis. If compression occurs at the level of T12 and L1/2, the vascular system may be involved. This often causes claudication during downhill walking, and is relieved with spinal flexion. Nerve root compression is more typical at L5/S1 and thus in difficult cases it may be necessary to obtain an MRI of the lumbosacral spine. Diagnosis is critical since therapy may range from simple physical therapy to surgical decompression if symptoms are severe or if motor paralysis exists.
II.C. Chronic Sensorimotor Distal Polyneuropathy (DPN)
Chronic sensorimotor distal polyneuropathy (DPN) is the most common and widely recognized form of diabetic neuropathy. The onset is usually insidious, following stress or initiation of therapy for diabetes. DPN may be either sensory or motor, and involve small fibers, large fibers, or both (40) . Initial neurologic evaluation should focus on detection of the specific part of the nervous system affected by diabetes. Most patients with DPN have a combination of both large and small nerve fiber involvement.
II.C.1. Large fiber neuropathies
A majority of neuropathies in older adults involve large fibers. Large fiber neuropathies may involve sensory and/or motor nerves, and most patients will present with a "glove and stocking" distribution of sensory loss(41).These tend to be the neuropathies of signs rather than symptoms. They are manifested by reduced vibration (often the first objective evidence of neuropathy) and position sense, weakness, muscle wasting and depressed tendon reflexes. Early in the course of the neuropathic process, multifocal sensory loss might also be found (Box 2). The symptoms may be minimal, such as a sensation of walking on cotton, floors feeling “strange”, inability to turn the pages of a book, or inability to discriminate among coins. In some patients, severe distal muscle weakness can accompany the sensory loss resulting in an inability to stand on the toes or heels.
However, little is known at the clinical and population levels about the role of age-related loss in peripheral nerve function to sarcopenia and loss of strength associated with aging. Loss of lean mass - or sarcopenia - is thought to account for much of the loss of strength and function in older adults(42,43).In addition to lower mass, aging muscle is characterized by loss of muscle fibers, predominantly type 2 fast twitch fibers, and an increase in grouping or “clustering” of type 1 fibers(44).These changes are thought to be due in part to disuse atrophy and in part to drop out of the anterior horn motor neuron at the level of the spinal cord. When the motor neuron is lost with disease (polio, ALS) or aging, remaining motor neurons can sprout new dendritic connections to “orphaned” muscle fibers. This reinnervation process may be responsible for the increase in grouping of type 1 fibers and may limit regaining type 2 fibers after loss due to atrophy. Although the innervation of muscle tissue is essential to its function, very little is known about the relative contribution of peripheral nerve function to muscle function and functional decline in community dwelling older adults. In diabetes, severe peripheral neuropathy is quite clearly related to muscle atrophy(45).By MRI scanning, diabetic neuropathy in type 1 diabetes is associated with a 50% reduction in muscle volume, with a high correlation between neuropathy score and muscle volume (r=−0.75, p<0.001)(46).Given the known atrophy and denervation in the pathophysiologic description of muscle aging, it is remarkable how little is known at the clinical and population levels about the role of age-related loss in nerve function to the age-related loss of muscle mass and strength. This neurogenic process may be a critical link in the pathogenesis of sarcopenia and mobility loss in old age.
Older adults with large fiber neuropathies have difficulty stabilizing their bodies when walking on irregular surfaces, with concomitant impairment in reaction time and balance(6).This lack of peripheral sensory input increases the risk of falling and fracture in these patients. In the Women’s Health and Aging study, women with diabetes reported difficulty in performing 14 of 15 daily tasks which included walking 2–3 blocks, lifting 10 pounds, using a telephone, and bathing(7).Failure to perform basic activities of daily living readily compromise an individual’s independence and quality of life, which increases mortality and morbidity in this susceptible population(47). Norfolk quality of life (QOL) tool is used to measure patients’ perception of the effects of diabetes and diabetic neuropathy(47).
Epidemiologic studies have found that older adults with poor peripheral nerve function have worse physical performance, balance, muscle density and bone density(22,23,48–50). Most of these associations were independent of diabetes status. A two-fold higher prospective decline in motor performance exists for older adults with distal symmetrical neuropathy(51).Clinical consequences of higher fall and fracture rates are also evident in older adults with peripheral nerve impairments. In a prospective cohort aged ≥70 years, those with loss of touch sensation in their feet had a 2.5 times greater risk of major injurious falls, including fractures, joint dislocations, lacerations requiring sutures, and intracranial injuries(52). In the Study of Osteoporotic Fractures, recurrent falling was related to worse vibration sense (age-adjusted OR=1.12, 95%CI: 1.05–1.19) and loss of touch sensation (age-adjusted OR=1.58, 95%CI: 1.34–1.87) in older women(53) .
In recent years, several inexpensive devices have been developed for the assessment of somatosensory function, including vibration, thermal energy, and light-touch perception. These instruments allow for the noninvasive assessment of cutaneous sensory functions, which correlate with specific neural fiber function. In addition to the above modalities, quantitative sensory tests (QST) are available for the assessment of pain threshold and cutaneous current perception(40).
Clinical manifestations of large fiber neuropathies
Impaired vibration perception and position sense
Depressed tendon reflexes
Dull (like a toothache), crushing or cramp-like pain in the bones of the feet
Sensory ataxia (waddling like a duck)
Wasting of small muscles of feet with hammertoes and weakness of hands and feet
Shortening of the Achilles tendon with equinus.
Increased blood flow to the foot (hot foot) with increased risk of Charcot neuroarthropathy.
II.C.2. Small fiber neuropathies
Small nerve fiber dysfunction usually occurs early and is often present without objective signs or electrophysiologic evidence of nerve damage(40). It manifests first in the lower limbs with symptoms of pain and hyperalgesia, followed by a loss of thermal sensitivity and reduced light-touch and pinprick sensation(54). Small unmyelinated C-fibers control pain sensation, warm thermal perception and autonomic function. A patient with early damage to these nerves may experience burning, dysesthetic pain, often accompanied by hyperalgesia, and allodynia. This pain is distinct from that of large fiber neuropathy, where the pain is usually described as deep and “gnawing.” Because peripheral sympathetic nerve fibers are also comprised of small, unmyelinated C-fibers, it is not surprising that pain is improved with sympathetic blocking agents (e.g. beta-blockers, calcium channel blockers).
It should be noted that dry, cracked skin and impaired skin blood flow in the feet, together with impaired sympathetic regulation of sweat glands and A-V shunt vessels in the feet, create a favorable environment for bacteria. In the absence of pain, which occurs with the depletion of substance P, patients may be led to believe that their neuropathy has subsided, when in fact it is progressing. These patients may also display decreased thermal pain thresholds, which may be due in part to the decrease in nerve growth factor (NGF) which maintains small fiber neurons. The clinical manifestations of small vs. large fiber neuropathies are summarized below:
Clinical manifestations of small fiber neuropathies
Prominent pain: burning and superficial and associated with allodynia i.e. interpretation of all stimuli as painful (e.g. touch)
Hypoalgesia late in the condition
Defective autonomic function with decreased sweating, dry skin, impaired vasomotion and blood flow and cold feet
Intact reflexes, motor strength
Silent electrophysiology
Reduced sensitivity to 1.0g Semmes Weinstein monofilament and pricking sensation using the Waardenberg wheel or similar instrument
Abnormal thresholds for warm thermal perception, neurovascular function, pain, quantitative sudorimetry and quantitative autonomic function tests
Increased risk of foot ulceration and subsequent gangrene
II.D. Differential diagnosis
Diabetes as the cause of neuropathy is diagnosed by exclusion of various other causes of neuropathy. In those patients with diabetes and neuropathy who present with symptoms of distal symmetric sensorimotor deficit, differential diagnosis should include: hereditary sensory neuropathies, B12 and folate deficiency, syphilis, Lyme disease, neuropathy associated with IgM monoclonal gammopathy of undetermined significance (IgM MGUS neuropathy), other paraneoplastic conditions, autoimmune diseases, and toxic neuropathies. In patients with one or more motor neurologic syndromes, chronic motor neuropathies, AIDP, CIDP, and IgG and IgA MGUS neuropathies should actively be sought.
