Abstract
Background and Aims
Most research on the widespread phenomenon of nectar robbing has focused on the effect of the nectar robbers' behaviour on host-plant fitness. However, attention also needs be paid to the characteristics of host plants, which can potentially influence the consequences of nectar robbing as well. A system of three sympatric Corydalis species sharing the same nectar-robbing bumble-bee was therefore studied over 3 years in order to investigate the effect of nectar robbing on host reproductive fitness.
Methods
Three perennial species of Corydalis were studied in the Shennongjia Mountain area, central China. Observations were conducted on visitor behaviour and visitation frequencies of nectar-robbers and legitimate pollinators.
Key Results
The results indicated that the effect of nectar robbing by Bombus pyrosoma varied among species, and the three species had different mating systems. Seed set was thus influenced differentially: there was no effect on seed set of the predominantly selfing C. tomentella; for the facultative outcrossing C. incisa, nectar robbing by B. pyrosoma had a positive effect; and nectar robbing had a significant negative effect on the seed set of outcrossing C. ternatifolia.
Conclusions
A hypothesis is proposed that the type of host-plant mating system could influence the consequences of nectar robbing on host reproductive fitness.
Key words: Nectar robbing, Corydalis, reproductive fitness, bumble-bee, Bombus pyrosoma, mating systems
INTRODUCTION
As a widespread phenomenon that occurs during anthesis of flowering plants, nectar robbing has been one of the focal points of plant reproductive ecological research over the last few decades (Maloof, 2001). In different nectar-robber–host systems diverse conclusions and inferences have been made regarding the consequences of nectar robbing on host reproduction (Maloof and Inouye, 2000; Newman and Thomson, 2005). Nectar robbers have often been described as ‘cheaters’ in some studies because they obtain a reward from the host without providing a pollination service (Darwin, 1872; Thomson, 1986; Bronstein, 1994; Richardson, 2004); as Inouye (1980) has pointed out, nectar robbers ‘generally are not pollinators’. This view is, however, being constantly revised by new experimental results; in some robber–host systems positive or neutral effects of nectar robbing on host fitness have been found, where seed set either increased or remained unchanged after nectar robbing. For example, Guitian et al. (1994) observed that carpenter bees robbing Petrocoptis grandiflora had a positive effect on seed set, and Navarro (2000) also detected a positive effect in the bumble-bee–Anthyllis vulneraria system. On the other hand, bumble-bees robbing Vicia faba (Newton and Hill, 1983) and bluebells (Morris, 1996) had no significant effect on female plant fitness. Maloof and Inouye (2000) surveyed 18 papers and found a similar level of positive, negative and neutral effects of nectar robbing on host fitness in different study systems. Although results based on such a limited survey can not provide firm evidence for the equal level of the three effects, they at least suggest that the influences of nectar robbing on host fitness are complicated.
What is the reason for the differing effects of nectar robbing on host fitness in different systems? Clues can be found by looking into previous research on the subject. The match between the size of the robber and the host flower is relevant: large nectar robbers such as carpenter bees and bumble-bees can have positive or neutral effects on the host depending on flower size. In research on 13 flowers visited by nectar-robbing carpenter bees, 12 of them had either a positive or a neutral effect, while small nectar robbers such as Trigona bees always induced negative effects (for review see Zhang et al., 2007b). These results are likely to be because robbers such as carpenter bees and bumble-bees are large enough to touch the floral sexual organs with their abdomen and cause pollination. Higashi et al. (1988) termed nectar robbers with pollination capability ‘robber-like pollinators’. In contrast, small-sized Trigona bees have no pollination capability. Roubik (1982) suggested that Trigona bees would have a negative effect because they drove away other visitors while robbing nectar. Even if robbers can provide a certain amount of pollination service in some cases, any positive effect may be counteracted by their damage to the corolla, nectary or ovules, and their influence on the sexual phase or the life span of the flowers (Zhang et al., 2007a). Moreover, nectar robbing may induce a shift of role of legitimate pollinators into secondary nectar robbers (they do not bite holes, but obtain nectar through the holes created by primary nectar robbers; see Inouye, 1983), or discourage visits of legitimate pollinators due to the decrease in the amount of nectar available as a result of robbing. Besides influencing host female fitness, nectar robbing could potentially enhance male fitness and increase the offspring outcrossing rate through forcing legitimate pollinators to fly further in search of nectar, thus expanding the pollen dispersal distance and neighbourhood size, and reducing geitonogamy (Zimmerman, 1985; Klinkhamer and de Jong, 1993; Maloof, 2001; Castro et al., 2008, 2009).
