Abstract
Background and Aims
The Marantaceae (550 spp.) is the most derived family in the order Zingiberales and exhibits a complex explosive pollination mechanism. To understand the evolutionary significance of this unique process of pollen transfer, comparative morphological and ecological studies were conducted in Gabon.
Methods
During a total stay of 11 months, 31 species of Marantaceae were investigated at different sites in Gabon. The study included analyses of floral diversity, observations on the pollinator spectrum as well as ecological measurements (e.g. nectar sugar concentration and volume).
Key Results
Analyses reveal five flower types based on flower size and pigmentation, spatial arrangement of the floral tube and presence/absence of nectar guides and conspicuous outer staminodes. Each type is associated with a specific functional pollinator group leading to the description of distinct pollination syndromes. The ‘small (horizontal)’ flowers are predominantly pollinated by small bees (Thrinchostoma spp., Allodapula ornaticeps), the ‘large (horizontal)’ and ‘medium-sized (horizontal)’ flowers by medium-sized bees (Amegilla vivida, Thrinchostoma bicometes), the ‘locked (horizontal)’ flowers by large bees (Xylocopa nigrita, X. varipes) and the ‘(large) vertical’ flowers by sunbirds.
Conclusions
The longevity of Marantaceae individuals and the omnipresence of their pollinators allowed the specialization to a given functional pollinator group. Intermediate ecological values, however, make occasional pollinator overlaps possible, indicating potential pathways of pollinator shifts. Similar radiation tendencies observed on other continents hint at similar selective pressures and evolutionary constraints.
Key words: Africa, floral diversity, functional pollinator groups, Gabon, Marantaceae, nectar, plant–animal interaction, pollination syndromes
INTRODUCTION
The Marantaceae are characterized by a unique pollination mechanism including proterandry, secondary pollen presentation and an irreversible style movement (Claßen-Bockhoff, 1991; Yeo, 1993; Kennedy, 2000; Locatelli et al., 2004). They are a pantropically distributed family of perennial herbs and lianas from the order Zingiberales (Kress, 1995). Flowers are asymmetrical and attract their pollinators with conspicuous staminodes and nectar. Still in the bud, the pollen is deposited onto the back of the ‘head’ of the style (Kennedy, 1978; Claßen-Bockhoff and Heller, 2008a). The style is then held under tension by the hooded staminode (Pischtschan and Claßen-Bockhoff, 2008). When the pollinator touches the trigger-like appendage of the hooded staminode, the style springs forward, scrapes off the pollen from the pollinator and places its own pollen onto the pollinator's mouth parts or into the proboscidial fossae. Each flower is open for a single day, having just one chance of being pollinated and dispersing its own pollen since the explosive pollination mechanism cannot be reset (Kunze, 1984; Claßen-Bockhoff, 1991; Kennedy, 2000).
In view of the high species number in Marantaceae (about 550 species, 31 genera; Andersson, 1998) compared with the low number in their sister group, the Cannaceae (ten species, one genus; Kubitzki, 1998), it is likely that the peculiar pollen transfer mechanism might have enhanced speciation in the family (Kennedy, 2000). However, too little is known about the pollination biology and sexual reproduction in the Marantaceae to reconstruct the evolutionary significance of the explosive style movement.
The least is known about the African species where not a single pollinator observation has been reported until now. Pollinator observations from the new world recorded small, medium-sized and large bee species and hummingbirds (Kennedy, 2000; Locatelli et al., 2004; Leite and Machado, 2007; Claßen-Bockhoff and Heller, 2008b) and in Asia Marantaceae are pollinated by small, medium-sized and large solitary bees (Halictidae, Amegilla, Xylocopa; Claßen-Bockhoff, 1991; Kato, 1996; Momose et al., 1998; Kennedy, 2000; Clausager and Borchsenius, 2003) and probably moths (assumed in Cominsia spp.).
In Africa there are approx. 40 species which fall into six separate clades, the basal Sarcophrynium clade (approx. 15 spp.), the more derived genus Marantochloa (approx. 16 spp., including Ataenidia) and four small clades with only one to three species each (Afrocalathea, Halopegia, Haumania, Thalia) (Dhetchuvi, 1996; Prince and Kress, 2006). The species are dominant in the understorey of lowland rainforests (‘Marantaceae forest’; Letouzey, 1968; De Foresta, 1990) where they often form large, probably purely clonal, stands (Dhetchuvi, 1996; Brncic, 2003). The geographic range extends from Senegal (West Africa) to Kenya (East Africa) and Madagascar. The centre of diversity is in Gabon with about 35 species (approx. 85%) that have highly overlapping distributional ranges (Dhetchuvi, 1996).
The present paper analyses the plant–pollinator interactions in African Marantaceae based on data collected during comprehensive field studies in Gabon. It represents the first survey on the pollination biology of about 30 of the 40 African Marantaceae, including species from all African genera (except Thalia). It provides information about floral diversity and attractants and the adaptation to different functional pollinator groups.
MATERIALS AND METHODS
Research site
Field investigations were conducted in Gabon, first from October 2004 to January 2005 during an explorative round trip to different sites of evergreen rainforest (Dibouka/Lastoursville, Libreville, Lope, Makokou, Mayumba, Mikongo, Sindara/Waka, Tchimbele/Monts de Cristal), then from October 2005 to January 2006 at the biological station Ipassa (0°31′N, 12°48′E) near Makokou and finally from October to December 2006 in Tchimbele (0°36·8′N, 10°24·0′E) in the Monts de Cristal mountain range. In Makokou and Monts de Cristal there are marked seasons, two rainy seasons (September to December and March to May) and two dry seasons (December to February and June to September; Leroux, 1975; Saint-Vil, 1977; Davis et al., 1994; Vande wegh, 2004).
Plant material
In total, 31 species were investigated of which 29 were encountered in Gabon and two exclusively in the greenhouse at Mainz University, Germany (Table 1). Sixteen species of Marantaceae were studied at Makokou (Ma), 18 species in the Monts de Cristal area (MC). Some species occurred in both locations. In total five new species have been identified (Hypselodelphys sp.1 nov., Marantochloa sp.1 nov., M. sp.2 nov., M. sp.3 nov., Thaumatococcus sp.1 nov.; A. C. Ley, unpubl. res.). Additional data of further species was obtained from individuals at Dibouka, Lope and Sibang.
Table 1.
List of species included in the present paper
| Species | Abbreviation | Site | Growth habit | Voucher |
|---|---|---|---|---|
| Afrocalathea rhizantha K. Schum. | Afro rhiz | Ma, MC | Perennial herb | Ley 7, 58, 164, 184, 214, 251, 263 (LBV, WAG) |
| Ataenidia conferta (Benth. in Benth. Hook f.) Milne-Redh. | Atae conf | Ma | Perennial herb | Ley 159 (LBV, WAG) |
| Halopegia azurea K. Schum. | Halo azur | Ma, MC | Perennial herb | Ley 24, 31, 43, 59, 68, 110, 138, 153, 165, 188, 274, 310 (LBV, WAG) |
| Haumania danckelmanniana (J. Br. & K. Schum.) Milne-Redh. | Haum danc | Ma, MC | Liana | Ley 2, 4, 6, 60, 62, 162 (LBV, WAG) |
| Haumania liebrechtsiana (De Wild. & Th. Dur.) J.Leonard | Haum lieb | MC | Liana | Ley 50, 51, 52, 53 (LBV, WAG) |
| Hypselodelphys hirsuta (Loes.) Koechlin | Hyps hirs | Ma, MC | Liana | Ley 156, 167, 174, 269 (LBV, WAG) |
| Hypselodelphys poggeana (K. Schum.) Milne-Redh. | Hyps pogg | Ma | Liana | Ley 168, 169 (LBV, WAG) |
| Hypselodelphys scandens Louis & Mullenders | Hyps scan | Ma | Liana | Ley 160 (LBV, WAG) |
| Hypselodelphys violacea (Ridley) Milne-Redh. | Hyps viol | Si | Liana | Ley 28 (LBV, WAG) |
| Hypselodelphys sp.1 nov. | Hyps sp.1 nov. | Lo | Liana | Ley 141, 125 (LBV, WAG) |
| Marantochloa congensis (K. Schum.) J.Leonard Mullenders | Mara cong | Lo, Ma, MC | Perennial herb | Ley 3, 18, 157, 163, 166, 240, 243 (LBV, WAG) |
| Marantochloa cordifolia (K. Schum.) Koechlin | Mara cord | Ma, MC | Perennial herb | Ley 170, 173 (LBV, WAG) |
| Marantochloa filipes (Benth. in Hook.) Hutch. | Mara fili | MC, Si | Perennial herb | Ley 30, 262 (LBV, WAG) |
| Marantochloa incertifolia Dhetchuvi | Mara ince | Ma, MC | Perennial herb | Ley 179, 182, 236, 248 (LBV, WAG) |
| Marantochloa leucantha (K. Schum.) Milne-Redh. | Mara leuc | MZ | Perennial herb | Ley 66 (LBV, WAG) |
| Marantochloa mannii (Benth.) Milne-Redh. | Mara mann | MZ | Perennial herb | Living collection, University of Mainz |
| Marantochloa monophylla (K. Schum.) D'Orey | Mara mono | Ma, MC | Perennial herb | Ley 183, 191, 197, 198, 203, 209, 217, 238, 249, 270 (LBV, WAG) |
| Marantochloa purpurea (Ridley) Milne-Redh. | Mara purp | Ma, Lo | Perennial herb | Ley 102, 140, 180 (LBV, WAG) |
| Marantochloa sp. 1 nov. | Mara sp. 1 nov. | MC | Perennial herb | Ley 268 (LBV, WAG) |
| Marantochloa sp. 2 nov. | Mara sp. 2 nov. | MC | Perennial herb | Ley 190, 194, 195, 204, 205, 207, 212, 235, 267 (LBV, WAG) |
| Marantochloa sp. 3 nov. | Mara sp. 3 nov. | MC | Perennial herb | Ley 256 (LBV, WAG) |
| Megaphrynium gabonense Koechlin | Mega gabo | Ma | Perennial herb | Ley 1, 9, 10, 11, 14, 23 (LBV, WAG) |
| Megaphrynium macrostachyum (Benth.) Milne-Redh. | Mega macr | MC, Si | Perennial herb | Ley 186, 260 255 (LBV, WAG) |
| Megaphrynium trichogynum Koechlin | Mega tric | Ma, MC, Di | Perennial herb | Ley 5, 22, 187, 221, 264, 271 (LBV, WAG) |
| Sarcophrynium brachystachyum (Benth.) K. Schum. | Sarc brac | MC | Perennial herb | Ley 189, 192, 193, 199, 211, 219, 220, 233 (LBV, WAG) |
| Sarcophrynium prionogonium (K. Schum.) K. Schum. | Sarc prio | MC | Perennial herb | Ley 55, 200, 208, 222, 223, 226, 227, 241, 244, 247, 265 (LBV, WAG) |
| Sarcophrynium schweinfurthianum (Kuntze) Milne-Redh. | Sarc schw | Ma | Perennial herb | Ley 224, 225, 232, 245, 246 (LBV, WAG) |
| Thalia geniculata L. | Thal geni | MZ | Perennial herb | living collection University of Mainz |
| Thaumatococcus daniellii (Benn.) Benth. | Thau dani | Di | Perennial herb | Ley 69, 96 (LBV, WAG) |
| Thaumatococcus sp.1 nov. | Thau sp.1 nov. | MC | Perennial herb | Ley 56, 201, 202, 218 (LBV, WAG) |
| Trachyphrynium braunianum (K. Schum.) Baker | Trach brau | Ma, Lo | Liana | Ley 103, 171, 172 (LBV, WAG) |
Di, Dibouka; Lo, Lope; Ma, Makokou; MC, Monts de Cristal; Mi, Mikongo; Si, Sibang – all in Gabon; MZ, Mainz greenhouse in Germany.
