Abstract
Background and Aims
Extended seed longevity in the dry state is the basis for the ex situ conservation of ‘orthodox’ seeds. However, even under identical storage conditions there is wide variation in seed life-span between species. Here, the effects of seed traits and environmental conditions at the site of collection on seed longevity is explored for195 wild species from 71 families from environments ranging from cold deserts to tropical forests.
Methods
Seeds were rapidly aged at elevated temperature and relative humidity (either 45°C and 60% RH or 60°C and 60% RH) and regularly sampled for germination. The time taken in storage for viability to fall to 50% (p50) was determined using Probit analysis and used as a measure of relative seed longevity between species.
Key Results
Across species, p50 at 45°C and 60% RH varied from 0·1 d to 771 d. Endospermic seeds were, in general, shorter lived than non-endospermic seeds and seeds from hot, dry environments were longer lived than those from cool, wet conditions. These relationships remained significant when controlling for the effects of phylogenetic relatedness using phylogenetically independent contrasts. Seed mass and oil content were not correlated with p50.
Conclusions
The data suggest that the endospermic seeds of early angiosperms which evolved in forest understorey habitats are short-lived. Extended longevity presumably evolved as a response to climatic change or the invasion of drier areas. The apparent short-lived nature of endospermic seeds from cool wet environments may have implications for re-collection and re-testing strategies in ex situ conservation.
Key words: Gene banks, seed ageing, seed longevity, taxonomic trends, climate, seed structure
INTRODUCTION
The fact that seeds of most species can be dried and stored from year-to-year has been exploited since the beginning of agriculture. Indeed, the ability of many orthodox seeds (sensu Roberts, 1973) to remain viable for tens or hundreds of years in dry storage (Walters et al., 2005; Daws et al., 2007), means that they also provide a convenient vehicle for the long-term ex situ conservation of plant germplasm.
The longevity of seeds held in dry storage is mainly determined by seed moisture content and storage temperature, with life-span increasing predictably with decreasing temperature and moisture content (Harrington, 1963, 1972; Ellis and Roberts, 1980). However, there are also wide inherent differences in seed longevity between species (Harrington, 1972; Priestley et al., 1985). For example, using the improved seed viability equations (derived from rapid ageing experiments at elevated temperatures and moisture contents; Ellis and Roberts, 1980), the predicted time for viability to decline from 97·7% to 84·1% for seeds stored under gene-bank conditions (−20°C after equilibration at 15% RH, 15°C) ranges from approx. 30 years for Ulmus carpinifolia to approx. 6000 years for Sorghum bicolor (Liu et al., 2008). Similarly, using the Avrami equation to model data from re-testing of 41 847 seed accessions from 276 species stored at the USDA National Center for Genetic Resources Preservation (NCGRP), Walters et al. (2005) predicted that there would be a difference of 626 years in the time for viability to fall to 50% between the shortest (Bromus sitchensis; 7 years) and longest lived accessions (Trifolium campestre; 633 years).
However, there is already evidence that some species produce seeds with much shorter longevity in dry storage. For example, seeds (with high initial viability) of Anemone nemorosa are predicted to survive <1 year under seed bank storage conditions (Ali et al., 2007). As there is an increasing aspiration to conserve seeds from wild plant species (Target VIII of the Global Strategy for Plant Conservation; SCBD, 2006), it is likely that more species will be found whose seeds have a similarly short life-span.
Understanding species differences in seed longevity is therefore crucial to the effective management of seed conservation collections because it underpins the selection of viability re-test intervals and hence regeneration or re-collection strategies. It is particularly critical for collections of wild plant species where, due to the genetic heterogeneity of wild plant populations, any significant decline in viability will result in the loss of genotypes from the accession (Walters, 2003). However, species-specific constants for the improved viability equations are only available for approx. 56 species (Liu et al., 2008) the majority of which are temperate species of agricultural importance. Generating constants is time consuming and involves the expenditure of tens-of-thousands of seeds per species. Even if the universal values for the temperature coefficients (Dickie et al., 1990) are accepted, carrying out the rapid ageing experiments needed to determine the other two parameters would still require thousands of seeds. Thus, species constants are unlikely ever to be experimentally determined for the majority of plant species. Consequently, predictions of seed longevity in gene-bank storage for threatened wild plant species are problematic. An alternative approach to actually determining longevity for individual species is to identify reliable and robust correlates of longevity and use these to develop general predictive models (Daws et al., 2006).
A number of studies have reported potential correlates of seed longevity in dry storage including seed mass, oil content, carbohydrate composition, taxonomy and climate (e.g. Horbowicz and Obendorf, 1994; Priestley, 1986; Pritchard and Dickie, 2003; Walters et al., 2005). However, a purported link between high oil content and short storage life-span has not been supported by recent analyses (Pritchard and Dickie, 2003; Walters et al., 2005; Bird, 2006).
Seed size may be used as a predictor of persistence in the natural environment, with small seeds generally showing greater persistence in the soil seed bank compared with larger seeds (Thompson et al., 1993; Funes et al., 1999; Cerabolini et al., 2003; Peco et al., 2003). It might be expected that seeds showing greater persistence in the soil would be long-lived in dry storage. This is supported by reports of a positive correlation between seed longevity measured in the laboratory under controlled (Long et al., 2008) or uncontrolled (Bekker et al., 2003) conditions and seed persistence in the soil. However, no such correlation was found when seed longevity under seed bank conditions was compared with seed persistence in soil (Walters et al., 2005), possibly because the stresses and protective mechanisms involved in conferring persistence in the soil are different to the stresses and protective mechanisms that make seeds resistant to ageing in long-term dry storage at low temperatures.
The most significant advance in understanding the underlying factors influencing seed longevity in dry storage comes from the analysis of the NCGRP data. Walters et al. (2005) suggested both a taxonomic and climatic component to inter-specific differences in longevity. Thus, seeds from some families were inherently short lived (e.g. Apiaceae, Brassicaceae) and others long-lived (e.g. Malvaceae and Chenopodiaceae) and species originating from cool, temperate climates tended to produce seeds with short life-spans and those from warm, arid environments seeds with long life-spans. However, these authors did not assess the relative importance of these factors.
In this paper is presented a predictive model of relative seed longevity, determined by analysing longevity data for 195 species sampled from accessions stored in the Royal Botanic Gardens (RBG) Kew, Millennium Seed Bank and representing diverse taxa and global-wide sampling. Due to the logistical and time-scale problems with comparing storage life-span under gene-bank conditions, seed longevity was determined using standardized rapid ageing conditions (Davies and Probert, 2004; Hay et al., 2006). These data are used to assess putative factors of significance in predicting seed longevity, including physical attributes of the seed such as mass and oil content and the climate of the area where seed collections originated. Relative embryo size (RES) across species has also been correlated with the developmental stage of the embryo (Forbis et al., 2002) and intra-specific studies have indicated that longevity increases during seed development (e.g. Hay and Probert, 1995; Ali et al., 2007). Therefore, we also consider whether differences in the relative size of the embryo are related to differences in storage life-span. In addition, since performance at sub-zero temperatures has to be extrapolated from rapid ageing conditions, we test whether our ageing data correlates with long-term (20-year) storage data for species held under gene-bank conditions.
Specifically, the following hypotheses are tested with the aim of developing a predictive model for seed longevity: (1) among seed traits, seed mass, oil content and RES will not be related to seed longevity; (2) climatic parameters (mean annual temperature and total annual rainfall) will not be related to seed longevity. In comparative analyses, species are not statistically independent, as demonstrated by Walters et al. (2005) for seed longevity. Therefore, not only will cross-species analyses be conducted but also phylogenetically independent contrasts will be conducted to test for an evolutionary association between the variables of interest and seed storage life-span.
MATERIALS AND METHODS
Species selection
High viability collections (≥85% germination) of 195 species with known germination requirements were identified from conservation collections held at the Millennium Seed Bank under international gene bank standard conditions of 15% RH and −20°C (FAO/IPGRI, 1994) for between 2 and 35 years (Table 1). Species were selected to give broad taxonomic and geographic coverage and were only included if seed longevity characteristics had not been studied previously. They represent a range of orders (30) and families (71), from 65°N (Finland) to 50°S (Chile) and 173°E (New Zealand) to 125°W (Canada).
