Abstract
Background and Aims
Apomictic plants maintain functional pollen, and via pollination the genetic factors controlling apomixis can be potentially transferred to congeneric sexual populations. In contrast, the sexual individuals do not fertilize apomictic plants which produce seeds without fertilization of the egg cells. This unidirectional introgressive hybridization is expected finally to replace sexuality by apomixis and is thought to be a causal factor for the wide geographical distribution of apomictic complexes. Nevertheless, this process may be inhibited by induced selfing (mentor effects) of otherwise self-incompatible sexual individuals. Here whether mentor effects or actual cross-fertilization takes place between diploid sexual and polyploid apomictic cytotypes in the Ranunculus auricomus complex was tested via experimental crosses.
Methods
Diploid sexual mother plants were pollinated with tetra- and hexaploid apomictic pollen donators by hand, and the amount of well-developed seed compared with aborted seed was evaluated. The reproductive pathways were assessed in the well-developed seed via flow cytometric seed screen (FCSS).
Key Results
The majority of seed was aborted; the well-developed seeds have resulted from both mentor effects and cross-fertilization at very low frequencies (1·3 and 1·6 % of achenes, respectively). Pollination by 4x apomictic pollen plants results more frequently in cross-fertilization, whereas pollen from 6x plants more frequently induced mentor effects.
Conclusions
It is concluded that introgression of apomixis into sexual populations is limited by ploidy barriers in the R. auricomus complex, and to a minor extent by mentor effects. In mixed populations, sexuality cannot be replaced by apomixis because the higher fertility of sexual populations still compensates the low frequencies of potential introgression of apomixis.
Key words: Apomixis, Ranunculus auricomus, evolution, geographical parthenogenesis, crossing experiments, flow cytometry
INTRODUCTION
Asexual reproduction is often thought to confer short-term evolutionary advantages by avoiding the 2-fold costs of sexuality: the cost of producing male individuals or organs, and avoiding the break-up of favourable gene combinations at meiosis (e.g. Bell, 1982). Nevertheless, a short-term success of asexual organisms is manifested in distribution patterns called geographical parthenogenesis, which describes the widespread phenomenon whereby asexual populations often have larger distribution ranges, tend to higher altitudes and latitudes, and are more abundant in previously glaciated areas than their sexual relatives (e.g. Van Dijk, 2003; Kearney, 2005; Hörandl, 2006).
Several hypotheses have been discussed to explain causality and evolutionary implications of geographical parthenogenesis: (a) indirect benefits from polyploidy and/or hybrid origin of asexual organisms (e.g. Kearney, 2005); (b) advantages of uniparental reproduction (Stebbins, 1950; Baker, 1967; Hörandl et al., 2008); (c) gene flow from apomicts to sexuals and introgression of apomixis into sexual populations (Mogie, 1992; Mogie et al., 2007); (d) production of generalists (general purpose genotypes; Baker and Stebbins, 1965; Lynch, 1984) vs. specialized genotypes (Vrijenhoek, 1984, 1994); or (e) biotic interactions with other organisms (Maynard Smith, 1978; Bell, 1982; Glesener and Tilman, 1987). These factors may have different impacts in various groups of organisms, but may also act synergistically (Hörandl, 2006). Climatic changes may provide opportunities for origins of asexual organisms, which arise mainly from hybrids of sexual species or from introgressive hybridization (Hörandl, 2009). We wanted to test specifically the potential of apomicts to swamp sexuality by introgressive hybridization, as predicted by theory (e.g. Mogie, 1992; Mogie et al., 2007).
In flowering plants, apomixis, the mode of reproduction via asexually formed seed (Asker and Jerling, 1992), is frequently connected to geographical parthenogenesis (Bierzychudek, 1985; Hörandl, 2006; Hörandl et al., 2008). The geographical phenomenon is mostly observed in cases of gametophytic apomixis (Richards, 1997), which comprises three functional steps: (1) the formation of an unreduced egg cell (via formation of an unreduced embryo sac); (2) the parthenogenetic development of the embryo, i.e. without fertilization of the egg cell; and (3) the formation of the endosperm, which provides nutritious tissue for the embryo. In the majority of apomictic plants, fertilization of the polar nuclei by one sperm nucleus is still necessary for the development of the endosperm (pseudogamy) because of genomic imprinting in the endosperm (e.g. Vinkenoog et al., 2003; Curtis and Grossniklaus, 2008). Male-sterile mutants may initially spread because of fitness advantages, but finally the scarcity of pollen drives the population to extinction. Male-sterile plants and hermaphrodites may co-exist under spatial conditions with a sufficiently large distance of pollen and seed dispersal (Stewart-Cox et al., 2005). Only a few taxa, mainly Asteraceae, are capable of autonomous (pollen-independent) endosperm formation, but nevertheless maintain pollen production. This may be explained by selection for clonal diversity, which is largely dependent on gene flow via the pollen function (Meirmans et al., 2006). For these reasons, the male function is maintained and viable pollen is still produced in almost all apomictic plants.
Apomixis is a heritable trait and under genetic control (e.g. Nogler, 1984; Grimanelli et al., 2001; Curtis and Grossniklaus, 2007). Hence the genetic factors controlling apomixis can be potentially transferred from an apomictic individual to the offspring of a sexual plant via pollen. Consequently, the offspring of the fertilized sexual individual will be at least partly apomictic. In contrast, the pollen of the sexual individuals will not fertilize the apomicts and will not result in the inheritance of the sexual wild type. Because of this unidirectional hybridization, apomixis has a 1·5-fold fitness advantage over sexuality, and in mixed populations apomixis is expected soon to come to fixation (Mogie, 1992). This model is based on a simple heritability mechanism of apomixis via a single genetic factor and all progeny expressing the trait. In fact, multilocus genetic control and epigenetic effects may actually alter expression in many apomictic plants (Curtis and Grossniklaus, 2007; Vijverberg and Van Dijk, 2007). In facultative apomicts, reverse gene flow from sexuals to apomicts and potential re-establishment of sexuality has to be considered. Nevertheless, Mogie's (1992) hypothesis provides a theoretical basis for testing actual gene flow from apomicts to sexuals in a model system with a single-locus control of apomixis.
