Abstract
Background
Subarachnoid hemorrhage (SAH) often causes a prolongation of the corrected QT (QTc) interval during the acute phase. The aim of the present study was to examine independent risk factors for QTc prolongation in patients with SAH by means of multivariate analysis.
Method
We studied 100 patients who were admitted within 24 hours after onset of SAH. Standard 12-lead electrocardiography (ECG) was performed immediately after admission. QT intervals were measured from the ECG and were corrected for heart rate using the Bazett formula. We measured serum levels of sodium, potassium, calcium, adrenaline (epinephrine), noradrenaline (norepinephrine), dopamine, antidiuretic hormone, and glucose.
Results
The average QTc interval was 466 ± 46 ms. Patients were categorized into two groups based on the QTc interval, with a cutoff line of 470 ms. Univariate analyses showed significant relations between categories of QTc interval, and sex and serum concentrations of potassium, calcium, or glucose. Multivariate analyses showed that female sex and hypokalemia were independent risk factors for severe QTc prolongation. Hypokalemia (<3.5 mmol/l) was associated with a relative risk of 4.53 for severe QTc prolongation as compared with normokalemia, while the relative risk associated with female sex was 4.45 as compared with male sex. There was a significant inverse correlation between serum potassium levels and QTc intervals among female patients.
Conclusion
These findings suggest that female sex and hypokalemia are independent risk factors for severe QTc prolongation in patients with SAH.
Keywords: female, hypokalemia, multivariate analysis, QT prolongation, subarachnoid hemorrhage
Introduction
Hypokalemia and many types of electrocardiographic (ECG) abnormality are observed during the acute phase of subarachnoid hemorrhage (SAH) [1-3]. Among ECG abnormalities, a prolongation of the QT interval, especially when associated with hypokalemia, deserves particular attention because it is frequently observed in those patients who develop life-threatening ventricular arrhythmias such as tor-sades de pointes [1,2,4]. However, the pathogeneses of these abnormalities during the acute phase of SAH have not yet been fully investigated.
It is commonly believed that hypokalemia identified during the acute phase of SAH is caused by a catecholamine surge following SAH [2]. In healthy male volunteers, Brown and coworkers [5] demonstrated that serum infused adrenaline (epinephrine) caused excessive activation of the sodium/potassium-ATPase (Na+/K+-ATPase; Na+/K+ pump) as a result of β2-adrenergic receptor stimulation; this resulted in trafficking of potassium ions from extracellular to intracellu-lar spaces, leading to a decrease in serum potassium levels.
Recent work showed that there were some relationships between serum catecholamine levels and QT intervals during the acute phase of SAH [1,6]. However, one cannot refute the possibility that the catecholamine surge may influence the QT interval indirectly via hypokalemia, because hypokalemia (as well as hypocalcemia and hypomagnesemia) can cause a prolongation in the QT interval [4,7-9].
In a previous preliminary study [3], we suggested that women may be more prone than men to develop both hypokalemia and prolongation in the corrected QT (QTc) interval on the first day of SAH. In the present prospective study, we assessed various possible risk factors for the QTc prolongation that is observed in SAH patients in order to examine independent risk factors by means of multivariate analysis.
Patients and method
Patients
We prospectively studied 100 patients admitted within 24 hours after onset of SAH to the National Defense Medical College Hospital between January 1997 and April 2002. Of these 100 patients, 36 were men and 64 were women; mean age on admission was 59.1 ± 11.8 years, with a range of 21–82 years. Neurologic condition on admission was evaluated according to the Hunt and Kosnik classification [10], and severity of SAH was assessed according to Fisher's classification [11]. The exclusion criteria included endocrino-logic disease, heart disease, renal failure, previous SAH or intracerebral hemorrhage, young age (<19 years), pregnancy, pacemaker rhythm, or taking any of the following drugs: digitalis, quinidine, procainamide, disopyramide, and diuretics. All patients had brain computed tomography studies compatible with their diagnoses. Each patient underwent a cerebral angiogram to identify a ruptured aneurysm, except for eight patients whose SAH were so severe that there was no indication for cerebral angiography. Patients with traumatic SAH or SAH of unknown origin, despite assessment by cerebral angiography, were also excluded from the study. Informed consent was obtained from the patients and/or their guardians.