Table 1.
Comparison between the proportion of seed collections per family displaying a significant (P < 0·05) drop in viability during 20-year storage at 15% RH and −20°C in the RBG Kew, Millennium Seed Bank, and the mean p50 recorded for that family from artificial ageing at 45°C and 60% RH (also shown are the ranks used for performance at −20°C and in artificial ageing that were used in the Spearman's Rank Correlation)
| Family (-aceae) | No. of collections tested after 20 years | Proportion of collections displaying no significant drop in germination after 20 years | Rank 1 | Mean p50 (d) | n | Rank 2 |
|---|---|---|---|---|---|---|
| Aizo- | 2 | 0·50 | 16 | 76·9 | 1 | 42 |
| Amaranth- | 64 | 0·84 | 41 | 38·7 | 4 | 22 |
| Api- | 29 | 0·55 | 20 | 54·4 | 2 | 35 |
| Aster- | 207 | 0·75 | 36 | 52·6 | 4 | 31 |
| Boragin- | 8 | 0·50 | 16 | 47·0 | 1 | 27 |
| Brassic- | 73 | 0·70 | 29 | 43·6 | 2 | 26 |
| Campanul- | 32 | 0·41 | 12 | 11·2 | 4 | 11 |
| Caprifoli- | 1 | 0·00 | 1 | 8·3 | 1 | 7 |
| Caryophyll- | 513 | 0·81 | 40 | 39·8 | 16 | 24 |
| Cercidiphyll- | 1 | 0·00 | 1 | 0·1 | 1 | 1 |
| Cist- | 30 | 0·93 | 46 | 78·2 | 1 | 43 |
| Convolvul- | 10 | 0·90 | 45 | 69·3 | 2 | 41 |
| Crassul- | 22 | 0·73 | 33 | 57·0 | 2 | 36 |
| Cucurbit- | 7 | 0·86 | 42 | 54·0 | 1 | 34 |
| Cupress- | 1 | 0·00 | 1 | 10·7 | 1 | 10 |
| Cyper- | 8 | 0·75 | 35 | 47·0 | 2 | 28 |
| Dipsac- | 9 | 0·78 | 38 | 59·3 | 3 | 38 |
| Eric- | 13 | 0·31 | 9 | 9·4 | 5 | 9 |
| Fab- | 5 | 0·76 | 37 | 83·5 | 6 | 45 |
| Gentian- | 51 | 0·71 | 32 | 19·2 | 6 | 15 |
| Gerani- | 5 | 0·80 | 39 | 82·8 | 2 | 44 |
| Gesneri- | 1 | 0·00 | 1 | 0·9 | 1 | 2 |
| Haemodor- | 2 | 0·50 | 16 | 41·7 | 1 | 25 |
| Hydrophyll- | 5 | 0·40 | 11 | 49·5 | 2 | 29 |
| Irid- | 9 | 0·67 | 26 | 53·7 | 1 | 32 |
| Junc- | 8 | 0·63 | 22 | 59·2 | 1 | 37 |
| Juncagin- | 1 | 0·00 | 1 | 5·6 | 1 | 5 |
| Lami- | 75 | 0·65 | 25 | 69·0 | 7 | 40 |
| Loas- | 5 | 0·60 | 21 | 9·0 | 2 | 8 |
| Lythr- | 3 | 0·67 | 26 | 14·0 | 1 | 12 |
| Melanthi- | 1 | 0·00 | 1 | 4·5 | 4 | 3 |
| Onagr- | 171 | 0·49 | 15 | 53·9 | 12 | 33 |
| Papaver- | 49 | 0·45 | 13 | 36·7 | 3 | 21 |
| Po- | 722 | 0·86 | 44 | 26·7 | 4 | 19 |
| Polemoni- | 4 | 0·50 | 16 | 22·0 | 3 | 17 |
| Polygon- | 20 | 0·70 | 30 | 38·7 | 1 | 23 |
| Portulac- | 11 | 0·73 | 33 | 51·1 | 1 | 30 |
| Primul- | 30 | 0·47 | 14 | 31·0 | 8 | 20 |
| Ranuncul- | 81 | 0·69 | 28 | 14·9 | 6 | 13 |
| Ros- | 56 | 0·86 | 42 | 21·8 | 2 | 16 |
| Rubi- | 10 | 0·70 | 30 | 16·0 | 3 | 14 |
| Saxifrag- | 3 | 0·33 | 10 | 4·6 | 2 | 4 |
| Scrophulari- | 101 | 0·63 | 24 | 25·4 | 4 | 18 |
| Solan- | 75 | 0·63 | 23 | 62·9 | 8 | 39 |
| Urtic- | 2 | 0·00 | 1 | 84·4 | 1 | 46 |
| Viol- | 2 | 0·00 | 1 | 6·3 | 2 | 6 |
Ageing protocol
Ageing experiments on samples from the targeted collections were carried out between 2001 and 2006. At the outset, all seed samples were aged according to a standardized protocol (Davies and Probert, 2004). First, in order to raise the moisture content of the seeds prior to ageing and to minimize the subsequent adjustment of moisture content when samples were transferred to the ageing conditions, ten samples of 50 seeds each were rehydrated at 47% RH at 20°C in open glass vials or Petri dishes; the vials/dishes were placed over a non-saturated solution of LiCl (anhydrous, Laboratory Reagent Grade; Fisher Scientific UK Ltd, Leics., UK) in distilled water held in a sealed 300 × 300 × 130 mm electrical enclosure box (Ensto UK Ltd, Southampton). At the end of the re-hydration period (14 d), seed equilibrium relative humidity (eRH) was checked using a sample of the equilibrating seeds or, if the volume of seeds was too small, a reference sample of Ranunculus sceleratus seeds that accompanied the test species during rehydration. The eRH was measured using a water activity measuring instrument which comprised a hygrometer sensor housed in an AW-DI0 water activity probe, used in conjunction with a HygroPalm 3 display unit (Rotronic Instruments UK Ltd, Crawley, UK). Once the test species was judged to have reached equilibrium, samples were transferred to a second electrical enclosure box, over a non-saturated solution of LiCl at 60% RH placed in a LEEC KIF compact incubator (LEEC Ltd, Nottingham, UK) at 45 ± 2°C. The RH generated by the LiCl in the box was checked at 4- to 6-week intervals by pipetting a sample of approx. 10 mL solution and placing it into the sample chamber of the water activity-measuring instrument described above. Samples were allowed to equilibrate for up to 2 h before returning the sample to the enclosure box. The bulk solution was adjusted if necessary, usually by adding distilled water, stirring and allowing the solution to equilibrate before rechecking the RH (Hay et al., 2008).
One sample of 50 seeds for each species was removed after each of 1, 2, 5, 9, 20, 30, 50, 75, 100 and 125 d for germination testing. Seeds were sown as two lots of 25 seeds each on 1% distilled water agar held in 55- or 90-mm-diameter Petri dishes (Bibby Sterilin Ltd, Staffordshire, UK) and placed in a LMS 250A or 600A cooled incubator (LMS Ltd, Sevenoaks, UK) at a temperature regime (constant or alternating) previously found to be optimal for germination of that accession in routine seed bank testing (see Appendix). Some of the accessions required additional treatments including the addition of 250 mg L−1 GA3 (Sigma-Aldrich Company Ltd, Dorset, UK), mechanical scarification of the seed coat (carried out prior to equilibration at 49% RH) or cold stratification (typically at 5°C for 56 d) (Appendix). Plates were regularly checked for germination and seeds scored as germinated once the radicle had reached ≥2 mm in length.
In the ageing experiments at 60% RH and 45°C, four species of Myrtaceae: Calothamnus crassus, C. graniticus, C. rupestris and Melaleuca diosmifolia were found to be extremely long-lived, requiring sampling up to 500 d to generate reliable survival curves. In order to speed up the ageing process for a further 20 species, which were also expected to be very long lived, ageing was carried out at 60% RH and 60°C. These species originated mainly from Australia and/or displayed serotiny. Sampling was carried out at 2, 5, 10, 15, 20, 25, 30, 40, 55 and 75 d and a germination test carried out as before.