During range expansions, apomicts have additionally a superior colonizing ability because of uniparental reproduction. The faster moving apomicts will build up a barrier against the slower moving sexual populations because the latter will always be pollinated mainly by the more abundant apomictic individuals. Consequently, sexuality would at best remain in geographically isolated relic populations but otherwise introgressed and replaced by apomixis (e.g. Mogie, 1992; Mogie et al., 2007). Side effects also have to be considered. Hybridization of apomictic lineages with sexually related species increases genotypic diversity of the former (Hörandl and Paun, 2007). Diversity of clones or genotypes, in turn, is an important prerequisite for colonizing various ecological niches and is another important causal factor of geographical parthenogenesis (Vrijenhoek, 1984, 1994; Hörandl, 2006).
Introgression of genetic factors controlling apomixis into sexual species is further thought to contribute to the taxonomic distribution of apomixis in angiosperms. Even if apomictic lineages would be short-lived, apomixis genes can be transferred to novel genetic backgrounds via introgression and could survive for long evolutionary time periods. This mechanism may, beside pre-adapations for apomixis, explain the observed clustering of apomixis in related genera of angiosperms at the tribal and subtribal level (Van Dijk and Vivjerberg, 2005).
The actual amount of introgression of apomixis into sexual individuals depends on various factors. First, actual amounts of female and male fitness may vary considerably among apomictic taxa. While pseudogamous taxa often have a lower female fitness compared with sexuals (e.g. Huber, 1988; Voigt et al., 2007; Hörandl, 2008), this may be the other way round in autonomous apomixis (e.g. Michaels and Bazzaz, 1986; Van Dijk, 2007). If we take fitness differences into account, and assume that half of the pollen of apomicts fertilizes sexual individuals and transfers apomixis to their offspring, then the frequencies of apomixis will increase in the population if
where fasex is the female fitness of asexual individuals and fsex that of the sexual individuals, and m is the respective male fitness quotient (Mogie, 1992). If m = 1, then the f quotient must only exceed 0·5, or, in other words, asexual seed set needs to exceed only half of that of the sexual individuals to increase the frequency of apomixis in the population. One questionable variable is the value of m, which is likely to be lower than 1 (Mogie et al., 2007); apomicts usually produce higher frequencies of aborted pollen than sexual species because of meiotic disturbances during microsporogenesis.
The 50 % fertilization success of apomictic pollen on sexual mother plants is a crucial variable for the success of introgression and cannot be taken for granted. It must be considered that sexual relatives of apomictic plants are most frequently diploid, whereas apomixis in flowering plants is almost exclusively connected to polyploidy (e.g. Asker and Jerling, 1992). Crosses between different ploidy levels are often unsuccessful because of endosperm incompatibilities (e.g. Nogler, 1984; Levin, 2002). Moreover, so-called mentor effects may block the cross-fertilization of sexual individuals caused by mixtures of self-pollen and apomictic pollen on their stigmas. Such mixtures may lead to a breakdown of self-incompatibility systems in outcrossing sexuals, and therefore to selfing (e.g. Richards, 1997). This mentor effect has been proved in many sexual, otherwise self-incompatible taxa of apomictic complexes (Tas and Van Dijk, 1999; Mráz, 2003; Brock, 2004). Sexual progeny derived from selfing will have an increase of homozygosity and may potentially suffer from inbreeding depression; nevertheless, selfing of sexuals will reduce frequencies of introgressed apomictic progeny. Finally, frequencies of cross-pollinations between sexuals and apomictic individuals may be limited by other pre-mating barriers to crossing, such as different flowering times, different frequencies of pollinator visits or microniche differentiation.
The apomictic Ranunculus auricomus complex provides a model system for studying various factors of geographical parthenogenesis. Most polyploid biotypes show aposporous apomixis, i.e. a somatic cell of the nucellus divides to form an unreduced, 8-nucleate embryo sac (Häfliger, 1943; Izmaiłow, 1967; Nogler, 1971, 1984). Apospory is heritable and controlled by a single dominant Mendelian factor A– which can be transferred to the offspring of sexual species via the pollen (Nogler, 1984). The unreduced egg cell develops parthenogenetically into an embryo, which is genetically identical to the mother plant. Pollination is necessary for fertilization of the endosperm; without pollination, seed set fails (Rutishauser, 1954; Izmaiłow, 1967; Hörandl, 2008). The pollen of apomictic plants is meiotically reduced, partly aborted, but remains to some extent functional (Häfliger, 1943; Izmaiłow, 1996; Hörandl et al., 1997). The apomictic biotypes are characterized by bad pollen quality, reduced petals and partly aborted fruits (Häfliger, 1943; Izmaiłow, 1996; Hörandl et al., 1997; Lohwasser, 2001; Hörandl, 2008). These developmental disturbances are usually thought to be a consequence of the hybrid origin of apomicts (e.g. Izmaiłow, 1967; Paun et al., 2006b). Apomixis in the R. auricomus complex is always connected to polyploidy.
The four sexual species known have small, partly disjunct distribution areas in central and southern Europe (Hörandl and Paun, 2007). Apomictic polyploid cytotypes have been reported throughout the range of the complex in the temperate to arctic zones of Europe and Asia (e.g. Jalas and Suominen, 1989). Cytological and histological investigations have confirmed apomixis throughout the range of the complex, thus confirming a pattern of geographical parthenogenesis. The causal factors still remain to be studied. When pollinators are available, apomictic biotypes have a significantly lower female reproductive fitness compared with sexuals because of high frequencies of aborted fruits (Lohwasser, 2001; Hörandl, 2008). Nevertheless, the mean female asexual seed set regularly exceeds 50 % of that of the sexuals, but germination rates of sexual or apomictic taxa are not significantly different (Hörandl, 2008). This infers that cross-fertilization could potentially lead to an introgression of apomixis into sexual populations, and finally replacement of sexual individuals. This process could have swamped sexual populations and contributed to the present predominance of apomictic lineages.