Marantaceae are perennial herbs and lianas with rhizomes (Table 1). Therefore it is highly difficult to identify genetically different individuals in the field. From the plagiotropic rhizomes groups of shoot systems and long petiolated leaves arise in distinct units which are called ‘individual unit’ here and to which they are referred in the present paper.
Species identification is based on available keys (Koechlin, 1964, 1965; Dhetchuvi, 1996), type descriptions and comparison with type specimens in various herbaria (BR, K, LBV, P, WAG). Vouchers are deposited at the National Herbarium in Libreville (LBV), Gabon, and at the Herbarium Vadense in Wageningen (WAG), The Netherlands (Table 1). Inflorescences and flowers were fixed in 70% ethanol for microscopic investigations. Photographs of inflorescences, flowers and flower details were taken on fresh material in the field with a Nikon Coolpix 995.
Floral diversity and attractants
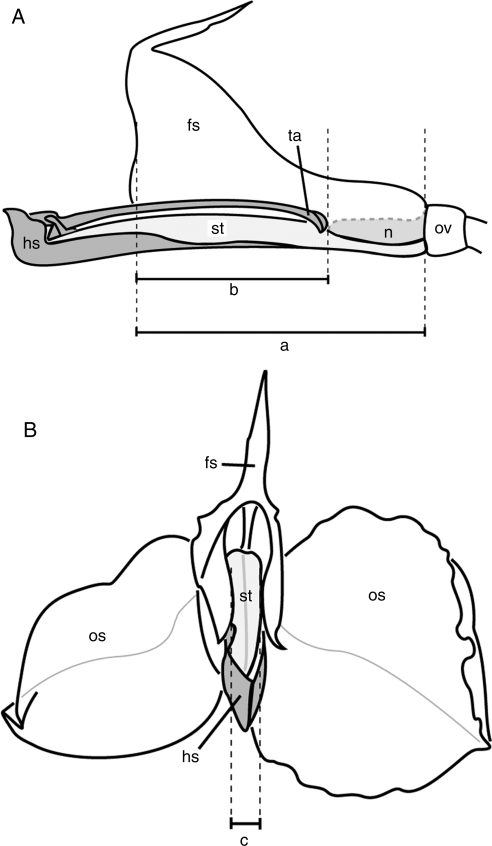
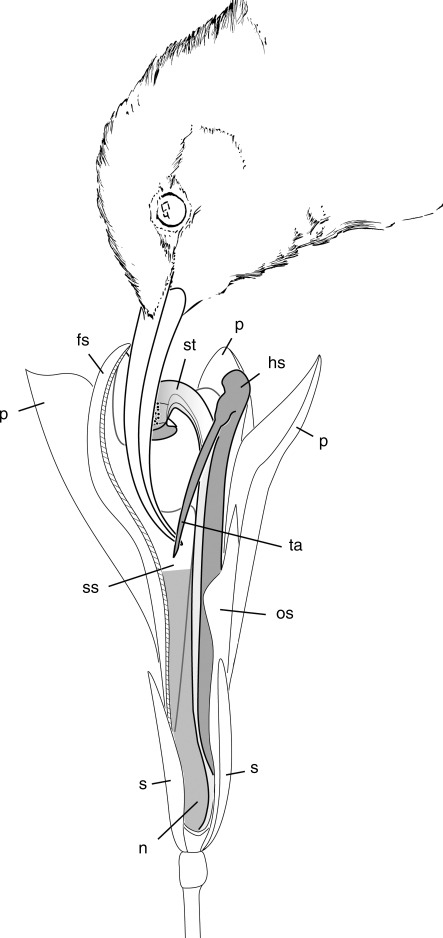
The length of the fleshy staminode (Fig. 1, a), the distance between flower entrance and nectar (b) and the width of the flower entrance (c) are of special importance for matching size of flower and pollinator. These length measurements were taken for each species on up to ten fresh flowers of different individuals with a millimetre-grid paper. To determine the distances from the flower entrance to the tip of the trigger the fleshy staminode was removed with a razor blade. This also allowed of the nectar level to be determinated. Additionally, flower colour was recorded.
Fig. 1.
Flower morphometric data exemplified on Hypselodelphys hirsuta: (A) lateral view; (B) frontal view fertile theca with appendage, sepals and petals removed. a, Length of fleshy staminode; b, distance between flower entrance and nectar; c, width of flower entrance. hs, hooded staminode (dark grey); fs, fleshy staminode; n, nectar (medium grey); os, outer staminode; ov, ovary; st, style (light grey); ta, trigger appendage.
Nectar concentration and volume was generally measured in the morning between 0600 h and 1000 h on about ten untriggered bagged flowers of different individuals per species. Nectar concentration was determined with an Eclipse refractometer (0–50%) especially designed for small quantities. The volume of the nectar per flower was determined by extracting subsequently all the nectar present with SIGMA microcapillary tubes (1 µL, 2 µL) and adding up the extracted volumes.
Pollinators
To determine the pollinators of each species, direct observations were conducted on untriggered flowers. Only animals that are able to trigger the flower were considered as potential pollinators. The observations were conducted for 8–42 h per species (in total 500·75 h of observation) preferentially at different times of the day, at different sites (Makokou, Mikongo, Monts de Cristal and Sibang/Libreville) and in consecutive years. For each visit, the time of the day, the visitor species, its time spent in the observed plant population, the duration and mode of flower-handling, the flowering stage of the flowers (bud, flower open or wilted) and whether the style was released or unreleased were recorded. From the observed visits over several days the average visitation rate, i.e. the number of visits per visitor species per plant population per hour was calculated. As the pollinators often but not always visited all open flowers in a row this is a slight over-estimation of the visitation frequency per flower.
As far as possible, the interactions between pollinators and flowers were documented on video tape with a digital video camera (Sony DCR-TRV30E). The videos are deposited at the Institut für Spezielle Botanik und Botanischer Garten, Johannes Gutenberg-Universität Mainz, Germany. They are the basis for the estimated average flower handling time per insect and Marantaceae species (see Supplementary Data, available online).
To measure the fitting of insects and flowers, an attempt was made to obtain a specimen sample of as many different insect pollinators as possible. The insects were identified and the proboscis length measured by C. Eardley (Pretoria, South Africa). Specimens of Lipotriches, Megachile and Thrinchostoma could not be identified to species level as these genera need to be revised first (C. Eardley, pers. com.). For each sample body length, head width and proboscis length were determined. To account for the maximal reach of the insect to suck the nectar the proboscis length was measured as the length of the glossa/galea in long-tongued bees (e.g. Amegilla). In short-tongued bees (e.g. Thrinchostoma) the lengths of prementum and mentum were added. Only specimens with a complete data set were used for statistical analyses. Based on these measurements pollinator species are clustered into four functional groups (small, medium-sized and large bees and sunbirds) assuming a similar behaviour on the flower and exertion of similar selection pressures (Fenster et al., 2004; see also Wilson et al., 2004).
In Afrocalathea rhizantha pollinator efficiency was analysed by marking individual flowers after style releases by birds were observed and recording their fruit-set hereafter.
Statistical analyses
The measurements on floral morphology, nectar and pollen (the latter taken from Ley, 2008) as well as the presence and absence data of distinct colours (white, violet, red, yellow, blue), nectar guides, conspicuous outer staminodes, open/obstructed flower entrances and the vertical/horizontal orientation of the flower were then subjected to a multidimensional scaling analysis. Analyses were performed on the whole and parts of the data set to increase resolution. All analyses confirmed the establishment of five floral types. For each floral type average and standard deviations of quantitative measurements were calculated. To determine whether data sets were significantly different from each other a non-parametric test (Kolmogorov–Smirnov) was applied as not all data sets were normally distributed. Further on, correlations between data sets were tested with a two-sided correlation analysis after Pearson. All analyses were conducted in SPSS Version 15.0.1.
RESULTS
Floral diversity
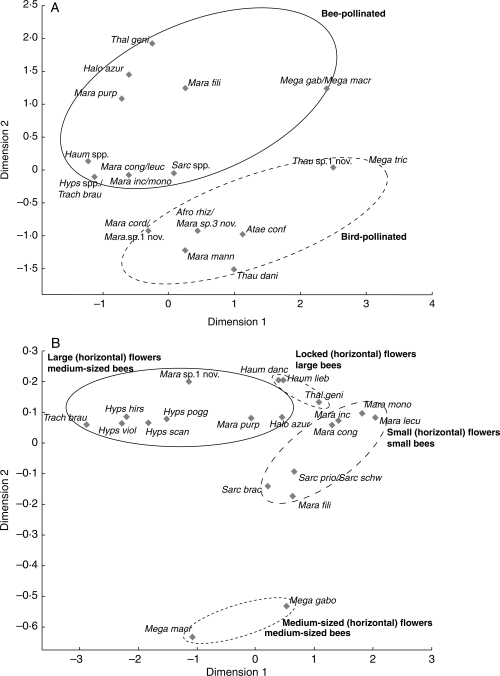
The African Marantaceae display a high floral diversity. Nevertheless, detailed observations suggest the establishment of five distinct flower types which is supported by multidimensional scaling analyses (Fig. 2) and confirmed by significant statistical differences in morphological and ecological characteristics between the suggested types (Fig. 3).
Fig. 2.
Multidimensional scaling analyses of pollination syndromes in African Marantaceae: (A) all species; (B) bee-pollinated species only. Data include: floral morphology, nectar volume and concentration and pollen load per theca (taken from Ley, 2008), presence/absence of distinct colours (white, violet, red, yellow, blue), nectar guides and conspicuous outer staminodes, open or locked flower entrances and the vertical or horizontal orientation of the flower. Circles summarize Marantaceae species pollinated by the same pollinator.
