Abstract
Background and Aims
Nitric oxide (NO) has been demonstrated to stimulate the activity of nitrate reductase (NR) in plant roots supplied with a low level of nitrate, and to affect proteins differently, depending on the ratio of NO to the level of protein. Nitrate has been suggested to regulate the level of NO in plants. This present study examined interactive effects of NO and nitrate level on NR activity in roots of tomato (Solanum lycocarpum).
Methods
NR activity, mRNA level of NR gene and concentration of NR protein in roots fed with 0·5 mm or 5 mm nitrate and treated with the NO donors, sodium nitroprusside (SNP) and diethylamine NONOate sodium (NONOate), and the NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide (cPTIO), were measured in 25-d-old seedlings.
Key Results
Addition of SNP and NONOate enhanced but cPTIO decreased NR activity in the roots fed with 0·5 mm nitrate. The opposite was true for the roots fed with 5 mm nitrate. However, the mRNA level of the NR gene and the protein concentration of NR enzyme in the roots were not affected by SNP treatment, irrespective of nitrate pre-treatment. Nevertheless, a low rate of NO gas increased while cPTIO decreased the NR activities of the enzyme extracts from the roots at both nitrate levels. Increasing the rate of NO gas further increased NR activity in the enzyme extracts of the roots fed with 0·5 mm nitrate but decreased it when 5 mm nitrate was supplied. Interestingly, the stimulative effect of NO gas on NR activity could be reversed by NO removal through N2 flushing in the enzyme extracts from the roots fed with 0·5 mm nitrate but not from those with 5 mm nitrate.
Conclusions
The effects of NO on NR activity in tomato roots depend on levels of nitrate supply, and probably result from direct interactions between NO and NR protein.
Key words: Nitric oxide, nitrate, nitrate reductase, post-translational regulation, tomato, Solanum lycocarpum
INTRODUCTION
Nitric oxide (NO), a freely diffusible and reactive gaseous compound, has been demonstrated to be an indispensable signal molecule involved in various physiological functions of plants (Lamattina et al., 2003; Neill et al., 2003). For example, NO has been found to improve plants' resistance to various biotic and abiotic stresses (e.g. against pathogens, Lamattina et al., 2003; salinity, Zhao et al., 2007b; antioxidant defence, Zhang et al., 2007) and to regulate organelle functions (e.g. mitochondria, Guo and Crawford, 2005; Gupta et al., 2005; Zemojtel et al., 2006), tissue development (e.g. maturation and senescence, Leshem et al., 1998; seed germination and root development, del Río et al., 2004) and secondary metabolism (e.g. cellulose synthesis, Correa-Aragunde et al., 2008). Recently, it was found that NO positively regulates the activity of nitrate reductase (NR) in roots of Chinese cabbage (Brassica chinensis) grown at 1 mm nitrate (Du et al., 2008).
Nitrate has also been known to regulate NR activity in plants (Crawford, 1995). NR activity was rapidly reduced when plants were subjected to nitrate starvation (Galangau et al., 1988; Kaiser and Huber, 2001) and was strongly induced upon the supply of nitrate (Shaner and Boyer, 1976; Lin et al., 1994), indicating the signalling role of nitrate in the regulation of NR. On the other hand, NR has been shown to be a major source of NO in plants (Yamasaki et al., 1999; Yamasaki and Sakihama, 2000; Meyer et al., 2005; Yamasaki, 2005). Therefore, nitrate concentration in the rooting medium could also affect the endogenous amount of NO in the plants via the mediation of NR activity. Studies on Arabidopsis thaliana and Vicia faba confirmed that the relative and absolute levels of nitrate and nitrite were key determinants in NR-induced production of NO (Vanin et al., 2004).
It has been demonstrated that the effect of NO on proteins is multiple and depends on the ratios between NO and protein levels. For example, during the nitrosylation of haemoglobin by NO, the equilibrium between NO bound to haems and thiols breaks when the initial NO:haemoglobin ratio exceeds a specific range (Gow and Stamler, 1998). Accordingly, the regulatory function of NO on NR might also vary with various levels of nitrate supply due to the alteration of endogenous NO level.
The present study used the NO donors and the NO scavenger to investigate the regulatory effect of NO on NR activity in tomato seedlings and how this regulation would be affected by the level of nitrate supply. We hypothesized that NO could affect NR activity in a plant fed with different levels of nitrate.
MATERIALS AND METHODS
Seeding culture
Seeds of tomato (Solanum lycocarpum ‘Zheza 809’) were germinated in a 0·5 mm CaSO4 solution. Four days after sowing, seedlings were transferred to an aerated hydroponic system containing nutrient solution (pH 6·8) with the following composition (in μm): H3BO3, 10; MnSO4, 0·5; ZnSO4, 0·5, CuSO4, 0·1; (NH4)6Mo7O24, 0·1; Fe-EDTA, 15; KH2PO4, 250; MgSO4, 500; K2SO4, 500; Ca(NO3)2, 500; (NH4)2SO4, 250. After 1 week, seedlings were transferred to the nutrient solutions with two different levels of nitrate: 0·5 mm (considered as the ‘low nitrate’ supply) and 5 mm (designated as the ‘high nitrate’ supply). Other nutrients were the same as above. Plants were grown in a controlled-environment room at a humidity of 60%, with a 12-h light period (photosynthetic photon flux density of 200 µmol m−2 s−1) and a 25°C/20°C light/dark temperature regime.