Recent evidence supports an autoimmune etiology for neuropathy in AIDS, Lyme disease, AIDP, CIDP, multifocal motor neuropathy, MGUS neuropathies and even diabetic polyneuropathy(29,41). Hence, an intensive work up for humoral immune mechanisms should be performed. If any of these conditions are found, the appropriate therapeutic regime for the specific disease must be instituted, before embarking on a regime of diabetic neuropathy management. It is not always possible to determine the exact cause of neuropathy if monoclonal gammopathy and diabetes coexist in the same patient. A course of intravenous immunoglobulin (IVIg) or immunosuppression should be attempted depending on the class of monoclonal antibody.
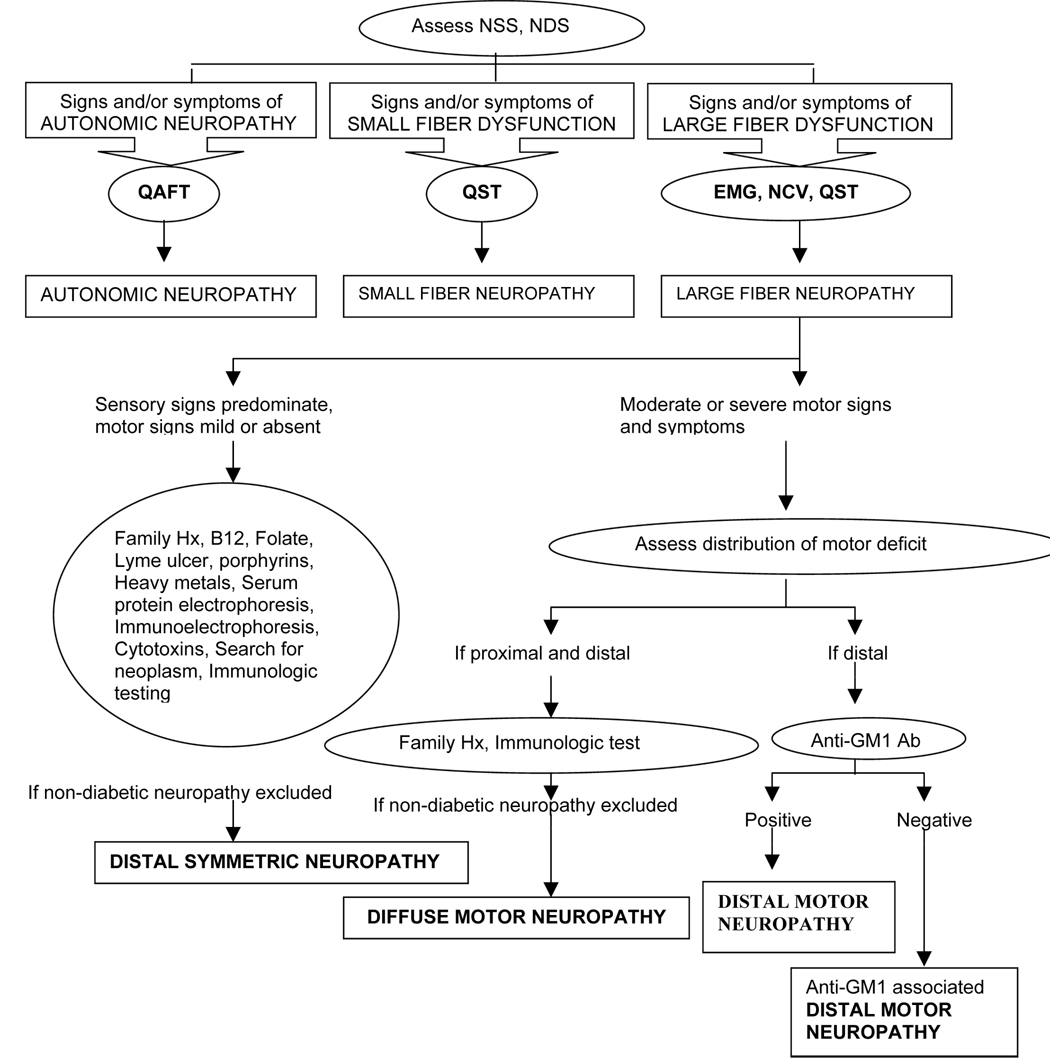
Nerve tissue biopsy may be helpful for excluding other causes of neuropathy and in the determination of predominant pathologic changes in patients with complex clinical findings as a means of dictating choice of treatment(39,55). Our laboratory performs nerve biopsies only when noninvasive neurological procedures fail to provide an answer and/or when extensive evaluation is necessary for scientific purposes(55). We expect a further increase in our dependence on histopathologic and ultrastructural examination of nerve tissue for differentiation of neuropathic syndromes, as our knowledge of pathophysiologic and clinical complexity among diabetic neuropathic variants increases. Figure 7 depicts a diagnostic algorithm for the assessment of neurologic deficit and classification of neuropathic syndromes.
Fig. 7.
A diagnostic algorithm for assessment of neurologic deficit and classification of neuropathic syndrome is given below. NSS; Neurological Symptom Score, NDS; Nerve Disability Score, QST; Quantitative Sensory Test, QAFT; Quantitative Autonomic Function Test, EMG; Electromyography, NCV; Nerve Conduction Velocity (1)
II.E. Charcot neuroarthropathy
Charcot neuroarthropathy is a progressive condition associated with prolonged neuropathy and characterized by pathological fracture, joint dislocation, and if left untreated, disabling joint deformity. The most common location for Charcot is in the foot. The prevailing theory of Charcot progression suggests that autonomic neuropathy causes increased blood flow to the extremities which increases bone resorption and causes osteopenia. Subsequent motor neuropathies cause muscular imbalance which place abnormal stress on the affected extremity. Sensory neuropathies prevent the patient from sensing abnormal changes in the joints and bones which may occur due to minor trauma, such as during walking(56). It is further hypothesized that Achilles tendon shortening due to destruction of collagen fibers may be due to accumulation of advanced glycation endproducts (AGEs)(57,58).
Patients with Charcot neuroarthropathy may present acutely with severe pain (or no pain if severe sensory neuropathy), a warm to hot swollen foot with increased skin blood flow (despite decreased warm sensory perception and vibration detection), and possible radiographic evidence of osteopenia. The acute Charcot foot can mimic cellulitis or, less commonly, deep vein thrombosis, so these should be first investigated. It should also be noted that radiographic findings can be normal in the acute phase, with subsequent films showing severe subluxation and/or fracture. Strict immobilization and protection of the foot using a total contact cast is the recommended approach to treating acute Charcot. Pain and inflammation respond to bisphosphonates (e.g. slow IV pamidronate infusion over 12 hours) within 3 to 4 weeks(59). It is worth noting that oral bisphosphonates may cause esophageal dysfunction and increase the risk of obstruction and perforation. Achilles tendon shortening producing equinus is correctable by surgical lengthening and may prevent further progression. Patient education, protective footwear, and routine foot care are required to prevent further complications such as foot ulceration. In cases of severe joint and bony destruction, reconstructive surgery is effective in salvaging the limb and improving mobility and quality of life(47).
III. MANAGEMENT OF NEUROPATHY
The high prevalence of certain subclinical diseases in the elderly may be associated with declines in peripheral nerve function. Importantly, these conditions are modifiable so early intervention on these risk factors may prevent peripheral nerve function declines and the subsequent clinical consequences associated with peripheral neuropathy. Vitamin B12 deficiency is a known cause of clinical neuropathy(60). However, the impact of marginally poor vitamin B12 levels, found in between 22–35% of community-dwelling older adults(61–63), on peripheral nerve decline is unknown. This has clear clinical implications for defining B12 replacement criteria(64). Nearly all peripheral arterial disease (PAD) in the elderly is subclinical, with 98% asymptomatic(65). An Italian study in community-dwelling elderly found an association of subclinical PAD and poor nerve function(66). This finding is of particular importance since 12% of older adults aged 70–79 years and 22% aged ≥80 years have subclinical PAD in the US. Subclinical PAD is underappreciated clinically, but highly preventable(67,68).