Data collection
Standard 12-lead ECG was performed immediately after admission. Two consecutive QT intervals were manually measured for all 12 leads of the ECG with the assessor blinded to the name and group of the patient. The QT intervals were measured from the beginning of the QRS complex to the visual return of the T wave to the isoelectric line, and were corrected for heart rate using the Bazett formula: QTc = QT/(square root of RR interval). The mean QTc interval was calculated from all QTc intervals measured. The intraob-server coefficient of variation was 1.5% and the interobserver coefficient of variation was 1.8%. Blood samples were obtained from the patients at the same time as ECG, and serum levels of sodium, potassium, calcium, adrenaline, noradrenaline (norepinephrine), dopamine, antidiuretic hormone, and glucose were measured.
Statistical analyses
In order to identify potential risk factors for severe QTc prolongation, univariate analysis was conducted using the χ2 test, Student's t test, or Mann–Whitney U test. The model was reduced using manual back and forth procedure, with variable selection based on the statistical significance of the estimates (P < 0.1). Multiple logistic regression analysis was then performed to identify independent risk factors for severe QTc prolongation. Pearson's correlation coefficient was used to assess the correlation between serum potassium levels and QTc intervals. Data are expressed as means ± SD. P < 0.05 was considered statistically significant and all analyses were performed using the StatView Version 5.0 statistical package (SAS Institute Inc, Cary, NC, USA).
Results
The QTc intervals on admission ranged from 328 to 629 ms in this series; it was less than 400 ms in eight patients, 400–450 ms in 28 patients, 450–500 ms in 48 patients, and more than 500 ms in 16 patients. The average QTc interval was 466 ± 46 ms. For the purposes of univariate analyses, the patients were stratified into two groups based on the QTc interval, with a cutoff line of 470 ms (Table 1).
Table 1.
Univariate analysis of various factors for predicting severe corrected QT prolongation in 100 SAH-patients
| QTc interval (ms) | |||
| Patient variable | <470 (n = 60) | ≥ 470 (n = 40) | P |
| Age (years) | 59.4 ± 12.1 | 58.7 ± 11.6 | NS |
| Sex (male : female) | 30 : 30 | 6 : 34 | 0.0004 |
| H & K grade | |||
| Grade 1 | 4 | 2 | NS |
| Grade 2 | 25 | 9 | |
| Grade 3 | 12 | 12 | |
| Grade 4 | 11 | 10 | |
| Grade 5 | 8 | 7 | |
| Fisher's classification | |||
| Group 2 | 5 | 2 | NS |
| Group 3 | 41 | 27 | |
| Group 4 | 14 | 11 | |
| Aneurysm site | |||
| AcomA | 16 | 5 | NS |
| ICA | 14 | 15 | |
| MCA | 13 | 10 | |
| Upper BA | 4 | 1 | |
| Others | 8 | 6 | |
| Unknown | 5 | 3 | |
| Sodium (mmol/l) | 139.7 ± 2.9 | 140.0 ± 3.0 | NS |
| Potassium (mmol/l) | 3.63 ± 0.42 | 3.21 ± 0.43 | < 0.0001 |
| Normal (≥ 3.5 mmol/l; n) | 39 | 10 | < 0.0001 |
| Hypokalemia (<3.5 mmol/l; n) | 21 | 30 | |
| Calcium (mmol/l) | 8.52 ± 0.39 | 8.29 ± 0.52 | <0.1 |
| Glucose (mg/dl) | 178 ± 49 | 207 ± 56 | < 0.02 |
| Adrenaline (pg/ml) | 783 ± 923 | 747 ± 937 | NS |
| Noradrenaline (pg/ml) | 1122 ± 718 | 1173 ± 548 | NS |
| Dopamine (pg/ml) | 37.4 ± 35.7 | 34.2 ± 18.0 | NS |
| ADH (pg/ml) | 73.9 ± 58.7 | 77.4 ± 29.4 | NS |
Where applicable, values are expressed as mean ± SD. ADH, antidiuretic hormone; BA, basilar artery; H & K, Hunt and Kosnik; ICA, internal carotid artery; MCA, middle cerebral artery; QTc, corrected QT; SAH, subarachnoid hemorrhage.