NO donors and scavenger treatments
Two different types of NO donors, SNP (sodium nitroprusside) and DEA/NONOate (diethylamine NONOate sodium), and the NO scavenger, cPTIO [2-(4-carboxyphenyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide] were used. After 2 weeks of growth at 0·5 mm or 5 mm nitrate, the seedlings were continuously grown in 0·5 mm or 5 mm nitrate nutrient solutions containing 0, 1, 2, 5 or 10 µm of SNP, DEA/NONOate and cPTIO, respectively.
Time-course study
According to the result of the above experiment, 5 µm of SNP and 10 µm of cPTIO were chosen to study the time-course response. The plants pre-cultured in 0·5 mm or 5 mm nitrate for 2 weeks were grown under continuous light conditions in the solution used in that experiment. The solutions were renewed every 3 h. Samples of roots were taken at 0, 3, 6, 12 and 24 h for determination of NR activity.
Determination of NR activity
NR activity was assayed as described by Poonnachit and Darnell (2004). The whole root system was excised from plants, and about 0·4 g of fresh tissue was placed in each test tube (two tubes per replicate per treatment). Five millilitres of assay solution, composed of 2% 1-propanol, 100 mm KH2PO4 (pH 7·5) and 30 mm KNO3, were added to each test tube. One tube per replicate per treatment was immediately filtered through Whatman No. 2 paper and used as the time 0 control. Samples were vacuum-infiltrated for 5 min and incubated in a shaking water bath at 25°C for 30 min in the dark. After incubation, the assay solution with roots was filtered and a 1-mL aliquot from each sample was removed to a new tube. One millilitre of sulfanilamide (1% w/v in 1·5 m HCl) and 1 mL N-(1-naphthyl)-ethylenediaminedihydrochloride (0·02% w/v in 0·2 m HCl) were added. The samples were incubated at room temperature for 30 min. The absorbance at 540 nm was determined with a spectrophotometer. The concentration of nitrite produced by NR in the reaction mixture was calculated by subtraction to the time 0 control. The activity of NR was expressed as μmol of NO2− produced per hour and per gram of fresh weight.
RT-PCR analyses
Root samples were frozen in liquid nitrogen immediately after collection and stored at −80°C. One hundred milligrams of tissues from three different plants were ground in liquid nitrogen and total RNA was isolated by Trizol (Invitrogen), and then first-strand cDNA was synthesized with total RNA by PrimeScript RT reagent kit (Takara). All RNA samples were checked for DNA contamination before cDNA synthesis. The NR mRNA was detected by the SYBR Green RT-PCR kit (Bioer) with NR gene-specific primers (forward primer CAAGCAATCCATCTCCCAT; reverse primer CATCTCTGTATCGTCTTCAGGA) and performed with an ABI 7300 real-time PCR System (Applied Biosystems, Foster City, CA, USA) with the following cycling conditions: 2 min at 94°C, 40 cycles of 94°C for 10 s, 60°C for 15 s, 72°C for 30 s. A pair of housekeeping genes of α-tubulin was used for a control PCR (forward primer CCTGAACAACTCATAAGTGGC; reverse primer AGATTGGTGTAGGTAGGGCG). For each determination of mRNA, three cDNA samples derived from three independent RNA extractions were analysed. All analyses were standard procedures of the PE 7300 detection system.
ELISA immuno-quantification of NR protein in the root
Extraction of soluble proteins and assay of NR protein concentration were conducted according to Leleu and Vuylsteker (2004). Briefly, NR proteins were extracted by 50 mm HEPES–KOH (pH 8·2) buffer containing 0·1 m NaCl, 1 mm EDTA, 5 mm cysteine, 1 µm leupeptin and 5 µm FAD, and then precipitated with 45% (NH4)2SO4. The precipitate was subsequently dissolved in 200 µL of the extraction buffer and quantified by ELISA procedure using a monoclonal mouse antibody directed against Cucurbita maxima (squash) NR and horseradish peroxidase-conjugated goat anti-mouse IgG and measured at 405 nm by an ELISA palter reader (BIO-RAD 680). Total protein was estimated by the Bradford procedure (Bradford, 1976).