The metabolic syndrome represents another prevalent risk factor for peripheral nerve impairments in the elderly. Prevalence of the metabolic syndrome in the U.S. is >40% in adults aged ≥60 years(69). It is a risk factor for peripheral neuropathy among diabetic adults(70,71)..In the Cardiovascular Health Study of older adults, participants with normal glucose metabolism or a mildly elevated impaired fasting glucose (IFG) had lower heart rate variability (HRV), a marker of cardiovascular autonomic neuropathy, in the presence of ≥2 components of the metabolic syndrome(69). In addition to the reduction of blood glucose levels, prevention and treatment of the other components of the metabolic syndrome (obesity, lipid abnormalities and high blood pressure) could be targeted to prevent peripheral nerve declines in older adults. Once the diagnosis of neuropathy has been made, therapy to reduce symptoms and prevent further progression should be initiated. Diabetic patients with large fiber neuropathies are incoordinate and ataxic and are 17 times more likely to fall than their non-neuropathic counterparts(72). Older subjects have a higher incidence of neuropathy than younger subjects, especially involving large fibers. It is vitally important to improve strength and balance in the patient with large fiber neuropathy. Older adults with and without neuropathy can benefit from high intensity strength training by increasing muscle strength, improving coordination and balance, and thus reducing fall and fracture risk(73,74). Low impact activities which emphasize muscular strength and coordination, and challenge the vestibular system, such as pilates, yoga, and Tai Chi may also be particularly helpful.
Strategies for management of large fiber neuropathies
Strength, gait, and balance training
Pain management as detailed below
Orthotics fitted with proper shoes to treat and/or prevent foot deformities
Tendon lengthening for equinus caused by achilles tendon shortening
Bisphophonates to treat osteopenia
Surgical reconstruction and full contact casting as necessary
Strategies for management of small fiber neuropathies
There are several simple measures that can protect the foot deficient in functional C-fibers from developing ulceration, and therefore, gangrene and amputation:
Foot protection is of the utmost importance. Wearing padded socks can promote ulcer healing and/or reduce the likelihood of developing one(75).
Supportive shoes with orthotics if necessary.
Regular foot and shoe inspection. Patients should inspect the plantar surface of their feet with a mirror on a daily basis. (Many are too obese to see their feet, let alone the undersurface).
Extreme caution to prevent heat injury. Patients should test the bathwater with a part of the body that is not insensate before plunging a numb foot into the water. Patients should also be cautioned against falling asleep in front of the fireplace with their insensate feet close to the fire.
Use emollient creams to moisturize dry skin and prevent cracking and infection.
III.A. Therapies aimed at pathogenic mechanisms
Retrospective and prospective studies have suggested a relationship between hyperglycemia and the development and severity of diabetic neuropathy, and significant effects of intensive insulin treatment on prevention of neuropathy(76). Intensive treatment of hyperglycemia in the elderly is controversial. Additionally, no epidemiologic or natural history study has defined the importance of late onset diabetes in aged populations and IFG as risk factors for nerve function decline in very old adults. Recent data from the Cardiovascular Health Study suggests that IFG is a risk factor for autonomic neuropathy in the elderly(77).
Studies in animal models and cultured cells provide a conceptual framework for the cause and treatment of diabetic neuropathy. However, the limited translational work in diabetic patients continues to generate debate over the cause(s) of human diabetic neuropathy and to date we have no effective long-term treatment. . Several clinical trials found that treating oxidative stress may improve peripheral and autonomic neuropathy in type 2 diabetic adults(19,78–80). Thiazolidinediones, which reduce hyperglycemia through reductions in insulin resistance and may also influence chronic inflammation, potentially impact pathways leading to peripheral neuropathy(81). Exciting emerging evidence indicates that fibrates and statins are protective for peripheral nerve function decline in type 2 diabetic adults(82). Older adults using statins show a greater benefit than younger adults due to their higher attributable risk of cardiovascular disease(83). However, the impact of statins on peripheral neuropathy in the elderly is not yet evaluated. A summary of the drugs that have been studied in clinical trials aimed at treating the pathogenic mechanisms of DPN are listed in Box 4.
Box 4.
Treatment Of Diabetic Neuropathy Based On Pathogenetic Mechanisms
| Abnormality | Compound | Aim of treatment | Status of RCTs |
|---|---|---|---|
| Polyol pathway ↑ | Aldose reductase inhibitors | Nerve sorbitol ↓ | |
| Sorbinil | Withdrawn (AE) | ||
| Tolrestat | Withdrawn (AE) | ||
| Ponalrestat | Ineffective | ||
| Zopolrestat | Withdrawn (marginal effects) | ||
| Zenarestat | Withdrawn (AE) | ||
| Lidorestat | Withdrawn (AE) | ||
| Fidarestat | Effective in RCTs, Trials ongoing | ||
| AS-3201 | Effective in RCTs, Trials ongoing | ||
| Epalrestat | Marketed in Japan | ||
| myo-Inositol ↓ | Myo-Inositol | Nerve myo-inositol ↑ | Equivocal |
| Oxidative stress ↑ | α–Lipoic acid, NutriNerve | Oxygen free radicals ↓ | Effective in RCTs, trials ongoing |
| Nerve hypoxia ↑ | Vasodilators | NBF ↑ | |
| ACE inhibitors | Effective in 1 RCT | ||
| Prostaglandin analogs | Effective in 1 RCT | ||
| phVEGF165 gene transfer | Angiogenesis ↑ | RCTs ongoing | |
| Protein kinase C ↑ | PKC β inhibitor | NBF ↑ | Phase II +ve* |
| (ruboxistaurin) | Phase III −ve* | ||
| C-peptide ↓ | C-peptide | NBF ↑ | Studies ongoing |
| Neurotrophism ↓ | Nerve growth factor (NGF) | Nerve regeneration growth ↑ | Ineffective |
| BDNF | Nerve regeneration, growth ↑ | Ineffective | |
| LCFA metabolism↓ | Acetyl-L-carnitine | LCFA accumulation ↓ | Ineffective |
| GLA synthesis ↓ | γ–Linolenic acid (GLA) | EFA metabolism ↑ | Withdrawn |
| NEG ↑ | Aminoguanidine | AGE accumulation ↓ | Withdrawn |
III.B. Therapy aimed at treating symptoms in patients with DPN
It is critical to discern the underlying condition in diabetic patients with pain. Physicians must be able to differentiate painful diabetic neuropathy from other unrelated or coexisting conditions in patients with diabetes. The most common of these are claudication, Morton’s neuroma, Charcot neuroarthropathy, fasciitis, osteoarthritis, and radiculopathy (Table 2).
Table 2.
Common Pain Syndromes Similar to Painful Diabetic Neuropathy
| Condition | Key Characteristics and Differentiating Features |
|---|---|
| Claudication | • Doppler ultrasonography confirms clinical diagnosis of arterial occlusion |
| • Diabetic patients may present with normal extremities and absent foot pulses | |
| • Peripheral arterial occlusion with underlying atherosclerosis | |
| • Usually intermittent, worsened by walking; remits with rest; other signs/symptoms suggest arterial insufficiency | |
| Morton’s neuroma | • Benign neuroma formation on third plantar interdigital nerve |
| • Generally unilateral | |
| • More frequent in women | |
| • Pain elicited when pressure is applied with the thumb between the first and fourth metatarsal heads | |
| Osteoarthritis | • Can be secondary to diabetes mellitus, but onset of pain is usually gradual and in 1 or 2 joints |
| • Differential diagnosis based on x-ray | |
| • Morning stiffness, diminished joint motion, and flexion contractures | |
| • Pain worsens with exercise and improves with rest | |
| • Radiculopathy can result | |
| Radiculopathy | • Can be caused by diabetes, but also from arthritis or metastatic disease |
| • Neurologic examinations and imaging can localize lesion site | |
| • Pain can occur in thorax, extremities, shoulder, or arm, depending on site of lesion | |
| Charcot neuroarthropathy | • May result from osteopenia due to increased blood flow following repeated minor trauma in individuals with diabetic neuropathy |
| • Warm to hot foot with increased skin blood flow | |
| • Decreased warm sensory perception, vibration detection | |
| Plantar fasciitis | • Pain in plantar region of the foot |
| • Tenderness along plantar fascia when ankle is dorsiflexed | |
| • Shooting or burning in the heel with each step | |
| • Worsening pain with prolonged activity | |
| • Often associated with calcaneal spur on radiography | |
| Tarsal tunnel syndrome | • Caused by entrapment of the posterior tibial nerve |
| • Pain and numbness radiate from beneath the medial malleolus to the sole | |
| • Clinical examination includes percussion, palpation for possible soft-tissue matter, nerve conduction studies, magnetic resonance imaging | |
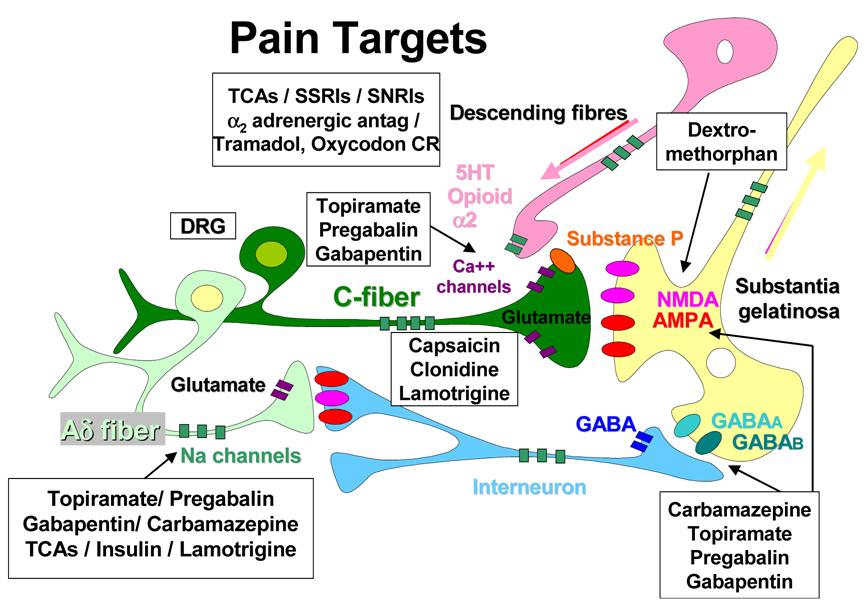
Treatment strategies should aim to decrease the afferent input, reduce local inflammation, suppress sympathetic fortification of the stimulus, reduce the impact of excitatory amino acids, alter the modulation of nociceptors, and suppress Na+ channel activity (Fig. 8).