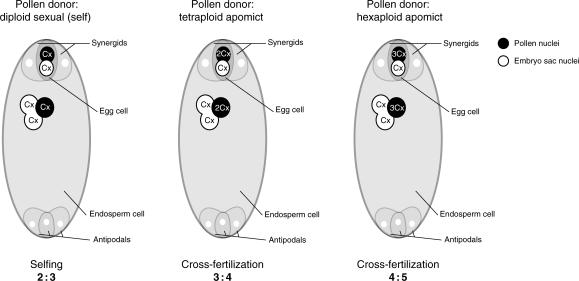
Here we wanted to test to what extent the pollination of diploid sexuals by polyploid apomicts actually results in cross-fertilization as a potential causal factor of the wide distribution of the apomictic complex. The diploid sexual species R. cassubicifolius and R. carpaticola were use here as mother plants and their sympatric polyploid apomictic hybrid derivatives were used as pollen donors; from this model system, information on fertility is available (Hörandl, 2008). To exclude other potential pre-mating barriers to crossing in natural populations, we tried an experimental approach with plants collected in the wild and cultivated in the experimental garden. We assessed whether hand-performed cross- and self-pollinations result in cross-fertilization and transfer of apomixis to sexuals, or rather in induced selfing (mentor effects). Seeds were analysed via flow cytometric seed screen (FCSS), a highly efficient method measuring the ratio of DNA content in the embryo compared with the endosperm arising from different ploidy levels. This ratio is informative about reproductive pathways and has been used in numerous studies for assessment of modes of reproduction and quantification of progeny (Matzk et al., 2000; Cáceres et al., 2001; Barcaccia et al., 2006, 2007; Krahulcová and Suda, 2006; Hufft Kao, 2007; Matzk, 2007; Talent and Dickinson, 2007; Voigt et al., 2007; Hörandl et al., 2008). In our experimental set-up, induced selfing would result in embryo:endosperm ratios of 2:3, while cross-fertilization would result in ratios of 3:4 and 4:5, respectively (Fig. 1). By including information on fitness and other reproductive parameters, such as self-compatibility and fitness of apomictic biotypes (Hörandl, 2008), we wanted to gain insights into the impact of these processes on the observed distribution patterns and on the evolution of the complex.
Fig. 1.
Scheme of the expected ratios of DNA content in the embryo compared with the endosperm on a diploid sexual mother plant with a reduced embryo sac. (A) Sexual reproduction or selfing; (B) cross-fertilization by a tetraploid pollen donor; (C) cross-fertilization by a hexaploid pollen donor (terminology after Greilhuber et al., 2005).
MATERIALS AND METHODS
Plant materials were collected from natural populations of Ranunculus (see Table 1 and Hörandl et al., 2000; Hörandl and Greilhuber, 2002; Paun et al., 2006a, b), and cultivated in the experimental garden of the Botanical Garden of the University of Vienna, in a half-shaded area under a large tree, resembling the natural forest habitats of the plants. Plants were selected to represent different ploidy levels and sexual vs. apomictic reproduction, according to information from earlier studies (Hörandl et al., 1997, 2000, 2008; Hörandl and Greilhuber, 2002; Paun et al., 2006a, b; Hörandl, 2008). For the sexual species, plants from three different populations were used as mother plants. On a few individuals, flow cytometric measurements were performed on leaves to confirm ploidy levels and pg values as determined in earlier studies. These measurements were in accordance with earlier DNA content measurements on these populations (see Table 1). In 2007, test runs on open-pollinated control plants of diploid sexual R. cassubicifolius revealed embryo : endosperm ratio peaks of 1·56–1·80. Cross-pollination of sexual individuals with pollen from apomicts revealed a clear case of a mentor effect, with an embryo:endosperm ratio of 1·56 (Hörandl et al., 2008). Here the aim was to try to quantify the occurrence of mentor effects vs. cross-fertilization on a larger data set, which was collected in spring 2008 (Table 2). Plants from one tetraploid population of R. × hungaricus (Hörandl et al., 2009), and three hexaploid populations of R. carpaticola × cassubicifolius (Paun et al., 2006b; Table 1) donated the apomictic pollen.
Table 1.
Materials used in this study
| Taxa (no. of individuals) | Chromosome no. ploidy level, somatic 1C-values (pg DNA) | Locality | Collectors, population number (herbarium) |
|---|---|---|---|
| Sexual mother plants | |||
| R. cassubicifolius (4) | 2n = 16 (2x)*, 3·11 pg | Austria, border Burgenland/Hungary, Hammer | Hörandl, 9562, and Paun, 46 (WU) |
| R. carpaticola (3) | 2n = 16 (2x), 3·06 pg† | Slovakia, Slovenské rudohorie, Revúca, hill Skalka | Hörandl, 8483 (WU) |
| R. carpaticola (7) | 2n = 16 (2x), 3·06 pg*, 2·93–3·05 pg | Slovakia, Slovenské rudohorie, Revúca, hill Pavlusove bane | Hörandl, 8486 (WU) |
| Apomictic pollen donor | |||
| R. carpaticola × cassubicifolius (2) | 48 (6x)*, 7·89–8·11 pg*,† | Slovakia, Liptovská kotlina, Ružomberok | Klimová, Paun and Hörandl, C40 (SAV) |
| R. carpaticola × cassubicifolius (1) | 48 (6x), 7·91–7·99* | C. Slovakia, Liptovská kotlina, Liptovský hrádok | Klimová, Paun and Hörandl, C44 (SAV) |
| R. carpaticola × cassubicifolius (1) | 48 (6x), 7·61–7·73 pg* | C. Slovakia, Turčianska kotlina, Vrútky-Piatrová | Klimová, Paun and Hörandl, C35 (SAV) |
| R. × hungaricus (1) | 32 (4x), 6·17 pg‡ | E. Slovakia, Nízke Beskydy, near NPR Humenská | Klimová, C10, 23.5.2003 (SAV) |
‡ E. Hörandl et al. (unpubl. res.; data J. Greilhuber).
Table 2.