Direct effects of NO gas and cPTIO on NR in enzyme extracts
The NR extracts were prepared using root tissues of plants which were pre-cultured in 0·5 mm or 5 mm nitrate for 2 weeks. Briefly, 400 g of roots were blended in 100 mL of 0·1 m HEPES–KOH buffer solution (pH 7·5), 5 mm EDTA, 10 mm cysteine with 5 g of PVPP and 14 mm mercaptoethanol containing anti-protease cocktail. The extract was filtered through Miracloth; 0·1 volume of 1% (w/v) protamine sulfate solution containing 6 mg of Tris mL−1 water was added and the extract was then centrifuged at 30 000 g for 15 min. The supernatant was adjusted to 45% saturation with solid (NH4)2SO4 and the precipitate collected by centrifugation. The pellet was dissolved in 10 mm HEPES–KOH buffer solution (pH 7·5) containing 1 mm EDTA and 1 mm cysteine. The NR was further purified on blue–Sepharose using a procedure described for corn NR (Campbell and Smarrelli, 1978).
To investigate the direct effects of NO gas on NR extracts, pure NO gas was first diluted by N2 to a final concentration of 0·04 µmol L−1, and then 1, 2 or 3 cm3 of the diluted NO gas was injected into the air-tight container containing NR solution. The reaction was initiated by injecting 10 mL of reaction solution containing 50 mm HEPES–KOH (pH 7·5), 100 µm NADH, 5 mm KNO3 and 2 mm EDTA, and shaken at 200 rpm at 25°C for 30 min. In addition, because the NR itself could produce NO via nitrite reduction (Yamasaki et al., 1999; Yamasaki and Sakihama, 2000; Meyer et al., 2005), the NO scavenger, cPTIO at a concentration of 0, 2 and 5 µm, was also directly added to the assay solution for NR determination. After incubation for 30 min with the NR extracts, the reactions of different treatments were stopped by addition of 500 µL of 30% trichloroacetic acid. After the addition of 1% sulfanilamide and 0·02% N-(1-naphtyl)-ethylene-diamine dihydrochloride, the concentration of nitrite in the reaction mixture was determined with a spectrophotometer at 540 nm. NR activities were estimated based on the quantities of NO2− formed by NR in the reaction mixture, which was calculated by subtraction of the negative control. The NR extracts with NR being denatured by trichloroacetic acid were used as the negative control.
To investigate whether the direct effects of NO on NR extracts are reversible, 3 cm3 of 0·04 µmol L−1 NO gas was injected into the air-tight container with NR solution, and shaken at 200 rpm at 25°C for 30 min. Then, the container was opened, flushed with N2 to remove NO gas, and 10 mL of reaction solution added for NR determination. After reacting for 30 min, the reaction was stopped by addition of 500 µL of 30% trichloroacetic acid, and nitrite concentration in the reaction mixture was determined as described above.
In situ measurement of NO in the root
In situ measurement of NO was performed in root tip and non-root tip with fluorescence microscopy (excitation 495 nm, emission 515 nm; Nikon Eclipse E600), using DAF-FM DA (3-amino, 4-aminomethyl-2′,7′-difluorescein, diacetate). Briefly, using a razor blade, the root tip and root segments (about 8 cm from root tip) were excised from the roots of plants that had previously grown in 0·5 mm or 5 mm nitrate for 2 weeks, and were then loaded for 15 min with 5 µm DAF-FM DA, a fluorescent NO indicator dye. The tissue was washed in HEPES–KOH buffer (pH 7·4) for 10 min before examination with a microscope.
Statistics
All statistical analyses were conducted with SAS software (SAS Institute, Cary, NC, USA). Means were compared by t-test at P < 0·05 in all cases.
RESULTS
Effect of NO on NR activity
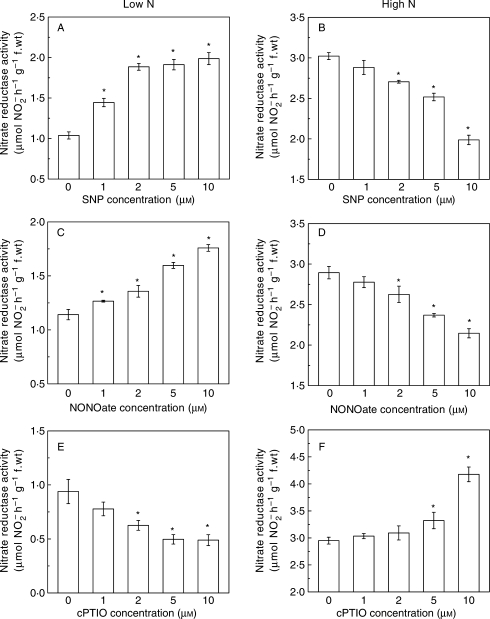
NR activities in roots fed with 0·5 mm nitrate were significantly enhanced by supplying 1–10 µm SNP (Fig. 1A) and NONOate (Fig. 1C) for 3 h. It increased as the concentration of SNP increased up to 2 µm and NONOate up to 10 µm, while it decreased as cPTIO increased (Fig. 1E). On the contrary, the reverse effects of treatments on NR activity were observed in the roots fed with 5 mm nitrate (Fig. 1B, D, F). These results indicate that the regulatory role of NO in NR activity depends on the level of nitrate supply in the rooting medium.
Fig. 1.