Determination of seed characteristics
The mean seed air dry weight (seeds equilibrated to 15% RH, 15°C) was calculated by weighing five lots of 50 seeds for each accession used in the study. Where available, whole seed oil contents for the species were obtained from either the Seed Information Database of the Royal Botanic Gardens Kew (Liu et al., 2008) or the Seed Oils Fatty Acid Database (http://sofa.bfel.de/).
RES was determined following the method of Forbis et al., (2002). Five seeds from each targeted accession were sectioned longitudinally. Subsequently, digital images were taken using a Stemi SV 11 Microscope (Carl Zeiss, Germany) and using Axiovision 3·1 (Carl Zeiss Vision), the area of the embryo was measured and expressed as a proportion of the total space inside the testa. However, since RES was skewed towards values close to 1·0 and could not be satisfactorily transformed to approximate the normal distribution, a discrete classification was used in all analyses where seeds were classified as endospermic when the RES is <1 and non-endospermic when the RES = 1.
Estimation of climate for seed-lot collection sites
Annual mean temperature and total annual precipitation values, based on data collated between 1950 and 2000, were obtained for the collection location of each seed lot by querying WORLDCLIM data (download version 1.4 http://www.worldclim.org/) at a maximum resolution of 30 arc-seconds (approx. 1 km) using the ‘Extract Values to Points’ tool in ESRI ArcMap (version 9.1).
Seed bank data analysis
For seed lots which have been held for >20 years at the Millennium Seed Bank, data for initial germination and germination after 20 years were extracted from the Millennium Seed Bank's Seed Bank Database. The highest percentage germination result in initial tests and after 20 years' storage were compared to test whether there had been a significant drop in viability during storage. Collections in which there had been a significant drop in germinability were identified by testing the null hypothesis of no difference between the two germination values. This was achieved by calculating the two-tailed probability corresponding to Z, the value of the normal deviate corresponding to the difference between the initial and final germination percentage values following the procedure outlined in Ellis et al. (1985).
As a result of limited overlap between both species and genera in this 20-year analysis and species in the artificial ageing tests, the proportion of species, showing a significant decline in viability over 20 years was calculated at the family level. This value was then correlated with the mean p50 for corresponding families (from rapid ageing) to test for a relationship between survival life-span under gene-bank and rapid ageing conditions.
Statistical analysis
Probit analysis of the seed ageing data was carried out using GenStat for Windows, Version 8 (VSN International Ltd, Oxford, UK) to estimate p50 (the time for viability to fall to 50%) through fitting of the basic seed viability equation:
| 1 |
where v is the viability in normal equivalent deviates (NED) at time p (days); Ki is the initial viability (NED) and σ is the standard deviation of the normal distribution of seed deaths in time.
For seeds aged at the higher temperature (60°C), an estimate of p50 at 45°C was calculated by applying a correction factor based on data for five species tested at both temperatures (three species tested in our laboratory and two species tested by collaborators, A. Martyn and D. Merritt). Across these five species there was an average 10·9 (±s.d. 5·7) fold difference in p50 between the two temperatures. Whilst the corrected values must be treated with caution, estimates of longevity (time for viability to fall by 1 NED) under the two different ageing environments calculated using the Seed Information Database (Liu et al., 2008), for seeds of other species for which viability constants for the Ellis and Roberts (1980) viability equations have been determined and for which seed oil contents are known (to estimate moisture contents under the two different ageing environments), suggest that this correction factor is appropriate.
Oil content was only available for 140 of 195 species. Consequently two analyses were conducted for both the multiple regression and phylogenetically independent contrasts (PIC) approaches. The first analysis included only the species for which oil content was available, the second all 195 species but excluding the oil content data. However, since oil content was the first term to be dropped from the regression model for the 140 species (i.e. it contributed least to explaining variation in p50) in both the cross-species and PIC approaches, only the multiple regression results for the analyses involving all 195 species are presented here. In the multiple regression analyses, the relationship between seed longevity (p50) and oil content (% dry weight basis), seed mass (mg), presence (1)/absence of endosperm (0), mean annual temperature (°C) and total annual rainfall (mm) was analysed using Minitab version 13 (Minitab Inc., State College, PA, USA). To ensure normality, p50 and seed mass were log10 transformed and oil content was arc-sine transformed in this and all other analyses. Total annual rainfall was transformed using a Box–Cox transformation with the optimum value of λ (0·30) determined using Minitab 13.
Regression models were constructed using backwards elimination. Initially all terms were included in the model and at each step in the procedure the variable with the smallest (non-significant) partial correlation was dropped from the model until only significant (P < 0·05) terms remained in the model (Sokal and Rohlf, 1995).
PICs (Felsenstein, 1985; Pagel, 1992) were used to analyse the relationship between seed longevity (as assessed by p50) and seed mass, oil content, the occurrence of endosperm, mean annual temperature and total annual rainfall. This approach is based on the logic of comparing pairs of species within a phylogeny that share an immediate common ancestor. The null hypothesis is that there is no correlation between changes in traits at the nodes. The package CAIC (Purvis and Rambaut, 1995) was used to generate contrasts. Within the phylogeny it was assumed that all branch lengths were the same: analyses of simulated data sets suggest that equal branch lengths may perform better than estimated branch lengths (Purvis et al., 1994). For analyses testing whether the transition from endospermic to non-endospermic seeds (or vice versa) is associated with changes in p50, the Brunch procedure, designed for discrete predictor variables, was used (Purvis and Rambaut, 1995). Contrasts from Brunch were analysed using a t-test on the mean of the contrasts: a mean significantly different to zero indicates correlated evolution between the traits of interest. For the continuous variables (seed mass, oil content, etc.) the Crunch procedure was used (Purvis and Rambaut, 1995). For Crunch, variables were either run individually, with a linear regression forced through the origin, subsequently fitted to the contrasts or simultaneously with multiple regression used to analyse the contrasts. For all phylogenetic analyses, the latest phylogeny available to (sub-) family level from the Angiosperm Phylogeny Group was used (APG II, 2003). However, due to the wide range of families and the lack of complete phylogenies to genus level for most families, a series of polytomies were created.
RESULTS
Variation in p50 between species
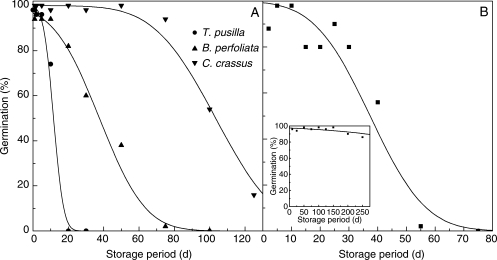
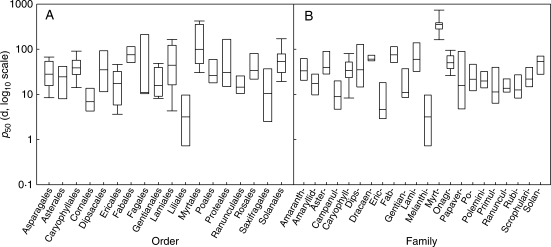
Across the 195 species, viability declined with increasing duration of the ageing treatment (for examples, see Fig. 1). However, there was wide variation across species in the time taken for viability to fall to 50%: p50 ranged between 0·1 d for Cercidiphyllum japonicum to 771 d for Calothamnus rupestris, respectively (both aged at 45°C and 60% RH; Appendix). Within orders, there was a wide span of longevities with p50 spanning more than an order of magnitude in some orders (e.g. Fagales and Lamiales). In addition, the Myrtales contained the longest lived species and the Liliales the shortest lived (Fig. 2A). Similarly there was wide variation in p50 between families, including in some cases within the same order (e.g. Myrtaceae and Onagraceae in the Myrtales with mean estimates for p50 of 366 d and 54 d, respectively; Fig. 2B).
Fig. 1.
Survival curves fitted by probit analysis for four of the species in this study: seeds of (A) Tofieldia pusilla, Blackstonia perfoliata and Calothamnus crassus aged at 60% RH and 45°C, and (B) Calothamnus rupestris aged at 60% RH, 60°C and 60% RH, 45°C (inset).
Fig. 2.