Fig. 8. Different mechanisms of pain and possible treatments.
C fibers are modulated by sympathetic input with spontaneous firing of different neurotransmitters to the dorsal root ganglia, spinal cord and cerebral cortex. Sympathetic blockers (e.g. clonidine) and depletion of axonal substance P used by C fibers as their neurotransmitter (e.g. by capsaicin) may improve pain. In contrast Ad fibers utilize Na+ channels for their conduction and agents that inhibit Na+ exchange such as antiepileptic drugs, tricyclic antidepressants and insulin may ameliorate this form of pain. Anticonvulsants (carbamazepine, gabapentin, pregabalin, topiramate) potentiate activity of g-aminobutyric acid, inhibit Na+ and Ca2+ channels and inhibit N-methyl-D-aspartate receptors and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors. Dextromethorphan blocks N-methyl-D-aspartate receptors in the spinal cord. Tricyclic antidepressants, selective serotonin reuptake inhibitors (e.g. fluoxetine), and serotonin and norepinephrine reuptake inhibitors inhibit serotonin and norepinephrine reuptake, enhancing their effect in endogenous pain-inhibitory systems in the brain. Tramadol is a central opioid analgesic. α2 antag, α 2 antagonists; 5HT, 5-hydroxytryptamine; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; DRG, dorsal root ganglia; GABA: g-aminobutyric acid; NMDA, N-methyl-D-aspartate; SNRIs, serotonin and norepinephrine reuptake inhibitors; SP, substance P; SSRIs, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants;(101)
Amitriptyline is prescribed for diabetic neuropathy(84), but anticholinergic side effects such as orthostatic hypotension and possible cardiac arrhythmias (84,85) warrant caution in its use. Contraindications to amitriptyline and other tricyclic antidepressants include cardiac conduction block, long QT syndrome, myocardial infarction within 6 months, ventricular arrhythmias and/or frequent premature ventricular contractions(85). Older adults with neuropathy are at risk for adverse events from tricyclic antidepressants especially stability, balance and cognitive problems(86). For this reason, patients over 40 years old should have a screening electrocardiogram prior to using these medications(86).
Other commonly used drug classes include analgesics (local, simple, and narcotic), antiarrhythmics, and antiepileptic drugs (Table 3)(85). Based on positive results from randomized, controlled trials and expert clinical opinion of members of the faculty of the Fourth International Conference on the Mechanisms and Treatment of Neuropathic Pain, recommendations for first-line medications for neuropathic pain include gabapentin, 5% lidocaine patch, opioid analgesics, tramadol hydrochloride, and tricyclic antidepressants(86). Consideration of the safety and tolerability of different therapies is important in avoiding adverse effects, a common result of treatment of neuropathic pain. Dosages must be titrated based on positive response, treatment adherence, and adverse events(86).
Table 3.
Drugs Approved by the FDA for Treatment of Neuropathic Pain Syndrome (adapted from [51])
| Medication | Indication | Beginning Dosages | Titration | Maximum Dosage | Duration of Adequate Trial |
|---|---|---|---|---|---|
| Gabapentin | Postherpetic neuralgia | 100–300 mg every night or 100–300 mg 3×/d | Increase by 100–300 mg 3×/d every 1–7 d as tolerated | 3600 mg/d (1200 mg 3 ×/d); reduce if low creatinine clearance | 3–8 wk for titration plus 1–2 wk at maximum tolerated dosage |
| Pregabaline | Postherpetic neuralgia | 50 mg three times a day | Increase upto 100 mg three times a day | 600 mg a day | Start with 50mg TID and increase upto 100mg TID over 1 week |
| Lamotrigine | Postherpetic neuralgia | 200–400 mg every night. | Start with 25 to 50 mg every other day and increase by 25 mg every week. | 500 mg a day | 3 to 5 wk for titration ad 1–2 wk at maximum tolerated dosage. |
| Carbamazepine** | Trigeminal neuralgia | 200 mg/d (100 mg bid) | Add up to 200 mg/d in increments of 100 mg every 12 h | 1200 mg/d | |
| 5% lidocaine patch | Postherpetic neuralgia | Maximum of 3 patches daily for a maximum of 12 hr | None needed | Maximum of 3 patches daily for a maximum of 12 hr | 2 wk |
| Opioid analgesics* | Moderate to severe pain | 5–15 mg every 4 hr as needed | After 1–2 wk, convert total daily dosage to long-acting medication as needed | No maximum with careful titration; consider evaluation by pain specialist at dosages exceeding 120–180 mg/d | 4–6 wk |
| Tramadol hydrochloride | Moderate to moderately severe pain | 50 mg 1 or 2×/d | Increased by 50–100 mg/d in divided doses every 3–7 d as tolerated | 400 mg/d (100 mg 4×/d); in patients older than 75 yr, 300 mg/d in divided doses | 4 wk |
| Tricyclic antidepressants (eg, nortriptyline hydrochloride or desipramine hydrochloride) | Chronic pain | 10–25 mg every night | Increase by 10–25 mg/d every 3–7 d as tolerated | 75–150 mg/d; if blood level of active drug and its metabolite is <100 ng/mL, continue titration with caution | 6–8 wk with at least 1–2 wk at maximum tolerated dosage |
| Duloxetine Serotonin/norepinephrine Reuptake inhibitor |
Diabetic neuropathic pain | 30 mg bid | Increase by 60 to 60 bid No further titration | 4 wk | |
| Fluoxetine Serotonin/norepinephrine Reuptake inhibitor |
Diabetic neuropathic pain | 30 mg bid | Increase by 60 to 60 bid. No further titration | 4 wk |
Dosages given are for morphine sulfate.
Source: Tegretol [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2003.
Anti-epileptic drugs (AEDs) have a long history of effectiveness in the treatment of neuropathic pain. Since 1993, nine new AEDs (felbamate, gabapentin, pregabalin, lamotrigine, topiramate, tiagabine, levetiracetam, oxcarbazepine, and zonisamide) have received FDA approval for the adjunctive treatment of partial seizures (87) (Table 3). Three of these drugs have also been approved for generalized seizures (felbamate, lamotrigine, topiramate) and three (felbamate, lamotrigine, oxcarbazepine) for monotherapy(87). Principal mechanisms of action include sodium channel blockade (felbamate, lamotrigine, oxcarbazepine, topiramate, zonisamide), potentiation of GABA activity (tiagabine, topiramate), calcium channel blockade (felbamate, lamotrigine, topiramate, zonisamide), antagonism of glutamate at N-methyl-D-aspartate (NMDA) receptors (felbamate, memantine, dextromethorphan) or α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) (felbamate, topiramate), and mechanisms of action still undetermined (gabapentin, pregabalin, levetiracetam). Only two drugs have been approved by the FDA for the treatment of painful diabetic neuropathy, Pregabalin and Duloxetine.