The SNP, NONOate and cPTIO-dose responses of nitrate reductase activity in roots of tomato. The seedlings were fed in 0·5 mm (A, C, E) and 5 mm nitrate (B, D, F) for 2 weeks and were then transferred to the nutrient solution containing different concentrations of nitric oxide donor SNP (A, B) or DEA/NONOate (C, D) or cPTIO (E, F). Nitrate reductase activity was measured 3 h after treatment. Error bars show s.d. (n = 5), asterisks denote a significant difference from the control at P < 0·05.
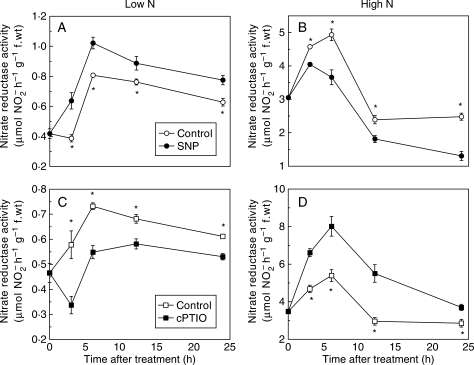
Time-course experiments were carried out to confirm the impact of NO on NR. NR activities in the roots fed with 0·5 mm nitrate were consistently higher in the SNP treatment and lower in the cPTIO treatment than in the control roots during treatment for 24 h (Fig. 2A, C). However, the NR activities in roots fed with 5 mm nitrate were consistently lower in the SNP treatment and higher in the cPTIO treatment compared with the control roots (Fig. 2B, D). Irrespective of treatment, NR activity generally increased with time in the first 6 h and then decreased (Fig. 2).
Fig. 2.
Time course of the effect of SNP and cPTIO on nitrate reductase activity in roots of tomato. The seedlings were fed with 0·5 mm (A, C) and 5 mm nitrate (B, D) for 2 weeks, and were then transferred to the nutrient solution without or with 5 µM SNP or 10 µM cPTIO under continuous light conditions. Nitrate reductase activity was measured at 0, 3, 6, 12 and 24 h. Error bars show the s.d. (n = 5), asterisks denote a significant difference from the control at P < 0·05.
Effect of NO on NR gene expression and NR protein accumulation
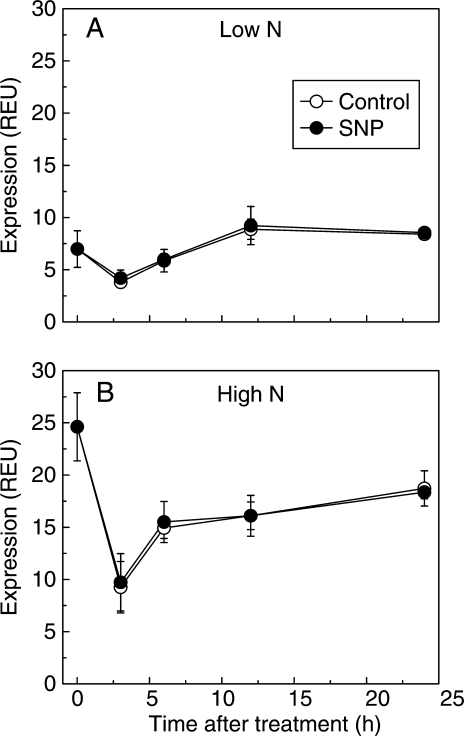
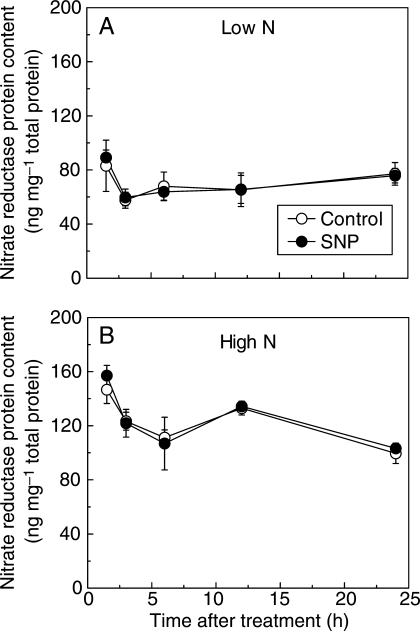
The molecular mechanism underlying the effect of NO on NR regulation in roots was also investigated. The SNP did not modify the mRNA levels of NR genes in the roots fed with 0·5 mm or 5 mm nitrate during the 24-h treatment. Irrespective of SNP treatment, the mRNA levels of NR genes decreased in the first 3 h and increased to maximum at 12 h for the roots fed with 0·5 mm nitrate and at 24 h for the roots fed with 5 mm nitrate (Fig. 3). In addition, although the concentration of NR protein fluctuated due to the photoperiod, it was not affected by SNP incubation in the root bathing solution containing 0·5 mm or 5 mm nitrate during treatment for 24 h (Fig. 4). The results indicate that NO regulates NR activity in the roots fed with different levels of nitrate at the post-translational level.