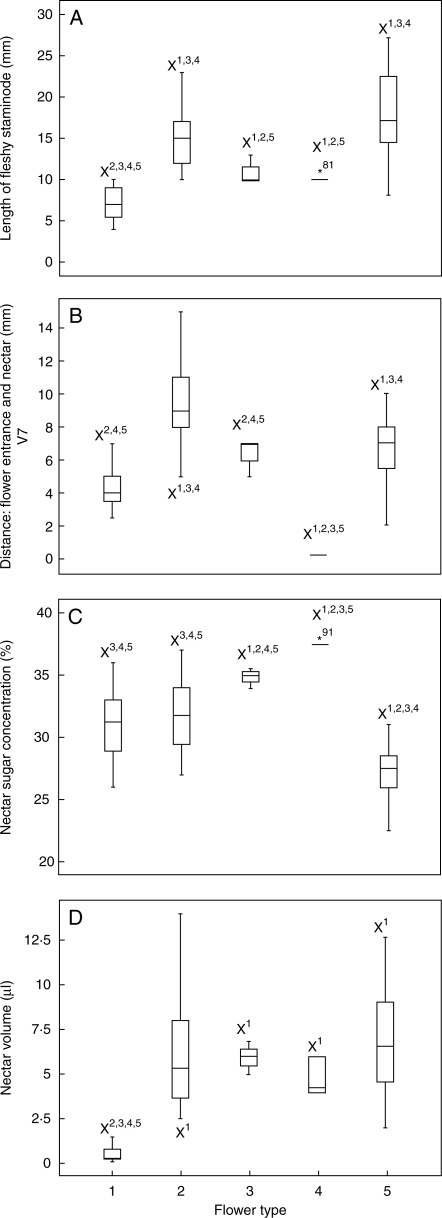
Fig. 3.
Flower morphological and ecological data summarized by flower type: 1, ‘small (horizontal)’ flower; 2, ‘large (horizontal)’ flower; 3, ‘medium-sized (horizontal)’ flower; 4, ‘locked (horizontal)’ flower; 5, ‘(large) vertical’ flower. (A) Length of fleshy staminode; (B) distance between flower entrance and nectar; (C) nectar concentration; (D) nectar volume. X, significantly different after non-parametric Kolmogorov–Smirnov test at a level of P < 0·01 towards floral types (see superscript numbers).
Small (horizontal) flowers (Fig. 4A)
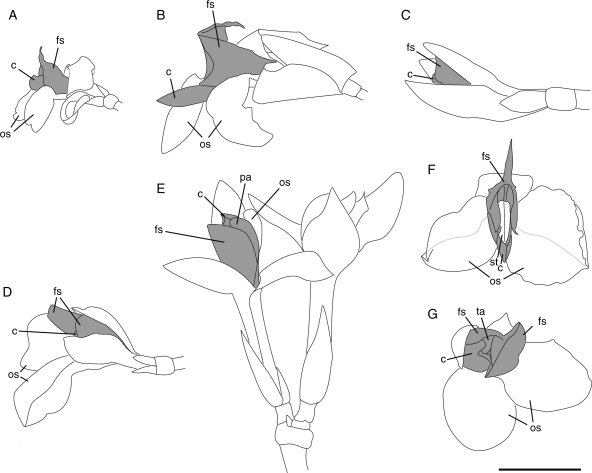
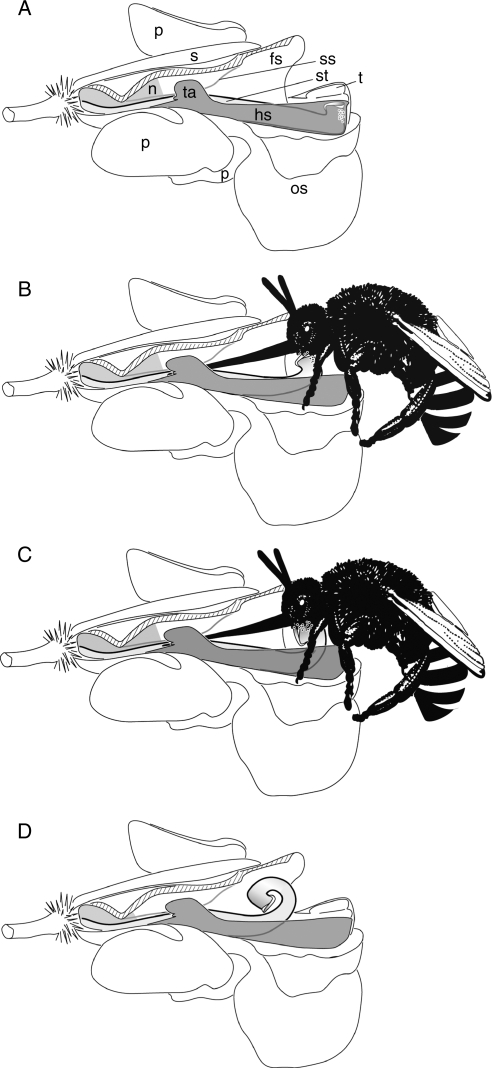
Fig. 4.
The five floral types (‘small’, ‘large’, ‘medium-sized’ and ‘vertical’ flowers) represented by Marantochloa congensis (A), Hypselodelphys hirsuta (B, F), Megaphrynium macrostachyum (C), Haumania danckelmanniana (D, G) and Thaumatococcus daniellii (E). (A–E) Side view in natural orientation in the inflorescence (E, flower pair); (F, G) frontal view. c, Unit composed of the style enveloped by the hooded staminode and the petaloid appendage of the fertile theca; fs, fleshy staminode; os, outer staminode; pa, petaloid appendage of fertile theca; st, style; ta, trigger appendage of the hooded staminode; grey, organs of inner androecial whorl. Scale bar = 1 cm.
The group includes eight species from two genera (Table 2) that generally have small (<10 mm in length) white flowers (Fig. 5A, B) except Marantochloa filipes with pink flowers. The flowers have horizontally orientated floral tubes, relatively large outer staminodes and a yellow trigger as nectar guide. No scent is discernable. The average length of the fleshy staminode is 6·83 ± 1·69 mm, the average distance to the nectar 4·30 ± 1·05 mm (n = 23; Fig. 3 and Table 2).
Table 2.
Flower morphometric data and pollinator observations
| Pollination (visits population−1 h−1) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bees |
Birds | Other visitors | ||||||||||||
| Small | Medium-sized |
Large |
||||||||||||
| Flower characteristics |
Halictidae | Apidae | Anthophoridae |
|||||||||||
| Flower colour | n | a (mm) | b (mm) | c (mm) | t | Thrinchostoma spp.2/Allodapula ornaticeps3 | Thrinchostoma bicometes | Amegilla vivida | Xylocopa nigrita | Xylocopa varipes | Megachile sp.12, M. sp.23 | Cyanomitra olivaceus cf. | Butterfly | |
| Small (horizontal) flowers | ||||||||||||||
| Mara cong | w | 7 | 7·35 ± 0·47 | 5·10 ± 0·25 | 1·00 | 10·75 | 0·52 ± 0·452 | |||||||
| Mara fili | r | 10 | 9·55 ± 0·5 | 3·30 ± 0·65 | 1·50 | 21·00 | 0·99 ± 0·772 | |||||||
| Mara ince (Ma) | w | 1 | 7·00 | 1·00 | 15·00 | 0·33 ± 0·072 | ||||||||
| Mara ince (MC) | w | 3 | 7·00 ± 0·00 | 5·00 ± 0·00 | 1·00 | ? | ||||||||
| Mara leuc | w | 5 | 4·88 ± 0·45 | 3·60 ± 0·65 | 1·00 | ? | ||||||||
| Mara mono | w | 3 | 5·66 ± 0·57 | 3·50 ± 0·86 | 1·00 | 14·50 | 1·10 ± 0·053 | |||||||
| Sarc brac | Rose | 2 | 11·00 ± 0 | 5·00 ± 0·00 | 1·00 | 12·25 | 1·74 ± 1·222 | |||||||
| Sarc prio | Rose | 4 | 9·50 ± 0·57 | 5·88 ± 0·62 | 1·00 | 24·75 | 3·00 ± 2·462 | |||||||
| Sarc schw | Beige | 2 | 9·50 ± 0·7 | 5·50 ± 0·00 | 1·00 | 12·25 | ? | # | ||||||
| Large (horizontal) flowers | ||||||||||||||
| Halo azur | bl | 15 | 10·06 ± 1·53 | 9·33 ± 1·03 | 2·00 | 26·00 | 0·67 ± 0·39 | 0·05 ± 0·13 | 0·41 ± 0·45 | |||||
| Hyps hirs | v | 8 | 18·75 ± 1·28 | 9·75 ± 1·14 | 2·00 | 26·00 | 0·49 ± 0·63 | 0·28 ± 0·58 | 0·79 ± 1·17 | |||||
| Hyps pogg | v | 9 | 16·50 ± 1·06 | 9·06 ± 2·10 | 2·00 | 13·00 | 0·82 ± 1·27 | 0·91 ± 1·31 | ||||||
| Hyps scan | v | 8 | 17·50 ± 1·19 | 11·75 ± 1·04 | 2·00 | 14·00 | 2·78 ± 1·68 | 1·874 ± 1·35 | ||||||
| Hyps viol | v | 3 | 19·00 ± 1·00 | 7·67 ± 1·52 | 2·00 | # | ||||||||
| Mara sp.1 nov. | w | 4 | 15·25 ± 0·5 | 9·00 ± 0·00 | 3·00 | 27·25 | 1·45 ± 1·43 | |||||||
| Mara purp | p | 7 | 11·78 ± 0·39 | 7·42 ± 1·13 | 2·00 | 9·75 | 3·66 ± 2·98 | 1·45 ± 1·05 | ||||||
| Trach brau | v | 3 | 21·00 ± 1 | 11·00 ± 1·00 | 2·00 | 8·00 | 2·14 ± 1·11 | 0·36 ± 0·51 | ||||||
| Medium-sized (horizontal) flowers | ||||||||||||||
| Mega gabo | r, y | 3 | 10·00 ± 0 | 7·00 ± 0·00 | 1·00 | 18·00 | 0·12 ± 0·13 | 1·89 ± 2·17 | 0·03 ± 0·052 | 0·32 ± 0·50 | 0·69 ± 0·56 | |||
| Mega macr | r, y | 5 | 15·20 ± 0·44 | 7·50 ± 0·70 | 1·00 | 19·00 | 0·33 ± 0·30 | 0·77 ± 0·16 | ||||||
| Locked (horizontal) flowers | ||||||||||||||
| Haum danc | w | 3 | 10·3 ± 0·12 | 2·00 ± 0·00 | 2·00 | 31·50 | 0·30 ± 0·46 | 0·66 ± 0·80 | 3·56 ± 4·153 | 0·06 ± 0·15 | 0·58 ± 0·86 | |||
| Haum lieb | w | 2 | 10·00 ± 0 | 2·00 ± 0·00 | 2·00 | ? | ||||||||
| Thal geni | v | 1 | 8·00 | 3·00 | 2·00 | |||||||||
| (Large) vertical flowers | ||||||||||||||
| Afro rhiz (MA) | w | 5 | 24·40 ± 2·07 | 4·25 ± 1·70 | 9·50 | 0·08 ± 0·17 | 0·0·22 ± 0·26 | |||||||
| Afro rhiz (MC) | w | 22·25 | 0·66 ± 0·45 | |||||||||||
| Atae conf | w, r br | 8 | 18·19 ± 0·65 | 7·69 ± 1·34 | 1·00 | 42·25 | # | 0·98 ± 0·82 | 0·29 ± 0·59 | |||||
| Mara cord | w, y | 10 | 18·75 ± 2·32 | 8·33 ± 1·37 | 2·00 | 7·75 | 0·36 ± 0·50 | 0·23 ± 0·32 | 0·52 ± 0·38 | |||||
| Mara sp.2 nov. | w | 5 | 17·70 ± 1·09 | 11·70 ± 0·97 | 2·50 | 19·50 | ? | |||||||
| Mara sp.3 nov. | w | 4 | 17·87 ± 1·43 | 8·26 ± 0·48 | 2·00 | 15·00 | 0·20 ± 0·17 | 1·11 ± 0·74 | ||||||
| Mega tric | r, y | 10 | 14·30 ± 0·94 | 8·81 ± 0·75 | 2·00 | 20·50 | 0·95 ± 1·12 | |||||||
| Thau dani | w | ? | ||||||||||||
| Thau sp.1 nov. | y | 5 | 25·00 ± 1 | 8·60 ± 0·54 | 2·50 | 20·00 | 0·56 ± 0·40 | |||||||
Flower measurements: a, length of fleshy staminode; b, distance between flower entrance and nectar; c, width of flower entrance; minute deviations not shown (for floral measurements see Fig. 1).
