Abstract
BACKGROUND:
An accurate assessment of potential lymph node metastasis is an important issue for the appropriate treatment of early gastric cancer. Minimizing the number of invasive procedures used in cancer therapy is critical for improving the patient’s quality of life.
OBJECTIVE:
To evaluate the clinicopathological features associated with lymph node metastasis of early gastric cancer in patients from a single institution in China.
METHODS:
A retrospective review of data from 410 patients surgically treated for early gastric cancer at the First Affiliated Hospital (Nanjing, China) between 1998 and 2007, was conducted. The clinicopathological variables associated with lymph node metastasis were evaluated.
RESULTS:
Lymph node metastasis was observed in 12.20% of patients. The macroscopic type, tumour size, location in the stomach, depth of gastric carcinoma infiltration, and presence of vascular or lymphatic invasion showed a positive correlation with the incidence of lymph node metastasis by univariate analysis. Multivariate analyses revealed histological classification, macroscopic type, tumour size, depth of gastric carcinoma infiltration, and the presence of vascular or lymphatic invasion to be significantly and independently related to lymph node metastasis. The depth of gastric carcinoma infiltration was the strongest predictive factor for lymph node metastasis. For intramucosal cancer, tumour size was the unique risk factor for lymph node metastasis. For submucosal cancer, histological classification and tumour size were independent risk factors for lymph node metastasis.
CONCLUSIONS:
Histological classification, macroscopic type, tumour size, depth of gastric carcinoma infiltration, and the presence of vascular or lymphatic invasion are independent risk factors for lymph node metastasis in patients with early gastric cancer in China. Minimal invasive treatment, such as endoscopic mucosal resection, may be possible for highly selected cancers.
Keywords: China, Early gastric cancer, Lymph node metastasis, Risk factor
Abstract
HISTORIQUE :
Il est important de procéder à un examen approfondi des métastases ganglionnaires potentielles pour traiter de façon appropriée le cancer de l’estomac peu avancé. Et pour améliorer la qualité de vie du patient, il est indispensable de réduire le nombre d’interventions effractives dans le traitement du cancer.
OBJECTIF :
Évaluer les caractéristiques clinicopathologiques des métastases ganglionnaires associées au cancer de l’estomac peu avancé chez des patients d’un établissement de santé chinois.
MÉTHODES :
Les auteurs ont réalisé une analyse rétrospective des données provenant de 410 patients traités chirurgicalement pour un cancer de l’estomac peu avancé, au premier hôpital affilié de Nanjing, Chine, entre 1998 et 2007. Ils ont évalué les variables clinicopathologiques associées aux métastases ganglionnaires.
RÉSULTATS :
Des métastases ganglionnaires ont été observées chez 12,20 % des patients. Selon l’analyse univariée, le type macroscopique, la taille de la tumeur, sa localisation dans l’estomac, la profondeur de l’infiltration du carcinome gastrique et la présence d’un envahissement vasculaire ou lymphatique se sont révélés en corrélation positive avec l’incidence des métastases ganglionnaires. Les analyses multivariées ont pour leur part révélé un lien significatif et indépendant entre la classification histologique, le type macroscopique, la taille de la tumeur, la profondeur de l’infiltration du carcinome gastrique et la présence d’un envahissement vasculaire ou lymphatique, d’une part, et les métastases ganglionnaires, de l’autre. La profondeur de l’infiltration du carcinome gastrique a été le plus solide prédicteur des métastases ganglionnaires. Dans le cas du cancer intramuqueux, la taille de la tumeur a été le seul facteur de risque de métastases ganglionnaires. Dans le cas du cancer sous-muqueux, la classification histologique et la taille de la tumeur ont été des facteurs de risque indépendants de métastases ganglionnaires.