Fig. 3.
Time course of the effect of SNP on mRNA level of nitrate reductase in roots of tomato. The seedlings were fed with 0·5 mm (A) and 5 mm nitrate (B) for 2 weeks, and were then transferred to the nutrient solution without or with 5 µm SNP under continuous light conditions. The mRNA level of nitrate reductase was analysed at 0, 3, 6, 12 and 24 h after treatment. Relative mRNA levels were calculated and normalized with respect to α-tubulin mRNA (= 100 REU). Error bars represent the s.d (n = 6); no significant differences from the control were observed.
Fig. 4.
Time course of the effect of SNP on nitrate reductase protein content in roots of tomato. Two-week-old seedlings were grown with 0·5 mm (A) and 5 mm (B) nitrate for 2 weeks, and were then transferred to the nutrient solution without or with 5 µm SNP under continuous light conditions. Nitrate reductase protein content was measured with ELISA at 1·5, 3, 6, 12 and 24 h after treatment. Error bars represent the s.d. (n = 6); no significant differences from the control were observed.
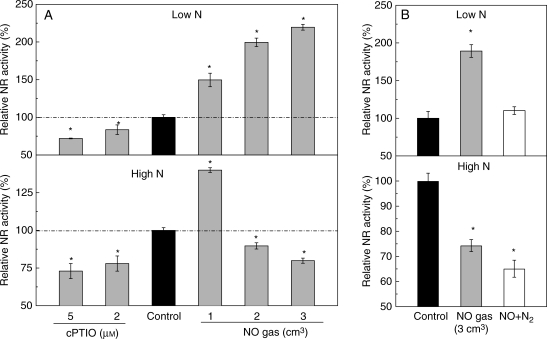
Direct effect of NO on NR activity
Because the concentration of NR protein was not modified by NO, the direct effect of NO on NR activity of the enzyme extracts was investigated. The NR activity of enzyme extracted from the roots fed with 0·5 mm nitrate was consistently increased along with the application rate of NO gas, and decreased with increasing concentrations of cPTIO (Fig. 5A). Unlike NR activity in fresh root samples, NR activity of the enzyme extracted from the roots fed with 5 mm nitrate was reduced with increasing concentration of cPTIO, and increased by a low rate of NO gas (1 cm3). However, further increasing the rate of NO gas resulted in significant inhibition of NR activity in the enzyme extracts (Fig. 5A). Interestingly, at the high rate of NO gas (3 cm3), the stimulative effect of NO on NR extracted from the roots fed with 0·5 mm nitrate could be reversed by NO removal through N2 flushing, whereas the inhibitory effect of NO on NR extracted from the roots fed with 5 mm nitrate could not be reversed by the same treatment (Fig. 5B).
Fig. 5.
Direct effects of NO on the nitrate reductase activity of tomato: (A) effects of NO gas and cPTIO on the nitrate reductase activity of enzyme extracts; (B) the reversible or irreversible effect of NO gas on the nitrate reductase activity of enzyme extracts. The enzyme was extracted from the roots fed with 0·5 (Low N) or 5 (High N) mm nitrate. The mixtures of enzyme extract and assay solution were directly incubated without (control) or with 1, 2 or 3 cm3 NO gas (0·04 µmol L−1) or 2 mm or 5 µm cPTIO. For the investigation of reversible or irreversible effect, the enzyme extract was pre-treated with 3 cm3 NO gas for 30 min, and then flushed with N2 and added assay solution (NO + N2) to initiate the enzyme reaction. The concentrations of NO2− in the mixtures were measured 30 min after enzyme reaction, and the enzyme activities were then calculated. Error bars show the s.d. (n = 5), asterisks denote a significant difference from the control at P < 0·05.
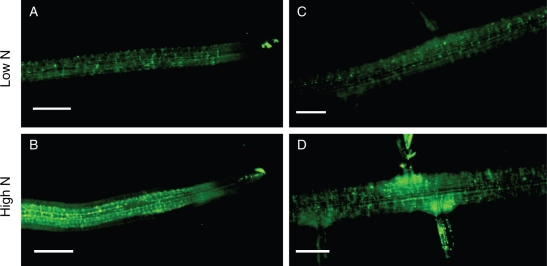
Effect of nitrate level on NO accumulation in roots
As NR is one of the major sources of NO synthesis in plants, NO concentrations were analysed in roots cultivated with different nitrate levels by fluorescent NO indicator dye DAF-FM DA. As shown in Fig. 6, the NO concentration in root tips and root segments of the plants fed with 5 mm nitrate was obviously higher than that fed with 0·5 mm nitrate.
Fig. 6.
The concentration of NO (brightness) in tomato roots fed with 0·5 mm (A, C) and 5 mm (B, D) nitrate. (A, B) The root tips, (C, D) the non-root tip regions (about 8 cm from root tip). The NO concentration was detected by fluorescence microscopy with DAF-FM DA (diaminofluorescein-FM diacetate). Scale bars = 1 mm.