Previous research has mostly focused on the direct or indirect (i.e. influencing legitimate pollinators' behaviour) effects of the behaviour of nectar robbers on host fitness. Little attention has been paid to the perspective that host-plant characteristics can also influence the consequence of nectar robbing (but see Stout et al., 2000; Arizmendi, 2001; Irwin and Maloof, 2002), which is manifested by the fact that the same or similar nectar robbers can have different types of effect on different host plants. For example, bumble-bees had a positive effect on Anthyllis vulneraria subsp. vulgaris (Navarro, 2000), but a negative effect on Ipomopsis aggregata (Irwin and Brody, 1999). In examples where carpenter bees are nectar robbers, positive effects were induced in Fouquieria splendens (Waser and Real, 1979) and Petrocoptis grandiflora (Guitian et al., 1994), but a negative effect was induced in Glechoma longituba (Zhang et al., 2007a, 2009). Even in study systems that are very similar, effects can be very different from each other, as in the robber–host systems of bumble-bees and several Corydalis species (Maloof, 2001; Higashi et al., 1988; Olesen, 1996). The characteristics of the nectar robbers alone clearly can not account for such a situation; hence, Burkle et al. (2007) predicted that plant mating system types would have a very close relationship with the effect of nectar robbing on host female fitness. This prediction was partially supported through their experiments on Delphinium nuttallianum and Linaria vulgaris sharing a common nectar-robbing bumble-bee, Bombus occidentalis. Delphinium nuttallianum is a self-compatible, but non-autogamously selfing, pollen-limited perennial whereas L. vulgaris is a self-incompatible perennial that is generally not pollen limited, and the two species shared the same nectar-robbing bumble-bee. Using plant material with large differences in traits in order to test the prediction may have some deficiencies because differential floral traits could significantly influence the outcome of nectar robbing (Castro et al., 2008). A comparison among congeners with similar floral traits yet different mating systems would be more appropriate.
We postulate that in similar systems of nectar robbers and hosts, the types of host mating systems, including stigmal traits, could determine the effects of robbing. Mating systems of selfing, facultative outcrossing, and outcrossing could have different effects on nectar robbing. To test this hypothesis, we chose three species with very similar floral morphology in the Corydalis genus, in which nectar robbing is common: C. tomentella, C. incisa and C. ternatifolia. According to our preliminary observation these sympatric species share the same nectar robber (a bumble-bee) and major legitimate pollinators (three bee species), but the percentage of their flowers that were robbed (nectar-robbing rate) and their seed sets differed significantly from one another. We speculated that these plant species have different types of mating systems and that these induced the subsequent differences. In this research we focused on (1) the nectar-robbing rates and seed set of the three species under natural conditions; (2) the behaviour of the nectar robber and the major legitimate pollinators; (3) the effects of nectar robbing on reproductive success in robbed flowers and unrobbed flowers; and (4) a primary examination of mating systems and the traits of stigmas of the three species. Further, we discuss the influence of the mating systems on the consequences of nectar robbing.
MATERIAL AND METHODS
Study system and site
The study was carried out in the Shennongjia Mountain area, central China, where species' biodiversity is very rich. About twenty species from the genus Corydalis are distributed in this area, being especially rich along stream banks and nearby slopes. The study site was along a stream valley at Muyu Town, Shennongjia Mountain (32°27′N, 110°26′E), approx. 1100–1300 m above sea level.
In a preliminary investigation, we found at least seven species of Corydalis along several hundred meters of the stream valley, with nectar robbing of different levels occurring in them. These species of Corydalis are common and are typical spring ephemerals, with a flowering season aprroximately from March to May in the study area. Each flower of these plants has a spur in which nectar collects. Although flowers opened progressively from the bottom to the top of an inflorescence, there was considerable overlap in the anthesis period of most of the flowers; on average, it took 3–4 d for all the flowers within an inflorescence to open. The entire flowering period of each species was about 15–20 d. Seeds mature from mid-May to early June, then they fall from the pods and are secondarily dispersed by ants (Wu et al., 1996). We chose three perennial species (C. tomentella, C. incisa, C. ternatifolia) with similar floral characters and plenty of individuals as study materials. A survey was conducted of individual height, flower size, number of flowers per florescence and nectar volume (see Table 1). According to our observations, except for occasional carpenter bees (Xylocopa sinensis) the nectar robber of these species was mainly Bombus pyrosoma – a short-tongue bumble-bee. The main legitimate pollinators included Apis cerana, Colletes arnicularis and Amegilla zonata. No other short-tongue bumble-bees were found to rob these plants in the area.
Table 1.