Results of crossings of diploid sexual plants and polyploid apomictic plants (well-developed seeds only) analysed by FCSS (mother plants designated by population number/individuals number)
| Mother plant | Pollen donor | Ploidy pollen plant | No. of good achenes | DNA content pg (1C) | Ratio of first peak to diploid pg value* | Histogram peaks | Mode of reproduction |
|---|---|---|---|---|---|---|---|
| 46/10 | 10/1 | 4x | 3 | 6·75 | 2·2 | 4x endosperm | Hybridization |
| 46/10 | 10/1 | 4x | 1 | 6·50 | 2·1 | 4x endosperm | Hybridization |
| 46/12 | 10/1 | 4x | 8 | 4·99 | 1·6 | 3x embryo, 4x endosperm | Hybridization |
| 46/03 | 10/1 | 4x | 1 | 6·53 | 2·1 | 4x endosperm | Hybridization |
| 46/03 | 10/1 | 4x | 1 | 5·97 | 1·9 | 4x endosperm | Hybridization |
| 8483/25 | 10/1 | 4x | 3 | 6·34 | 2·1 | 4x endosperm | Hybridization |
| 8483/25 | 10/1 | 4x | 1 | 6·30 | 2·1 | 4x endosperm | Hybridization |
| 8483/05 | 10/1 | 4x | 4 | 7·00 | 2·3 | 4x endosperm | Hybridization |
| 8483/05 | 10/1 | 4x | 6 | n.a. | n.a. | 4x endosperm | Hybridization |
| 8486/12 | 10/1 | 4x | 2 | n.a. | n.a. | 4x endosperm | Hybridization |
| 46/17 | 40/1 | 6x | 1 | 10·80 | 3·5 | 6–7x endosperm | Hybridization |
| 8483/25 | 10/1 | 4x | 2 | 5·18 | 1·7 | 3x endosperm | Mentor |
| 46/06 | 44/3 | 6x | 4 | 5·35 | 1·7 | 3x endosperm | Mentor |
| 46/06 | 44/3 | 6x | 2 | 5·18 | 1·7 | 3x endosperm | Mentor |
| 46/06 | 44/3 | 6x | 1 | 5·38 | 1·7 | 3x endosperm | Mentor |
| 8486/22 | 40/1 | 6x | 3 | n.a. | n.a. | 2x embryo, 3x endosperm | Mentor |
| 8486/12 | 10/1 | 4x | 1 | n.a. | n.a. | 4x, 8x, 12x and 16x | cf. hybridization |
| 46/14 | 40/5 | 6x | 12 | n.a. | n.a. | Approx. 16x endosperm | cf. hybridization |
Each measurement represents the pooled seed set of 1–3 flowers. Flowers with zero seed set are not included here. Cases without a pg value were measured without standards; see text.
* Measured from leaves, see Table 1.
Self-incompatibility of the diploid sexual species has been assessed in an earlier study by a pollinator exclusion test on the same plants as used here, plus on other individuals of the same populations (Hörandl, 2008). For the introgression study, flowers were enclosed from the bud stage until fruit maturity in cellophane bags, and bags were opened only for controlled hand-pollinations with tetraploid or hexaploid apomictic pollen plants (Table 1). For this purpose, plants were taken indoors to avoid possible influence from airborne pollen during the manipulations. One flower of an apomictic polyploid individual was gently rubbed on the flowers of a sexual individual during the period of stigma receptivity. Because of the close vicinity of anthers and carpels in flowers, this manipulation produced a mix of self and foreign pollen on the stigma, as happens under natural conditions. At fruit maturity, the total fruits (developed plus aborted achenes) were collected, and achenes were counted to assess retrospectively the total number of ovules per flower according to the methods of Hörandl (2008). In well-developed achenes, the pericarp was removed, and the seed (testa + embryo + endosperm tissue) was prepared for flow cytometry. Many achenes appeared to be empty, and the frequency of aborted seeds compared with the total number of achenes was calculated both for flowers and for the total number of ovules to get an estimate of the total rates of seed abortion, mentor effects and interploidal cross-fertilization. Around 100 achenes from three flowers were germinated on wet filter paper at room temperature, but failed to germinate completely, confirming an overall very low rate of seed set (see Results).
Well-developed seeds were analysed via FCSS in order to assess the reproductive pathways. The seeds and the tissue of the standard organisms were chopped according to Galbraith et al. (1983) in Otto I buffer (Otto et al., 1981) using a razor blade. After filtration through a 30 µm mesh and incubation with RNase A (0·15 mg mL−1) at 37 °C for 30 min, Otto II buffer (Otto et al., 1981) containing propidium iodide (PI; final concentration 50 µg mL−1; Greilhuber et al., 2007) was added. Staining was carried out at 7 °C from 1 h up to overnight. For measurement, a CyFlow ML flow cytometer (Partec, Muenster, Germany) equipped with a green laser (520 nm, Cobolt Samba, Cobolt AB, Stockholm, Sweden) was used. Seeds were either used singly or were pooled (Table 2). Data calculation was performed on the resulting histograms either from the peaks of embryo:endosperm ratios, or by evaluating pg values measured from external standards (Pisum sativum ‘Kleine Rheinländerin’, 1C = 4·42 pg DNA; Greilhuber and Ebert, 1994). The expected embryo: endosperm ratios after selfing and outcrossing are illustrated in Fig. 1. For an estimate of the potential for introgression according to Mogie's formula, we used mean values of seed set of diploid sexual plants and the hexaploid apomictic plants from 3 years from the data set of Hörandl (2008) as fitness measures.
RESULTS
Altogether seed set was very low after all experimental pollinations. From a total of 49 flowers, 1960 achenes were produced (mean: 50·3 achenes per flower), but only 69 (3·5 %) of them had normally developed seed that could be analysed via FCSS (Table 2). Surprisingly, many achenes had a well-developed hardened and brown pericarp, but were empty inside; this is in contrast to seed abortion in bagged self-incompatible plants. Here even the pericarp was not developed, and achenes remained dwarfish, yellow and empty (Hörandl, 2008). In the experiments presented here, even those flowers with some well-developed achenes had very low amounts of seed set, ranging from one to 12 achenes per flower. The percentage of well-developed achenes per collective fruit ranged from 0·8 to 31·7 %.
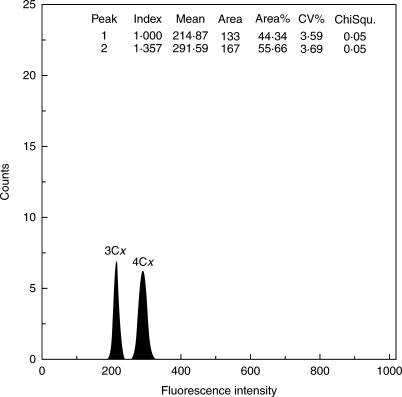
Flow cytometric histograms revealed ideally two peaks for embryo and endosperm, as shown in Fig. 2. In some measurements, the embryo peak was not clearly visible, but only a single endosperm peak, sometimes accompanied by smaller G2 peaks of the growing endosperm, was observed. This can be explained by the delayed development of the embryo compared with the endosperm in the R. auricomus complex, as described in detail by Izmaiłow (1967): most egg cells start to divide only 11 d after pollination; at this time, the endosperm already has 500–1000 nuclei. Even at the fruit stage, the small embryo may often not provide enough tissue to be detected by flow cytometry, especially if single achenes are being analysed. Nevertheless, the ploidy level of the endosperm peak could be assessed via DNA content. Genome size in the endosperm ranged from 4·99 to 5·38 pg (triploid), from 6·30 to 7·00 pg (tetraploid) and up to 10·80 pg (hexa- to heptaploid); Table 2.