CONCLUSION :
La classification histologique, le type macroscopique, la taille de la tumeur, la profondeur de l’infiltration du carcinome gastrique et la présence d’un envahissement vasculaire ou lymphatique sont des facteurs de risque indépendants de métastases ganglionnaires chez les patients présentant un cancer de l’estomac peu avancé en Chine. Un traitement minimalement effractif, comme la résection muqueuse endoscopique, est envisageable pour certains cancers précis.
Gastric cancer remains one of the most prevalent causes of cancer-related death in China. Early gastric carcinoma (EGC) is defined as a lesion of the stomach confined to the mucosa and/or submucosa, regardless of lymph node metastatic status and tumour size (1). The detection of EGC has increased worldwide in recent years because of advances in endoscopic techniques and equipment (2). The five-year survival rate for EGC is more than 90%, and lymph node metastasis (LNM) is one of the most important prognostic factors for EGC (3). The five-year survival rate was reported to be 87.3% in patients with involvement of the regional lymph node and 94.2% in those without lymph node involvement (4). In the past 20 years, most surgeons considered D2 lymphadenectomy (dissection of all group I and group II lymph nodes) to be the standard and optimal surgical procedure for patients with EGC. Even total gastrectomy and D3 lymphadenectomy (dissection of all group I, group II and group III lymph nodes) with combined resection of other organs have been used to achieve curative resection (5–7). Excellent curative treatment for patients with EGC has been obtained with regional lymphadenectomy. However, the LNM rate of EGC is reported to be approximately 11% to 18% (3,8,9), and approximately 70% to 80% of patients will undergo overtreatment with D2 lymphadenectomy (7). Recently, less invasive treatments have been performed for EGC, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) (10,11). Because of recent advances in surgical instrumentation and techniques, laparoscopic procedures have been suggested to be an alternative treatment for EGC (4,12). Such methods of limited surgery should be considered when deciding on a treatment method for EGC.
All of these factors contribute to the necessity of an accurate assessment of a potential LNM when determining the appropriate treatment for EGC, which in turn, improves the five-year survival rate of patients and their quality of life. The present retrospective study was designed to investigate the clinicopathological characteristics of EGC at a single institution in China and to identify the factors associated with LNM. Using these predictive factors for LNM, criteria for the medical management of patients with EGC may be established and appropriate therapy obtained.
METHODS
A consecutive series of 410 patients underwent surgery as an initial treatment for solitary EGC between January 1998 and December 2007 at the Department of General Surgery in the First Affiliated Hospital, Nanjing Medical University, Nanjing, China. Gastric carcinomas were classified using the Japanese Classification of Gastric Carcinoma (13). EGC was defined as a tumour invading the the mucosa or submucosa (ie, T1), and all participants had been pathologically diagnosed with T1 EGC and had undergone potentially curative gastrectomy. Data were retrieved from operative and pathology reports.
The stomach was anatomically divided into three portions: the upper one-third, the middle one-third and the lower one-third. The postoperative clinicopathological parameters that were evaluated included age (45 years or younger; older than 45 years); sex; tumour size (smaller than 2 cm; or 2 cm and larger); tumour location in the stomach (lower one-third, middle one-third, upper one-third); macroscopic type (elevated, flat or depressed); depth of gastric carcinoma infiltration (intramucosal, submucosal); histological classification (differentiated [well differentiated, moderately differentiated or papillary]; or undifferentiated [poorly differentiated, signet-ring cell or mucinous]), and the presence of vascular or lymphatic invasion.
Statistical analysis
Clinicopathological findings were compared using t tests or Pearson χ2 tests. A multivariate logistic regression analysis was performed to identify factors that were independently associated with LNM. P<0.05 was considered to be statistically significant.
RESULTS
Clinical features of patients
Between January 1998 and December 2007, a consecutive series of 3186 patients with gastric cancer underwent gastrectomy at the First Affiliated Hospital (Nanjing, China) in which EGC comprised 12.87% (410 of 3186 cases). Of these patients, 295 were men and 115 were women, ranging in age from 29 to 89 years. Undifferentiated cancer was more common among women (44.35%) than among men (20.34%; P=0.02). Of the patients with EGC, 50 had histologically proven LNM – an LNM rate of 12.20%.