t, hours of observation (total = 500·75 h)
bl, blue; br, bract; p, purple; r, red; v, violet; w, white; y, yellow.
Dominant pollinators are indicated in bold; #, single observation; ?, assumed pollinator.
The superscript numbers in the ‘Bees’ columns correspond with the superscript numbers behind the data in the column, e.g. for Thrinchostoma spp.2 all numbers in the same column with the same superscript number, e.g. 0·52+0·452, concern Thrinchostoma spp. only and not Allodapula ornaticeps.
Fig. 5.
The five pollination syndromes in African Marantaceae: (A–C) ‘small’ flowers and small bees; (D, E) ‘large’ flowers and medium-sized bees; (F) ‘medium-sized’ flowers and medium-sized bees; (G, H) ‘locked’ flowers and large bees; (J–L) ‘vertical’ flowers and sunbirds. (A) Marantochloa congensis; (B) Thrinchostoma sp. on Sarcophrynium prionogonium; (C) pollen deposition in the proboscidial fossae of Thrinchostoma sp.; (D) Amegilla vivida on Hypselodelphys poggeana; (E) Thrinchostoma bicometes on Halopegia azurea – note the long three-part proboscis; (F) Amegilla vivida hovering in front of Megaphrynium macrostachyum – note the absence of conspicuous outer staminodes as landing platforms; (G) Haumania danckelmanniana – note the yellow nectar guides around the closed flower entrance; (H) Xylocopa nigrita on Haumania danckelmanniana; (I) pollen deposition on the mouth parts of Xylocopa varipes; (J) sunbird visiting Thaumatococcus sp.1 nov.; (K) sunbird visiting Afrocalathea rhizantha; (L) sunbird visiting the hanging flowers of Megaphrynium trichogynum. Scale bar = 1 cm.
Large (horizontal) flowers (Fig. 4B, F)
Nine species from four genera (Table 2, plus Hypselodelphys sp.1 nov.) belong to this type. They generally have large pink, violet, violet–white (Fig. 5D) or bluish (Fig. 5E) flowers with horizontally arranged tubes and conspicuous outer staminodes. Yellow nectar guides are formed by the trigger appendage or the petaloid appendage of the fertile theca, the yellow hooded staminode or yellow spots on the fleshy staminode. No scent is discernable. The average length of the fleshy staminode is 15·75 ± 3·40 mm, the average distance to the nectar 9·52 ± 2·27 mm (n = 39; Fig. 3, Table 2).
Medium-sized (horizontal) flowers (Fig. 4C)
Two species from the genus Megaphrynium belong to this type (Table 2). The floral tubes are horizontally arranged. The outer staminodes are highly reduced. They are small and lanceolate. The flowers of M. macrostachyum can be either violet–white or yellow with a dark-red floral tube (Fig. 5F), the flowers of M. gabonense are pink and yellow. The average length of the fleshy staminode is 11·33 ± 1·41 mm, the average distance to the nectar 5·72 ± 1·30 mm (n = 9; Fig. 3 and Table 2).
Locked (horizontal) flowers (Fig. 4D, G)
The three species of this type from the genera Haumania (Table 2) and Thalia have white or dark violet flowers with large outer staminodes (Fig. 5G). Haumania species have conspicuously yellow nectar guides originating from the petaloid appendage of the fertile theca and the fleshy and hooded staminodes. Their trigger appendage completely obstructs the flower entrance (Fig. 5G) and is made of a firm tissue. The flowers exhibit a strong sweet scent. The average length of the fleshy staminode is 9·83 ± 0·92 mm, the average distance to the nectar 2·17 ± 0·41 mm (n = 6) (Fig. 3 and Table 2).
(Large) vertical flowers (Fig. 4E)
The nine species from five genera belonging to this type (Table 2) generally have large yellow (Fig. 5J), yellow to red (Fig. 5L) or white flowers (Fig. 5K) with long, thin floral tubes. The latter are usually longer than 10 mm except in Marantochloa mannii, Megaphrynium trichogynum and Marantochloa sp.2 nov. which have a tube length of 4–6 mm. Flowers are vertically arranged and have no nectar guides (except Marantochloa cordifolia, Marantochloa sp.2 nov.) and no discernable scent. Ataenidia conferta and Marantochloa mannii have white flowers surrounded by conspicuous red bracts. The outer staminodes in this type are usually large except in Megaphrynium trichogynum and Thaumatococcus spp. where they are highly reduced, providing the flower with a superficial actinomorphic symmetry. The tissue of these latter flowers is exceptionally hard and rubber-like. Megaphrynium trichogynum stands out by having hanging flowers with straight petals that are neither reflexed nor bent outwards as in all other species. The average length of the fleshy staminode over all species is 18·11 ± 4·49 mm, the average distance to the nectar 7·55 ± 3·63 mm (n = 40; Fig. 3 and Table 2).
Nectar reward
In the morning, before visitation, nectar is found up to the proximal end of the trigger appendage (see Fig. 1). Nectar volume ranges across all investigated species, on average between 0·3 and 11 µL (Table 3) and is significantly correlated with floral tube length (Spearman-rho correlation coefficient = 0·724, n = 76, P < 0·001).
Table 3.
Nectar sugar concentration and volume
| Sugar concentration (%) |
Volume (μL) |
|||
|---|---|---|---|---|
| n | mean ± s.d. | n | mean ± s.d. | |
| Small (horizontal) flowers | ||||
| Mara cong | 6 | 28·58 ± 0·93 | 5 | 0·37 ± 0·12 |
| Mara fili | 10 | 32·15 ± 0·63 | 10 | 0·80 ± 0·00 |
| Mara ince (Ma) | 0 | 2 | <1 | |
| Mara ince (MC) | 1 | 32·00 | 1 | 0·40 |
| Mara leuc | 10 | 32·95 ± 0·65 | 5 | 0·40 ± 0·10 |
| Mara mono | 8 | 30·68 ± 1·34 | 3 | 0·30 ± 0·00 |
| Sarc brac | 10 | 32·95 ± 0·87 | 6 | 0·55 ± 0·12 |
| Sarc prio | 7 | 33·92 ± 1·37 | 8 | 1·17 ± 0·41 |
| Sarc schw | <1 | |||
| Large (horizontal) flowers | ||||
| Halo azur | 15 | 28·13 ± 0·74 | 5 | 4·33 ± 0·94 |
| Hyps hirs | 12 | 34·17 ± 1·52 | 6 | 5·61 ± 1·66 |
| Hyps pogg | 10 | 33·70 ± 1·49 | 4 | 9·50 ± 1·91 |
| Hyps scan | 13 | 35·73 ± 1·17 | 4 | 8·50 ± 3·78 |
| Hyps viol | 0 | 0 | ||
| Mara sp.1 nov. | 11 | 29·81 ± 1·00 | 8 | 6·53 ± 1·87 |
| Mara purp | 11 | 31·27 ± 2·03 | 10 | 2·68 ± 0·30 |
| Trach brau | 20 | 30·50 ± 2·92 | 1 | 4 |
| Medium-sized (horizontal) flowers | ||||
| Mega gabo | 10 | 37·10 ± 1·44 | 3 | 6·26 ± 0·46 |
| Mega macr | 10 | 34·95 ± 1·79 | 1 | 5 |
| ‘Locked (horizontal)’ flowers | ||||
| Haum danc | 24 | 36·04 ± 4·67 | 5 | 5·90 ± 1·24 |
| Haum lieb | 10 | 40·70 ± 2·71 | 2 | 4·15 ± 0·21 |
| Thal geni | 0 | 0 | ||
| (Large) vertical flowers | ||||
| Afro rhiz | 10 | 25·35 ± 2·68 | 9 | 6·74 ± 2·81 |
| Atae conf | 27 | 26·94 ± 1·30 | 10 | 5·78 ± 1·67 |
| Mara cord | 12 | 30·46 ± 1·19 | 3 | 5·06 ± 2·65 |
| Mara mann | 11 | 27·27 ± 0·65 | 3 | ∼8·00 |
| Mara sp.2 nov. | 10 | 27·09 ± 0·33 | 10 | 5·85 ± 1·10 |
| Mara sp.3 nov. | 14 | 30·25 ± 1·71 | 3 | ∼8·00 |
| Mega tric | 12 | 27·70 ± 1·28 | 4 | 11 ± 1·15 |
| Thau dani | 0 | 0 | ||
| Thau sp.1 nov. | 12 | 27·04 ± 0·63 | 4 | 11 ± 1·15 |
n, number of flowers.
Nectar sugar concentration ranges between 25% and 40% (Table 3). The values for nectar concentration are significantly different between all types except between the ‘small’ and the ‘large’ flowers (Fig. 3). The highest concentration is found in the ‘locked’ flowers (37·41 ± 2·49%), followed by the ‘medium-sized’ flowers (35·03 ± 2·00%), the ‘large’ flowers (31·21 ± 2·95%), the ‘small’ flowers (31·25 ± 2·14%) and the ‘vertical’ flowers (25·69 ± 3·01%), respectively.