Univariate analysis
Univariate analyses demonstrated that there were significant relationships between the category of QTc interval and patient sex (Table 1). Significant relationships were also identified between category of QTc interval and the serum concentrations of potassium, calcium, or glucose (Table 1). Severe QTc prolongation (470 ms or more; n = 40) was more frequent among female SAH patients (53.1%; P = 0.0004) than among male patients (16.7%). The patients with severe QTc prolongation had lower serum potassium levels (3.21 ± 0.43 mmol/l; P < 0.0001) than did patients with a QTc interval of less than 470 ms (3.63 ± 0.42 mmol/l). Hypokalemia was defined as serum potassium level below 3.5 mmol/l. Severe QTc prolongation was more frequently found among SAH patients with hypokalemia (58.8%; P < 0.0001) than among SAH patients with normokalemia (20.4%). Patients with severe QTc prolongation also had lower serum calcium levels (8.29 ± 0.52 mmol/l; P < 0.1) as compared with patients with a QTc interval of less than 470 ms (8.52 ± 0.39 mmol/l). The concentration of serum glucose was significantly higher in patients with a severely prolonged QTc interval (207 ± 56 mg/dl; P < 0.02) as compared with patients with a QTc interval of less than 470 ms (178 ± 49 mg/dl).
There was, however, no relation between category of QTc interval and patient age, Hunt and Kosnik grade, Fisher's classification for computed tomography, site of ruptured aneurysm, or the serum concentration of sodium, adrenaline, noradrenaline, dopamine, or antidiuretic hormone. There were significant inverse correlations between serum potassium level and serum concentrations of adrenaline, noradrenaline, dopamine, and antidiuretic hormone (r = -0.712, -0.573, -0.432, -0.442, respectively). Furthermore, female SAH patients had lower potassium levels (3.36 ± 0.50 mmol/l; P = 0.001) than did male SAH patients (3.65 ± 0.36 mmol/l).
Multivariate analysis
The multivariate analyses showed that female sex and hypokalemia were independent risk factors for severe QTc prolongation in patients with SAH. In the first analysis, the serum potassium level was considered a continuous variable (analysis 1, Table 2). An increase of 1 mmol/l in serum potassium was associated with a relative risk of 0.13 (95% confidence interval 0.04–0.43; P < 0.001) for severe QTc prolongation, while female sex was associated with a relative risk of 3.69 (95% confidence interval 1.27–10.73; P < 0.02) as compared with male sex. A second analysis (analysis 2, Table 2) revealed that hypokalemia was associated with a relative risk of 4.53 (95% confidence interval 1.79–11.50; P < 0.002) as compared with normokalemia, while the relative risk with female sex was 4.45 (95% confidence interval 1.55–12.78; P < 0.01) as compared with male sex.
Table 2.
Multivariate analysis of various factors for predicting severe corrected QT prolongation in 100 subarachnoid hemorrhage patients
| Analyses | Relative risk (95% CI) | P |
| Analysis 1 | ||
| Female sex | 3.69 (1.27–10.73) | <0.02 |
| Serum potassium (for each 1 mmol/l) | 0.13 (0.04–0.43) | <0.001 |
| Analysis 2 | ||
| Female sex | 4.45 (1.55–12.78) | <0.01 |
| Hypokalemia (serum potassium <3.5 mmol/l) | 4.53 (1.79–11.50) | <0.002 |
Potential prognostic factors were selected from Table 1. CI, confidence interval.