Box plots of p50 in (A) orders and (B) families. Only orders and families with three or more species tested are shown. Boxes span the 25th to 75th percentiles; whiskers span the 5th to 95th percentiles.
For the 46 families that overlapped between rapid ageing and 20 years' seed bank storage, there was a highly significant correlation between the proportion of species in a family exhibiting a significant decline in viability after 20 years storage under gene-bank conditions and mean p50 at the family level (Spearman's Rank Correlation, rS = 0·527, d.f. = 44, P < 0·001; Table 1).
Correlates of p50
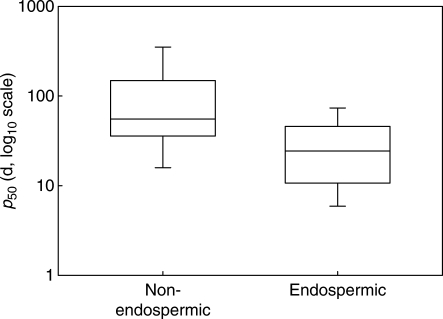
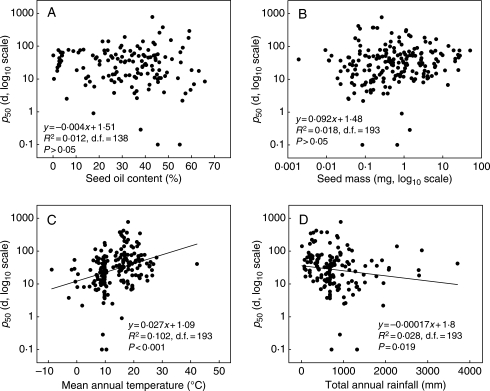
Endospermic seeds were significantly shorter lived than non-endospermic seeds (mean p50 values of 20·3 d vs. 65·7 d, one-way ANOVA, P < 0·001; Fig. 3). However, neither of the additional seed characteristics (seed mass and oil content) was significantly related to p50 (linear regression, R2 ≤ 0·02, P > 0·05; Fig. 4A, B). There was a significant positive relationship between mean annual temperature and p50 (linear regression, R2 = 0·102, P < 0·001), species from warmer environments tending to have a greater p50 (Fig. 4C). There was also a significant negative relationship between total annual precipitation (Box–Cox transformed data, λ = 0·30) and p50 (linear regression, R2 = 0·051, P = 0·002), species from drier environments tending to have a greater p50 (Fig. 4D).
Fig. 3.
Box plots comparing p50 for endospermic and non-endospermic seeds. Boxes span the 25th to 75th percentiles; whiskers span the 5th to 95th percentiles.
Fig. 4.
Relationship between p50 and (A) oil content, (B) seed mass, (C) annual temperature, and (D) annual rainfall.
Multiple regression for all 195 species, including the presence/absence of endosperm, log10 (seed mass), mean annual temperature and total annual precipitation (Box–Cox transformed, λ = 0·30) as the independent variables and p50 as the dependent variable, explained a significant proportion of the variation in p50 (R2 = 0·279, F = 18·39, P < 0·001). Only the presence/absence of endosperm, mean annual temperature and total rainfall remained when using a backwards model selection procedure (Table 2). This model explained a significant proportion of the variation in time for viability to fall to 50% (R2 = 0·278, F = 24·5, P < 0·001) such that:
where p50 is the time for viability to fall to 50% (days), E is the presence (1) or absence (0) of endosperm, AT is mean annual temperature (°C) and AR is annual rainfall (mm).
Table 2.
Model selection for the effects of endosperm, seed mass, mean annual temperature and total annual rainfall on p50 in the multiple regression analysis
| Term | Coefficient | t-value |
|---|---|---|
| Full model | ||
| Constant | 2·060 | 10·58*** |
| Endosperm | −0·459 | −5·86*** |
| Mass | 0·025 | 0·56 n.s. |
| Temperature | 0·021 | 3·83*** |
| Rainfall | −0·070 | −3·36*** |
| Reduced model | ||
| Constant | 2·059 | 10·59*** |
| Endosperm | −0·459 | −5·86*** |
| Temperature | 0·021 | 4·05*** |
| Rainfall | −0·081 | −3·51*** |
Terms in bold are those that were dropped from the model at each stage in the model selection process.
***, P < 0·001; n.s., not significant P > 0·05
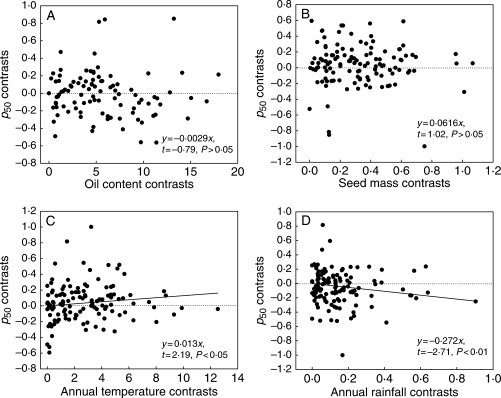
Accounting for phylogenetic relatedness indicated that the presence of endosperm was significantly associated with lower values of p50 (1-sample t-test, t = −2·36, P < 0·05). There was no relationship between contrasts in oil content and p50 (P > 0·05, Fig. 5A) or contrasts in seed mass and p50 (P > 0·05, Fig. 5B). However, there were significant relationships between contrasts in temperature and p50 (positive) and rainfall and p50 (negative) (P < 0·05; Fig. 5C, D). Simultaneously generating contrasts for all three continuous variables (excluding oil content) and p50 in Crunch and then conducting multiple regression on the contrasts showed a significant relationship (F = 2·70, P < 0·05). Partial correlation showed a significant negative correlation between p50 and annual rainfall contrasts (β = −0·286, t = −2·29, P < 0·05) and no correlation between p50 and either seed mass (β = 0·029, t = 0·4, P = 0·687) or mean annual temperature (β = 0·01, t = 1·03, P = 0·304).
Fig. 5.
Relationship between p50 contrasts and (A) oil content contrasts, (B) seed mass contrasts, (C) annual temperature contrasts, (D) annual rainfall contrasts. Contrasts were generated in CAIC and the regressions were forced through the origin.
DISCUSSION
The present data indicate variation in seed longevity spanning four orders of magnitude under identical storage conditions. This variation was related to seed structure and the climate of the area from where the species were collected. In addition, it was found that survival of rapid ageing, at the family level, was correlated with survival under gene-bank conditions. These findings may enable predictions of likely performance under gene-bank conditions based on information regarding climate and seed structure.
Seed mass, lipid content and p50
Among seed characteristics, it was found that, in agreement with other studies (Priestley, 1986; Walters et al., 2005; Bird, 2006), there was no relationship between seed longevity and oil content. Nonetheless, it is possible that lipid composition is important in determining longevity since the degree of fatty acid saturation will impact on the potential for lipid peroxidation which has been considered to be a primary reaction in ageing contributing to free-radical production and subsequent attack on other macro-molecules (Benson, 1990). This remains to be demonstrated.
Similarly, seed mass was not correlated with longevity as concluded elsewhere for a range of crop species (Pritchard and Dickie, 2003; Walters et al., 2005). Previous reports have indicated that smaller seeds tend to persist longer in the natural environment (Thompson et al., 1993; Funes et al., 1999); however, this may be more to do with smaller seeds requiring particular environmental triggers for germination than greater longevity per se.
Walters et al. (2005) reported a lack of correlation between longevity under seed bank conditions and seed persistence in a temperate mesic soil, whereas Long et al. (2008) found a positive correlation between seed longevity under rapid ageing conditions (45°C and 60% RH) and seed persistence in soil. These apparently contradictory findings might be explained by the different soils investigated in the two studies. The comparatively warm dry soils used by Long and co-workers were certainly much closer to the conditions of the rapid ageing tests compared with the very different conditions of seed bank storage and temperate mesic soil investigated by Walters and co-workers.