Risk factors for LNM by univariate and multivariate analysis
Table 1 presents a comparison of clinicopathological findings among patients with lymph node-negative and lymph node-positive tumours. A univariate analysis of the clinicopathological characteristics for LNM revealed macroscopic type, tumour size, location in the stomach, depth of gastric carcinoma infiltration, and the presence of vascular or lymphatic invasion to be significant factors.
TABLE 1.
Comparison of clinicopathological findings among patients with lymph node-negative and lymph node-positive tumours
| Characteristic |
Tumours |
χ2 | P | ||
|---|---|---|---|---|---|
| Lymph node-negative (n=360) | Lymph node-positive (n=50) | Lymph node-positive, % | |||
| Age, years | |||||
| ≤45 | 63 | 7 | 10 | 0.3777 | 0.5389 |
| >45 | 297 | 43 | 12.64 | ||
| Men | 261 | 34 | 11.53 | 0.1074 | 0.7432 |
| Women | 99 | 16 | 13.91 | ||
| Histolological classification | |||||
| Differentiated | 268 | 31 | 10.37 | 3.3772 | 0.0661 |
| Undifferentiated | 92 | 19 | 17.12 | ||
| Macroscopic type | |||||
| Elevated | 53 | 1 | 1.89 | 8.1695 | 0.0043 |
| Flat | 77 | 7 | 8.33 | ||
| Depressed | 230 | 42 | 15.44 | ||
| Tumour size | |||||
| <2 cm | 223 | 16 | 6.69 | 14.8508 | 0.0001 |
| ≥2 cm | 137 | 34 | 19.88 | ||
| Tumour location in the stomach | |||||
| Upper one-third | 89 | 5 | 5.32 | 4.2358 | 0.0396 |
| Middle one-third | 109 | 15 | 12.10 | ||
| Lower one-third | 162 | 30 | 15.63 | ||
| Depth of gastric carcinoma infiltration | |||||
| Intramucosal | 216 | 13 | 5.68 | 18.2333 | <0.0001 |
| Submucosal | 144 | 37 | 20.44 | ||
| Vascular or lymphatic invasion | |||||
| Negative | 347 | 42 | 10.80 | 11.5687 | 0.0007 |
| Positive | 13 | 8 | 38.1 | ||
Data presented as number of patients (n) unless indicated otherwise
Multivariate analysis revealed that histological classification, macroscopic type, tumour size, depth of gastric carcinoma infiltration, and the presence of vascular or lymphatic invasion were independent risk factors for LNM. The depth of gastric carcinoma infiltration was the most powerful risk factor for LNM (P=0.0005) (Table 2).
TABLE 2.
Multivariate logistic regression analysis of potential risk factors for lymph node metastasis in patients with early gastric cancer
| Characteristic | P | OR | 95% CI |
|---|---|---|---|
| Histological classification | 0.0400 | 2.058 | 1.034–4.098 |
| Macroscopic type | 0.0085 | 2.339 | 1.242–4.403 |
| Tumour size | 0.0030 | 2.750 | 1.409–5.365 |
| Depth of gastric carcinoma infiltration | 0.0005 | 3.589 | 1.754–7.342 |
| Vascular or lymphatic invasion | 0.0489 | 2.849 | 1.005–8.076 |
Stratification analysis of risk factors for LNM by depth of invasion
Stratification analysis of risk factors for LNM was performed according to the depth of gastric carcinoma infiltration. For intramucosal cancer, tumour size was the unique risk factor for LNM. The rate of LNM for tumours smaller than 2 cm in size was 3.29% (five of 152), but it increased to 10.39% (eight of 77) for tumours larger than 2 cm in size (P=0.0371).
For submucosal cancer, histological classification, tumour size, location in the stomach, and vascular or lymphatic invasion were significant factors for LNM (Table 3). Multivariate analysis revealed histological classification and tumour size to be independent risk factors for LNM in patients with a submucosal cancer (Table 4).