Functional pollinator groups
Within the bees, three functional groups can be distinguished according to the morphometric data of their mouth parts, body lengths and head width (Tables 1 and 4). The length of the mouth parts and the width of the head are significantly different between the groups at the P < 0·05 level (Kolmogorov–Smirnov test) and correlate with the distance from the floral entrance to the nectar (Spearman-rho correlation coefficient = 0·822, n = 19, P < 0·001). Small bees (Thrinchostoma spp., Allodapula ornaticeps) are characterized by a narrow head and body (head: 2·3 ± 0·2 mm; n = 9) and short mouth parts (3·80–6·35 mm), medium-sized bees (Amegilla vivida, Thrinchostoma spp.) by slightly longer mouth parts (10·00–13·90 mm) and by heads and bodies that are at least twice as broad as in the small bees (5·0 ± 0 mm; n = 6). Large and heavy bees (Xylocopa spp.) have comparable head widths (6·00 ± 0 mm; n = 2) and mouth part lengths (10 ± 0 mm; n = 2) whereas Megachile spp. have extremely short mouth parts (0·3 ± 0 mm; n = 2). The beaks of the sunbirds represent the longest mouth parts between the groups of pollinators (approx. 20 mm).
Table 4.
Morphometric data of pollinators and nectar-robbers
| Mean size of pollinator (mm)* |
Mean length of proboscis (mm) |
||||||
|---|---|---|---|---|---|---|---|
| Insects | FG | n | Body length | Head width | Prementum + mentum | Glossa/galea | Total |
| Halictidae | |||||||
| Thrinchostoma sp.1 on Marantochloa congensis | s | 2 | 14·0 ± 0·0 | 2·5 ± 0·0 | 2·5 ± 0·007 | 2·9 (n = 1) | 6·35 |
| Thrinchostoma sp.1 on Sarcophrynium brachystachyum | s | 2 | 11·5 ± 0·7 | 2·1 ± 0·10 | 2·5 ± 0·0 | 1·5 (n = 1) | 4·0 |
| Thrinchostoma sp.2 on Marantochloa filipes (Si) | s | 2 | 9·0 ± 0·0 | 2·1 ± 0·07 | 2·3 ± 0·0 | ? | > 2·3 |
| Thrinchostoma sp.3 on Marantochloa filipes (MC) | s | 1 | 10·0 | 2·0 | 1·9 | 1·9 | 3·8 |
| Thrinchostoma sp.4 on Sarcophrynium prionogonium | s | 1 | 16·0 | 2·5 | 2·5 | 1·5 | 4·0 |
| Thrinchostoma bicometes (Enderlien, 1903) | m | 1 | 20·0 | 3·0 | 5·2 | 8·7 | 13·9 |
| Lipotriches sp. | 1 | 12·0 | 3·0 | 1·9 | 0·1 | 1·9 | |
| Apidae | |||||||
| Amegilla vivida (Smith) | m | 5 | 14·6 ± 1·9 | 5·0 ± 0·0 | ∼5/5·86 ± 0·43 | ∼10 | |
| Allodapula ornaticeps Michener | s | 1 | 7·0 | 2·0 | 3·90 | 3·9 | |
| Anthophoridae | |||||||
| Xylocopa varipes Smith | l | 2 | 20·0 ± 0·0 | 6·0 ± 0·0 | ∼5/5·0 ± 0 | ∼10 | |
| Xylocopa nigrita (Fabricius) | l | 35·0† | |||||
| Megachilidae | |||||||
| Megachile sp.1 | m | 1 | 15·0 | 5·0 | 0·3 | 0·3 | |
| Megachile sp.2 | m | 1 | 22·0 | 5·0 | 0·3 | 0·3 | |
| Sunbird | |||||||
| Probably olive sunbird Cyanomitra olivaceus | b | 130–150 | ∼20 | ||||
FG, functional group: s, small bees; m, medium-sized bees; l, large bees; b, birds; n, number of individuals measured; MC, Monts de Cristal; Si, Sibang.
For undetermined Thrinchostoma spp. the flower visitors are mentioned; for the remaining species, see Table 2.
*, Length measurements taken by C. D. Eardley.
† After Eardley (1983).
All pollinator groups are active from sunrise to sunset. No constant activity pattern with higher or lower frequencies at special hours of the day can be defined. Instead, visitation is found to be highly variable within and between different days and localities (see Table 2).
Plant–pollinator interactions
Different bee species and one sunbird species were observed to trigger the African Marantaceae (Table 2). Plant species that were observed at different localities and in different years (Haumania danckelmanniana, Hypselodelphys hirsuta, Marantochloa filipes, Megaphrynium macrostachyum) always show the same functional pollinator groups. Butterflies and further unidentified insects also visited the flowers but were never found to trigger a flower.
Bee pollination
Bees pollinate all flowers of the ‘small’, ‘large’ and ‘locked’ flowers. These flowers provide a landing platform with their large and conspicuous outer staminodes (Fig. 4). Flowers that are twisted and present their outer staminodes in an unusual position are less frequently visited. While perching on the staminodes the bees are always forced into the same position so that they can get pollen precisely deposited into the proboscidial fossae (Fig. 5C). However, in flowers of Hypselodelphys, pollen is occasionally found on the fleshy staminode, indicating that not all pollen grains were deposited on the bee.
Bees also visit the flowers of the ‘medium-sized’ flowers. Here, however, large conspicuous outer staminodes are lacking (Fig. 4C). Amegilla bees either settle on the back-folded petals or hover in front of the flowers when sucking nectar. The large carpenter bees (Xylocopa spp.) have to use the second flower of a pair as a landing and perching site. They first suck the nectar from one flower and then turn around to feed from the other.
Small bees
At least four different species of the genus Thrinchostoma (Halictidae; Fig. 5B) and Allodapula ornaticeps (Apidae) visit the small flowers of the ‘small’ flowers (Table 4; see videos in Supplementary Data, available online). The bee species have a long proboscis compared with their body length (Table 4). Consequently, they have to lean back to retract the proboscis and fold it up after nectar sucking. Before leaving the flower they brush their proboscis. However, this remains incomplete, as an individual captured with an extended proboscis showed pollen grains in its proboscidial fossae (Fig. 5C). Besides in the proboscidial fossae, Thrinchostoma spp. have occasionally been observed carrying large yellow pollen loads on their hind limbs. Handling time per flower by the small insects is on average 11·41 ± 11·39 s (n = 94; see videos in Supplementary Data).
While individual Sarcophrynium brachystachyum and S. prionogonium flowers are visited repeatedly during the day, flowers of Marantochloa incertifolia, M. monophylla, M. congensis (Fig. 5A) and M. filipes are visited only once. They are generally triggered on the first visit and frequently fall off 1–3 h afterwards.
Medium-sized bees
Amegilla vivida (Fig. 5D). This species was observed exclusively on large (except Halopegia azurea) and medium-sized flowers. This bee species is a very fast flying insect, highly aware of any disturbance in its surroundings. Occasionally even small insects drive these bees away. In general, Amegilla bees visit all open flowers of an individual plant, thereby flying the shortest distances. To find further open flowers, they also visit non-flowering inflorescences that had open flowers a few hours or even a day before. Having found a flower, the bees extend their proboscis (glossa approx. 4–5 mm long) still in flight and hover in front of the flower before landing on the outer staminodes (Fig. 5D, see videos in Supplementary Data). Only in Megaphrynium species do they hover during the whole process of nectar uptake as a landing device is missing. During the landing process the proboscis is inserted into the flower tube and then the head is pushed forward until the fleshy staminode circumvents further penetration (Fig. 6). When the bee's proboscis touches the trigger appendage and releases the explosive movement of the style the bee sometimes startles for a moment, retracts its proboscis momentarily but keeps on foraging by further extending the galea to reach the nectar. Handling time per flower is rather short with an average of 3·50 ± 3·90 s (n = 46; see videos in Supplementary Data). Individual flowers are visited repeatedly during the day by different Amegilla vivida individuals. The style is sometimes only triggered after up to eight visits, but almost all flowers are released by noon. Nevertheless, insects continue to visit the flowers irrespectively until dusk. Amegilla bees were also observed on, for example, Decellandra barteri (Melastomataceae) and Bertieria sp. (Rubiaceae) and when they visited Marantaceae species they sometimes carried conspicuous yellow pollen loads on their hind legs.
Fig. 6.
Bee-pollination exemplified by Marantochloa purpurea and Amegilla vivida. (A) Flower before insect visit (hatched, cut open to allow insight into floral tube): style (st) straight, held under tension by the hooded staminode (hs) bearing the trigger appendage (ta); stiff swelling (ss) of the fleshy staminode (fs) blocking access to the nectar (n, light grey). os, Outer staminode; p, petal; s, sepal; t, thecae already wilted. (B) Style release after the trigger appendage is deflected by the pollinator; transfer of foreign pollen from the proboscidial fossae of the insect into the stigmatic cavity. (C) Own pollen from the pollen plate at the back of the stylar head is placed into the proboscidial fossae of the insect. (D) After visitation the style is completely and irreversibly rolled up inside the fleshy staminode.
Medium-sized bees: Thrinchostoma bicometes (Fig. 5E)
This species visits Halopegia azurea in slow-running creeks and swamps. This bee is also a fast flier with a long but thinner body than Amegilla vivida (Table 4). It constantly moves back and forth between individual plants at a height of 20 cm above the water level. Before landing it flies around in the given Halopegia population. Then it lands abruptly on the spacious lower outer staminode of a selected flower, inserts its proboscis into the floral tube and sucks the nectar. Handling time per flower is only a few seconds (n = 4, 12·25 ± 6·65 s; see videos in Supplementary Data). Before leaving it has to lean backwards to fully extract the three-part proboscis that has to be folded up into the proboscidial fossae underneath the long ‘snout’ (Fig. 5E). The style is released immediately during the first visit. Triggering is accompanied by a short tentative upwards movement of the lower outer staminode. After triggering, this outer staminode starts to fold up slowly. After about 1 h the flower entrance of the respective flower is totally closed and no further insect visit is possible.
Large bees: Xylocopa nigrita (Fig. 5H) and Xylocopa varipes (Fig. 5I)
These species visit Haumania danckelmanniana and Megaphrynium gabonense. Xylocopa bees are large (Table 4) and heavy and often the entire inflorescence moves under their weight when they land on the flowers (see videos in Supplementary Data). Handling time for a single flower takes about 5 s (n = 45, 4·06 ± 2·09 s, range 2–10 s). In Haumania danckelmanniana the bees hold onto the external staminodes, in Megaphrynium gabonense they sit on one of the two flowers of a flower pair while pushing their proboscis into the other. They visit up to ten flowers per minute and stay up to 10 min in a single Haumania danckelmanniana plant or in a large population of Megaphrynium gabonense where they fly from one inflorescence to the next. A heavy pollen load accumulates around the whole proboscis of the Xylocopa bees that was never observed to be cleaned during subsequent flower visits (Fig. 5I). An unidentified Xylocopa species has also once been documented visiting subsequent flowers of Ataenidia conferta.