Pregabalin produced significant improvements in pain scores within 1 week of treatment, which persisted for 6–12 weeks in four randomized controlled trials including 146–724 patients with diabetic neuropathy(88–91). Adverse events included dose related somnolence, ataxia and confusion, peripheral edema and constipation. A recent Canadian study evaluated cost-effectiveness of pregabalin vs. gabapentin for the treatment of painful DN concluding that pregabalin was more cost effective when compared with gabapentin(92).
Lamotrigine (200 to 400 mg daily) is an anticonvulsant with dual-action inhibition of neuronal hyperexcitability. Two randomized, placebo-controlled studies including 720 patients showed that the drug was inconsistently effective for the treatment of pain when compared with placebo, although it was generally safe and well tolerated(93).
In addition to providing efficacy against epilepsy, these new AEDs may also be effective in treating neuropathic pain. For example, the AED lamotrigine may decrease hyperexcitability in dorsal horn spinal neurons by inhibiting glutamate release-2 mechanisms and decrease spontaneous activity in regenerating primary afferent nerve fibers(94). In addition, the “wind-up” phenomenon caused by nerve injury and the kindling that occurs in hippocampal neurons in patients with mesial temporal sclerosis both enlist activation of NMDA receptors (95) which can be affected by felbamate(87).
The evidence supporting the use of antiepileptic drugs for the treatment of PN continues to evolve. Patients who have failed one anticonvulsant may respond to another, as drugs in this class often have different mechanisms of action(86). When these mechanisms are understood, it may prove beneficial to combine drugs for a synergistic effect. For example, a sodium channel blocker such as lamotrigine may be used with a glutamate antagonist such as felbamate. In addition, certain drugs may possess multiple mechanisms of action which increases its likelihood of success (e.g. topiramate). If pain is divided according to its derivation from different nerve fiber types (e.g. Aδ vs C-fiber), spinal cord or cortical, then different types of pain should respond to different therapies (Fig. 9).
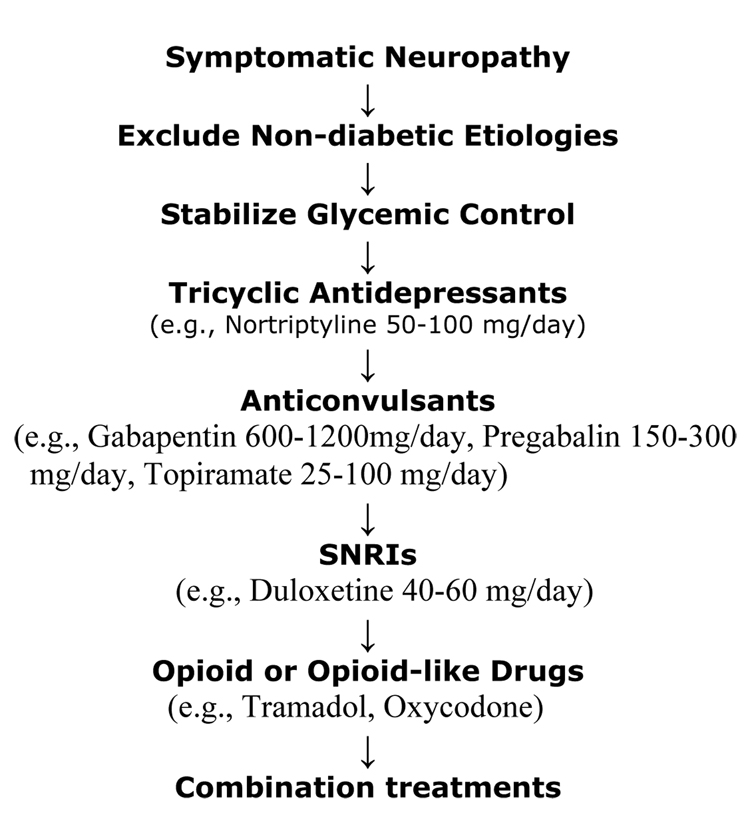
Figure 9. Algorithm for the Management of Symptomatic Diabetic Neuropathy.
Non-pharmacological, topical, or physical therapies can be useful at any time (capsaicin, acupuncture, etc.). The only two drugs approved by in the US for the treatment of painful diabetic neuropathy are pregabalin and duloxetine. However, based on the NNT (number needed to treat), tricyclic antidepressants are the most cost-effective ones. SNRIs: serotonin and norepinephrine reuptake inhibitors.(101)
Protein kinase C (PKC) activation is a critical step in the pathway to diabetic microvascular complications. It is activated by both hyperglycemia and disordered fatty-acid metabolism resulting in increased production of vasoconstrictive, angiogenic, and chemotactic cytokines including transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), endothelin (ET-1), and intercellular adhesion molecules (ICAMs). A multinational, randomized, phase-2, double blind, placebo-controlled trial with ruboxistaurin (a PKC-β inhibitor) failed to achieve the primary endpoints although significant changes were observed in a number of domains(96). Nevertheless, in a subgroup of patients with less severe DN (sural nerve action potential greater than 0.5 µV) at baseline and clinically significant symptoms, a statistically significant improvement in symptoms and vibratory detection thresholds was observed in the ruboxistaurin-treated groups as compared with placebo(97). A smaller, single center study recently published showed improvement in symptom scores, endothelium dependent skin blood flow measurements and quality of life scores in the ruboxistaurin treated group(98). These studies and the NATHAN studies have pointed out the change in natural history of DN with the advent of therapeutic lifestyle change, statins and ACE inhibitors, which have slowed the progression of DN and drastically changed the requirements for placebo-controlled studies.
While it would be preferable to rely on FDA-approved medications for the treatment of PN, no drugs have yet received an indication for this purpose. As shown in Table 3, only a few drugs, including 2 AEDs, have received FDA approval for the treatment of chronic neuropathic pain syndrome(87). Carbamazepine has FDA approval for the treatment of trigeminal neuralgia, and is effective in controlling the lightning pain of DN and both gabapentin and lidocaine 5% patch (86) are approved for postherpetic neuralgia(86).
III.C. Special considerations
Carbamazepine, a Na+ channel blocker, is effective against trigeminal neuralgia but is being replaced with the safer Oxcarbazine which is useful for “lightning” type pains. Lamotrigine may cause skin rashes if titrated up too rapidly and Gabapentin, whose action still remains obscure and may cause serious CNS side effects, has failed in one of three studies and causes weight gain. Dextromethorphan, an NMDA receptor antagonist was relatively weak and its successor Memantine has not undergone successful trials. Topical capsaicin (3 teaspoons cayenne pepper + 1 jar cold cream) depletes substance P but is difficult to use and can be dangerous if it contacts mucous membranes. Results from topical lidocaine or it oral equivalent mexilitine are equivocal. The anticonvulsant drug, Topiramate, has been used successfully to treat pain in diabetic patients and also promotes weight loss and restful sleep, suggesting that the drug may have other beneficial effects apart from relieving pain(99). Tramadol and oxycodone are weak opiods which have also shown to be effective but require careful titration and observation.
Another type of pain, Aδ pain, is described as a more deep-seated ache which does not often respond to the medications above. Several different agents have been used with varying success. Continuous intravenous insulin infusion without blood glucose lowering may be useful in these patients. The patient is admitted in the evening and usual diabetes treatment is instituted and a regular meal plan followed. NaCl is administered intravenously. In the morning, insulin is infused in a dose of 0.8–1.0 units hourly. Pain reduction usually occurs within 48 hours at which time the insulin infusion is discontinued. If this measure fails there are several medications available that may abolish the pain.