TABLE 3.
Factors associated with lymph node metastasis in patients with submucosal cancer
| Characteristic | Node-negative (n=144) | Node-positive (n=37) | Node-positive, % | χ2 | P |
|---|---|---|---|---|---|
| Histological classification | |||||
| Differentiated | 117 | 24 | 17.02 | 4.4344 | 0.0352 |
| Undifferentiated | 27 | 13 | 32.50 | ||
| Macroscopic type | |||||
| Elevated | 22 | 1 | 4.55 | 3.2711 | 0.0705 |
| Flat | 22 | 6 | 21.43 | ||
| Depressed | 100 | 30 | 23.08 | ||
| Tumour size | |||||
| <2 cm | 76 | 11 | 12.64 | 6.0016 | 0.0143 |
| ≥2 cm | 68 | 26 | 27.66 | ||
| Tumour location in the stomach | |||||
| Upper one-third | 43 | 5 | 10.42 | 4.9990 | 0.0254 |
| Middle one-third | 45 | 11 | 19.64 | ||
| Lower one-third | 56 | 21 | 27.27 | ||
| Vascular or lymphatic invasion | |||||
| Negative | 134 | 30 | 18.29 | 4.5814 | 0.0323 |
| Positive | 19 | 10 | 34.48 | ||
Data presented as number of patients (n) unless indicated otherwise
TABLE 4.
Multivariate logistic regression analysis of potential risk factors for lymph node metastasis in patients with submucosal cancer
| Characteristic | P | OR | 95% CI |
|---|---|---|---|
| Histological classification | 0.0186 | 0.370 | 0.162–0.847 |
| Tumour size | 0.0084 | 0.341 | 0.153–0.759 |
DISCUSSION
EGC accounts for only a small proportion of the advanced gastric cancers in China. In the present series, EGC comprised 12.87% of all synchronous patients with gastric cancer. In the western hemisphere, EGC accounts for 4% to 16% of all gastric carcinoma cases (14), while in Japan, the proportion of EGC is approximately 30% to 50% (15). Obviously, one of the most urgent issues is to improve the detection of EGC in China and in the western hemisphere.
Previous studies have reported that LNM is one of the most important prognostic factors for EGC, with an incidence of 5.7% to 20.0% (16). Roviello et al (3) reported a 10-year survival rate for EGC of 92% for early stage cases, 82% and 73% for tumours with one to three and four to six positive nodes, repectively, and dropped to 27% for tumours with more than six positive metastatic nodes. The results of the study by An et al (17) revealed that the survival rate for intramucosal cancers was significantly higher than that of submucosal cancers because of the lower incidence of LNM in intramucosal lesions. The incidence of nodal involvement has been reported to range from 2.6% to 4.8% in intramucosal cancers and 16.5% to 23.6% in submucosal cancers (3,8,9). Similarly, the present study demonstrated lymph node involvement rates of 5.68% for intramucosal cancers and 20.44% for submucosal cancers.
Several studies have been performed to evaluate the risk of LNM in EGC and to establish the most appropriate treatment strategy. Several prognostic factors including sex, depth of invasion, tumour size, gross appearance, vascular invasion or lymphatic permeation, and perineural involvement have been demonstrated to be related to LNM in EGC (15–18). In the current analysis of a consecutive series of EGC cases, multivariate analyses revealed that histological classification, macroscopic type, tumour size, depth of infiltration, and presence of vascular or lymphatic invasion are significantly and independently related to LNM, with the depth of infiltration being the strongest predictor for LNM. Among these factors, submocosal invasion, vascular or lymphatic invasion, and tumour diameter greater than 2.0 cm have been reported as risk factors in previous studies (15–17). Our results revealed a high correlation in the incidence of LNM with tumour size and submucosal invasion (r=1.00). This finding may be explained by the tendency of larger EGCs to invade the submucosal layer (15). Regarding histological classification, a significant correlation has been demonstrated between undifferentiated tumours and the incidence of LNM in EGC (17,19). Uniquely, our study revealed macroscopic type as an independent risk factor for LNM; however, we did not find any correlation between macroscopic type and histological classification, or between tumour size and submocosal invasion. Furthermore, the correlation was not demonstrated between macroscopic type and LNM in the stratification analysis according to the depth of infiltration. Therefore, the value of macroscopic type as predictor for LNM in EGC needs further investigation.