Correlation between serum potassium level and corrected QT interval
There was a significant inverse correlation between serum potassium level and QTc interval (Pearson's correlation coefficient r = -0.439, P < 0.0001; Fig. 1a) among all SAH patients. Furthermore, we evaluated the impact of sex on the correlation between serum potassium levels and QTc interval. Among female SAH patients, there was a significant inverse correlation between serum potassium levels and QTc interval (r = -0.474, P < 0.0001; Fig. 1c). On the other hand, there was no significant correlation between the serum potassium levels and QTc intervals (r = -0.009; Fig. 1b) among male SAH patients.
Figure 1.
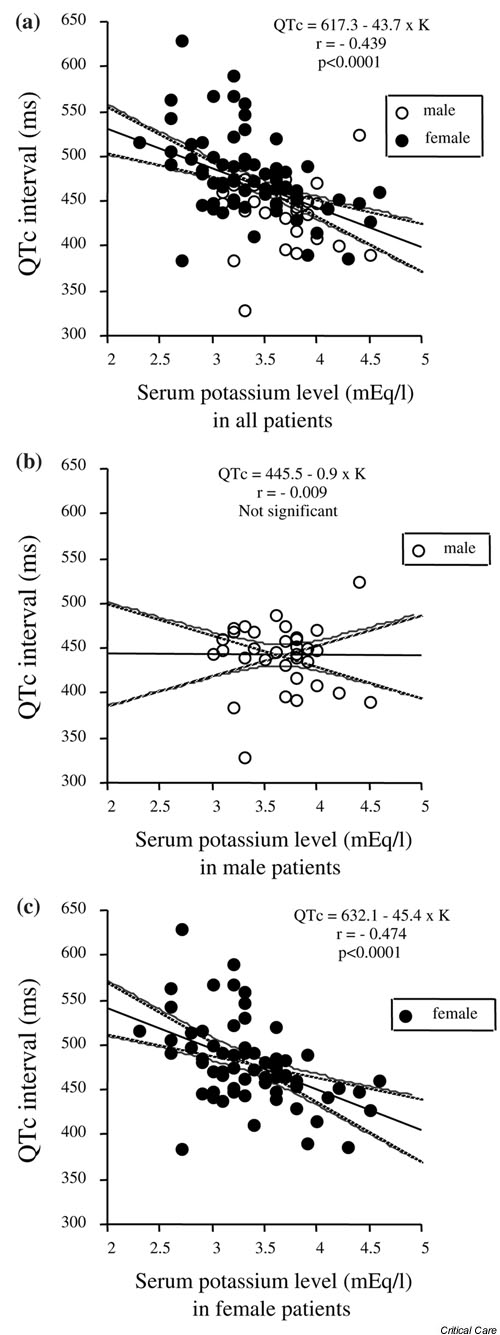
Correlation between serum potassium levels and corrected QT (QTc) intervals in 100 patients with subarachnoid hemorrhage (SAH). (a) There was a significant inverse correlation between serum potassium levels and QTc intervals (Pearson's correlation coefficient r = -0.439, P < 0.0001) among all SAH patients. Furthermore, we evaluated the impact of sex on the correlation between serum potassium levels and QTc interval. (c) Among female SAH patients there was a significant inverse correlation between serum potassium levels and QTc intervals (r = -0.474; P < 0.0001). (b) On the other hand, there was no significant correlation between serum potassium levels and QTc intervals among male SAH patients.
Discussion
The present findings suggest that female sex and hypokalemia are independent risk factors for severe QTc prolongation in patients with SAH. Recent clinical observations and experimental data indicate sex-specific differences in various ECG abnormalities. Several in vitro and in vivo experimental studies [12-14] have shown that female sex is a strong risk factor for drug-induced long QT interval and cardiac arrhythmias. In a study of healthy young individuals, Stramba-Badiale and coworkers [15] demonstrated that females had a longer QTc interval than did males. Furthermore, recent evidence suggested that women are more prone than men to develop torsades de pointes during administration of cardiovascular drugs that prolong cardiac repolarization [9,16].