Fig. 2.
Flow cytometry histogram (fluorescence intensity vs. number of particles) of seeds (mother plant R. cassubicifolius 46/12) showing a 3Cx embryo and a 4Cx endosperm peak, indicating cross-fertilization by the 4x apomictic pollen donator.
In five measurements, FCSS revealed a triploid endosperm peak as can result only from haploid pollen. Two such cases were obtained after pollination by a tetraploid and four after pollination by a hexaploid pollen donor (Table 2). The diploid embryo peak was clearly visible in two histograms; in the other cases, triploid endosperm could be inferred from somatic C-values (Table 1). We infer from these observations that the seed originated from induced self-fertilization (mentor effects) by the plant's own pollen. From the total number of achenes produced, 1·3 % resulted in such selfed diploid sexual seed.
Increased ploidy levels of the endosperm were observed in 13 measurements altogether; in 11 of them, the expected ploidy level of endosperm after cross-fertilization was observed. In most cases, a tetraploid endosperm was revealed after pollination by a tetraploid pollen donor (Table 2); the expected triploid embryo peak was sometimes visible (Fig. 2). Only in one flower with a single seed was a 6–7x endosperm (most probably hexaploid) observed after pollination by a hexaploid pollen donor. In this case, we suppose that 4x pollen originated from a disturbed meiosis of the apomictic plant and fertilized both embryo and the 2x endosperm. Alternatively, the embryo sac of the sexual was unreduced and 3x pollen fertilized a 4x endosperm. In any case, cross-fertilization is inferred. Seeds that have resulted from cross-fertilizations developed only in 1·6 % of the total number of achenes. In two samples, unexpected high ploidy levels were observed (one sample of single seeds with 4x, 8x and 16x peaks and one pooled sample of 12 seeds with a peak at approx. 16x). It is supposed that endomitotic endosperm has developed after fertilization by pollen of the apomicts, or that unreduced gametes have been involved. These seeds have probably also resulted from hybridization, but represented just 0·7 % of the total seed set.
DISCUSSION
Our results confirm the utility of FCSS for assessment of modes of reproduction (Matzk et al., 2000; Matzk, 2007; Hörandl et al., 2008). Intraspecific variation of C-values in the parental individuals explains the minor deviations of observed embryo:endosperm ratios from the exact mathematical ratios of 2:3 for sexual selfing and 3:4 for cross-fertilization (Table 2).
The results confirm the occurrence of mentor effects in the R. auricomus complex, but at very low frequencies. In experimental crosses of Taraxacum, mentor effects were observed in 89 % of the offspring after pollination of diploid sexual plants with pollen from triploid apomicts (Tas and Van Dijk, 1999). The authors suggested that aneuploid pollen or interploidal crosses may cause the breakdown of the self-incompatibility system. Our data also show a more frequent occurrence of mentor effects after pollination by hexaploid than by tetraploid pollen donors (Table 2). For a generalization, however, more data and statistical tests would be needed. The functional background of mentor effects is still not well understood, and studies need to be carried out to determine whether a greater difference in ploidy levels, a certain allelic composition or the genetic distance of parental individuals causes the breakdown of self-incompatibility systems. Mentor effects have been mainly reported in heterospecific crosses and can be facilitated by inactive or dead pollen (de Nettancourt, 2001). The hexaploid apomictic cytotype used here is probably a hybrid derivative of the sexual species (Paun et al., 2006b), and not a distant taxon. It is more likely that the high percentages of aborted pollen in apomicts and the different ploidy levels induce a mentor effect in our study taxa. In Ranunculus, several pollen tubes grow in parallel in competition, such that only one pollen tube reaches the micropyle (Rendle and Murray, 1988). The higher frequencies of good pollen grains of sexual individuals probably confer a quantitative advantage of self-pollen tubes by increasing the likelihood of self-fertilization compared with apomictic pollen tubes.
The frequencies of seed derived from mentor effects remain strikingly low. This may be explained by an incomplete breakdown of the self-incompatibility system. Ranunculus probably has a gametophytic self-incompatibility system (Hörandl, 2008), where mentor effects are less likely (de Nettancourt, 2001). Details of pollen tube growth and inhibition, however, need to be studied. Another possible reason for low seed set may be early abortion of selfed seed because of inbreeding depression (Tas and Van Dijk, 1999). However, mentor effects probably have a rather limited impact on the genetic population structure of diploid sexuals. In natural populations of dandelions, mentor effects occur at even lower frequencies than in experimental crosses (e.g. Brock, 2004). A potential reduction of fitness of sexuals because of inbreeding depression needs to be studied, but the impact of induced selfing on population genetic structure is probably negligible. The phenomenon may instead be of importance by inhibiting introgression of apomixis into sexual individuals.
The frequencies of actual cross-fertilized seeds were also very low even after enforced crossing, inferring that actual introgression of apomixis genes into sexual populations occurs much more rarerly than theoretically expected. The exclusion of pre-mating crossing barriers in the experimental set-up infers that incompatibilities between cytotypes probably act as a strong barrier to crossing (Nogler, 1984). Possible reasons for failure of seed set are manifold. First, percentages of good pollen in apomictic R. auricomus are usually very low (Izmaiłow, 1996; Hörandl et al., 1997). As discussed above, low frequencies of viable pollen tubes may be a disadvantage for the success of pollination by apomicts. This disadvantage could theoretically be compensated by higher frequencies of apomictic individuals in a natural population, thus increasing the total quantity of apomictic pollen. Secondly, seed set may fail because of endosperm imbalance. In angiosperms, a 2 : 1 ratio of the maternal to the paternal genome in the endosperm is optimal for endosperm development because of genomic imprinting; shifts of this ratio result in developmental disturbances or even seed abortion (Spielmann et al., 2003; Vinkenoog et al., 2003). Apomictic plants have evolved various modifications of reproduction to maintain this ratio (e.g. Savidan, 2007). Fertilization of diploid endosperm nuclei by diploid or triploid apomictic pollen may cause failure of endosperm development and consequently abortion of the embryo. In R. auricomus, the 2 : 1 ratio in the endosperm may be conserved, because even tetraploid pseudogamous plants tend to maintain it by using both pollen nuclei for fertilization of the endosperm (Rutishauser, 1954; Talent and Dickinson, 2007). The endosperm problem of pseudogamous plants brings further minority cytotype exclusion principles into play (Levin, 1975, 2002): a rare apomictic individual in a population will be mainly pollinated with haploid pollen of the dominating sexual individuals, resulting in reduced fertility. The minority cytotype will consequently have problems in establishing in a population. Introgression of apomixis probably follows a single-locus model of inheritance of apospory (Nogler, 1984). Uncoupling of apospory and parthenogenesis is possible, but extremely rare (Nogler, 1995). Theoretically, this infers that unreduced egg cells of apomictic plants may also occasionally be fertilized by sexual pollen. Such hybrids are extremely rare even in experimental crossings (Nogler, 1995). Moreover, expression of apomixis is dosage dependent. Haploid sexual pollen transfers only one copy of the wild-type allele (A+) to a polyploid apomict that might have more copies of the allele controlling apospory (A–). To what extent fertilization by sexuals actually re-establishes sexual phenotypes in the offspring needs to be studied. It is more likely that such processes contribute to the origin of novel apomictic genotypes. Altogether we face an unexpected complexity when estimating actual introgression of apomixis into sexuality.