Endosperm, environment, taxonomy and p50
Whilst some of the variation in p50 is not explained by the present model, the analysis clearly demonstrates that species with endosperm (i.e. comparatively small embryos), from cool wet environments tend to be short-lived in dry storage. Moreover, these relationships are significant when accounting for the potentially confounding effects of phylogenetic relationships (using PICs). Similarly, Walters et al. (2005) observed that seeds of species that originated in moist, temperate geographic regions such as Europe tended to have shorter life-spans compared with seeds of species originating in warm arid regions (Australia, South Asia). In further support of the proposition that species from hot dry environments are more likely to have comparatively long-lived seeds, the survival and germination of 200-year-old seeds of three South African species has been recently reported (Daws et al., 2007). Conversely, Ali et al. (2007) reported that endospermic seeds of Anemone nemorosa from the UK have very limited seed longevity (the time taken for viability to drop to 5% at 45°C and 60% RH ranged from approx. 2 d to 14 d): this species typically occurs in cool damp woodland environments.
In an evolutionary context, Forbis et al. (2002) attributed RES to the degree of embryo development at the time of dispersal with small embryos being the ancestral state in the angiosperms. Field and Arens (2005) suggested that early angiosperms evolved in disturbed, moist understorey environments such as montane forests and along riverbanks. Thus, the present data suggest that early angiosperms had short-lived seeds (in dry storage), which is likely to be consistent with their limited exposure to drying. Thus, we hypothesize that prolonged longevity in the dry state evolved as an adaptation to either climatic drying, or the invasion of hot, dry environments where seeds might be expected to persist in the dry state for irregular multi-year intervals between rainfall events sufficient for successful establishment.
Figure 2 suggests that seed life-span is also a characteristic of particular taxonomic groupings, as reported previously (Priestley et al., 1985; Walters et al., 2005). For example, species investigated in the Myrtales were typically long-lived while those in the Liliales were short lived. Such patterns were also evident at the family level. For example, the Campanulaceae, Ericaceae and Melanthiaceae were consistently short lived (p50 ranged from 0·1 d to 22 d) whereas Myrtaceae were consistently long lived (p50 ranged from 152 d to 771 d). Within some families, p50 varied considerably, yet genera within those families showed relatively little variation in longevity. For example, in the Primulaceae, p50 ranged from 4 d to 140 d but the five species of Primula tested were all short lived (p50 ranged from 6 d to 22 d; cf. Fig. 2B and Appendix). All five species of Gentiana were also short lived (p50 ranged from 8 d to 13 d) whereas Blackstonia perfoliata and Centaurium erythraea, also Gentianaceae, were significantly longer lived (p50 of 38 d and 37 d, respectively). Hay et al. (2006) also found little variation in p50 within the genus Rhododendron. Thus, for these consistently short-lived genera, the longevity of one species is likely to be a good predictor of con-generics. However, these taxonomic trends may result from related species sharing similar traits such as seed structure, and habitats, and hence having a similar longevity. Interestingly, the presence of endosperm and the environmental variables were still significantly related to p50 when these phylogenetic effects were controlled using PIC analysis, supporting the hypothesis that correlated evolution between p50 and other seed traits and climate has resulted in these apparent taxonomic trends.
Implications for gene banks
A significant challenge for seed-bank managers is ensuring collections maintain high levels of viability to avoid loss of genotypes from the population while storing seeds from increasingly diverse taxa, many of which have not been studied in any way (at least in terms of seed biology). Thus, predictions of seed longevity based on seed characteristics and species' ecology would be of significant benefit to the seed banking community. Current guidelines (Rao et al., 2006) recommend that base collections stored according to international standards (FAO/IPGRI, 1994) be tested for viability after 5 or 10 years of storage depending on initial viability and whether the seeds have poor longevity. However, there is increasing evidence that seeds of some exceptionally short-lived species such as Anemone nemorosa may lose viability within a year or two (Ali et al., 2007). Conversely, seeds that are likely to be extremely long lived in storage, such as some of the Myrtaceae species tested here, might currently be tested more frequently than necessary, involving the expenditure of seeds which could represent valuable germplasm (in the sense that limited numbers of seeds are available and recollection is difficult). Here, for the first time, data have highlighted that seed structure and climate of origin are important indicators of seed longevity. Thus species from cool, moist environments, particularly those with small embryos relative to the size of the seed, may need to be tested more frequently than non-endospermic seeds from hot dry environments.
In contrast to Walters et al. (2005), who investigated correlates of seed longevity under gene-bank conditions, seeds in the current study were aged at elevated temperatures and RH. Potential advantages of this approach include the application of a standardized protocol to a wide range of germplasm enabling rapid generation of data: this protocol was applied to 195 species from 30 orders and 71 families. However, while the causes of seed death in gene-bank and rapid-ageing conditions may not be the same, families with species whose seeds aged rapidly in artificial ageing also showed evidence of more rapid seed viability loss during seed bank storage, with a significant reduction in viability over 20 years storage at −20°C. Moreover, collections from genera, such as Primula, predicted to be consistently short lived in comparative ageing tests also showed significant declines in viability after 20 years' storage in the seed bank.
The present data have shown that seed longevity is related to seed structure and climate of origin. Short-lived seeds are more likely to possess small embryos and originate from cool wet regions. Although we have not been able to explain some of the variation in p50 and we have not considered intra-specific variation in seed longevity resulting from genetic or environmental influences or seed maturity, we can now predict which species are more likely to be short lived in storage. The rapid-ageing test described here offers seed-bank managers a means of assessing the potential longevity of seed collections of such species under seed-bank conditions, thereby enabling the selection of appropriate viability retest intervals and thus better management of conservation collections.
ACKNOWLEDGEMENTS
We are grateful to Tiziana Ulian who helped with initial data analysis; Hannah Davies, Wendy Bastable, Kirsty Brazil and Rosemary Newton who carried out the controlled ageing tests and embryo measurements; Amelia Martyn and David Merritt for sharing data on two Australian species and many other Millennium Seed Bank partners who have taken an active interest and directly or indirectly supported this work. Financial support was provided by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew is supported by grant-in-aid from Defra.
APPENDIX
Details of species used in the study: classification to family level (following APG, 2003), country of origin, time to 50% viability loss (p50) in the ageing conditions (45°C, 60% RH), and germination conditions used. Species in bold were aged at 60°C, 60% RH (p50 shown for these is an estimate based on the measured p50 at 60°C multiplied by a factor of 10·9; see text for details).