However, little is known about the mechanism responsible for these sex related differences in the QTc interval or in the occurrence of torsades de pointes. Several studies have indicated that sex hormones play an important role in these sex differences. Drici and coworkers [17] showed that ovariectomy resulted in a shorter QT interval, and high doses of sex hormones lengthened the QT interval. In addition, Haseroth and coworkers [18] demonstrated that estrogen replacement therapy significantly prolonged QTc intervals in post-menopausal women. However, several other studies reported that sex hormones did not affect the QT interval in healthy women [19,20]. Various factors may be involved in the sex-specific difference in ventricular repolarization (e.g. sex-related differences in cardiac ionic channels). Female rabbit ventricular myocytes had a significantly slower rapid component of the delayed rectifier than did male cells, which might contribute to the gender difference in the QT interval [12]. In fact, most drugs that prolong the QTc interval have been shown to block cardiac potassium channels, especially the rapid component of the delayed rectifier [21,22].
Women are more susceptible to hypokalemia, especially that due to diuretics [23,24] or SAH [3]. Considering that hypokalemia causes a prolongation in the QTc interval [4,7-9,25], the sex-specific difference in ventricular repolarization described above might result from the sex-related difference in serum potassium levels. It is interesting that serum potassium levels did not affect QTc interval among male SAH patients, whereas there was a significant inverse correlation between serum potassium levels and QTc interval among female patients in the present study.
One mechanism that may account for this sex-specific difference in serum potassium levels is a sex-specific difference in the activity of the Na+/K+-ATPase (Na+/K+ pump), as reported by Lasker and coworkers [26]. Those investigators found that Na+/K+–ATPase activity in red blood cells was higher in females than in males, thus leading to a sex-specific difference in the Na+/K+ balance in both the intracellular and extracellular spaces. Another possible explanation is a difference in the total exchangeable body potassium. Kleinfeld and coworkers [27] demonstrated that elderly women were more vulnerable to hypokalemia, and indicated that this was possibly due to age- and sex-associated differences in body mass composition, which might result in a physiologically low total exchangeable body potassium.
An inverse correlation between the serum catecholamines and potassium levels suggests that a catecholamine surge following SAH plays an important role in the pathogenesis of hypokalemia during the acute phase of SAH. Recent evidence suggested that adrenaline-induced hypokalemia resulted from stimulation of a β-adrenoceptor linked to membrane Na+/K+-ATPase, causing potassium influx [5,28]. On the other hand, there was no correlation between serum cate-cholamine level and QTc interval in the present series. These findings indicate that a catecholamine surge following SAH does not play a direct role in the pathogenesis of the QTc prolongation in patients with SAH. Interestingly, Reid and coworkers [28] showed that serum infused-adrenaline caused not only hypokalemia but also a prolongation in the QTc interval in normal volunteers, which appears to be highly consistent with the present findings in patients with SAH.
The therapeutic implications of the present findings are clear. When a patient with SAH is hospitalized during the acute phase of SAH, as a rule potassium-free intravenous fluids should not be used as initial fluid therapy, particularly in female patients. Furthermore, careful ECG monitoring is needed to detect critical cardiac arrhythmias as soon as possible, and avoidance of drugs that prolong ventricular repolarization (quinidine, procainamide, and disopyramide) in patients with SAH, especially female patients, is mandatory. The findings reported here also suggest an important new direction for further research, focusing on the detection of critical cardiac arrhythmias in order to prevent sudden death at an early stage in SAH.
There are some limits to the conclusions that can be drawn from the present study. The study lacks data on ECG changes after admission in the SAH patients, which can only be assessed by means of continuous Holter monitoring. It was therefore impossible to consider the influence of QTc prolongation on the occurrence of critical cardiac arrhythmias such as torsades de pointes. Further studies are required to investigate the pathophysiologic basis for the QTc prolongation and to assess ECG changes after admission in patients with SAH.