Characteristics of the three study species of Corydalis together with their nectar-robbing rate and seed set under natural conditions in the three study years
| Nectar robbing rate (%) |
Seed set (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Floral colour | No. flowers per florescence | Floral size (cm) | 2004 | 2005 | 2006 | 2004 | 2005 | 2006 |
| C. tomentella | yellow | 8–14 | 1·4–1·7 | 29·2 | 46·5 | 34·9 | 97·3 | 95·8 | 96·1 |
| C. incisa | purple | 12–23 | 2·0–2·5 | 41·6 | 71·1 | 47·8 | 61·8 | 72·4 | 66·6 |
| C. ternatifolia | red | 7–10 | 1·9–2·4 | 44·3 | 77·6 | 51·8 | 49·7 | 45·1 | 51·9 |
Nectar-robbing rate and reproductive success under natural conditions
In order to assess the relationship between female plant reproduction and natural levels of nectar robbing, the study community was observed from late-March to late-April in 2004, 2005 and 2006. For each species 30–40 well-developed individuals with several blooming inflorescences were chosen for observation. One inflorescence (>7 flowers) on each individual was marked for examination of nectar-robbing rate and seed set. The flowers opened sequentially from bottom to top of the inflorescence and top flowers were smaller and less frequently robbed, so nectar-robbing rates were recorded for the five lower-most flowers on the inflorescence. Robbed flowers were easy to detect from the holes or slits visible in the spurs. Marked inflorescences were collected 7–10 d later, before any capsule dehiscence. For each plant, percentage of seed set (number of seeds produced divided by the total number of ovules per plant) and total seed set were calculated as measures of female plant reproduction.
Behavioural observations of nectar robbers and legitimate pollinators
Observations were conducted on visitor behaviour and visitation frequencies of the nectar robbers and legitimate pollinators on the three species in the study populations on 24 and 25 March and 1, 5 and 6 April, 2005, each of which were sunny days. On each day five individuals were randomly chosen for observation, each located in a different patch of peak-blooming plants with more than 20 individuals of the target species, and one inflorescence per individual was marked. We observed the marked plants for four periods of 20 min each day (0840–0900, 1100–1120, 1400–1420, 1700–1720 h), and recorded visitation frequency, visitor behaviour, and the differences among the three species.
Differences in seed set for robbed and unrobbed flowers
Nectar robbing may affect reproductive success not only at whole-plant level but also at the individual flower level (Irwin and Brody, 1999). In order to examine the effect of nectar robbing on female reproduction at the flower level, we also marked another inflorescence on the individuals mentioned above in 2004 and 2005. About one-third of all the flowers on each inflorescence were cut off from the top in order to prevent an effect on reproductive success of the lower flowers due to resource limitation, and the remainder of the inflorescences were rendered as either having all flowers robbed or all flowers unrobbed by the following treatments. First, these inflorescences were divided into two sets. The first set, with approx. 8–10 inflorescences per species, was open to all visitors and the flowers were subsequently robbed robbed, at different rates. The other set, with about 5–7 inflorescences per species, was protected from nectar robbing by covering the nectar spurs with a ‘collar’ to deter robbers. The collars were made of small pieces of translucent oilpaper. A pilot experiment showed that this method deterred only nectar robbers and did not deter legitimate pollinators from visiting. After 3–4 d, the unrobbed flowers from the first set were all cut, and only robbed flowers were left to fruit. Capsules were collected 7–10 d after anthesis to record seed set.
Examination of mating system type
In 2006 we randomly selected 40 young inflorescences from different individual plants of each species. Open flowers were cut off and two treatments were conducted on two groups from the same species: (1) 20 inflorescences were caged by fine mesh until the flowers withered in order to prevent insect visits; and (2) 20 inflorescences were caged by fine mesh until the flowers opened, and then five flowers from the lower part of the inflorescence were hand-pollinated using fresh pollen from individuals at least 10 m away following emasculation of these flowers. Methods were similar to those used by Maloof (2000). After artificial pollination, other flowers were cut off and only pollinated flowers were caged until they withered. Traditional breeding studies usually involve completely emasculating the flowers (Schoen and Lloyd, 1992), but in the case of Corydalis plants this is impractical: because the anthers are appressed to the stigma, and the anthers dehisce prior to, or simultaneously with, the opening of the outer petals, anthers cannot be removed from an open flower without moving pollen onto the stigma. This type of secondary pollen presentation is very common in the genus (Maloof, 2000). Removing the anthers from a bud would require cutting open both the outer and the inner petals; the validity of pollination treatments after such damage would be dubious. For that reason, although we carried out emasculation in the outcrossing treatment, it is possible that some self-pollen could become attached to the stigma before emasculation. Therefore, we labelled this treatment as ‘outcross + self-pollinated’. Capsules were collected 7–10 d after anthesis in order to record seed set. Data for an open-pollination array used the results of natural conditions mentioned above.