If we try to estimate the basic potential for introgression by using Mogie's formula with data gained from experimental approaches, we get the following values (values for female fitness fasex/fsex taken from mean numbers of well-developed achenes per flower in Hörandl, 2008, multiplied by frequencies of cross-fertilized seed set as observed here, and male fitness m set as 1): 17·047/27·419 + 0·016 = 0·638. That is, even if we leave complexity of external crossing and inheritance patterns in natural populations aside, and even if we assume 100 % male fertility, the value remains <1 which means that frequencies of apomixis will decrease in our model system.
The value above might still overestimate the amount of introgression for natural populations, where pre-pollination crossing barriers may additionally keep sexual and apomictic cytotypes separated. In two mixed populations of diploid sexual R. notabilis and tetraploid R. variabilis, ploidy determinations and isoenzyme studies did not support a hypothesis of frequent hybridization between apomictic and sexual cytotypes. In this case, different flowering times and different habitat preferences of the sexual and apomictic taxa may limit cross-pollinations and inhibit introgression of apomixis (Hörandl et al., 2000). The taxa used for the crosses here do not differ in their phenology, but they do have slightly different habitat preferences: sexual R. cassubicifolius and R. carpaticola grow preferentially on forest floors, whereas the apomictic hybrid taxa also occur in meadows. Finally, frequencies of pollinator visits may differ between sexual and apomictic taxa; in the R. auricomus complex, apomictic plants are visited less frequently by insects, probably because of the partly aborted petals (Steinbach and Gottsberger, 1994).
We conclude that introgression of apomixis into sexuals via unidirectional hybridization is not an important causal factor for geographical parthenogenesis in the R. auricomus complex. Sexual populations should be able to maintain sexuality as long as female fitness is higher than that of apomicts, and as long as population size and availability of pollinators suffice to maintain outcrossing. These conclusions are supported by the general rarity of triploids in the complex (Jalas and Suominen, 1989) and the rarity of mixed populations. Apomictic lineages may have originated either from hybridization of sexual taxa (Paun et al., 2006b) or from facultative sexuality within apomicts, which is suggested from population genetic structure (Paun et al., 2006a; Hörandl et al., 2009). The main advantage of apomictic populations is probably the ability for uniparental reproduction, which is provided by pseudogamy and self-compatibility of apomictic taxa (Hörandl, 2008). This feature may allow for rapid colonization of novel or moderately disturbed habitats, such as meadows (Paun et al., 2006a; Hörandl and Paun, 2007). The occupation of potential novel niches by apomicts may inhibit range expansions of sexual populations and limit their distribution to ancient relic areas. In these populations, however, sexuality remains because of higher fitness (Hörandl, 2008).
The generalization of the model, however, needs to be studied. First, introgression of apomixis should be facilitated if sexuals have the same ploidy level as apomicts. Secondly, in interploidal crosses, a relaxation of endosperm balance may help to produce triploid offspring. Insensitivity against endosperm imbalance is expected in autonomous apomicts, where no sperm nuclei are used for fertilization of the polar nuclei. This mode of reproduction is present in most Asteraceae, and indeed introgression of apomixis into sexual populations has been observed (Brock, 2004). Such a case may also occur in Ranunculus kuepferi, an alpine apomict showing geographical parthenogenesis (Huber, 1988). Here FCSS and population genetic studies suggest frequent formation of triploid apomicts in the diploid–tetraploid hybrid zone, and various types of ploidy level in the endosperm (Cosendai and Hörandl, unpubl. res.). We conclude that modelling approaches have to consider the actual modes of reproduction before generalizations can be made.
ACKNOWLEDGEMENTS
We thank Katarína Klimová and Ovidiu Paun for help in material collections in Slovakia, and two anonymous referees for valuable suggestions. This work was supported by the Austrian Research Foundation (FWF), project P19006-B03, and by the Austrian Academy of Sciences (ÖAW), Commission for Interdisciplinary Ecological Studies (P2007-03), both granted to E.H. Funding to pay the Open Access publication charges for this article was provided by the Austrian Research Foundation (FWF).