Competing interests
None declared.
Key messages
• A prolongation in QTc interval is often observed during the acute phase of SAH
• Female sex and hypokalemia are independent risk factors for severe QTc prolongation in patients with SAH
• The clinical implication of this is that, when a patient with SAH is hospitalized during the acute phase of SAH, as a rule potassium-free intravenous fluids should not be used for initial fluid therapy, particularly in female patients
• In patients with SAH, careful ECG monitoring is needed to detect critical cardiac arrhythmias as soon as possible, and avoidance of drugs that prolong ventricular repolarization (quinidine, procainamide, and disopyramide) is mandatory, especially in female SAH patients
Abbreviations
ECG = electrocardiogram/electrocardiography; Na+/K+-ATPase = sodium/potassium ATPase; QTc = corrected QT; SAH = subarachnoid hemorrhage.
Acknowledgments
Acknowledgements
We thank Jana Dunbar, MS (Division of Neurosurgery, Medical College of Virginia, Richmond, USA), for her assistance in statistical analyses and the editorial process.
References
- Andreoli A, di Pasquale G, Pinelli G, Grazi P, Tognetti F, Testa C. Subarachnoid hemorrhage: frequency and severity of cardiac arrhythmias. A survey of 70 cases studied in the acute phase. Stroke. 1987;18:558–564. doi: 10.1161/01.str.18.3.558. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kongable GL, Kassell NF. Electrocardiographic abnormalities after nontraumatic subarachnoid hemorrhage. J Neurosurg Anesthesiol. 1994;6:156–162. doi: 10.1097/00008506-199407000-00002. [DOI] [PubMed] [Google Scholar]
- Fukui S, Otani N, Katoh H, Tsuzuki N, Ishihara S, Ohnuki A, Miyazawa T, Nawashiro H, Shima K. Female gender as a risk factor for hypokalemia and QT prolongation after subarach-noid hemorrhage. Neurology. 2002;59:134–136. doi: 10.1212/wnl.59.1.134. [DOI] [PubMed] [Google Scholar]
- Machado C, Baga JJ, Kawasaki R, Reinoehl J, Steinman RT, Lehmann MH. Torsade de pointes as a complication of subarachnoid hemorrhage: a critical reappraisal. J Electrocardiol. 1997;30:31–37. doi: 10.1016/s0022-0736(97)80032-5. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2-receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414–1419. doi: 10.1056/NEJM198312083092303. [DOI] [PubMed] [Google Scholar]
- Darbar D, Smith M, Morike K, Roden DM. Epinephrine-induced changes in serum potassium and cardiac repolarization and effects of pretreatment with propranolol and diltiazem. Am J Cardiol. 1996;77:1351–1355. doi: 10.1016/S0002-9149(96)00204-4. [DOI] [PubMed] [Google Scholar]
- Akita M, Kuwahara M, Tsubone H, Sugano S. ECG changes during furosemide-induced hypokalemia in the rat. J Electrocardiol. 1998;31:45–49. doi: 10.1016/s0022-0736(98)90006-1. [DOI] [PubMed] [Google Scholar]
- Janeira LF. Torsades de pointes and long QT syndromes. Am Fam Physician. 1995;52:1447–1453. [PubMed] [Google Scholar]
- Schwartz JB. The electrocardiographic QT interval and its prolongation in response to medications: differences between men and women. J Gend Specif Med. 2000;3:25–28. [PubMed] [Google Scholar]
- Hunt WE, Kosnik EJ. Timing and perioperative care in intracranial aneurysm surgery. Clin Neurosurg. 1974;21:78–89. doi: 10.1093/neurosurgery/21.cn_suppl_1.79. [DOI] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Liu XK, Katchman A, Drici MD, Ebert SN, Ducic I, Morad M, Woosley RL. Gender difference in the cycle length-dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther. 1998;285:672–679. [PubMed] [Google Scholar]