| Species name | Family (-aceae) | Country of origin | p50 (d) | Germination pre-treatments | Germination conditions (°C) |
|---|---|---|---|---|---|
| Acacia etbaica Schweinf. | Fab- | Kenya | 90·1 | Mechanical scarification with a scalpel | 20 |
| Allocasuarina acutivalvis (F.Muell.) L.A.S.Johnson | Casuarin- | Australia | 202·0 | 10 | |
| Allocasuarina campestris (Diels) L.A.S.Johnson | Casuarin- | Australia | 221·0 | 15 | |
| Alnus glutinosa (L.) Gaertn. | Betul- | England | 10·1 | 25 | |
| Alonsoa acutifolia Ruiz & Pav. | Scrophulari- | Peru | 17·1 | 15 | |
| Amaranthus caudatus L. | Amaranth- | England | 70·6 | 20 | |
| Anagallis arvensis L. | Primul- | Eygpt | 140·0 | 20 | |
| Anagallis foemina Mill. | Primul- | England | 44·0 | 25† | |
| Aquilegia formosa Fisch. ex DC. | Ranuncul- | USA | 12·7 | 25 | |
| Astronium fraxinifolium Schott | Anacardi- | Brazil | 19·0 | 20 | |
| Atriplex bunburyana F.Muell. | Amaranth- | Australia | 17·4 | 15 | |
| Atriplex eardleyae Aellen | Amaranth- | Australia | 30·0 | 20 | |
| Betula nana L. | Betul- | Scotland | 10·6 | 30 | |
| Blackstonia perfoliata (L.) Huds. | Gentian- | England | 37·6 | 20 | |
| Brassica napus L. | Brassic- | England | 65·1 | 20 | |
| Caiophora lateritia Benth. | Loas- | England | 4·3 | 20 | |
| Calceolaria hypericina Poepp. ex Benth. | Scrophulari- | Chile | 26·7 | 15 | |
| Calluna vulgaris (L.) Hull | Eric- | England | 17·6 | 35/20*,† | |
| Calothamnus crassus (Benth.) Hawkeswood | Myrt- | Australia | 375·0 | 15 | |
| Calothamnus graniticus Hawkeswood | Myrt- | Australia | 256·3 | 20 | |
| Calothamnus robustus Schauer | Myrt- | Australia | 374·0 | 15 | |
| Calothamnus rupestris Schauer | Myrt- | Australia | 771·0 | 15 | |
| Calotropis procera (Aiton) W.T.Aiton | Asclepiad- | Morocco | 18·5 | 30 | |
| Campanula pyramidalis L. | Campanul- | Hrvatska | 3·9 | 20 | |
| Campanula rapunculoides L. | Campanul- | Bulgaria | 23·0 | 20 | |
| Carex hystericina Muhl. ex Willd. | Cyper- | USA | 67·8 | 25 | |
| Centaurium erythraea Rafn | Gentian- | England | 36·9 | 15 | |
| Cephalaria joppica (Spreng.) Beg. | Dipsac- | Lebanon | 128·3 | 15 | |
| Cercidiphyllum japonicum Siebold & Zucc. | Cercidiphyll- | South Korea | 0·1 | 35/20* | |
| Chamaecrista mimosoides (L.) Greene | Fab- | Burkina Faso | 63·5 | Mechanical scarification with a scalpel | 25 |
| Chenopodium auricomum Lindl. | Amaranth- | Australia | 36·7 | 25/10* | |
| Cistus ladanifer L. | Cist- | Spain, | 78·2 | 15 | |
| Clarkia bottae (Spach) F.H.Lewis & M.E.Lewis | Onagr- | USA | 44·7 | 15 | |
| Clarkia lewisii P.H.Raven & D.R.Parn. | Onagr- | USA | 52·8 | 15 | |
| Clarkia rubicunda (Lindl.) F.H.Lewis & M.E.Lewis | Onagr- | USA | 49·4 | 25/10* | |
| Convolvulus crenatifolius Ruiz & Pav. | Convolvul- | Peru | 103·4 | 23/9* | |
| Conyza sumatrensis (Retz.) E.Walker | Aster- | Indonesia, Sulawesi | 35·5 | 20 | |
| Cooperia drummondii Herb. | Amaryllid- | USA | 8·0 | 20 | |
| Cooperia pedunculata Herb. | Amaryllid- | USA | 18·2 | 20 | |
| Cortusa matthioli L. | Primul- | England | 3·7 | 20 | |
| Cotula coronopifolia L. | Aster- | New Zealand | 105·0 | 25 | |
| Crassula clavata N.E.Br. | Crassul- | South Africa | 77·2 | 20 | |
| Cryptantha watsonii (A. Gray) Greene | Boragin- | USA | 47·0 | 10 | |
| Cucumis pustulatus Naudin ex Hook. f. | Cucurbit- | Kenya | 54·0 | 35/20* | |
| Cuscuta campestris Yunck. | Convolvul- | France | 35·3 | 25 | |
| Cyperus luzulae (L.) Retz. | Cyper- | Peru | 26·3 | 35/20* | |
| Dasylirion lucidum Rose | Dracaen- | Mexico | 73·9 | 25 | |
| Dasylirion wheeleri S.Watson ex Roth. | Dracaen- | USA | 60·3 | 35/20* | |
| Dipsacus fullonum L. | Dipsac- | England | 14·6 | 15 | |
| Dissotis elliotii Gilg | Melastomat- | Burkina Faso | 46·5 | 20 | |
| Elymus canadensis L. | Po- | USA | 25·4 | 20 | |
| Ephedra fragilis Desf. | Ephedr- | Tunisia | 45·9 | 20 | |
| Epilobium ciliatum Raf. | Onagr- | USA | 34·6 | 20 | |
| Eucalyptus gracilis F.Muell. | Myrt- | Australia | 364·0 | 20 | |
| Ferula communis L. | Api- | Morocco | 75·1 | 10 | |
| Ficus exasperata Vahl | Mor- | Malawi | 76·6 | 20 | |
| Ficus salicifolia Vahl | Mor- | Oman | 33·8 | 20 | |
| Filipendula vulgaris Moench | Ros- | England | 16·3 | 15 | |
| Fouquieria splendens Engelm. in Wisl. | Fouquieri- | USA | 34·8 | 15 | |
| Galium mollugo L. | Rubi- | Norway | 8·3 | 20 | |
| Garidella nigellastrum L. | Ranuncul- | England | 28·9 | 23/9*,† | |
| Genista umbellata Clos | Fab- | Spain | 184·8 | Mechanical scarification with a scalpel | 15 |
| Gentiana depressa D.Don | Gentian- | Hungary | 8·6 | 20 | |
| Gentiana gracilipes Turrill | Gentian- | England | 11·0 | 20 | |
| Gentiana purdomii C.Marquand | Gentian- | Finland | 8·0 | 20 | |
| Gentiana pyrenaica L. | Gentian- | Germany | 13·0 | 25 | |
| Gentiana waltonii Burkill | Ranuncul- | Austria | 9·1 | 15 | |
| Geranium lucidum L. | Gerani- | England | 113·5 | 15 | |
| Geranium robertianum L. | Gerani- | France | 52·1 | 20 | |
| Geum aleppicum Jacq. | Ros- | Canada | 27·2 | 20 | |
| Gilia capitata Sims | Polemini- | England | 32·2 | 20 | |
| Glaucium corniculatum (L.) Rudolph | Papaver- | Morocco | 89·5 | 35/20*,† | |
| Gomphocarpus fruticosus (L.) W.T.Aiton | Asclepiad- | Botswana | 41·0 | 25/15* | |
| Habranthus tubispathus (L'Hér.) Traub | Amaryllid- | USA | 8·9 | 20 | |
| Hakea candolleana Meisn. | Prote- | Australia | 166·0 | 35/20 | |
| Hakea leucoptera R.Br. | Prote- | Australia | 14·9 | 35/20 | |
| Hyoscyamus niger L. | Solan- | England | 32·0 | 30† | |
| Hyptis suaveolens (L.) Poit. | Lami- | Nepal | 138·8 | 20 | |
| Isopyrum fumarioides L. | Ranuncul- | England | 9·8 | 20 | |
| Jasione montana L. | Campanul- | England | 10·7 | 15 | |
| Juncus xiphioides E.Mey. | Junc- | USA | 59·2 | 15 | |
| Kalanchoe lanceolata (Forssk.) Pers. | Crassul- | Kenya | 36·8 | 25 | |
| Kniphofia rooperi (T.Moore) Lem. | Asphodel- | RSA | 26·4 | 25/10* | |
| Leonurus sibiricus L. | Lami- | Argentina | 32·2 | 25/15* | |
| Lepidium montanum Nutt. | Brassic- | USA | 22·2 | 15 | |
| Leptospermum ericoides A.Rich. | Myrt- | New Zealand | 152·0 | 25 | |
| Leucaena leucocephala (Lam.) de Wit | Fab- | Yemen | 75·2 | Mechanical scarification with a scalpel | 20 |
| Lobelia giberroa Hemsl. | Campanul- | Kenya | 7·1 | 15 | |
| Ludwigia alternifolia L. | Onagr- | USA | 24·1 | 35/20* | |
| Ludwigia leptocarpa (Nutt.) H.Hara | Onagr- | USA | 50·2 | 35/20* | |
| Lychnis viscaria L. | Caryophyll- | Denmark | 6·5 | 20 | |
| Lythrum salicaria L. | Lythr- | England | 14·0 | 23/9*,† | |
| Meconopsis horridula Hook.f. & Thomson | Papaver- | England | 4·8 | 20 | |
| Melaleuca diosmifolia Andrews | Myrt- | Australia | 289·0 | 15 | |
| Melaleuca eleuterostachya F.Muell. | Myrt- | Australia | 346·0 | 25 | |
| Melaleuca thyoides Turcz. | Myrt- | Australia | 420·0 | 25 | |
| Melicytus ramiflorus Forster & Forster f. | Viol- | New Zealand | 0·3 | 15 | |