LITERATURE CITED
- Asker SE, Jerling L. Apomixis in plants. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Berkeley: California Press; 1982. [Google Scholar]
- Baker HG. Support for Baker's law – as a rule. Evolution. 1967;21:853–856. doi: 10.1111/j.1558-5646.1967.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Baker HG, Stebbins GL. The genetics of colonizing species. New York: Academic Press; 1965. [Google Scholar]
- Barcaccia G, Arzenton F, Sharbel TF, Varotto S, Parrini P, Lucchin M. Genetic diversity and reproductive biology in ecotypes of the facultative apomict Hypericum perforatum L. Heredity. 2006;96:322–334. doi: 10.1038/sj.hdy.6800808. [DOI] [PubMed] [Google Scholar]
- Barcaccia G, Bäumlein H, Sharbel T. Apomixis in St. John's wort (Hypericum perforatum L.) an overview and glimpse towards the future. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 259–280. [Google Scholar]
- Bierzychudek P. Patterns in plant parthenogenesis. Experientia. 1985;41:1255–1264. doi: 10.1007/978-3-0348-6273-8_9. [DOI] [PubMed] [Google Scholar]
- Brock MT. The potential for genetic assimilation of a native dandelion species, Taraxacum ceratophorum (Asteraceae), by the exotic congener T. officinale. American Journal of Botany. 2004;91:656–663. doi: 10.3732/ajb.91.5.656. [DOI] [PubMed] [Google Scholar]
- Cáceres ME, Matzk F, Busti A, Pupilli F, Arcioni S. Apomixis and sexuality in Paspalum simplex: characterization of the mode of reproduction in segregating progenies by different methods. Sexual Plant Reproduction. 2001;14:201–206. doi: 10.1007/s00497-001-0109-1. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. Amphimixis and apomixis: two sides of the same coin. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 37–62. [Google Scholar]
- Curtis MD, Grossniklaus U. Molecular control of autonomous embryo and endosperm development. Sexual Plant Reproduction. 2008;21:79–88. [Google Scholar]
- Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983;220:1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Glesener RR, Tilman D. Sexuality and the components of environmental uncertainty, clues from geographic parthenogenesis in terrestrial animals. American Naturalist. 1987;112:169–673. [Google Scholar]
- Greilhuber J, Ebert I. Genome size variation in Pisum sativum. Genomics. 1994;37:646–655. doi: 10.1139/g94-092. [DOI] [PubMed] [Google Scholar]
- Greilhuber J, Lysák MA, Doležel J, Bennett MD. The origin, evolution and proposed stabilisation of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany. 2005;94:255–260. doi: 10.1093/aob/mci019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greilhuber J, Temsch EM, Loureiro JCM. Nuclear DNA content measurement. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Weinheim: Wiley-VCH; 2007. pp. 67–101. [Google Scholar]
- Grimanelli D, Leblanc O, Perotti E, Grossniklaus U. Developmental genetics of gametophytic apomixis. Trends in Genetics. 2001;17:597–604. doi: 10.1016/s0168-9525(01)02454-4. [DOI] [PubMed] [Google Scholar]
- Häfliger E. Zytologisch-embryologische Untersuchungen pseudogamer Ranunkeln der Auricomus-Gruppe. Berichte der Schweizer Botanischen Gesellschaft. 1943;53:317–379. [Google Scholar]
- Hörandl E. The complex causality of geographical parthenogenesis. New Phytologist. 2006;171:525–538. doi: 10.1111/j.1469-8137.2006.01769.x. [DOI] [PubMed] [Google Scholar]
- Hörandl E. Evolutionary implications of self-compatibility and reproductive fitness in the apomictic Ranunculus auricomus polyploid complex (Ranunculaceae) International Journal of Plant Science. 2008;169:1219–1228. doi: 10.1086/591980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E. Geographical parthenogenesis: opportunities for asexuality. In: Schön I, Martens K, Van Dijk PJ, editors. Lost sex. Heidelberg: Springer; 2009. in press. [Google Scholar]
- Hörandl E, Greilhuber J. Diploid and autotetraploid sexuals and their relationships to apomicts in the Ranunculus cassubicus group: insights from DNA content and isozyme variation. Plant Systematics and Evolution. 2002;234:85–100. [Google Scholar]
- Hörandl E, Paun O. Patterns and sources of genetic diversity in apomictic plants: implications for evolutionary potentials and ecology. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 169–154. [Google Scholar]
- Hörandl E, Dobeš C, Lambrou M. Chromosomen- und Pollenuntersuchungen an österreichischen Sippen des Ranunculus auricomus-Komplexes. Botanica Helvetica. 1997;107:195–209. [Google Scholar]
- Hörandl E, Greilhuber J, Dobeš C. Isozyme variation and ploidy levels within the apomictic Ranunculus auricomus complex: evidence for a sexual progenitor species in southeastern Austria. Plant Biology. 2000;2:53–62. [Google Scholar]
- Hörandl E, Cosendai A-C, Temsch E. Understanding the geographic distributions of apomictic plants: a case for a pluralistic approach. Plant Ecology and Diversity. 2008;1:309–320. doi: 10.1080/17550870802351175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörandl E, Greilhuber J, Klimová K, et al. Reticulate evolution and taxonomic concepts in the Ranunculus auricomus complex (Ranunculaceae): insights from morphological, karyological and molecular data. Taxon. 2009 in press. [PMC free article] [PubMed] [Google Scholar]
- Huber W. Natürliche Bastardierungen zwischen weißblühenden Ranunculus-Arten in den Alpen (Natural hybridizations between white-flowered species of Ranunculus in the Alps) [German with English abstract] Veröffentlichungen des Geobotanischen Institutes der ETH Zürich. 1988;100:1–160. [Google Scholar]
- Hufft Kao R. Asexuality and the coexistence of cytotypes. New Phytologist. 2007;175:764–772. doi: 10.1111/j.1469-8137.2007.02145.x. [DOI] [PubMed] [Google Scholar]
- Izmaiłow R. Macrosporogenesis in the apomictic species Ranunculus cassubicus. Acta Biologica Cracoviensia. 1967;8:183–195. [Google Scholar]
- Izmaiłow R. Reproductive strategy in the Ranunculus auricomus complex (Ranunculaceae) Acta Societatis Botanicorum Poloniae. 1996;65:167–170. [Google Scholar]
- Jalas J, Suominen J. Atlas Florae Europaeae. 8. Nymphaeaceae to Ranunculaceae. Helsinki: The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo; 1989. [Google Scholar]
- Kearney M. Hybridization, glaciation and geographical parthenogenesis. Trends in Ecology and Evolution. 2005;20:495–502. doi: 10.1016/j.tree.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Krahulcová A, Suda J. A modified method of flow cytometric seed screen simplifies the quantification of progeny classes with different ploidy levels. Biologia Plantarum. 2006;50:457–460. [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Levin DA. The role of chromosomal change in plant evolution. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lohwasser U. Biosystematische Untersuchungen an Ranunculus auricomus L. (Ranunculaceae) in Deutschland. Dissertationes Botanicae. 2001;343:1–220. [Google Scholar]