- Lu HR, Marien R, Saels A, De Clerck F. Are there sex-specific differences in ventricular repolarization or in drug-induced early afterdepolarizations in isolated rabbit purkinje fibers? J Cardiovasc Pharmacol. 2000;36:132–139. doi: 10.1097/00005344-200007000-00018. [DOI] [PubMed] [Google Scholar]
- Lu HR, Remeysen P, Somers K, Saels A, De Clerck F. Female gender is a risk factor for drug-induced long QT and cardiac arrhythmias in an in vivo rabbit model. J Cardiovasc Electrophysiol. 2001;12:538–545. doi: 10.1046/j.1540-8167.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- Stramba-Badiale M, Locati EH, Martinelli A, Courville J, Schwartz PJ. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24-h Holter recordings. Eur Heart J. 1997;18:1000–1006. doi: 10.1093/oxfordjournals.eurheartj.a015357. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.cir.94.6.1471. [DOI] [PubMed] [Google Scholar]
- Haseroth K, Seyffart K, Wehling M, Christ M. Effects of prog-estin-estrogen replacement therapy on QT-dispersion in post-menopausal women. Int J Cardiol. 2000;75:161–165. doi: 10.1016/S0167-5273(00)00317-X. [DOI] [PubMed] [Google Scholar]
- Saba S, Link MS, Homoud MK, Wang PJ, Estes NA., III Effect of low estrogen states in healthy women on dispersion of ventricular repolarization. Am J Cardiol. 2001;87:354–356. doi: 10.1016/S0002-9149(00)01377-1. [DOI] [PubMed] [Google Scholar]
- Larsen JA, Tung RH, Sadananda R, Goldberger JJ, Horvath G, Parker MA, Kadish AH. Effects of hormone replacement therapy on QT interval. Am J Cardiol. 1998;82:993–995. doi: 10.1016/S0002-9149(98)00523-2. [DOI] [PubMed] [Google Scholar]
- Ben-David J, Zipes DP. Torsades de pointes and proarrhythmia. Lancet. 1993;341:1578–1582. doi: 10.1016/0140-6736(93)90708-o. [DOI] [PubMed] [Google Scholar]
- Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111:1088–1093. doi: 10.1016/0002-8703(86)90010-4. [DOI] [PubMed] [Google Scholar]
- Clark BG, Wheatley R, Rawlings JL, Vestal RE. Female preponderance in diuretic-associated hypokalemia: a retrospective study in seven long-term care facilities. J Am Geriatr Soc. 1982;30:316–321. doi: 10.1111/j.1532-5415.1982.tb05620.x. [DOI] [PubMed] [Google Scholar]
- Toner JM, Ramsay LE. Thiazide-induced hypokalaemia; prevalence higher in women. Br J Clin Pharmacol. 1984;18:449–452. doi: 10.1111/j.1365-2125.1984.tb02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota M, Ohtani H, Hanada E, Kotaki H, Sawada Y, Iga T. Effects of hypokalaemia on arrhythmogenic risk of quinidine in rats. Life Sci. 1998;62:2159–2169. doi: 10.1016/S0024-3205(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Lasker N, Hopp L, Grossman S, Bamforth R, Aviv A. Race and sex differences in erythrocyte Na+, K+, and Na+-K+-adenosine triphosphatase. J Clin Invest. 1985;75:1813–1820. doi: 10.1172/JCI111894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfeld M, Borra S, Gavani S, Corcoran A. Hypokalemia: are elderly females more vulnerable? J Natl Med Assoc. 1993;85:861–864. [PMC free article] [PubMed] [Google Scholar]
- Reid JL, Whyte KF, Struthers AD. Epinephrine-induced hypokalemia: the role of beta adrenoceptors. Am J Cardiol. 1986;57:23F–27F. doi: 10.1016/0002-9149(86)90884-2. [DOI] [PubMed] [Google Scholar]