DISCUSSION
In higher plants, reduction of nitrate to nitrite by the NR enzyme is the first committed step in the nitrate assimilation pathway (Buchanan et al., 2000). Therefore, regulation of NR plays a key role in nitrate assimilation. This present study showed that the addition of the NO donors, SNP and NONOate, stimulated and NO scavenger, cPTIO, inhibited NR activity of tomato roots fed with 0·5 mm nitrate, whereas the reverse was true for the roots fed with 5 mm nitrate (Figs 1 and 2), suggesting that NO mediates the NR activity in plant roots differently, depending on the level of nitrate supply.
It has been suggested that NR is regulated by signals at the transcriptional or post-translational level (Buchanan et al., 2000). In the present research, it was found that the mRNA levels of NR genes of the roots fed with both 0·5 mm and 5 mm nitrate were reduced at the beginning of the 24-h light regime, and then increased (Fig. 3). This photoperiod-induced change is similar to the result found in maize roots treated with a long light regime (Bowsher et al., 1991). In addition, the concentration of NR protein also fluctuated due to the photoperiod (Fig. 4). However, as shown in Figs 3 and 4, the mRNA levels of the NR gene and accumulations of NR protein in the roots fed with both 0·5 mm and 5 mm nitrate were not affected by the exogenous NO donors. Furthermore, the NO scavenger cPTIO decreased, but the NO donors increased the NR activity of the enzyme extracts where the NR was isolated and the transcription and translation were interrupted (Fig. 5A). Therefore, we conclude that the regulatory effect of NO on NR activity occurs at the post-translational level.
The effect of NO and cPTIO on NR activity in the NR protein extracts was inconsistent with the effect on NR activity in the roots fed with 5 mm nitrate (Figs 1 and 5A). Such a discrepancy might be due to the difference in basal NO levels between plant roots and the NR protein extracts. In the intact roots, NO can be easily visualized by fluorescent indicator dye DAF-FM DA, and the basal NO level in the 5 mm nitrate treatment was much higher than that in the 0·5 mm nitrate treatment (Fig. 6). In comparison, the basal level of NO was not detectable in the NR protein extracts using the DAF-FM DA dye (data not shown; following the method of Graziano and Lamattina, 2007). Clearly, the concentration of NO in the NR protein extracts was far lower than that in the intact roots. It has been demonstrated that the effect of NO on proteins is multiple and depends on the ratios between NO and protein (Gow and Stamler, 1998). Therefore, the difference in NO concentration between the roots fed with 0·5 mm or 5 mm nitrate might also be the reason why NO regulated NR activity differently when different levels of nitrate were supplied. This deduction was further implied by the finding that increasing the amount of added NO gas significantly inhibited NR activity of the enzyme extracted from the roots fed with 5 mm nitrate (Fig. 5A).
It has been demonstrated that NO could have a reversible or an irreversible effect on proteins. For example, the activities of tobacco catalase and ascorbate peroxidase were reversibly inhibited by NO (Clark et al., 2000). In contrast, the inhibitory effect of NO on the hydroxylation activity of rat cytochrome P450 was irreversible (Minamiyama et al., 1997). Here, it was found that the stimulative effect of NO on NR activity of the enzyme extracted from the roots fed with 0·5 mm nitrate could be quickly reversed after NO removal, whereas the inhibitory effect of NO on NR activity of the enzyme extracted from the roots fed with 5 mm nitrate was irreversible (Fig. 5B). The actual mechanism of how NO directly affects NR protein warrants further investigation at the biochemical level.
Another question which remains open is why the NO regulates NR activity differently in different nitrate growth conditions. In the present study, 0·5 mm nitrate in the growth solution did not meet the requirement for optimal growth of tomato. Subsequently, the plants need to increase NR activity to enhance nitrate assimilation, which may be the reason why NO enhanced NR activity in the low-nitrate condition. However, there appears to be a logical problem for this explanation as NO concentration was lower in the roots of 0·5 mm than 5 mm nitrate-fed plants (Fig. 6). This contradictory observation may be explained by the following: the nitrate level used in the present research is not extremely low, while it has been demonstrated that the NO level in roots fed with extremely low nitrate (0·01 mm) was also increased (Zhao et al., 2007a). Hence, it can be predicted that the NO level in the root would increase when the level of actual nitrate supply was both lower and higher than the level for optimal plant growth. The deduction is similar to that of another element essential for nutrition in plants – iron (Fe) – of which it has been demonstrated that deficiency and excessive supply can both result in an increase in NO levels in plants (Arnaud et al., 2006; Graziano and Lamattina, 2007). In Fe-deficient conditions, the increased NO enhances the systems related to Fe uptake (Graziano and Lamattina, 2007), while it avoids oxidative stress by mediating ferritin gene expression in excessive Fe conditions (Arnaud et al., 2006).