- Lynch M. Destabilizing hybridization, general-purpose genotypes and geographic parthenogenesis. Quarterly Review of Biology. 1984;59:257–290. [Google Scholar]
- Matzk F. Reproduction mode screening. In: Doležel J, Greilhuber J, Suda J, editors. Flow cytometry with plant cells. Analysis of genes, chromosomes, and genomes. Weinheim: Wiley-VCH; 2007. pp. 131–152. [Google Scholar]
- Matzk F, Meister A, Schubert I. An efficient screen for reproductive pathways using mature seeds of monocots and dicots. The Plant Journal. 2000;21:97–108. doi: 10.1046/j.1365-313x.2000.00647.x. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. The evolution of sex. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- Meirmans PG, DEN Nijs HCM, Van Tienderen PH. Male sterility in triploid dandelions: asexual females vs asexual hermaphrodites. Heredity. 2006;96:45–62. doi: 10.1038/sj.hdy.6800750. [DOI] [PubMed] [Google Scholar]
- Michaels HJ, Bazzaz FA. Resource allocation and demography of sexual and apomictic Antennaria parlinii. Ecology. 1986;67:27–36. [Google Scholar]
- Mogie M. The evolution of asexual reproduction in plants. London: Chapman and Hall; 1992. [Google Scholar]
- Mogie M, Britton NF, Stewart-Cox JA. Asexuality, polyploidy and the male function. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 195–214. [Google Scholar]
- Mráz P. Mentor effects in the genus Hieracium s.str. (Compositae, Lactuceae) Folia Geobotanica. 2003;38:345–350. [Google Scholar]
- de Nettancourt D. Incompatibility and incongruity in wild and cultivated plants. 2nd edn. Berlin: Springer; 2001. [Google Scholar]
- Nogler GA. Genetik der Aposporie bei Ranunculus auricomus s.l. W. Koch. I. Embryologie. Berichte der Schweizer Botanischen Gesellschaft. 1971;81:139–179. [Google Scholar]
- Nogler GA. Genetics of apospory in apomictic Ranunculus auricomus: 5. Conclusion. Botanica Helvetica. 1984;94:411–423. [Google Scholar]
- Nogler GA. Genetics of apomixis in Ranunculus auricomus. VI. Epilogue. Botanica Helvetica. 1995;105:111–115. [Google Scholar]
- Otto F, Oldiges H, Göhde W, Jain VK. Flow cytometric measurement of nuclear DNA content variations as a potential in vivo mutagenicity test. Cytometry. 1981;2:189–191. doi: 10.1002/cyto.990020311. [DOI] [PubMed] [Google Scholar]
- Paun O, Greilhuber J, Temsch E, Hörandl E. Patterns, sources and ecological implications of clonal diversity in apomictic Ranunculus carpaticola (Ranunculus auricomus complex, Ranunculaceae) Molecular Ecology. 2006a;15:897–910. doi: 10.1111/j.1365-294X.2006.02800.x. [DOI] [PubMed] [Google Scholar]
- Paun O, Stuessy TF, Hörandl E. The role of hybridization, polyploidization and glaciation in the origin and evolution of the apomictic Ranunculus cassubicus complex. New Phytologist. 2006b;171:223–236. doi: 10.1111/j.1469-8137.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- Rendle H, Murray BG. Breeding systems and pollen tube behaviour in compatible and incompatible crosses in New Zealand species of Ranunculus L. New Zealand Journal of Botany. 1988;26:467–471. [Google Scholar]
- Richards AJ. Plant breeding. London: Chapman and Hall; 1997. [Google Scholar]
- Rutishauser A. Die Entwicklungserregung des Endosperms bei pseudogamen Ranunculusarten. Mitteilungen der Naturfreunde-Gesellschaft Schaffhausen. 1954;25:1–45. [Google Scholar]
- Savidan Y. Apomixis in higher plants. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 15–22. [Google Scholar]
- Spielmann M, Vinkenoog R, Scott RJ. Genetic mechanisms of apomixis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358:1095–1103. doi: 10.1098/rstb.2003.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950. [Google Scholar]
- Steinbach K, Gottsberger G. Phenology and pollination biology of five Ranunculus species in Giessen, Central Germany. Phyton. 1994;34:203–218. [Google Scholar]
- Stewart-Cox JA, Britton NF, Mogie M. Space mediates coexistence of females and hermaphrodites. Bulletin of Mathematical Biology. 2005;67:1273–1302. doi: 10.1016/j.bulm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Talent N, Dickinson TA. Endosperm formation in aposporous Crataegus (Rosaceae, Spiraeoideae, tribe Pyreae): parallels to Ranunculaceae and Poaceae. New Phytologist. 2007;173:231–249. doi: 10.1111/j.1469-8137.2006.01918.x. [DOI] [PubMed] [Google Scholar]
- Tas ICQ, Van Dijk PJ. Crosses between sexual and apomictic dandelions (Taraxacum). I. The inheritance of apomixis. Heredity. 1999;83:707–714. doi: 10.1046/j.1365-2540.1999.00619.x. [DOI] [PubMed] [Google Scholar]
- Van Dijk PJ. Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philosophical Transactions of the Royal Society B: Biological Sciences. 2003;358:1113–1121. doi: 10.1098/rstb.2003.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk PJ. Potential and realized costs of sex in dandelions, Taraxacum officinale s.l. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 215–233. [Google Scholar]
- Van Dijk PJ, Vijverberg K. The significance of apomixis in the evolution of the angiosperms:a reappraisal. In: Bakker F, Chatrou L, Gravendeel B, Pelser PB, editors. Plant species-level systematics: new perspectives on pattern and process. Ruggell, Liechtenstein: ARG-Gantner; 2005. pp. 101–116. [Google Scholar]
- Vijverberg K, Van Dijk PJ. Genetic linkage mapping of apomixis loci. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 136–166. [Google Scholar]
- Vinkenoog R, Bushell C, Spielman M, Adams S, Dickinson HG, Scott RJ. Genomic imprinting and endosperm development in flowering plants. Molecular Biotechnology. 2003;25:149–184. doi: 10.1385/MB:25:2:149. [DOI] [PubMed] [Google Scholar]
- Voigt ML, Melzer M, Rutten T, Mitchell-Olds T, Sharbel TF. Gametogenesis in the apomictic Boechera holboellii complex: the male perspective. In: Hörandl E, Grossniklaus U, Van Dijk PJ, Sharbel T, editors. Apomixis: evolution, mechanisms and perspectives. Ruggell, Liechtenstein: ARG-Gantner; 2007. pp. 235–258. [Google Scholar]
- Vrijenhoek RC. Ecological differentiation among clones: the frozen niche variation model. In: Woermann K, Loeschcke V, editors. Population biology and evolution. Berlin: Springer; 1984. pp. 217–231. [Google Scholar]
- Vrijenhoek RC. Unisexual fish: model systems for studying ecology and evolution. Annual Review of Ecology, Evolution, and Systematics. 1994;25:71–96. [Google Scholar]