Previous research has demonstrated that the per-flower pollen/ovule ratio (P/O ratios) of flowering plants is closely related to the mating system of the plant and can be an indicator of the mating system type (Cruden, 1977). Therefore, in order to confirm the results obtained above regarding mating systems, we also examined the pollen/ovule ratios of the three species. Two old buds and two open flowers on each inflorescence from the two treatment groups were stored in small bottles with fixation liquid (alcohol:acetic acid, 3:1) in order to count pollen and ovule production per flower. The stigmas were later removed with fine forceps and squashed on microscope slides in basic fuchsin gel. The number of pollen grains adhering to the stigma was counted under a compound microscope in the lab (see Kearns and Inouye, 1993). In addition, to supplement the identification of mating systems we also examined the stigmal morphology, and pollen deposition and germination on the stigmas of the three species.
Data analysis
A two-way ANOVA test was used to compare the nectar-robbing rate among species and years, with species as the fixed factor and year as the random factor. Regression analysis was used to compare the relationship between the nectar-robbing rate and host seed set. One-way ANOVA was also used to test the seed set between robbed and unrobbed flowers as well as between different treatments. Data were log-transformed to adjust the variances. The significance level was set at P = 0·05, and all statistical analyses were performed using the SPSS (13·0) statistical package.
RESULTS
Nectar-robbing rate and reproductive success under natural conditions
Under natural conditions, the average nectar-robbing rate in each year for C. tomentella ranged from 29·2–46·5 %; for C. incisa it was 44·3–77·6 %; and for C. ternatifolia it was 41·8–71·1 % (Table 1). The results showed that C. tomentella had a lower robbing rate than the other two species (F1,202 = 7·715, F1,200 = 5·223, P < 0·01), which were not different from each other (F1,204 = 1·012, P > 0·05). A two-way ANOVA test indicated significant differences in nectar-robbing rate among species (F2,303 = 7·324, P < 0·01) and among years (F2,303 = 3·417, P < 0·05), but there was no significant interaction of species and years (Table 2). There was a significant difference in seed set among species under natural conditions (F2,303 = 14·826, P < 0·001): C. tomentella had a higher seed set than the other two species (F1,202 = 11·217, F1,200 = 19·435, P < 0·001), which were also different from each other (F1,204 = 5·162, P < 0·01). A correlation test showed that the relationship between nectar-robbing rate and host seed set was insignificant for each species (rsp1 = 0·418, rsp2 = 0·559, rsp3 = 0·581; all P > 0·05).
Table 2.
Effects of species and years on nectar-robbing rate in Corydalis. Differences in nectar-robbing rate were analysed using a two-way ANOVA test, with species as the fixed factor and year as the random factor
| d.f. | Mean-square | F | P | |
|---|---|---|---|---|
| Species | 2 | 9·325 | 7·324 | 0·008 |
| Year | 2 | 6·249 | 3·417 | 0·024 |
| Species × year | 4 | 2·617 | 1·546 | 0·129 |
| Error | 682 |
Behaviour observations of the nectar robber and the legitimate pollinators
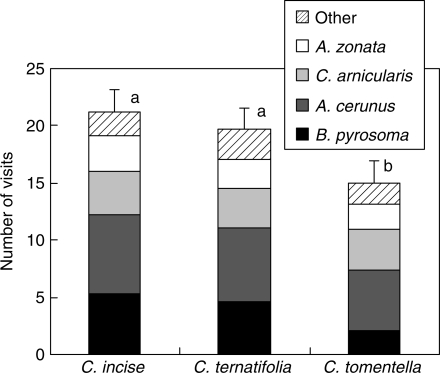
The observations suggested that the visitation frequencies of Bombus pyrosoma to the individual inflorescences in the four observation periods were different, and with total mean number (±s.e.) of visits of 5·3 for C. incise (±2·8, n = 25), 4·6 for C. ternatifolia (±2·4, n = 25), and 2·1 for C. tomentella (±2·0, n = 25). Visitation frequency differed significantly among the three hosts (F2,72 = 5·113, P < 0·01). Nectar robbers often started from the lower part of the inflorescence to suck nectar after piercing the spur, then went on upward but paid no attention to the buds. The nectar-robbing B. pyrosoma were also sometimes observed collecting pollen from C. incisa flowers, which may contribute to pollination to some degree. Nectar robbers also visited previously robbed inflorescences but stayed for less time and visited fewer flowers. Visits by legitimate pollinators (the three bee species A. cerana, C. arnicularis, A. zonata) differed slightly in number, and together with B. pyrosoma accounted for 80 % of the overall visits to the Corydalis species (Fig. 1).
Fig. 1.