On the other hand, although NO is an indispensable signal molecule involved in various physiological functions, high levels of NO are toxic to plants (Shapiro, 2005). The toxic dose of NO to plants is highly species specific. For example, cultured cells of soybean showed no increase in cell death upon exposure to 500 µm SNP (Delledonne et al., 1998), whereas 1 µm SNP was sufficient to induce cell death in haploid Pacific yew (Taxus brevifolia) cultures (Pedroso and Magalhaes, 2000). Tomato plants have been found to be more sensitive to the presence of NO in the atmosphere than other species (Capron and Mansfield, 1976). In addition, it was found that the gene expression of haemoglobin, an endogenous NO scavenger (Perazzolli et al., 2006), was rapidly induced when the nitrogen-deficient tomato plants were transferred to the growth medium containing 4·8 mm nitrate (Wang et al., 2003), which is similar to the nitrate level (5 mm) used in the present research, implying the necessity of NO scavenging in this nitrate condition. In this present study, it was found that the concentration of NO in tomato roots fed with 5 mm was obviously higher than those fed with 0·5 mm (Fig. 6). Therefore, the inhibitory function of NO on NR activity in the high-nitrate treatment may be beneficial for tomato plants to alleviate NO toxicity by reducing NO synthesis from NR. This hypothesis will be the focus of future research.
Conclusions
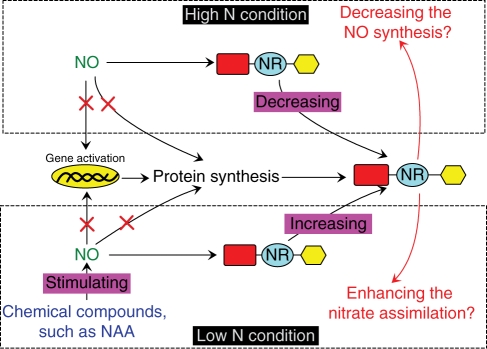
The present study demonstrated that NO could differentially regulate NR activity at the post-translational level. Besides, in previous research, it has been demonstrated that NO was also the downstream signal of some chemical compounds, such as NAA (naphthaleneacetic acid), in NR activity stimulation when the nitrate supply is relatively low (1 mm) (Du et al., 2008). Taking the results demonstrated in the present and previous studies together, we propose the following model to summarize the role of NO in regulating NR activity (Fig. 7). When the nitrate supply is relatively low, NR activity is stimulated by the basal endogenous NO or the NO induced by chemical compounds via a post-translational regulatory pathway. However, when nitrate is supplied at high levels, NR activity is also inhibited by basal endogenous NO via a post-translational regulatory pathway. The functions of this differential regulation mechanism of NO on NR in plant physiological processes remain unknown, and future research is warranted to elucidate them.
Fig. 7.
Model proposed for the effect of NO on nitrate reductase activity (NRA). In low-level nitrate supply conditions, NRA was stimulated by the basal endogenous NO or the NO induced by chemical compounds via a post-translational regulatory pathway. However, in high-level nitrate supply conditions, the NRA was inhibited by the basal endogenous NO also via a post-translational regulatory pathway. The role of the physiological processes on nitrate assimilation and NO synthesis in conditions of low and high levels of nitrate supply speculated on above should be further studied. A red ‘ × ’ in the figure denotes that the pathway is not acted on by NO, and ‘NR’ denotes the enzyme protein of nitrate reductase.
ACKNOWLEDGEMENTS
This work was financially supported by the State Key Development Program for Basic Research of China (grant nos. 2009CB119003 and 2007CB109305), the Natural Science Foundation of China (NSFC, nos. 30871590), the Major Research Program of Zhejiang Province (no. 2008C12061-1), and China Postdoctoral Science Foundation funded project (grant no. 20080440204).