Large bees: Megachile
Different species of Megachile visit the same Marantaceae species as the Xylocopa bees. The slightly smaller Megachile sp.1 was observed on Megaphrynium gabonense, the larger Megachile sp.2 on Haumania danckelmanniana (Table 2). The bees are of an intermediate body size between Amegilla and Xylocopa and have extremely short mouth parts (Table 4). The frequency of visitation was highly variable in Makokou and the bees were totally absent during the observations in Monts de Cristal. The individuals fly from one flower to the next and stay for several minutes in one Haumania danckelmanniana individual. Their handling per flower takes on average 8 s (n = 11, 8·00 ± 2·68 s). However, it could not be confirmed whether they were really able to trigger the flower.
Sunbird pollination
Sunbirds (Nectariniidae) (Fig. 5J–L), probably the olive sunbird (Cyanomitra olivaceus), are the exclusive visitors of the ‘vertical’ flowers (Fig. 7). Only once was an Amegilla sp. observed on Marantochloa cordifolia and a Xylocopa sp. on Ataenidia conferta. The birds are faster than the insect pollinators with a handling time per flower of <1 s to a maximum of 2 s (see videos in Supplementary Data). Within 1 min they enter a given population, choose a few flowers, hold on to a nearby petiole or hover in front of the flower, intrude their beak into the tubes, suck the nectar, change to the next flower and finally leave the population rather quickly. The birds are able to reach the nectar from any side of the flowers and therefore the pollen is not always deposited on the same spot on the beak but all around it (see videos in Supplementary Data). Occasionally, the birds clean their beak after having visited all flowers of an individual unit. In Ataenidia conferta leftovers of pollen can sometimes be found on the fleshy staminodes. Pollination efficiency tests on Afrocalathea rhizantha yield a definite fruit-set of 64·70% after visitation by birds (n = 17).
Fig. 7.
Pollination of the vertically arranged bird-pollinated flower of Thaumatococcus sp.1 nov. The flower is cut open (hatched) to allow view inside the floral tube. The olive sunbird (Cyanomitra olivaceus cf.) intrudes its beak into the flower tube thereby deflecting the trigger appendage which leads to the rolling-in movement of the style and the subsequent pollen deposition onto the beak. The nectar is sucked by elongating the tongue (not shown). fs, Fleshy staminode; hs, hooded staminode (dark grey); n, nectar (medium grey); os, outer staminodes (highly reduced); p, petal; s, sepal, ss, stiff swelling of fleshy staminode; st, style (light grey); ta, trigger appendage; dots, pollen grains.
Visitation rate is highest at rich blooming shoot systems where the birds visit all open flowers consecutively. However, the birds are also capable of regularly finding the single, small and inconspicuous white flowers of Marantochloa sp.3 nov. hidden between large leaves. Furthermore, they visit and release Afrocalathea rhizantha flowers early in the morning before they are entirely open.
Megaphrynium trichogynum is the only African species of the Marantaceae with hanging flowers and floral tubes elongated by straight petals (Fig. 5L). As their inflorescences are arranged near the ground the birds perch on the petioles of the densely arranged leaves, pull the flower towards them and stick the beak deep into the flower tube. As already observed in Hypselodelphys spp. pollinated by Amegilla vivida, styles are not always triggered at the first visit.
The sunbirds also visit and subsequently trigger the flowers of a few species from the other four floral types of the African Marantaceae (see Table 2) and are additionally seen on flowers of other plant families (e.g. Zingiberaceae). All bird-visited flowers have a relatively high amount of nectar and a landing site or at least a strong inflorescence which counterbalances the introduced beak while the bird is hovering in front of the flower.
Pollinator visitation rate
The average visitation rate by pollinators differs between the flower types. It is higher in those types that are predominantly bee pollinated (‘small’ flowers: 153 h of observation, 1·38 ± 2·13 visits h−1; ‘medium-sized’ flowers: 37 h of observation, 1·56 ± 2·04 visits h−1; ‘large’ flowers: 124 h of observation, 1·41 ± 1·79 visits h−1; ‘locked’ flowers: 31·5 h of observation, 3·87 ± 3·73 visits h−1) than in the exclusively bird-pollinated ‘vertical’ flowers (108 h of observation, 0·68 ± 1·07 visits h−1).
DISCUSSION
This article presents for the first time pollinator observations on the African herbs and lianas from the family Marantaceae. These species are confined to the understorey of tropical lowland rainforest and both bee and bird pollination could be documented.
Pollination syndromes
The concept of pollination syndromes is based on observations of the close association of distinct flower types with specific functional pollination groups and is a recurrent subject in the literature across entirely different plant groups (Heinrich, 1975; Schemske, 1981; Gottsberger, 1989; Sakai et al., 1999; Specht et al., 2001; Kay and Schemske, 2003). Whereas specialization was formerly treated based merely on floral morphology (Blütenstile in Vogel, 1954) more recent studies have been more specific in differentiating between morphological, ecological and functional specialization (Fenster et al., 2004; Ollerton et al., 2007). Summarizing the documented differentiation of syndromes in the African Marantaceae they are based primarly on flower morphological specializations and energetic adaptations towards the pollinators. The degree of ecological specialization is surprisingly low.
Bee-pollination syndromes
Small bees and ‘small’ flowers
For the observed unique pollination of these small flowers by small bees energetic exclusion of larger animals might be the important factor. Although the inflorescences of some of these species would be strong enough to support larger animals the extremely small amount of nectar of an intermediate concentration might be unprofitable for larger bees and birds (Willmer and Stone, 2004). For the small bees, however, this quantity seems to be just profitable though not sufficient so that the bees are still forced to visit several flowers which in turn ensures the plant's pollen transfer. The exclusion of the large bees is advantageous for the small bees as in the absence of these competitors the available nectar volume is higher.
Although the nectar quantity is comparatively small in these flowers the average handling time per flower by the Thrinchostoma bees is on average twice as long as by the large bees on larger flowers with larger nectar quantities. This might be caused by a reduced intake rate due to their smaller size and narrow proboscis (Harder, 1986; Borrell and Krenn, 2006).
The eight species with small flowers are pollinated by five different bee species which are hardly overlapping in the species they visit so that the first impression is that of a species-specific relationship. However, as the plant and the bee species share similar habitat characteristics (Dhetchuvi, 1996; Ley, 2008) this specificity might disappear in the light of more profound studies.
Medium-sized bees and ‘medium-sized’ and ‘large’ flowers
The specific adaptation of the ‘medium-sized’ and ‘large’ flowers to larger bees is the distance to the nectar. It is too far for the short proboscis of the small bees. Additionally, some species such as Marantochloa purpurea and M. sp.1 nov. also exclude very large and heavy carpenter bees and birds probably because the hanging inflorescences are too slender for these bees and birds to land on. All other species of this floral type provide stout inflorescences and (occasional) observations of large bees (for Hypselodelphys: L. White, Wildlife Conservation Society, Gabon and S. Schoenecker, Gutenberg-Universität Mainz, Germany, pers. com.) and birds visiting their flowers do exist.
The restricted occurrence of Halopegia azurea in shallow slow-running creeks and its bluish flowers presented just above the water level might play a role in its unique and strict relationship with Thrinchostoma bicometes.
Large bees and ‘locked’ flowers
The species with ‘locked’ flowers flower predominantly in open habitats, in clearings and at forest edges, thereby matching the preferences of their dominant pollinators – the Xylocopa bees (for Thalia geniculata, see Davis, 1987; Appanah, 1990; Claßen-Bockhoff, 1991; Yeo, 1993). As the individuals bloom richly and produce high nectar sugar concentrations they provide the bees with a high quantity of food in a short time (Roubik et al., 1995). This enables the latter to cover their extremely high metabolic costs caused by a high wing load and flight speed (Louw and Nicolson, 1983; Gerling et al., 1989). Additionally, the bees seem to be able to collect pollen as in this exceptional case it is deposited onto the proboscis and not into the proboscidial fossae (Fig. 5I). However, the large quantity of pollen grains deposited onto the bee (Fig. 5I) and the scarcity of observed grooming behaviour (see videos in Supplementary Data available online) still provides a successful pollen transfer between flowers.
Smaller bees might be excluded from Haumania spp. flowers due to their locked flower entrance. Probably considerable strength is needed to push the covering leaves of the fleshy staminode aside and to deflect the trigger appendage. The short distance to the nectar also makes the flowers attractive to the short-tongued Megachile sp.2; however, it remains unclear whether this bee is a pollinator or a nectar thief as no pollen deposition on the insect could yet be testified. Their considerable strength certainly allows them to harvest the nectar (see Westerkamp, 1993).
Bird-pollination syndrome
The large vertical flowers show characteristics that are often associated with bird pollination such as yellow and red colours [Vogel, 1954; see also C. timothei, C. lutea and C. platystachya (Kennedy, 2000; Claßen-Bockhoff and Heller, 2008b)] and large quantities of low concentrated nectar (Percival, 1974; Baker, 1975; Stiles, 1976; Roubik et al., 1995; Nicolson, 2002; Nicolson and Fleming, 2003). However, white also plays an important role in African bird-pollinated species (see Afrocalathea rhizantha, Ataenidia conferta, Marantochloa cordifolia, M. sp.2 nov., M. sp.3 nov.; see also Vogel, 1954; Wester and Claßen-Bockhoff, 2007) stressing the fact that colour is far less correlated with specific pollinators than previously assumed (see also Momose et al., 1998). The vertically arranged bracts of Ataenidia conferta additionally collect rainwater, possibly rendering the inflorescences an important water supply for birds. Ataenidia conferta and Megaphrynium trichogynum are characterized by a high number of simultaneously open flowers which decreases the energy-consuming movements of the birds (Westerkamp, 1990). Correspondingly, massively blooming populations also yield the highest visitation rates (compare with Gentry, 1974; Stephenson, 1979). Handling time by birds is much shorter than by any of the bee species. However, due to the extremely rapid pollen transfer (Claßen-Bockhoff, 1991) pollen deposition should be independent of handling time (see also, for example, Ollerton et al., 2007). Thus the latter remains a function of optimal intake rate by the respective pollinator (see Borell and Krenn, 2006) and should not influence the fitness of the plant. Pollen of these flowers is deposited onto the bird's beak (Fig. 5J, K); its occasional cleaning does not totally impede pollen transfer as proven by the direct fruit-set experiments.
Bees are probably excluded from these flowers by the vertical arrangement of their floral tubes that might not fit the forward operation of their proboscis (Nilsson et al., 1985). In addition, in Megaphrynium trichogynum, decapitated insects (A. C. Ley, pers. obs.) illustrate that these animals are not able to handle the vigorous style movement of these large flowers. The ground near inflorescences of these species in closed forests probably does not closely match the habitat preferences of Xylocopa bees (Louw and Nicolson, 1983; Gerling et al., 1989).