Visitation frequency of nectar robbers and legitimate pollinators to three Corydalis species over an average of four observation periods in one day. Values are means (+s.e.), and different letter indicate a significant difference between species.
It was also observed that although a small number (<20 %) of legitimate pollinators (A. cerana) shifted to being secondary nectar robbers after flowers in the three study species had been robbed, most pollinators still visited flowers in a legitimate way, and did not differentiate between robbed and unrobbed flowers.
Seed set of robbed and unrobbed flowers
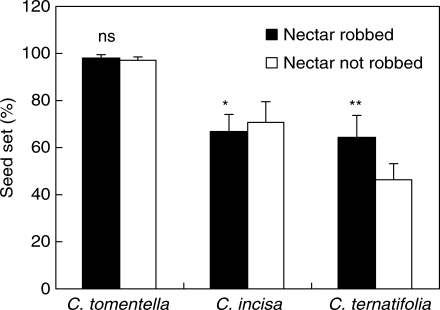
The effect of nectar robbing on host seed set was not consistent among species. For C. tomentella the seed set of robbed flowers (97·2 ± 0·6 %, n = 73) was not significantly different from the seed set of unrobbed flowers (98·4 ± 0·4 %, n = 118; F1,189 = 0·312, P > 0·05). Seed set of C. incisa for robbed flowers was 70·7 % (±8·8, n = 115) and for unrobbed flowers it was 66·6 % (±7·7, n = 119); the increase in host seed set of 4 % indicated a marginally positive effect of nectar robbing (F1,232 = 1·145, P = 0·042). For C. ternatifolia seed set fpr robbed flowers was 46·3 % (±7·3, n = 120) while that of unrobbed flowers was 64·2 % (±9·3, n = 116), so nectar robbing decreased host seed set significantly by 18 % (F1,234 = 25·459, P < 0·001). Nectar robbing in C. incisa and C. ternatifolia had contrasting effects between the two species (Fig. 2).
Fig. 2.
Seed set of robbed flowers and unrobbed flowers for the three study species of Corydalis. Values are means (+s.e.). ns, P > 0·05; *, P < 0·05; **, P < 0·01.
Examination of mating system
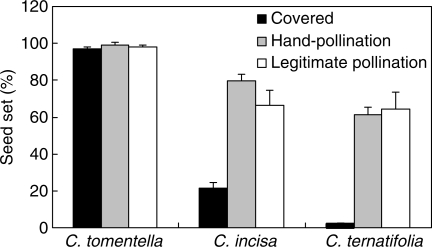
The seed set of C. tomentella was high when pollinators were excluded (96·9 ± 0·3 %, n = 99), and also high in open-pollinated (98·4 ± 0·4 %, n = 178) and artificially pollinated flowers (97·1 ± 0·3 %, n = 100), with no significant differences among the three values (F2,375 = 0·317, P > 0·05), which indicates that the species has a selfing mating system. The seed set of C. incisa without pollinators was low (21·4 ± 2·5 %, n = 97), and was higher when flowers were artificially pollinated (79·8 ± 4·3 %, n = 92) or open pollinated (66·6 ± 7·7 %, n = 141), which indicates that the mating system of this species is facultative outcrossing. For C. ternatifolia seed set without pollinators was very low (2·2 ± 0·3 %, n = 100), but higher in the artificial-pollination treatment (61·3 ± 4·1 %, n = 99) and highest when flowers were open pollinated (64·2 ± 9·3 %, n = 127), which indicates its mating system is outcrossing (Fig. 3).
Fig. 3.
Seed set for the study species under three polination treatments, as indicated. Values are means (+s.e.).
An examination of per-flower pollen and ovule production indicated that the P/O ratio differed among the three species (Table 3). Based on the criteria of mating systems identified by P/O ratios that Cruden (1977) suggested, it is clear that the mating system of C. ternatifolia is outcrossing, C. incisa is a facultative outcrosser and C. tomentella a facultative selfer.
Table 3.
Numbers of pollen and ovules in three species of Corydalis
| Species | No. pollen in single flower* | No. ovules in single flower* | P/O |
|---|---|---|---|
| C. tomentella | 8983 ± 2344 | 24·4 ± 3·6 | 368 |
| C. incisa | 33925 ± 2142 | 15·5 ± 2·2 | 2188 |
| C. ternatifolia | 50084 ± 3921 | 13·7 ± 2·1 | 3710 |
* Values are means ± s.e., n = 30.