| Mentzelia lindleyi Torr. & A.Gray | Loas- | England | 13·7 | 15 | |
| Myrica serrata Lam. | Myric- | Swaziland | 11·0 | 25/10* | |
| Narthecium ossifragum (L.) Huds. | Melanthi- | England | 0·1 | Warm stratification (28 d at 25°C) followed by cold stratification (56 d at 5°C) | 15 |
| Navarretia squarrosa (Eschsch.) Hook. & Arn. | Polemini- | Canada | 19·8 | 20 | |
| Nemophila menziesii Hook. & Arn. | Hydrophyll- | USA | 51·8 | 15 | |
| Neohymenopogon parasiticus (Wall.) Bennet | Rubi- | Nepal | 27·0 | 25 | |
| Nicotiana glutinosa L. | Solan- | England | 177·7 | 20 | |
| Nicotiana tabacum L. | Solan- | England | 18·5 | 20 | |
| Nolina texana S. Watson | Dracaen- | USA | 55·4 | 15 | |
| Oenothera epilobiifolia Kunth | Onagr- | Colombia | 71·5 | 20 | |
| Oenothera fallax Renner em Rostanski | Onagr- | France | 99·6 | 25 | |
| Oenothera kunthiana (Spach) Munz | Onagr- | Mexico | 76·2 | 15 | |
| Oenothera rosea L'Hér. ex Aiton | Onagr- | USA | 52·5 | 20 | |
| Oenothera texensis P.H.Raven & D.R.Parn. | Onagr- | USA | 37·3 | 15 | |
| Olsynium junceum (E.Mey. ex C.Presl) Goldblatt | Irid- | Chile | 53·7 | 15 | |
| Oxychloris scariosa (F.Muell.) Lazarides | Po- | Australia | 53·4 | 20 | |
| Paederia grandidieri Drake | Rubi- | Madagascar | 12·5 | 25 | |
| Papaver orientale L. | Papaver- | Turkey | 15·8 | 20 | |
| Paspalum conjugatum P.J.Bergius | Po- | 18·1 | 25 | ||
| Phacelia viscida Torr. | Hydrophyll- | USA | 47·2 | 20† | |
| Philadelphus lewisii Pursh | Hydrange- | USA | 6·9 | Cold stratification (56 d at 5°C) | 20 |
| Phycella scarlatina Ravenna | Amaryllid- | Chile | 16·7 | 15 | |
| Physalis pubescens L. | Solan- | England | 26·5 | 25 | |
| Pieris formosa D.Don | Eric- | England | 3·6 | 20 | |
| Pinus maximinoi H.E.Moore | Pin- | Nicaragua | 6·7 | 25/15* | |
| Poa bulbosa L. | Po- | Greece | 10·0 | 15 | |
| Podotheca gnaphalioides Graham | Aster- | Australia | 43·8 | 15 | |
| Polemonium pauciflorum S.Watson | Polemini- | England | 14·0 | 20/10* | |
| Portulaca oleracea L. | Portul- | Colombia | 52·3 | 25 | |
| Primula acaulis (L.) Hill | Primul- | England | 21·6 | 20 | |
| Primula cortusoides L. | Primul- | England | 10·7 | 25 | |
| Primula magellanica Lehm. | Primul- | Chile | 8·0 | 20/10*,† | |
| Primula polyneura Franch. | Primul- | England | 5·8 | 20 | |
| Primula veris L. | Primul- | England | 11·9 | 20 | |
| Pterostyrax hispida Siebold & Zucc. | Stryr- | England | 25·1 | 5 | |
| Pycnostachys deflexifolia Baker | Lami- | Kenya | 139·6 | 25 | |
| Ranunculus acris L. | Ranuncul- | England | 15·2 | 35/20* | |
| Ranunculus sceleratus L. | Ranuncul- | England | 13·7 | 25/10* | |
| Regelia inops (Schauer) Schauer | Myrt- | Australia | 314·0 | 15 | |
| Rhododendron campanulatum D.Don. | Eric- | Nepal | 2·2 | 15 | |
| Rhododendron micranthum Turcz. | Eric- | South Korea | 4·6 | 20 | |
| Rhododendron mucronulatum Turcz. | Eric- | England | 18·8 | 25 | |
| Rhodophiala advena (Ker Gawl.) Traub | Amaryllid- | Chile | 23·3 | 20 | |
| Rhodophiala advena (Ker Gawl.) Traub | Amaryllid- | Chile | 30·8 | 15 | |
| Rhodophiala bagnoldii (Herb.) Traub | Amaryllid- | Chile | 29·7 | 25 | |
| Rhodophiala bagnoldii (Herb.) Traub | Amaryllid- | Chile | 12·5 | 25 | |
| Ribes alpinum L. | Grossulari- | England | 10·5 | 15 | |
| Ribes sanguineum Pursh | Grossulari- | USA | 30·6 | 25/10* | |
| Rogeria longiflora (Royen) Gay ex Dc. | Pedali- | RSA | 189·6 | 25 | |
| Rumex crispus L. | Polygon- | England | 38·7 | 20 | |
| Ruschia barnardii L.Bolus | Aizo- | RSA | 76·9 | 20 | |
| Sagina procumbens L. | Caryophyll- | England | 52·3 | 20 | |
| Salvia disermas L. | Lami- | RSA | 59·9 | 20 | |
| Samolus ebracteatus Kunth | Primul- | USA | 45·9 | 20 | |
| Saponaria officinalis L. | Caryophyll- | France | 17·6 | Cold stratification (28 d at 5°C) | 25/10* |
| Saxifraga cespitosa L. | Saxifrag- | Wales | 6·7 | 15 | |
| Saxifraga petraea L. | Saxifrag- | Italy | 2·5 | 15 | |
| Scabiosa prolifera L. | Dipsac- | Portugal | 35·1 | 10 | |
| Senna occidentalis (L.) Link | Fab- | Zimbabwe | 75·7 | Mechanical scarification with a scalpel | 20 |
| Silene alba (Mill.) E.H.L.Krause | Caryophyll- | England | 65·4 | 25/10* | |
| Silene colorata Poir. | Caryophyll- | Italy | 32·1 | 20 | |
| Silene compacta Fisch. ex Hornem. | Caryophyll- | Greece | 51·9 | 25/10* | |
| Silene conica L. | Caryophyll- | Greece | 61·4 | 20 | |
| Silene dioica (L.) Clairv. | Caryophyll- | Sweden | 22·3 | 20 | |
| Silene gallica L. | Caryophyll- | Spain | 114·7 | 20 | |
| Silene italica (L.) Pers. | Caryophyll- | Greece | 40·5 | 20 | |
| Silene mellifera Boiss. & Reut | Caryophyll- | Spain | 27·5 | 15 | |
| Silene noctiflora L. | Caryophyll- | Canada | 27·4 | 25/10* | |
| Silene nutans L. | Caryophyll- | Bulgaria | 35·5 | 20 | |
| Silene otites (L.) Wibel | Caryophyll- | Germany | 9·1 | 10 | |
| Silene vulgaris (Moench) Garcke | Caryophyll- | Germany | 43·2 | 15 | |
| Solanum lasiophyllum Humb. & Bonpl. ex Dunal | Solan- | Australia | 58·0 | 35/20* | |
| Solanum panduraeforme Dunal | Solan- | Botswana | 70·7 | 35/20* | |
| Solanum quadriloculatum F.Muell. | Solan- | Australia | 70·0 | 35/20* | |
| Spergularia marina (L.) Griseb. | Caryophyll- | Argentina | 28·8 | 10 | |
| Streptocarpus cyaneus S.Moore | Gesneri- | Swaziland | 0·9 | 20 | |
| Swainsona colutoides F.Muell. | Fab- | Australia | 12·0 | 15 | |
| Talinum paniculatum (Jacq.) Gaertn. | Portulac- | Argentina | 51·1 | 20 | |
| Tetradenia riparia (Hochst.) Codd | Lami- | RSA | 7·7 | 20 | |
| Teucrium botrys L. | Lami- | England | 41·5 | 20 | |
| Teucrium polium L. | Lami- | Morocco | 63·6 | 15 | |
| Thuja plicata Donn ex D.Don | Cupress- | 10·7 | 35/20* | ||
| Tofieldia calyculata (L.) Wahlenb. | Melanthi- | Bosnia | 2·6 | Cold stratification (56 d at 5°C) | 20† |
| Tofieldia pusilla Pers. | Melanthi- | Scotland | 11·7 | Cold stratification (56 d at 5°C) | 20 |
| Torilis radiata Moench | Api- | Lebanon | 33·8 | 20 | |
| Triglochin maritima L. | Juncagin- | England | 5·6 | 20 | |
| Urtica pilulifera L. | Urtic- | Lebanon | 84·4 | 15 | |
| Usteria guineensis Willd. | Logani- | Burkina Faso | 40·1 | 25 | |
| Valerianella dentata (L.) Pollich | Valerian- | England | 56·6 | 10† | |
| Veronica verna L. | Scrophulari- | New Zealand | 14·0 | 15 | |
| Viola lutea Huds. | Viol- | England | 12·2 | 20 | |
| Waitzia nitida (Lindl.) Paul G.Wilson | Aster- | Australia | 25·9 | 15 | |
| Weigela subsessilis L.H.Bailey | Caprifoli- | South Korea | 8·3 | Cold stratification (56 d at 5°C) | 33/19* |
| Withania somnifera (L.) Dunal | Solan- | Kenya | 49·5 | 20 | |
| Xerophyta humilis (Baker) T.Durand & Schinz | Vellozi- | Botswana | 18·5 | 25 | |
| Xiphidium caeruleum Aubl. | Haemodor- | Ecuador | 41·7 | 25 | |
| Yucca angustissima Engelm. ex Trelease | Agav- | USA | 42·1 | 20 | |
| Zigadenus elegans Pursh | Melanthi- | Canada | 3·8 | 5 |
* The elevated temperature was applied for 8 h each day with light provided during the warm phase.