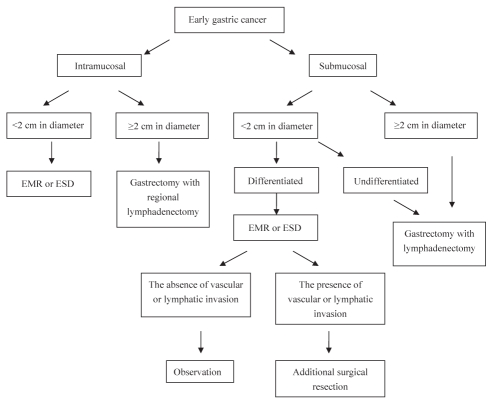
To establish a more practical criterion for the medical management of EGC, a stratification analysis according to invasion depth was performed. For intramucosal cancer, tumour size was the unique risk factor for LNM. For submucosal cancer, histological classification and tumour size were significant and independent risk factors for LNM. Because surgical resection of the stomach may be associated with poorer outcome in terms of quality of life (20), we suggest using a therapeutic pathway for management of EGC that reduces the need for more extensive surgical procedures based on our results (Figure 1). The empirical indications for EMR have been established (10,21). ESD techniques allow for large en bloc resections and precise histological staging by direct submucosal dissection (10). According to our results, these indications are suitable for the majority of Chinese patients with intramucosal EGC; however, EMR or ESD may not be ideal for intramucosal EGCs that are larger than 2 cm because of the high rate of LNM; therefore, a gastrectomy with a limited lymph node dissection is recommended. For invasive submucosal gastric cancers, we suggest that gastrectomy with lymphadenectomy be the standard treatment because of the higher rate of LNM, even if the gastric lesion was completely excised by EMR or ESD. However, if the lesion is smaller than 2 cm in diameter and histologically well differentiated, EMR or ESD may be performed and a careful postoperative histological study is of the utmost importance. When lymphatic permeation or vascular invasion is recognized in postresection specimens, additional surgical resection of the stomach with lymphadenectomy is recommended (15).
Figure 1).
Suggested management algorithm for patients with early gastric carcinoma. EMR Endoscopic mucosal resection; ESD Endoscopic submucosal dissection
In our suggested management algorithm (Figure 1), determination of invasion depth is the first step. Endoscopic ultrasound and computed tomography have been shown to be useful stage adjuncts. However, the diagnostic accuracy achieved by endoscopic ultrasound cannot be considered to be reliable enough to guide the surgical approach. Even in Japan, endoscopic ultrasound is not routinely used for staging purposes because it is not sensitive enough to detect microscopic invasion of the submucosa (22). Thus, a retrospective histological review of EMR or ESD specimens is very helpful.
CONCLUSION
The independent predictive factors for LNM in EGC in a series of Chinese patients were determined. Based on these observations, we proposed a therapeutic pathway for management of EGC. Further prospective studies are warranted to evaluate the efficacy of this therapeutic strategy. Surgeons should determine the extent of therapy needed when considering the possibility of LNM.