Though bird pollination has not yet been observed in Marantochloa sp.2 nov. it is highly likely because growth form and flower morphology resemble those of the bird-pollinated Afrocalathea rhizantha (Fig. 5K). Both species occur sympatrically in the Monts de Cristal area. However, in contrast to Afrocalathea rhizantha, the nectar guide-possessing flowers of Marantochloa sp.2 nov. might also be pollinated by bees (see Manning and Goldblatt, 2005). A similar case is given by the two bird-pollinated species, Marantochloa cordifolia and M. sp.3 nov. Both examples might hint as well to a recent shift in pollination from bee to bird pollination (see also Kay and Schemske, 2003) as towards an intermediate pollination system by bees and birds (bimodal systems in Manning and Goldblatt, 2005). Further examples are found in the predominantly bee-pollinated species that are also occasionally visited by birds.
Specific advantages of bird pollination over bee pollination could not be found in the African Marantaceae. There is no limitation of the plant species to specific habitats or altitudinal zones that would be advantageous for bird pollination (see Kay and Schemske, 2003), and the floral constancy is not known to be greater in birds than in the observed bees. In contrast, birds are known to be rather opportunistic foragers, not constant on one species (Stiles, 1978). The sparse flowering of the respective Marantaceae species would not support their daily energy requirements anyway. Nevertheless, fruit set in bee- and bird-pollinated species of the same breeding system and fruit size is comparable (Ley, 2008), although visitation rate is lowest in bird-pollinated species.
General comments on the specialization to functional pollinator groups
The limited number of functional pollinator groups involved in the pollination of African Marantaceae, out of the wealth of potential pollinators of the tropical lowland forest, demonstrates the degree of specialization of the Marantaceae flowers. Some potential pollinator groups are filtered out by the unique and complex floral morphology, including zygomorphy (see also Kunze, 1984; Ley, 2008) and having nectar hidden in narrow floral tubes (see also Ollerton et al., 2007). Furthermore, nectar is generally the sole reward, as the pollen load is small (see Ley, 2008; compare Cruden, 1977) and hidden. Variation in floral size, colour and orientation establishing distinct floral types leave finally only a few specific bee and bird species to successfully visit each of these floral types.
Both partners are highly correlated in size. Equal tendencies of size-adaptations between flower and pollinator have also been observed in the American Marantaceae (Kennedy, 1978). A spectacular example is given by Calathea crotalifera (Marantaceae) whose length of the floral tube varies over its range depending on the proboscis length of the co-occurring pollinator species (in Kennedy, 2000; see also Ollerton et al., 2007).
Besides size-adaptation, the relative positioning of the insect to the flower is crucial to increase the precision of the transfer of the small pollen load (Ley, 2008). In the bee-pollinated species this is controlled by secondary zygomorphy (see Kunze, 1984), induced by the presence of large outer staminodes serving as a landing platform (for Zingiberaceae and Costaceae, see also Sakai et al., 1999). The latter leads the bees to always handle flowers in the same way whereby the locality of pollen deposition and uptake is identical between conspecific flowers. The additional effect of those traits in facilitating floral access is underlined by the reduced visitation rate of twisted flowers. In bird-pollinated flowers the outer staminodes are highly reduced in size and/or number, potentially due to a loss in function (see Walker-Larsen and Harder, 2000).
Such a close match between flower and pollinator can only be established between reliably present partners (plant and pollinators) allowing the plant time to optimize its pollen transfer by adapting its floral traits to pollinator body characteristics (‘the most effective pollinator principle’; see Stebbin, 1970). The observed pollinators (Amegilla, Halictidae, Xylocopa, sunbirds) are known as reliable long-distance pollinators (Gess and Gess, 1996; Momose et al., 1998; Gathmann and Tscharntke, 2002) which are constantly present over several months or even all year round and are at present widely distributed in tropical Africa (Eardley, 1983; C. D. Eardley and R. Urban, unpubl. res.). In reverse, individuals in Marantaceae are large and have many reproductive seasons during their lifetime so that both partners can rely on the presence of the counterpart (Waser et al., 1996; Manning and Goldblatt, 2005).
However, the degree of specialization differs between the floral types described here with regard to their ecological as well as to their functional specialization (for definition, see Ollerton et al., 2007). Whereas, for example, species of the ‘small’ flowers are pollinated by only one functional pollination group (high functional specialization) consisting of numerous small bee species (low ecological specialization), the species of the ‘large’ flowers are pollinated by two different functional groups (reduced functional specialization) each containing only one species (high ecological specialization). Megaphrynium gabonense is so far the only species known to be visited by three different functional pollination groups (medium-sized, large bees and birds). Regarding the high morphological specialization of the Marantaceae flower it is surprising to find such a low functional and/or ecological specialization in these species.
The overlaps between different functional pollinator groups in visiting the same floral type of Marantaceae seem to be correlated with similarities in the requirements of different pollinators (e.g. large bees and birds need large amounts of nectar) and with overlaps in reward characteristics of different floral types (e.g. similar nectar concentration) (see also Waser et al., 1996). Thereby, overlaps are restricted to medium-sized and large pollinators and the respective flower types (Schemske and Horvitz, 1984; Locatelli et al., 2004). A high degree of specialization is, in particular, reached in species with specific handling requirements. Spectacular examples from America are closed flowers (Kennedy, 1978) or the flowers of the genus Calathea with extremely long floral tubes (Kennedy, 2000).
Approximately the same functional pollinator groups are known in Asian Costaceae, Marantaceae and Zingiberaceae (Momose et al., 1998; Sakai et al., 1999; Specht et al., 2001) and in neotropical Costaceae and Marantaceae (Kay and Schemske, 2003; hummingbird versus Euglossine bee, Kennedy, 2000). These associations indicate some universality in evolutionary selection pressures (see also Sakai et al., 1999) and flower architectural effects. However, there is a difference in ecological specialization between the continents (compare Schemske and Horvitz, 1984; Locatelli et al., 2004). Species seem to be far less ecologically specialized in America than in Africa. This might be due to a greater species diversity in hummingbirds and Euglossine bees in the neotropics in comparison to blue banded bees (Amegilla spp.) and sunbirds in the palaeotropics (Sakai et al., 1999; Borrow and Demey, 2004; Eardley and Urban, 2009).
In the future the here described relationships between flower and pollinator should be analysed towards the question whether the invoked adaptations really lead to a higher efficiency in the pollen transfer. The latter could be the main reason why the explosive pollination mechanism of the Marantaceae might have fostered the increased speciation in Marantaceae (about 550 species, 31 genera; Andersson, 1998) in comparison to its sister family Cannaceae (ten species, one genus; Kubitzki, 1998) as hypothesized by Kennedy (2000). Current data sets are still contradictory in that matter. While the highly complex pollination mechanism, together with the small pollen load, might suggest such a highly efficient pollen transfer between flowers, evaluations of natural fruit set, pollination experiments (Ley, 2008) and pollen counts on stigmas (Kennedy, 1983), seem to provide evidence for the opposite fact.
CONCLUSIONS
The data presented give a first comprehensive insight into the floral diversity and pollination of African Marantaceae. For the first time, perennial herbs and lianas were subject to such an analysis in Africa. The detection of a diverse pollinator fauna including first records of bird pollination in African Marantaceae and the observations of specific adaptations between flowers and pollinators lead to the hypothesize that pollinators have played an important role in the speciation of this family (see also Ley and Claßen-Bockhoff, 2009).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank the Institut de Recherche en Écologie Tropicale (IRET) which runs the biological station Ipassa at Makokou, the National Herbarium in Libreville (LBV) and the Wildlife Conservation Society Gabon (WCS) for logistical support. We are very grateful to L. Ngok (LBV), J. Wieringa and J. van Valkenberg (both WAG) for their help overcoming all the administrative obstacles and to the field guides J. Bekale, T. Essone and P. Nguema (all three in Monts de Cristal) for looking after our safety in the forest. Further thanks are directed to C. Eardley (Pretoria, South Africa) for identifying the pollinating insects and to L. Klöckner (Mainz) for refining our illustrations. Research permits (no. 0016/06/PM-MEPNRT/CENAREST/CS/PN) were granted by the Scientific Commission of National Parks Gabon. Financial support was given by the Deutsche Forschungsgemeinschaft (DFG: Cl 81/11-1, 81/11-2) and the Deutscher Akademischer Austauschdienst (D/04/39396).