IV. CONCLUSIONS
Diabetic neuropathy is a heterogeneous disease with diverse pathology. Recognition of the clinical homologue of these pathological processes is the first step in achieving the appropriate form of intervention. Treatment should be individualized such that the particular manifestation and underlying pathogenesis of each patient’s unique clinical presentation is considered. In older adults, special care should be taken to manage pain while optimizing daily function and mobility, with the fewest adverse side effects from medication. Older adults are at great risk for falling and fractures due to instability, weakness and require strength exercises, coordination training. Ultimately agents that address large fiber dysfunction will be essential if we are to reduce the gross impairment of QOL and ADLs that neuropathy visits upon the older person with diabetes.
Box 3.
Differential diagnosis of distal symmetric polyneuropathy
| Type | Syndrome |
|---|---|
| Congenital/Familial | Charcot Marie Tooth |
| Traumatic | Entrapment Syndromes |
| Inflammatory | Sarcoidosis |
| Leprosy | |
| Lyme Disease | |
| HIV | |
| Neoplastic | Carcinoma - paraneoplastic syndromes |
| Myeloma, Amyloid | |
| Reticuloses, leukemias, lymphomas | |
| Metabolic/Endocrine | Diabetes mellitus |
| Uremia | |
| Pernicious Anemia (B12 deficiency) | |
| Hypothyroidism | |
| Porphyria (Acute Intermittent) | |
| Vascular | Diabetes, vasculitis |
| Toxic | Alcohol |
| Heavy metals (lead, mercury, arsenic) | |
| Hydrocarbons, chemotherapeutic drugs | |
| Autoimmune | Diabetes |
| PLA syndrome | |
| Chronic Inflammatory Demyelinating Neuropathy | |
| Multifocal Motor Neuropathy | |
| Guillain Barre Syndrome |
Figure 6.
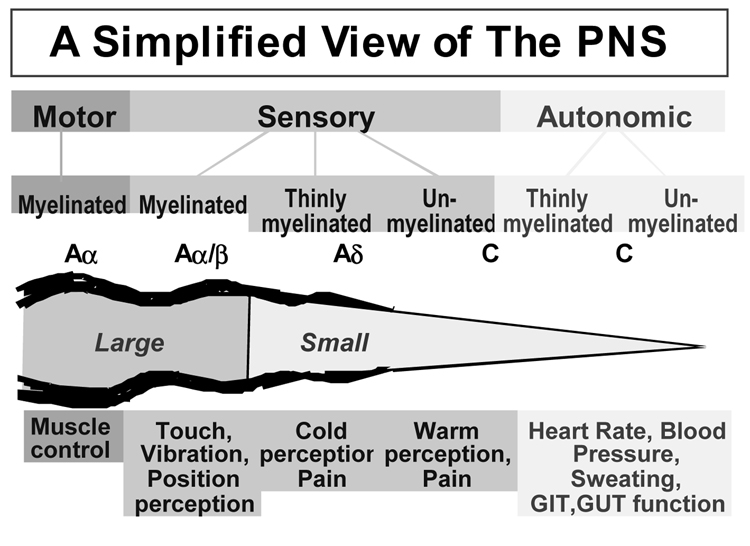
Schematic presentation of the physiologic function of different nerve fibers: Aα fibers are large myelinated fibers, in charge of motor functions and muscle control. Aα/β fibers are large myelinated fibers too, with sensory functions such as perception to touch, vibration and position. Aδ fibers are small myelinated fibers, in charge of pain stimuli and cold perception. C fibers can be myelinated or unmyelinated and have both sensory (warm perception and pain) and autonomic functions (blood pressure and heart rate regulation, sweating, etc.) GIT, GastroIntestinal Tract; GUT, GenitoUrinary Tract.(101)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Aaron I. Vinik, Strelitz Diabetes Institutes, Department of Internal Medicine, Eastern Virginia Medical School, 855 West Brambleton Avenue, Norfolk, VA 23510, vinikai@evms.edu, Phone 757-446-5912 FAX 757-446-5975.
Elsa S. Strotmeyer, Center for Aging and Population Health, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, strotmeyere@edc.pitt.edu.
Abhijeet A Nakave, Strelitz Diabetes Institutes, Department of Internal Medicine, Eastern Virginia Medical School, 855 West Brambleton Avenue, Norfolk, VA 23510, nakavea@evms.edu.
Chhaya V Patel, Strelitz Diabetes Institutes, Department of Internal Medicine, Eastern Virginia Medical School, 855 West Brambleton Avenue, Norfolk, VA 23510, patelcv@evms.edu.
Reference List
- 1.Vinik AI, Mitchell BD, Leichter SB, Wagner AL, O'Brian JT, Georges LP. Epidemiology of the Complications of Diabetes. In: Leslie RDG, Robbins DC, editors. Diabetes: Clinical Science in Practice. Cambridge, United Kingdom: Cambridge University Press; 1995. pp. 221–287. [Google Scholar]
- 2.Knuiman M, Welborn T, McCann V, Stanton K, Constable I. Prevalence of diabetic complications in relation to risk factors. Diabetes. 1986;35:1332–1339. doi: 10.2337/diab.35.12.1332. [DOI] [PubMed] [Google Scholar]
- 3.Young MJ, Boulton AJM, MacLeod AF, Williams DRR, Sonksen PH. A multicenter study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36:1–5. doi: 10.1007/BF00400697. [DOI] [PubMed] [Google Scholar]
- 4.Holzer SE, Camerota A, Martens L, Cuerdon T, Crystal P, Zagari M. Costs and duration of care for lower extremity ulcers in patients with diabetes. Clin Ther. 1998;20:169–181. doi: 10.1016/s0149-2918(98)80044-1. [DOI] [PubMed] [Google Scholar]
- 5.Caputo GM, Cavanagh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med. 1994;331:854–860. doi: 10.1056/NEJM199409293311307. [DOI] [PubMed] [Google Scholar]
- 6.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85:245–252. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Maty SC, Fried LP, Volpato S, Williamson J, Brancati FL, Blaum CS. Patterns of disability related to diabetes mellitus in older women. J Gerontol A Biol Sci Med Sci. 2004;59:148–153. doi: 10.1093/gerona/59.2.m148. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JK, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004;52:1532–1537. doi: 10.1111/j.1532-5415.2004.52418.x. [DOI] [PubMed] [Google Scholar]
- 9.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 10.Vinik AI, Erbas T. Recognizing and Treating Diabetic Autonomic Neuropathy. Cleveland Clinic Journal of Medicine. 2001;68(11):928–944. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 11.Vinik A, Mehrabyan A. Diagnosis and Management of Diabetic Autonomic Neuropathy. Comprehensive Therapy. 2003;29(23):130–145. doi: 10.1007/s12019-003-0017-4. [DOI] [PubMed] [Google Scholar]
- 12.Lindenlaub T, Sommer C. Cytokines in sural nerve biopsies from inflammatory and non-inflammatory neuropathies. Acta Neuropathol. 2003;105:593–602. doi: 10.1007/s00401-003-0689-y. [DOI] [PubMed] [Google Scholar]
- 13.Jude EB, Abbott CA, Young MJ, Anderson SG, Douglas JT, Boulton AJ. The potential role of cell adhesion molecules in the pathogens of diabetic neuropathy. Diabetologia. 1998;41:330–336. doi: 10.1007/s001250050911. [DOI] [PubMed] [Google Scholar]
- 14.Brownlee M. Advanced products of nonenzymatic glycosylation and the pathogenesis of diabetic complications. In: Rifkin H, Porte D, editors. Diabetes Mellitus: Theory and Practice. 1st ed. New York Amsterdam, London: Elsevier; 1990. p. 279. [Google Scholar]
- 15.Laghi PF, Pastorelli M, Beermann U, de CS, Gallo S, Blardi P, Di PT. Peripheral neuropathy associated with ischemic vascular disease of the lower limbs. Angiology. 1996;47:569–577. doi: 10.1177/000331979604700605. [DOI] [PubMed] [Google Scholar]
- 16.Young MJ, Veves A, Smith JV, Walker MG, Boulton AJ. Restoring lower limb blood flow improves conduction velocity in diabetic patients. Diabetologia. 1995;38:1051–1054. doi: 10.1007/BF00402174. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 18.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schutte K, Kerum G, Malessa R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999;22:1296–1301. doi: 10.2337/diacare.22.8.1296. [DOI] [PubMed] [Google Scholar]
- 20.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES. Body mass index, diabetes, and C-reactive protein among U.S. adults. Diabetes Care. 1999;22:1971–1977. doi: 10.2337/diacare.22.12.1971. [DOI] [PubMed] [Google Scholar]
- 23.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 24.Frohlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 25.Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, Jaross W, Hanefeld M. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism. 2002;51:743–749. doi: 10.1053/meta.2002.32804. [DOI] [PubMed] [Google Scholar]
- 26.Vinik A, Mehrabyan A, Colen L, Boulton A. Focal entrapment neuropathies in diabetes. Diabetes Care. 2004;27:1783–1788. doi: 10.2337/diacare.27.7.1783. [DOI] [PubMed] [Google Scholar]
- 27.Wilbourn AJ. Diabetic entrapment and compression neuropathies. In: Dyck PJ, Thomas PK, editors. Diabetic Neuropathy. Philadelphia: Saunders; 1999. pp. 481–508. [Google Scholar]