† GA3 at 250 mg L−1 was included in the agar germination medium.
LITERATURE CITED
- Ali N, Probert R, Hay F, Davies H, Stuppy W. Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research. 2007;17:155–163. [Google Scholar]
- APG II. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnaean Society. 2003;141:399–436. [Google Scholar]
- Bekker RM, Bakker JP, Ozinga WA, Thompson K. Seed traits: essential for understanding seed longevity. Aspects of Applied Biology. 2003;69:1–9. [Google Scholar]
- Benson EE. Free radical damage in stored germplasm. Rome: International Board for Plant Genetic Resources; 1990. [Google Scholar]
- Bird AE. Thermal methods and comparative seed longevity in the genus Ranunculus L. UK: University of London; 2006. PhD Thesis. [Google Scholar]
- Cerabolini B, Ceriani RM, Caccianiga M, De Andreis R, Raimondi B. Seed size, shape and persistence in soil: a test on Italian flora from Alps to Mediterranean coasts. Seed Science Research. 2003;13:75–85. [Google Scholar]
- Davies H, Probert R. Protocol for comparative seed longevity testing sheet. London: Royal Botanic Gardens, Kew; 2004. www.kew.org/msbp/scitech/publications/comparative_longevity.pdf . [Google Scholar]
- Daws MI, Garwood NC, Pritchard HW. Prediction of desiccation sensitivity in seeds of woody species: a probabilistic model based on two seed traits and 104 species. Annals of Botany. 2006;97:667–674. doi: 10.1093/aob/mcl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws MI, Davies J, Vaes E, van Gelder R, Pritchard HW. Two-hundred-year seed survival of Leucospermum and two other woody species from the Cape Floristic region, South Africa. Seed Science Research. 2007;17:73–79. [Google Scholar]
- Dickie JB, Ellis RH, Kraak HL, Ryder K, Tompsett PB. Temperature and seed storage longevity. Annals of Botany. 1990;65:197–204. [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. Principles and methodology. Rome: International Board for Plant Genetic Resources; 1985. Handbook of seed technology for genebanks. Vol. I. [Google Scholar]
- FAO/IPGRI. Genebank standards. Rome: Food and Agriculture Organisation of the United Nations/International Plant Genetic Resource Institute; 1994. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Field TS, Arens NC. Form, function and environments of the early angiosperms: merging extant phylogeny and ecophysiology with fossils. New Phytologist. 2005;166:383–408. doi: 10.1111/j.1469-8137.2005.01333.x. [DOI] [PubMed] [Google Scholar]
- Forbis TA, Floyd SK, de Queiroz A. The evolution of embryo size in angiosperms and other seed plants: implications for the evolution of seed dormancy. Evolution. 2002;56:2112–2125. doi: 10.1111/j.0014-3820.2002.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Funes G, Basconcelo S, Diaz S, Cabido M. Seed size and shape are good predictors of seed persistence in soil in temperate mountain grasslands of Argentina. Seed Science Research. 1999;9:341–345. [Google Scholar]
- Harrington JF. The value of moisture-resistant containers in vegetable seed packaging. Californian Agricultural Station Bulletin. 1963;792:1–23. [Google Scholar]
- Harrington JF. Seed storage and longevity. In: Kozlowski TT, editor. Seed biology. III. New York, NY: Academic Press; 1972. pp. 145–245. [Google Scholar]
- Hay FR, Probert RJ. The effect of different drying conditions and maturity on desiccation tolerance and seed longevity in Digitalis purpurea L. Annals of Botany. 1995;76:639–647. [Google Scholar]
- Hay F, Klin J, Probert R. Can a post-harvest ripening treatment extend the longevity of Rhododendron L. seeds? Scientia Horticulturae. 2006;111:80–83. [Google Scholar]
- Hay F, Adams J, Manger K, Probert R. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology. 2008;36:737–746. [Google Scholar]
- Horbowicz M, Obendrof RL. Seed desiccation tolerance and storability: dependence on flatulence-producing oligosaccharides and cyclitols – review and survey. Seed Science Research. 1994;4:385–405. [Google Scholar]
- Liu K, Eastwood RJ, Flynn S, Turner RM, Stuppy WH. Seed information database (release 7.1) 2008. http://www.kew.org/data/sid .
- Long RL, Panetta FD, Steadman KJ, et al. Seed persistence in the field may be predicted by laboratory-controlled aging. Weed Science. 2008;56:523–528. [Google Scholar]
- Pagel MD. A method for the analysis of comparative data. Journal of Theoretical Biology. 1992;156:431–442. [Google Scholar]
- Peco B, Traba J, Levassor C, Sánchez AM, Azcárate FM. Seed size, shape and persistence in dry Mediterranean grass and scrublands. Seed Science Research. 2003;13:87–95. [Google Scholar]
- Priestley DA. Seed aging. Ithaca, NY: Comstock Publishing Associates; 1986. [Google Scholar]
- Priestley DA, Cullinan VI, Wolfe J. Differences in seed longevity at the species level. Plant, Cell & Environment. 1985;8:557–562. [Google Scholar]
- Pritchard HW, Dickie JB. Predicting seed longevity: the use and abuse of seed viability equations. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. pp. 655–721. [Google Scholar]
- Purvis A, Rambaut A. Comparative analysis by independent contrast (CAIC): an Apple Macintosh application for analysing comparative data. Computer Applications in the Biosciences. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Purvis A, Gittleman JL, Luh H-K. Truth or consequences: effects of phylogenetic accuracy on two comparative methods. Journal of Theoretical Biology. 1994;167:293–300. [Google Scholar]
- Rao NK, Hanson J, Dulloo ME, Ghosh K, Nowell D, Larinde M. Manual of seed handling in genebanks. Handbooks for genebanks no. 8. Rome: Bioversity International; 2006. [Google Scholar]
- Roberts EH. Predicting the storage life of seeds. Seed Science and Technology. 1973;1:499–514. [Google Scholar]
- SCBD (Secretariat of the Convention on Biological Diversity) Global strategy for plant conservation. 2006. http://www.cbd.int/gspc .
- Sokal RR, Rohlf FJ. Biometry. 3rd edn. New York, NY: Freeman and Co; 1995. [Google Scholar]
- Thompson K, Band SR, Hodgson JG. Seed size and shape predict persistence in soil. Functional Ecology. 1993;7:236–241. [Google Scholar]
- Walters C. Optimising seed banking procedures. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. pp. 723–743. [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research. 2005;15:1–20. [Google Scholar]