LITERATURE CITED
- Arnaud N, Murgia I, Boucherez J, Briat JF, Cellier F, Gaymard F. An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. Journal of Biological Chemistry. 2006;281:23579–23588. doi: 10.1074/jbc.M602135200. [DOI] [PubMed] [Google Scholar]
- Bowsher CG, Long DM, Oaks A, Rothstein SJ. Effect of light/dark cycles on expression of nitrate assimilatory genes in maize shoots and roots. Plant Physiology. 1991;95:281–285. doi: 10.1104/pp.95.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and molecular biology of plants. Rockville, MD: American Society of Plant Physiologists; 2000. [Google Scholar]
- Campbell WH, Smarrelli J. Purification and kinetics of higher plant NADH:nitrate reductase. Plant Physiology. 1978;61:611–616. doi: 10.1104/pp.61.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron TM, Mansfield TA. Inhibition of net photosynthesis in tomato in air polluted with NO and NO2. Journal of Experimental Botany. 1976;27:1181–1186. [Google Scholar]
- Clark D, Durner J, Navarre DA, Klessig DF. Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Molecular Plant–Microbe Interactions. 2000;13:1380–1384. doi: 10.1094/MPMI.2000.13.12.1380. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Lombardo C, Lamattina L. Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytologist. 2008;179:386–396. doi: 10.1111/j.1469-8137.2008.02466.x. [DOI] [PubMed] [Google Scholar]
- Crawford NM. Nitrate: nutrient and signal for plant growth. The Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Xia YJ, Dixon R, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Du ST, Zhang YS, Lin XY, Wang Y, Tang CX. Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.) Plant, Cell & Environment. 2008;31:195–204. doi: 10.1111/j.1365-3040.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- Galangau F, Daniel-Vedele F, Moureaux T, Dorbe MF, Leydecker MT, Caboche M. Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiology. 1988;88:383–388. doi: 10.1104/pp.88.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM. Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. The Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Stoimenova M, Kaiser WM. In higher plants, only root mitochondria, but not leaf mitochondria reduce nitrite to NO, in vitro and in situ. Journal of Experimental Botany. 2005;56:2601–2609. doi: 10.1093/jxb/eri252. [DOI] [PubMed] [Google Scholar]
- Kaiser WK, Huber SC. Post-translational regulation of nitrate reductase: mechanism, physiological relevance and environmental triggers. Journal of Experimental Botany. 2001;52:1981–1989. doi: 10.1093/jexbot/52.363.1981. [DOI] [PubMed] [Google Scholar]
- Lamattina L, García-Mata, Graziano M, Pagnussat G. Nitric oxide: the versatility of an extensive signal molecule. Annual Review of Plant Biology. 2003;54:109–136. doi: 10.1146/annurev.arplant.54.031902.134752. [DOI] [PubMed] [Google Scholar]
- Leleu O, Vuylsteker C. Unusual regulatory nitrate reductase activity in cotyledons of Brassica napus seedlings: enhancement of nitrate reductase activity by ammonium supply. Journal of Experimental Botany. 2004;55:815–823. doi: 10.1093/jxb/erh088. [DOI] [PubMed] [Google Scholar]
- Leshem YY, Wills RBH, Ku VVV. Evidence for the function of the free radical gas-nitric oxide (NO) as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiology and Biochemistry. 1998;36:825–833. [Google Scholar]
- Lin Y, Hwang CF, Brown JB, Cheng CL. 5′-Proximal regions of Arabidopsis nitrate reductase genes direct nitrate-induced transcription in transgenic tobacco. Plant Physiology. 1994;106:477–484. doi: 10.1104/pp.106.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Lea US, Provan F, Kaiser WM, Lillo C. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynthesis Research. 2005;83:181–189. doi: 10.1007/s11120-004-3548-3. [DOI] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, Inoue M. Irreversible inhibition of cytochrome P450 by nitric oxide. The Journal of Pharmacology and Experimental Therapeutics. 1997;283:1479–1485. [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytologist. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Pedroso MC, Magalhaes JR. Nitric oxide induces cell death in Taxus cells. Plant Science. 2000;157:173–180. doi: 10.1016/s0168-9452(00)00278-8. [DOI] [PubMed] [Google Scholar]
- Perazzolli M, Romero-Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. Journal of Experimental Botany. 2006;57:479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- Poonnachit U, Darnell R. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Annals of Botany. 2004;93:399–405. doi: 10.1093/aob/mch053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Río LA, Corpas FJ, Barroso JB. Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry. 2004;65:783–792. doi: 10.1016/j.phytochem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Shaner DL, Boyer JS. Nitrate reductase activity in maize (Zea mays L.) leaves. I. Regulation by nitrate flux. Plant Physiology. 1976;58:499–504. doi: 10.1104/pp.58.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AD. Nitric oxide signaling in plants. Vitamins and Hormones. 2005;72:339–398. doi: 10.1016/S0083-6729(05)72010-0. [DOI] [PubMed] [Google Scholar]
- Vanin AF, Svistunenko DA, Mikoyan VD, et al. Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. Journal of Biological Chemistry. 2004;279:24100–24107. doi: 10.1074/jbc.M312601200. [DOI] [PubMed] [Google Scholar]
- Wang YH, Kochian LV, Doyle JJ, Garvin DF. Two tomato non-symbiotic haemoglobin genes are differentially expressed in response to diverse changes in mineral nutrient status. Plant, Cell & Environment. 2003;26:673–680. [Google Scholar]
- Yamasaki H. The NO world for plants: achieving balance in an open system. Plant, Cell & Environment. 2005;28:78–84. [Google Scholar]
- Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Letters. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends in Plant Science. 1999;4:128–129. doi: 10.1016/s1360-1385(99)01393-x. [DOI] [PubMed] [Google Scholar]
- Zemojtel T, Fröhlich A, Palmieri MC, et al. Plant nitric oxide synthase: a never-ending story? Trends in Plant Science. 2006;11:524–525. doi: 10.1016/j.tplants.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Jiang MY, Zhang JH, et al. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytologist. 2007;175:36–50. doi: 10.1111/j.1469-8137.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- Zhao DY, Tian QY, Li LH, Zhang WH. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. Annals of Botany. 2007a;100:497–503. doi: 10.1093/aob/mcm142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Tian QY, Zhang WH. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiology. 2007b;144:206–217. doi: 10.1104/pp.107.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]