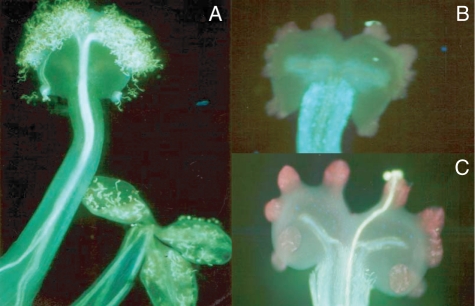
For C. tomentella most flowers autonomously self-pollinated after anthesis through pollen germination on anthers, so their stigmas were always observed to be covered by many germinated pollen grains, and there was no obvious membrane on the stigma mastoid (Fig. 4A). The amount of germinated pollen on stigmas of C. incisa varied, and there was a thin, pink membrane on the mastoids (Fig. 4B). Germinated pollen was less common on stigmas of C. ternatifolia, with only one or a few mastoids with pollen deposition and germination. The mastoids of C. ternatifolia were covered with a thick, pink membrane (Fig. 4C).
Fig. 4.
Stigmatic characteristics, pollen deposition and germination, and mastoid membrane in three study species of Corydalis. (A) Pollen germinates in an anther and autogamy occurs in C. tomentella. (B) Stigma mastoids with a thin, pink membrane in C. incisa. (C) Stigma mastoids with a thick, pink membrane in C. ternatifolia.
DISCUSSION
Nectar robbing is widespread both geographically and taxonomically (Irwin and Maloof, 2002); almost every plant species with floral tubes or nectar spurs suffers from nectar-robbing (Maloof and Inouye, 2000), and the effects on host fitness may modify plant community structure (see review by Zhang et al., 2007b). Host plants may suffer from robbing by the same or different nectar robbers with varying rates under different temporal and spatial conditions (Irwin and Maloof, 2002). The effect of nectar robbing on host fitness is not only complicated (Stout et al., 2000; Irwin and Maloof, 2002), but also unpredictable (Newman and Thomson, 2005; Zhang et al., 2009). However, the effect of host-plant mating system types on the results of nectar robbing is being recognized (Burkle et al., 2007), which provides a new perspective for us to study the phenomenon of nectar robbing.
Variations of nectar-robbing rate and seed set among species and years
The three species in this study differed significantly in nectar-robbing rates. We speculate that this is related to the floral size and nectar production per flower of the species, which we have examined in a previous study (see Table 1). Corydalis tomentella was not only the smallest in size but also had the least nectar production, so the nectar-robbing rate was also the lowest. The other two species had similar floral size and nectar production per flower and consequently insignificant differences in nectar-robbing rates were observed. Nectar production is usually positively related to floral size (Fenster et al., 2006), and the amount of nectar produced directly influences the rate of nectar-robbing on the host. The difference in nectar-robbing rate among years may be caused by the influence of climate or other factors on the populations of nectar robbers, as has been reported in other research (González and Valdivia, 2005). As for the lack of a significant correlation between nectar-robbing rate and seed set at the inflorescence or individual plant level, we presume that the reallocation of reproductive resources among flowers at different positions on inflorescence or individual plant may be relevant. There is evidence that reproductive resources in plants can be reallocated among flowers produced at different places and times (Ashman, 1992; Kliber and Eckert, 2004); however, at the individual flower level, the seed set varied significantly between robbed and unrobbed flowers in C. incisa and C. ternatifolia, indicating that nectar robbing could influence the seed set in some species (see Fig. 3). Thus the effect of nectar robbing on host fitness may be different at the individual plant level and at the flower level. In addition, in some study systems nectar robbing only occurred during a specific period within the flowering season, not throughout it (Zhang et al., 2007a), which was also observed in the present study and is worth further investigation.
Mating system of host and effects of nectar robbing on host fitness
The identification of mating system types based on P/O ratios was consistent with that based on our artificial experiments. We observed that the pollen of C. tomentella could germinate on self-anthers, and autonomous self-pollination occurred (Fig. 4A), so it is easy to understand the neutral effect of nectar robbing on this species. Results from C. incisa indicated a facultative outcrossing, similar to C. caseana (see Maloof, 2001); although it was self-compatible, with pollinators excluded the seed set was much lower than when outcross-pollinated. The membrane on stigma mastoids may block self-pollination or self-fertilization to some degree (see Fig. 4B). These stigmatic cuticles must be ruptured (as in the case of Medicago spp.; Kreitner and Sorensen, 1985) before the pollen tubes can enter the stigma. Visiting insects can effectively destroy the membrane so that pollinator-facilitated pollination is more successful. Bombus pyrosoma may help to rupture the stigma membrane, thus acting as a robber-like pollinator for C. incisa.