- 28.Kapritskaya Y, Novak C, Mackinnon S. Prevalence of smoking, obesity, diabetes mellitus and thyroid disease in patients with carpal tunnel syndrome. Ann Plast Surg. 2002;48(3):269–279. doi: 10.1097/00000637-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Vinik AI, Holland MT, LeBeau JM, Liuzzi FJ, Stansberry KB, Colen LB. Diabetic neuropathies. Diabetes Care. 1992;15:1926–1975. doi: 10.2337/diacare.15.12.1926. [DOI] [PubMed] [Google Scholar]
- 30.Leedman PJ, Davis S, Harrison LS. Diabetic amyotrophy. Reassessment of the clinical spectrum. Aust.N.Z.J.Med. 1988;18:768–773. doi: 10.1111/j.1445-5994.1988.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 31.Barohn RJ, Sahenk Z, Warmolts JR, Mendell JR. The Bruns-Garland syndrome (Diabetic amyotrophy) Arch Neurol. 1991;48:1130–1135. doi: 10.1001/archneur.1991.00530230038018. [DOI] [PubMed] [Google Scholar]
- 32.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. 1995 doi: 10.1002/ana.410380607. [article online], [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 34.Vinik AI, Pittenger GL, Milicevic Z, Cuca J. Autoimmune Mechanisms in the Pathogenesis of Diabetic Neuropathy. In: Eisenbarth RG, editor. Molecular Mechanisms of Endocrine and Organ Specific Autoimmunity. 1st ed. Georgetown: Landes Company; 1998. pp. 217–251. [Google Scholar]
- 35.Steck AJ, Kappos L. Gangliosides and autoimmune neuropathies: classification and clinical aspects of autoimmune neuropathies. J Neurol Neurosurg Psychiatry. 1994;57 Suppl:26–28. doi: 10.1136/jnnp.57.suppl.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinik A. Diagnosis and Management of Diabetic Neuropathy. Clinics in Geriatric Medicine. 1999;15:293–319. [PubMed] [Google Scholar]
- 37.Milicevic Z, Pittenger GL, Stansberry KB, Vinik AI. Raised anti-ganglioside GM1 antibody (GM1 Ab) titers in a subset of patients with distal symmetric polyneuropathy (DSPN) (Abstract) Diabetes. 1997;46:125A. doi: 10.1007/s001250050834. [DOI] [PubMed] [Google Scholar]
- 38.Sharma K, Cross J, Farronay O, Ayyar D, Sheber R, Bradley W. Demyelinating neuropathy in diabetes mellitus. Arch Neurol. 2002;59:758–765. doi: 10.1001/archneur.59.5.758. [DOI] [PubMed] [Google Scholar]
- 39.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol. 1995;52:1053–1061. doi: 10.1001/archneur.1995.00540350039015. [DOI] [PubMed] [Google Scholar]
- 40.Vinik AI, Suwanwalaikorn S, Stansberry KB, Holland MT, McNitt PM, Colen LE. Quantitative measurement of cutaneous perception in diabetic neuropathy. Muscle Nerve. 1995;18:574–584. doi: 10.1002/mus.880180603. [DOI] [PubMed] [Google Scholar]
- 41.Yu RK, Ariga T, Kohriyama T, Kusonoki S, Maeda Y, Myatani N. Autoimmune mechanisms in peripheral neuropathies. Ann Neurol. 1990;27 Suppl1:S30–S35. doi: 10.1002/ana.410270709. [DOI] [PubMed] [Google Scholar]
- 42.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 43.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 44.Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 45.Bus SA, Yang QX, Wang JH, Smith MB, Wunderling R, Cavanag PR. Intrinsic Muscle Atrophy and Toe Deformity in the Diabetic Neuropathic Foot. Diabetes Care. 2002;V25:1444–1450. doi: 10.2337/diacare.25.8.1444. [DOI] [PubMed] [Google Scholar]
- 46.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–2385. doi: 10.2337/diacare.27.10.2382. [DOI] [PubMed] [Google Scholar]
- 47.Vinik EJ, Hayes RP, Oglesby A, Bastyr E, Barlow P, Ford-Molvik SL, Vinik AI. The development and validation of the Norfolk QOL-DN, a new measure of patients' perception of the effects of diabetes and diabetic neuropathy. Diabetes Technol Ther. 2005;7:497–508. doi: 10.1089/dia.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 48.Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25:43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 49.Strotmeyer ES, Cauley JA, Schwartz AV, de RN, Resnick HE, Zmuda JM, Shorr RI, Tylavsky FA, Vinik AI, Harris TB, Newman AB. Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2006;21:1803–1810. doi: 10.1359/jbmr.060725. [DOI] [PubMed] [Google Scholar]
- 50.Lauretani F. Axonal degeneration affects muscle density in older men and women. Neurobiology of Aging. 2006;27:1145–1154. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inzitari M, Carlo A, Baldereschi M, Pracucci G, Maggi S, Gandolfo C, Bonaiuto S, Farchi G, Scafato E, Carbonin P, Inzitari D. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: the Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2006;54:318–324. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 52.Koski K, Luukinen H, Laippala P, Kivela SL. Risk factors for major injurious falls among the home-dwelling elderly by functional abilities. A prospective population-based study. Gerontology. 1998;44:232–238. doi: 10.1159/000022017. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25:1749–1754. doi: 10.2337/diacare.25.10.1749. [DOI] [PubMed] [Google Scholar]
- 54.Zhuang HX, Snyder CK, Pu SF, Ishii DN. Insulin-like growth factors reverse or arrest diabetic neuropathy: effects on hyperalgesia and impaired nerve regeneration in rats. Exp Neurol. 1996;140:198–205. doi: 10.1006/exnr.1996.0129. [DOI] [PubMed] [Google Scholar]
- 55.Said G, Goulon-Goreau C, Lacroix C, Moulonguet A. Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol. 1994;35:559–569. doi: 10.1002/ana.410350509. [DOI] [PubMed] [Google Scholar]
- 56.Young MJ, Marshall M, Adams JE, Selby P, Boulton AJM. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. 1995;18:34–38. doi: 10.2337/diacare.18.1.34. [DOI] [PubMed] [Google Scholar]
- 57.Haslbeck KM, Bierhaus A, Erwin S, Kirchner A, Nawroth P, Schlotzer U, Neundorfer B, Heuss D. Receptor for advanced glycation endproduct (RAGE)-mediated nuclear factor-kappaB activation in vasculitic neuropathy. Muscle Nerve. 2004;29:853–860. doi: 10.1002/mus.20039. [DOI] [PubMed] [Google Scholar]
- 58.Grant WP, Sullivan R, Sonenshine DE, Adam M, Slusser JH, Carson KA, Vinik AI. Electron microscopic investigation of the effects of diabetes mellitus on the Achilles tendon. J Foot Ankle Surg. 1997;36:272–278. doi: 10.1016/s1067-2516(97)80072-5. [DOI] [PubMed] [Google Scholar]
- 59.Anderson JJ, Woelffer KE, Holtzman JJ, Jacobs AM. Bisphosphonates for the treatment of Charcot neuroarthropathy. J Foot Ankle Surg. 2004;43:285–289. doi: 10.1053/j.jfas.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Baik HW, Russell RM. Vitamin B12 deficiency in the elderly. Annu Rev Nutr. 1999;19:357–377. doi: 10.1146/annurev.nutr.19.1.357. [DOI] [PubMed] [Google Scholar]
- 61.Stone KL, Bauer DC, Sellmeyer D, Cummings SR. Low serum vitamin B-12 levels are associated with increased hip bone loss in older women: a prospective study. J Clin Endocrinol Metab. 2004;89:1217–1221. doi: 10.1210/jc.2003-030074. [DOI] [PubMed] [Google Scholar]
- 62.Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20:921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 63.Tucker KL. Low plasma vitamin B12 is associated with lower BMD: the Framingham osteoporosis study. Journal of Bone and Mineral Research. 2005;20:152–158. doi: 10.1359/JBMR.041018. [DOI] [PubMed] [Google Scholar]
- 64.Eussen SJ, de Groot LC, Clarke R, Schneede J, Ueland PM, Hoefnagels WH, van Staveren WA. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Arch Intern Med. 2005;165:1167–1172. doi: 10.1001/archinte.165.10.1167. [DOI] [PubMed] [Google Scholar]