LITERATURE CITED
- Andersson L. Marantaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. IV. Flowering plants. Monocotyledons. Alismatanae and Commelinanae (except Gramineae) Berlin: Springer Verlag; 1998. pp. 278–474. [Google Scholar]
- Appanah S. Plant–pollinator interactions in the Malaysian rain forests. In: Bawa KS, Hadley M, editors. Reproductive ecology of tropical forest plants. Paris/Lancaster: Unesco/Parthenon Publishing Group; 1990. pp. 85–102. [Google Scholar]
- Baker HG. Sugar concentrations in nectars from hummingbird flowers. Biotropica. 1975;7:37–41. [Google Scholar]
- Borrell BJ, Krenn HW. Nectar feeding in long-proboscid insects. In: Herrel A, Speck T, Rowe N, editors. Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. Boca Raton, FL: CRC Press; 2006. pp. 185–211. [Google Scholar]
- Borrow N, Demey R. Birds of western Africa. Oxford: Princeton University Press; 2004. [Google Scholar]
- Brncic T. Oxford University; 2003. Ecology and patch dynamics of Megaphrynium macrostachyum (Benth.) Milne-Redh. (Marantaceae) in the south-west Central African Republic. PhD Thesis. [Google Scholar]
- Claßen-Bockhoff R. Untersuchungen zur Konstruktion des Bestäubungsapparates von Thalia geniculata (Marantaceen) Botanica Acta. 1991;104:183–193. [Google Scholar]
- Claßen-Bockhoff R, Heller A. Floral synorganisation and secondary pollen presentation in four Marantaceae from Costa Rica. International Journal of Plant Science. 2008a;169:745–760. [Google Scholar]
- Claßen-Bockhoff R, Heller A. Style release experiments in four Marantaceae from the Golfo Dulce area in Costa Rica. Stapfia. 2008b;88:557–571. [Google Scholar]
- Clausager K, Borchsenius F. The Marantaceae of Sabah, northern Borneo. Kew Bulletin. 2003;58:647–678. [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Davis MA. The role of flower visitors in the explosive pollination of Thalia geniculata (Marantaceae), a Costa Rican marsh plant. Bulletin of the Torrey Botanical Club. 1987;114:134–138. [Google Scholar]
- Davis SD, Heywood VH, Hamilton AC. Centres of plant diversity: a guide and strategy for their conservation. World Wildlife Fund for Nature and IUCN; 1994. [Google Scholar]
- De Foresta H. Origine et évolution des savanes intramayombiennes (R.P. du Congo). II. Apport de la botanique forestière. In: Lanfranchi R, Schwartz D, editors. Paysages quaternaires de l'Afrique Centrale Atlantique. Pointe-Noire: ORSTOM; 1990. [Google Scholar]
- Dhetchuvi JB. Taxonomie et phytogéographie des Marantaceae et des Zingiberaceae de ĺAfrique Centrale (Gabon, Congo, Zaire, Rwanda et Brundi) Université Libre de Bruxelles; 1996. PhD Thesis. [Google Scholar]
- Eardley CD. A taxonomic revision of the genus Xylocopa Latreille (Hymenoptera: Anthophoridae) in southern Africa. Entomology Memoir, Department of Agriculture, Republic of South Africa. 1983 No. 58. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annual Review of Ecology and Systematics. 2004;35:375–403. [Google Scholar]
- Gathmann A, Tscharntke T. Foraging ranges of solitary bees. Journal of Animal Ecology. 2002;71:757–764. [Google Scholar]
- Gentry AH. Flowering phenology and diversity in tropical Bignoniaceae. Biotropica. 1974;6:54–68. [Google Scholar]
- Gerling D, Velthuis HHW, Hefetz A. Bionomics of the large carpenter bees of the genus Xylocopa. Annual Review of Entomology. 1989;34:163–190. [Google Scholar]
- Gess FW, Gess SK. Nesting and flower visiting of some southern African Anthophorini (Hymenoptera: Apoidea: Apidae: Apinae) Annals of the Cape Province Museums/Natural History. 1996;19:347–373. [Google Scholar]
- Gottsberger G. Beetle pollination and flowering rhythm of Annona spp. (Annonaceae) in Brazil. Plant Systematics and Evolution. 1989;167:165–187. [Google Scholar]
- Harder LD. Effects of nectar concentration and flower depth on flower handling efficiency of bumble bees. Oecologia. 1986;69:309–315. doi: 10.1007/BF00377639. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Energetics of pollination. Annual Review of Ecology and Systematics. 1975;6:139–170. [Google Scholar]
- Kato M. Plant–pollinator interactions in the understorey of a lowland mixed dipterocarp forest in Sarawak. American Journal of Botany. 1996;83:732–743. [Google Scholar]
- Kay KM, Schemske DW. Pollinator assemblages and visitation rates for 11 species of neotropical Costus (Costaceae) Biotropica. 2003;35:198–207. [Google Scholar]
- Kennedy H. Berkeley, CA: University of California Press; 1978. Systematics and pollination of the “closed-flowered” species of Calathea (Marantaceae). [Google Scholar]
- Kennedy H. Calathea insignis (Hoja Negra, Hoja de Sal, Bijagua, Rattlesnake Plant) In: Janzen DH, editor. Costa Rican natural history. Chicago, IL: University of Chicago Press; 1983. pp. 204–207. [Google Scholar]
- Kennedy H. Diversification in pollination mechanisms in the Marantaceae. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne: CSIRO; 2000. pp. 335–343. [Google Scholar]
- Koechlin J. Scitaminales. In: Aubreville A, editor. Flore du Gabon. Paris: Muséum National d'Histoire Naturelle; 1964. pp. 91–158. [Google Scholar]
- Koechlin J. Scitaminales. In: Aubreville A, editor. Flore du Cameroun. Paris: Muséum National d'Histoire Naturelle; 1965. pp. 3–157. [Google Scholar]
- Kress WJ. Phylogeny of the Zingiberanae: morphology and molecules. In: Rudall PJ, Cribb DF, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. London: Royal Botanical Gardens, Kew; 1995. pp. 443–460. [Google Scholar]
- Kubitzki K. Cannaceae. In: Kubitzki K, editor. Families and genera of vascular plants. 4. Flowering plants: monocotyledons Alismatanae and Commelinanae (except Gramineae) Berlin: Springer-Verlag; 1998. pp. 103–106. [Google Scholar]
- Kunze H. Vergleichende Studien an Cannaceen - und Marantaceenblüten. Flora. 1984;175:301–318. [Google Scholar]
- Leite AV, Machado IC. Reproductive phenology, floral biology and pollinators of two sympatric species of Marantaceae from an Atlantic forest fragment, northeastern Brazil. Revista Brasileira de Botânica. 2007;30:221–231. [Google Scholar]
- Leroux M. Num. Sp. Climatologie Tropicale. CEGET/CNRS, Université de Bordeaux; 1975. Climatologie dynamique de l'Afrique: travaux et documents de géographie tropicale 19; pp. 88–111. [Google Scholar]
- Letouzey R. Étude phytogéographique du Cameroun. Paris: Editions Paul Lechevalier; 1968. no. 69. [Google Scholar]
- Ley AC. Evolutionary tendencies in African Marantaceae: evidence from floral morphology, ecology and phylogeny. Germany: University of Mainz; 2008. PhD Thesis. [Google Scholar]
- Ley AC, Claßen-Bockhoff R. Evolution in African Marantaceae: evidence from phylogenetic, ecological and morphological studies. American Journal of Botany. 2009 (in press). [Google Scholar]
- Locatelli E, Machado IC, Medeiros P. Saranthe klotzschiana (Koer.) Eichl. (Marantaceae) e seu mecanismo explosivo de polinizacao. Revista Brasiliera de botanica. 2004;27:757–765. [Google Scholar]
- Louw GN, Nicolson SW. Thermal, energetic and nutritional considerations in the foraging and reproduction of the carpenter bee Xylocopa capitata. Journal of the Entomological Society of Southern Africa. 1983;46:227–240. [Google Scholar]
- Manning JC, Goldblatt P. International Journal of Plant Sciences. Vol. 166. Iridaceae: Crocoideae) and the development of bimodal pollination strategies; 2005. Radiation of pollination systems in the cape genus Tritoniopsis; pp. 459–474. [Google Scholar]
- Momose K, Yumoto T, Nagamitsu T, et al. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant–pollinator community in a lowland dipterocarp forest. American Journal of Botany. 1998;85:1477–1501. [PubMed] [Google Scholar]
- Nicolson SW. Pollination by passerine birds: why are the nectars so dilute? Comparative Biochemistry and Physiology, B. 2002;131:645–652. doi: 10.1016/s1096-4959(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Nicolson SW, Fleming PA. Energy balance in the whitebellied sunbird, Nectarinia talatala: constraints on compensatory feeding, and consumption of supplementary water. Functional Ecology. 2003;17:3–9. [Google Scholar]
- Nilsson LA, Jonsson L, Rason L, Randrianjohany E. Pollination of Plectranthus vestitus (Lamiaceae) by trap-lining hovering bees in Madagascar. Plant Systematics and Evolution. 1985;150:223–236. [Google Scholar]
- Ollerton J, Killick A, Lamborn E, Watts S, Whiston M. Multiple meanings and modes: on the many ways to be a generalist flower. Taxon. 2007;56:717–728. [Google Scholar]
- Percival MS. Floral ecology of costal scrub in southeast Jamaica. Biotropica. 1974;6:104–129. [Google Scholar]
- Pischtschan E, Claßen-Bockhoff R. Setting-up tension in the style of Marantaceae. Plant Biology. 2008;10:441–450. doi: 10.1111/j.1438-8677.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- Prince LM, Kress WJ. Phylogenetic relationships and classification in Marantaceae: insights from plastid DNA sequence data. Taxon. 2006;55:281–296. [Google Scholar]
- Roubik DW, Yanega D, Aluja S M, Buchmann SL, Inouye DW. On optimal nectar foraging by some tropical bees (Hymenoptera: Apidae) Apidologie. 1995;26:197–211. [Google Scholar]
- Saint-Vil J. Les climats du Gabon. Annales de l'Université Nationale du Gabon. 1977;1:101–125. [Google Scholar]
- Sakai S, Kato M, Inoue T. Three pollination guilds and variation in floral characteristics of Bornean gingers (Zingiberaceae and Costaceae) American Journal of Botany. 1999;86:646–658. [PubMed] [Google Scholar]
- Schemske DW. Floral convergence and pollinator sharing in two bee-pollinated tropical herbs. Ecology. 1981;62:946–954. [Google Scholar]
- Schemske DW, Horvitz CC. Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science. 1984;235:519–521. doi: 10.1126/science.225.4661.519. [DOI] [PubMed] [Google Scholar]
- Specht CD, Kress WJ, Stevenson DW, DeSalle R. A molecular phylogeny of Costaceae (Zingiberales) Molecular Phylogenetics and Evolution. 2001;21:333–345. doi: 10.1006/mpev.2001.1029. [DOI] [PubMed] [Google Scholar]
- Stebbins GL. Adaptative radiation of reproductive characteristics in angiosperms. I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Stephenson AG. An evolutionary examination of the floral display of Catalpa speciosa (Bignoniaceae) Evolution. 1979;33:1200–1209. doi: 10.1111/j.1558-5646.1979.tb04773.x. [DOI] [PubMed] [Google Scholar]
- Stiles FG. Taste preferences, color preferences, and flower choice in hummingbirds. Condor. 1976;78:10–26. [Google Scholar]
- Stiles FG. Ecological and evolutionary implications of bird pollination. American Zoologist. 1978;18:715–727. [Google Scholar]
- Vande wegh JP. Forêts d'Afrique centrale. La nature et l'homme. Tielt, Belgique: Editions Lannoo SA; 2004. ECOFAC. [Google Scholar]
- Vogel S. Blütenbiologische Typen als Elemente der Sippengliederung dargestellt anhand der Flora Südafrikas. Botanische Studien. 1954;1:1–338. [Google Scholar]
- Walker-Larsen J, Harder LD. The evolution of staminodes in angiosperms: patterns of stamen reduction, loss and functional re-invention. American Journal of Botany. 2000;87:1367–1384. [PubMed] [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology. 1996;77:1043–1060. [Google Scholar]
- Wester P, Classen-Bockhoff R. Floral diversity and pollen transfer mechanisms in bird-pollinated Salvia species. Annals of Botany. 2007;100:401–421. doi: 10.1093/aob/mcm036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerkamp Chr. Bird-flowers: hovering versus perching exploitation. Botanica Acta. 1990;103:366–371. [Google Scholar]
- Westerkamp Chr. The co-operation between the asymmetric flower of Lathyrus latifolius (Fabaceae-Vicieae) and its visitors. Phyton (Horn, Austria) 1993;33:121–137. [Google Scholar]
- Willmer PG, Stone GN. Behavioral, ecological, and physiological determinants of the activity patterns of bees. Advances in the Study of Behavior. 2004;34:347–466. [Google Scholar]
- Wilson P, Castellanos MS, Hogue JN, Thomson JD, Armbruster WS. A multivariate search for pollination syndromes among penstemons. Oikos. 2004;104:345–361. [Google Scholar]
- Yeo P. Secondary pollen presentation: form, function and evolution. Plant Systematics and Evolution. 1993;(Suppl. 6):1–268. [Google Scholar]