The effect of nectar robbing on C. ternatifolia was significant and negative. Treatments applied to C. ternatifolia indicated that it is a self-incompatible or obligatory outcrossing species, similar to C. cava (see Olesen, 1996). Its pollen grains can not germinate on its own stigma, because there is a section of membrane on mastoids of stigmas that can restrain pollen germination (see pink coloration in Fig. 4C). We identified that the membrane contained protein through fluoroscopy (method of Yang and Zhou, 2001). For these flowers successful pollination required legitimate pollinators to take away the self-pollen secondarily presented on the stigma, to destroy the membrane on the mastoids and thus reveal them for outcross-pollen reception. Moreover, the shift of role between pollen collector and nectar robber could also greatly influence host fitness in the facultative outcrossing and outcrossing species (see also Olesen, 1996).
We agree with Burkle et al. (2007) that both pollination biology and plant mating system need to be considered in order to understand and predict the ecological outcomes of plant–animal interactions. In our study, nectar robbing had a marginally positive effect on the reproductive success of the facultative outcrossing C. incisa whereas in D. nuttallianum, which has a similar mating system, nectar robbing was found by Burkle et al. (2007) to have a negative effect on reproduction. Although these two species share a common mating system, they differ greatly in their pollination environment. Reproduction of D. nuttallianum was often limited by pollen availability (Burkle et al., 2007), but in C. incisa that stress was less strong, which accounts for the differential effect of nectar robbing on the reproduction of the two species.
In conclusion, we found that nectar robbing can have variable effects on female plant reproduction. This variation can be explained, at least in part, by the species' mating system. To our knowledge this is the first comparative study of nectar robbing of closely related species sharing the same nectar robbers, and we have related host mating-system types to the effects of nectar robbing on host fitness. Future studies that incorporate the activity of pollinators and plant life history traits into plant–robber interactions are likely to provide important ecological and evolutionary insights.
ACKNOWLEDGEMENTS
We would like to thank Liao Kuo, Wang XueYi, Shi XiaoChuan and Chen Lei for their assistance in the field. Zhao ZhiGang assisted in preparation of the manuscript and two anonymous reviewers provided very helpful comments. This work was supported by a grant from Specialized Research Fund for the Doctoral Program of Higher Education.
LITERATURE CITED
- Arizmendi MC. Multiple ecological interactions: nectar robbers and hummingbirds in a highland forest in Mexico. Canadian Journal of Zoology. 2001;79:997–1006. [Google Scholar]
- Ashman T-L. Indirect costs of seed production within and between seasons in a gynodioecious species. Oecologia. 1992;92:266–272. doi: 10.1007/BF00317374. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. Our current understanding of mutualism. The Quarterly Review of Biology. 1994;69:31–51. [Google Scholar]
- Burkle LA, Irwin RE, Newman DA. Predicting the effects of nectar robbing on plant reproduction: implications of pollen limitation and plant mating system. American Journal of Botany. 2007;94:1935–1943. doi: 10.3732/ajb.94.12.1935. [DOI] [PubMed] [Google Scholar]
- Castro S, Silveira P, Navarro L. Consequences of nectar robbing for the fitness of a threatened plant species. Plant Ecology. 2008;199:201–208. [Google Scholar]
- Castro S, Silveira P, Navarro L. Floral traits variation, legitimate pollination, and nectar robbing in Polygala vayredae (Polygalaceae) Ecological Research. 2009;24:47–55. [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. The effects of cross and self-fertilization in the vegetable kingdom. London: Murray; 1872. [Google Scholar]
- Fenster CB, Cheely G, Dudash MR, Reynolds RJ. Nectar reward and advertisement in hummingbird-pollinated Silene virginica (Caryophyllaceae) American Journal of Botany. 2006;93:1800–1807. doi: 10.3732/ajb.93.12.1800. [DOI] [PubMed] [Google Scholar]
- González PL, Valdivia CE. Direct and indirect effects of nectar robbing on the pollinating behaviour of Patagona gigas (Trochilidae) Biotropica. 2005;37:693–696. [Google Scholar]
- Guitian JJ, Sanchez M, Guitian P. Pollination ecology of Petrocoptis grandiflora Rothm. (Caryophyllaceae): a species endemic to the north west part of the Iberian Peninsula. Botanical Journal of the Linnean Society. 1994;115:19–27. [Google Scholar]