- 65.Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, Wolfson SK the Cardiovascular Health Study (CHS) Collaborative Research Group. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 66.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: the InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 67.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 68.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295:547–553. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 69.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 70.Costa LA, Canani LH, Lisboa HR, Tres GS, Gross JL. Aggregation of features of the metabolic syndrome is associated with increased prevalence of chronic complications in Type 2 diabetes. Diabet Med. 2004;21:252–255. doi: 10.1111/j.1464-5491.2004.01124.x. [DOI] [PubMed] [Google Scholar]
- 71.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen M, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with Type II diabetes. Diabeologia. 2001;44:1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 72.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 73.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA. 1994;272:1909–1914. doi: 10.1001/jama.1994.03520240037038. [DOI] [PubMed] [Google Scholar]
- 74.Liu-Ambrose T, Khan KM, Eng JJ, Janssen PA, Lord SR, McKay HA. Resistance and agility training reduce fall risk in women aged 75 to 85 with low bone mass: a 6-month randomized, controlled trial. J Am Geriatr Soc. 2004;52:657–665. doi: 10.1111/j.1532-5415.2004.52200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray H, Veves A, Young M, Richie D, Boulton A. Role of experimental socks in the care of the high risk diabetic foot. A multi-center patient evaluation study. American group for the study of experimental hosiery in the diabetic foot. Diabetes Care. 1993;16:1190–1192. doi: 10.2337/diacare.16.8.1190. [DOI] [PubMed] [Google Scholar]
- 76.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1:252–263. [Google Scholar]
- 77.Stein PK, Barzilay JI, Domitrovich PP, Chaves PM, Gottdiener JS, Heckbert SR, Kronmal RA. The relationship of heart rate and heart rate variability to non-diabetic fasting glucose levels and the metabolic syndrome: the Cardiovascular Health Study. Diabet Med. 2007;24:855–863. doi: 10.1111/j.1464-5491.2007.02163.x. [DOI] [PubMed] [Google Scholar]
- 78.Ziegler D, Schatz H, Conrad F, Gries FA, Ulrich H, Reichel G. Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20:369–373. doi: 10.2337/diacare.20.3.369. [DOI] [PubMed] [Google Scholar]
- 79.Ruhnau KJ, Meissner HP, Finn R, Reljanovic M, Lobisch M, Schutte K, Nehrdich D, Tritschler H, Mehnert H, Ziegler D. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabet Med. 1999;16(12):1040–1043. doi: 10.1046/j.1464-5491.1999.00190.x. [DOI] [PubMed] [Google Scholar]
- 80.Valensi P, Le DC, Richard JL, Farez C, Khodabandehlou T, Rosenbloom RA, LeFante C. A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy. A preliminary report. J Diabetes Complications. 2005;19:247–253. doi: 10.1016/j.jdiacomp.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 81.Viberti G. Thiazolidinediones-benefits on microvascular complications of type 2 diabetes. J Diabetes Complications. 2005;19:168–177. doi: 10.1016/j.jdiacomp.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 82.davis t, et al. Lipid-lowering Therapy Protects Against Peripheral Sensory Neuropathy in Type 2 Diabetes. Diabetes supplement. 2007 [Google Scholar]
- 83.Jacobson TA. Overcoming 'ageism' bias in the treatment of hypercholesterolaemia : a review of safety issues with statins in the elderly. Drug Saf. 2006;29:421–448. doi: 10.2165/00002018-200629050-00005. [DOI] [PubMed] [Google Scholar]
- 84.Coppini DV, Young PJ, Weng C, MacLeod AF, Sonksen PH. Outcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control study. Diabet Med. 1998;15:765–771. doi: 10.1002/(SICI)1096-9136(199809)15:9<765::AID-DIA663>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 85.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159:1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 86.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 87.LaRoche SM, Helmers SL. The new antiepileptic drugs: scientific review. JAMA. 2004;291:605–614. doi: 10.1001/jama.291.5.605. [DOI] [PubMed] [Google Scholar]
- 88.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254–263. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 90.Richter RW, Portenoy R, Sharma U, LaMoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6:253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Frampton JE, Scott LJ. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–2820. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- 92.Tarride JE, Gordon A, Vera-Llonch M, Dukes E, Rousseau C. Cost-effectiveness of pregabalin for the management of neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia: a Canadian perspective. Clin Ther. 2006;28:1922–1934. doi: 10.1016/j.clinthera.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 93.Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Wilks K, Quessy S, Blum D, Grainger J, White J, Silver M. Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain. 2007;128:169–179. doi: 10.1016/j.pain.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 94.Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology. 2001;57:505–509. doi: 10.1212/wnl.57.3.505. [DOI] [PubMed] [Google Scholar]
- 95.Backonja MM. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002;59:S14–S17. doi: 10.1212/wnl.59.5_suppl_2.s14. [DOI] [PubMed] [Google Scholar]
- 96.Vinik A, Bril V, Kempler P, Litchy W, Dyck P, Tesfaye S, Price K, Bastyr E the MBBQ Study. Treatment of symptomatic diabetic peripheral neuropathy with protein kinase CB inhibitor ruboxistaurin mesylate during a 1-year randomized, placebo-controlled, double-blind clinical trial. Clinical Therapeutics. 2005;27:1164s–1180s. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 97.Vinik AI, Bril V, Litchy WJ, Price KL, Bastyr EJ., III Sural sensory action potential identifies diabetic peripheral neuropathy responders to therapy. Muscle Nerve. 2005 doi: 10.1002/mus.20423. [DOI] [PubMed] [Google Scholar]
- 98.Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ, III, Wolka AM, Vinik AI. A 6-Month, Randomized, Double-Masked, Placebo-Controlled Study Evaluating the Effects of the Protein Kinase C-{beta} Inhibitor Ruboxistaurin on Skin Microvascular Blood Flow and Other Measures of Diabetic Peripheral Neuropathy. Diabetes Care. 2007;30:896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 99.Raskin P, Donofrio P, Rosenthal N, Hewitt D, Jordan D, Xiang J, Vinik A. Topiramate vs placebo in painful diabetic neuropathy: Analgesic and metabolic effects. 2004 doi: 10.1212/01.wnl.0000137341.89781.14. [article online], [DOI] [PubMed] [Google Scholar]
- 100.Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. 1997;46 Suppl 2:S54–S57. doi: 10.2337/diab.46.2.s54. [DOI] [PubMed] [Google Scholar]
- 101.Aaron Vinik MDCCMDANMaCPM: Diabetic Neuropathies. 2007 [article online], Available from http://endotext.org/diabetes/diabetes35/diabetesframe35.htm.
- 102.Barney Y, Bossmeyer R, Kokomen E. Neurological manifestations of aging. 1990 doi: 10.1093/geronj/32.4.411. [article online], [DOI] [PubMed] [Google Scholar]
- 103.Maki BE, Holliday PJ, Fernie GR. Aging and postural control. A comparison of spontaneous- and induced-sway balance tests. J Am Geriatr Soc. 1990;38:1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 104.Potvin AR, Syndulko K, Tourtellotte WW, Lemmon JA, Potvin JH. Human neurologic function and the aging process. J Am Geriatr Soc. 1980;1:1–9. doi: 10.1111/j.1532-5415.1980.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 105.Boulton A, Vinik A, Arezzo J, Bril V, Feldman E, Freeman R, Malik R, Maser R, Sosenko J, Ziegler D American Diabetes Association. Position Statement: Diabetic Neuropathies. 2005 doi: 10.2337/diacare.28.4.956. [article online], [DOI] [PubMed] [Google Scholar]
- 106.Cameron NE, Eaton SEM, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]