REFERENCES
- 1.Kajitani T. The general rules for the gastric cancer study in surgery and pathology. I. Clinical classification. Jpn J Surg. 1981;11:127–39. doi: 10.1007/BF02468883. [DOI] [PubMed] [Google Scholar]
- 2.Huguier M, Ferro L, Barrier A. Early gastric carcinoma: Spread and multicentricity. Gastric Cancer. 2002;5:125–8. doi: 10.1007/s101200200022. [DOI] [PubMed] [Google Scholar]
- 3.Roviello F, Rossi S, Marrelli D, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: A multicenter Italian study. J Surg Oncol. 2006;94:275–80. doi: 10.1002/jso.20566. [DOI] [PubMed] [Google Scholar]
- 4.Noh SH, Hyung WJ, Cheong JH. Minimally invasive treatment for gastric cancer: Approaches and selection process. J Surg Oncol. 2005;90:188–93. doi: 10.1002/jso.20228. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda Y, Saku M, Kawanaka H, et al. Prophylactic lymph node dissection for early gastric cancer invading submucosa. Hepatogastroenterology. 2004;51:887–90. [PubMed] [Google Scholar]
- 6.Borie F, Plaisant N, Millat B, Hay JM, Fagniez PL. Appropriate gastric resection with lymph node dissection for early gastric cancer. Ann Surg Oncol. 2004;11:512–7. doi: 10.1245/ASO.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Nitti D, Marchet A, Mammano E, et al. Extended lymphadenectomy (D2) in patients with early gastric cancer. Eur J Surg Oncol. 2005;31:875–81. doi: 10.1016/j.ejso.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Pelz J, Merkel S, Horbach T, Papadopoulos T, Hohenberger W. Determination of nodal status and treatment in early gastric cancer. Eur J Surg Oncol. 2004;30:935–41. doi: 10.1016/j.ejso.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181–5. doi: 10.1002/jso.20018. [DOI] [PubMed] [Google Scholar]
- 10.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929–42. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 11.Abe N, Watanabe T, Sugiyama M, et al. Endoscopic treatment or surgery for undifferentiated early gastric cancer? Am J Surg. 2004;188:181–4. doi: 10.1016/j.amjsurg.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 12.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Japanese Gastric Cancer Association Japanese classification of gastric carcinoma – 2nd English edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 14.Borie F, Millat B, Fingerhut A, Hay JM, Fagniez PL, De Saxce B. Lymphatic involvement in early gastric cancer. Arch Surg. 2000;135:1218–23. doi: 10.1001/archsurg.135.10.1218. [DOI] [PubMed] [Google Scholar]
- 15.Okabayashi T, Kobayashi M, Nishimori I, et al. Clinicopathological features and medical management of early gastric cancer. Am J Surg. 2008;195:229–32. doi: 10.1016/j.amjsurg.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Otsuji E, Kuriu Y, Ichikawa D, Ochiai T, Okamoto K, Yamagishi H. Prediction of lymph node metastasis by size of early gastric carcinoma. Hepatogastroenterology. 2007;54:602–5. [PubMed] [Google Scholar]
- 17.An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: Analysis of a single institutional experience. Ann Surg. 2007;246:749–53. doi: 10.1097/SLA.0b013e31811f3fb7. [DOI] [PubMed] [Google Scholar]
- 18.Xu YY, Huang BJ, Sun Z, Lu C, Liu YP. Risk factors for lymph node metastasis and evaluation of reasonable surgery for early gastric cancer. World J Gastroenterol. 2007;13:5133–8. doi: 10.3748/wjg.v13.i38.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CY, Chen JT, Chen GH, Yeh HZ. Lymph node metastasis in early gastric cancer: Clinicopathological analysis. Hepatogastroenterology. 2002;49:1465–8. [PubMed] [Google Scholar]
- 20.Blazeby JM, Metcalfe C, Nicklin J, Barham CP, Donovan J, Alderson D. Association between quality of life scores and short-term outcome after surgery for cancer of the oesophagus or gastric cardia. Br J Surg. 2005;92:1502–7. doi: 10.1002/bjs.5175. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: Relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35–8. doi: 10.1007/s10120-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 22.Reshamwala PA, Darwin PE. Endoscopic management of early gastric cancer. Curr Opin Gastroenterol. 2006;22:541–5. doi: 10.1097/01.mog.0000239870.04457.80. [DOI] [PubMed] [Google Scholar]