- Higashi S, Ohara HA, Matsuo K. Robber-like pollinators: overwintered queen bumblebees foraging on Corydalis ambigua. Ecological Entomology. 1988;13:411–418. [Google Scholar]
- Inouye DW. The terminology of floral larceny. Ecology. 1980;61:1251–1252. [Google Scholar]
- Inouye DW. The ecology of nectar robbing. In: Bentley B, Elias T., editors. The biology of nectaries. New York: Columbia University Press; 1983. pp. 153–173. [Google Scholar]
- Irwin RE, Brody AK. Nectar-robbing bumblebees reduce the fitness of Ipomopsis aggregata (Polemoniaceae) Ecology. 1999;80:1703–1712. [Google Scholar]
- Irwin RE, Maloof JE. Variation in nectar robbing over time, space, and species. Oecologia. 2002;133:525–533. doi: 10.1007/s00442-002-1060-z. [DOI] [PubMed] [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Niwot, CO: University Press of Colorado; 1993. [Google Scholar]
- Kliber A, Eckert CG. Sequential decline in allocation among flowers within inflorescences: proximate mechanisms and adaptive significance. Ecology. 2004;85:1675–1687. [Google Scholar]
- Klinkhamer PGL, de Jong TJ. Attractiveness to pollinators: a plant's dilemma. Oikos. 1993;66:180–184. [Google Scholar]
- Kreitner GL, Sorensen EL. Stigma development and the stigmatic cuticle of Medicago scutellata. Canadian Journal of Botany. 1985;63:813–818. [Google Scholar]
- Maloof JE. Reproductive biology of a North American subalpine plant: Corydalis caseana A.Gray ssp. brandegei (S.Watson) G.B. Ownbey. Plant Species Biology. 2000;15:281–288. [Google Scholar]
- Maloof JE. The effects of a bumble bee nectar robber on plant reproductive success and pollinator behaviour. American Journal of Botany. 2001;88:1960–1965. [PubMed] [Google Scholar]
- Maloof JE, Inouye DW. Are nectar robbers cheaters or mutualists? Ecology. 2000;81:2651–2661. [Google Scholar]
- Morris WF. Mutualism denied? Nectar-robbing bumble bees do not reduce female or male success of bluebells. Ecology. 1996;77:1451–1462. [Google Scholar]
- Navarro L. Pollination ecology of Anthyllis vulneraria subsp vulgaris (Fabaceae): nectar robbers as pollinators. American Journal of Botany. 2000;87:980–985. [PubMed] [Google Scholar]
- Newman DA, Thomson JD. Effects nectar robbing on nectar dynamics and bumblebee foraging strategies in Linaria vulgaris (Scrophulariaceae) Oikos. 2005;110:309–320. [Google Scholar]
- Newton SD, Hill GD. Robbing of field bean flowers by the short-tongued bumble bee Bombus terrestris L. Journal of Apicultural Research. 1983;22:124–129. [Google Scholar]
- Olesen JM. From naivete to experience: Bumblebee queens (Bombus terrestris) foraging on Corydalis cava (Fumariaceae) Journal of the Kansas Entomological Society. 1996;69:274–286. [Google Scholar]
- Richardson SC. Are nectar-robbers mutualists or antagonists? Oecologia. 2004;139:246–254. doi: 10.1007/s00442-004-1504-8. [DOI] [PubMed] [Google Scholar]
- Roubik DW. The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology. 1982;63:354–360. [Google Scholar]
- Schoen DJ, Lloyd DG. Self-and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. International Journal of Plant Sciences. 1992;153:381–393. [Google Scholar]
- Stout JC, Allen JA, Goulson D. Nectar robbing, forager efficiency and seed set: bumblebees foraging on the self-incompatible plant Linaria vulgaris (Scrophulariaceae) Acta Oecologica. 2000;21:277–283. [Google Scholar]
- Thomson JD. Pollen transport and deposition by bumble bees in Erythronium: influence of floral nectar and bee grooming. Journal of Ecology. 1986;74:329–342. [Google Scholar]
- Waser NM, Real LA. Effective mutualism between sequentially flowering plant species. Nature. 1979;281:670–672. [Google Scholar]
- Wu ZY, Zhuang X, Su ZY. The systematic evolution of Corydalis in relation to florogenesis and floristic regionalization in the world. Acta Botanica Yunnanica. 1996;18:241–267. [Google Scholar]
- Yang HY, Zhou C. Experimental researches on sexual plant reproduction. Wuhan, China: Wuhan University Press; 2001. [Google Scholar]
- Zhang YW, Gituru RW, Wang Y, Guo YH. Nectar robbing of carpenter bee and its effects on the reproductive fitness of Glechoma longituba (Lamiaceae) Plant Ecology. 2007a;193:1–13. [Google Scholar]
- Zhang YW, Wang Y, Guo YH. Effects of nectar robbing on plant reproduction and evolution. Frontiers of Biology in China. 2007b;2:443–449. [Google Scholar]
- Zhang YW, Yang CF, Zhao JM, Guo YH. Selective nectar robbing in a gynodioecious plant (Glechoma longituba) enhances female advantage. Journal of Evolutionary Biology. 2009;22:527–535. doi: 10.1111/j.1420-9101.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Cook S. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. American Midland Naturalist. 1985;113:84–91. [Google Scholar]