Abstract
BACKGROUND:
The Canadian health care system is mandated to provide reasonable access to health care for all Canadians regardless of age, sex, race, socioeconomic status or place of residence. In the present study, the impact of place of residence in Nova Scotia on access to cardiac catheterization and long-term outcomes following an acute myocardial infarction (MI) were examined.
METHODS:
All patients with an acute MI who were hospitalized between April 1998 and December 2001 were identified. Place of residence was defined by postal code and separated into three categories: metropolitan area (MA); nonmetropolitan urban area (UA); and rural area (RA). Rates of and waiting times for cardiac catheterization were determined, as were risk-adjusted long-term rates of mortality and readmission to the hospital.
RESULTS:
A total of 7351 patients were hospitalized with an acute MI during the study period. Rates of cardiac catheterization differed across the three groups (MA 45.6%, UA 37.3%, RA 37.3%; P<0.0001), as did mean waiting times (MA 15.0 days, UA 32.1 days, RA 28.7 days) (P<0.0001). After adjusting for differences among patients, residence in either UA or RA emerged as an independent predictor of lower rates of cardiac catheterization (UA: hazard ratio [HR] 0.77, P<0.0001; RA: HR 0.75, P<0.0001), greater waiting times (UA: an additional 14.1 days, P<0.0001; RA: an additional 10.8 days, P<0.0001) and increased long-term rates of readmission (UA: HR 1.24, P=0.0001; RA: HR 1.12, P=0.04).
CONCLUSION:
In patients admitted with an acute MI, residence outside of an MA was associated with diminished rates of cardiac catheterization, longer waiting times and increased rates of readmission. Despite universal health care coverage, Canadians are subject to significant geographical barriers to cardiac catheterization with associated poorer outcomes.
Keywords: Acute myocardial infarction, Catheterization, Epidemiology, Health outcomes, Morbidity, Mortality, Outcomes research
Abstract
HISTORIQUE :
Le système de soins de santé canadien a l’obligation de fournir un accès raisonnable aux soins de santé à tous les Canadiens, peu importe leur âge, leur sexe, leur race, leur statut socioéconomique ou leur lieu de résidence. Dans la présente étude, les auteurs ont analysé l’impact du lieu de résidence sur l’accès au cathétérisme cardiaque et sur le suivi à long terme après un infarctus aigu du myocarde (IAM) en Nouvelle-Écosse.
MÉTHODES :
Tous les patients victimes d’un IAM qui ont été hospitalisés entre avril 1998 et décembre 2001 ont été recensés. Le lieu de résidence a été défini par le code postal et regroupé en trois catégories : région métropolitaine (RM), région urbaine non métropolitaine (RU) et région rurale (RR). Les taux de cathétérismes cardiaques et les temps d’attente pour cette intervention ont été vérifiés, tout comme les taux de mortalité et de ré-hospitalisation à long terme ajustés selon le risque.
RÉSULTATS :
En tout, 7 351 patients ont été hospitalisés pour IAM au cours de la période étudiée. Les taux de cathétérismes cardiaques ont différé entre les trois groupes (RM 45,6 %, RU 37,3 %, RR 37,3 %) (p < 0,0001), tout comme les temps d’attente (RM 15,0 jours, RU 32,1 jours, RR 28,7 jours) (p < 0,0001). Après ajustement pour tenir compte de différences parmi les patients, le fait de résider en RU ou RR s’est avéré être un prédicteur indépendant de taux moindres de cathétérismes cardiaques (RU : rapport des cotes [RC] 0,77, p < 0,0001; RR : RC 0,75, p < 0,0001), de temps d’attente plus longs (RU : 14,1 jours de plus, p < 0,0001; RR : 10,8 jours de plus (p < 0,0001) et de taux de réhospitalisation à long terme plus élevés (RU : RC 1,24, p = 0,0001, RR : RC 1,12, p = 0,04).
CONCLUSION :
Chez les patients admis pour IAM, le fait de résider à l’extérieur d’une RM a été associé à des taux moindres de cathétérismes cardiaques, à des temps d’attente plus long et à un taux plus élevé de réhospitalisation. Malgré le principe d’universalité des soins de santé, les Canadiens sont en butte à des obstacles géographiques qui nuisent à leur accès au cathétérisme cardiaque et assombrissent leur pronostic.
The Canadian public health care system is a universal health care system mandated to provide reasonable access to quality health care for all Canadians regardless of age, sex, race, socioeconomic status or place of residence (1). However, a growing number of studies have shown that significant inequalities may exist within such a system, especially in relation to access to invasive cardiac care following an acute myocardial infarction (MI) (2–13). Alter et al (2) demonstrated that increases in neighbourhood income from the lowest to the highest quintile were associated with a 23% increase in rates of cardiac catheterization following an acute MI and a 45% reduction in waiting times in the province of Ontario. Similarly, Rodrigues et al (3) established marked regional variation in rates of cardiac procedures in patients who experienced an acute MI in the province of Quebec. Seidel et al (10) demonstrated that patients living at greater distances from cardiac catheterization facilities in Alberta had lower adjusted rates of cardiac catheterization. While it is becoming increasingly evident that inequalities exist in access to cardiac care across Canada, little is known regarding the effect of such inequalities on long-term rates of mortality and readmission to the hospital.
In provinces such as Quebec, Ontario and Alberta, invasive cardiac services are provided by multiple cardiac catheterization and revascularization centres. By contrast, Nova Scotia, with a population of less than one million people, possesses a single, centralized, tertiary cardiac care centre that offers cardiac catheterization, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) services for the entire province. Thus, Nova Scotia provides an ideal setting in which to examine the impact of place of residence on rates of cardiac catheterization following admission for acute MI and to explore its effect on long-term rates of all-cause mortality and readmission to the hospital.
METHODS
Data sources
Data were obtained from the Improving Cardiovascular Outcomes in Nova Scotia (ICONS) database, a population-based, province-wide clinical registry. From October 15, 1997 until present, this registry has captured information on all hospitalizations across Nova Scotia for selected cardiovascular diseases including acute MI (14,15). In addition to possessing detailed data relating to patient demographic characteristics and comorbid illnesses, the ICONS database contains information regarding discharge drug prescriptions, in-hospital and out of hospital procedure use (cardiac catheterization, PCI, CABG and heart valve surgery) and outcome data such as all-cause mortality and readmission to the hospital for various cardiac problems including nonfatal MI, unstable angina or congestive heart failure (CHF). Data concerning all-cause mortality were obtained from the Nova Scotia Vital Statistics database.
Study population
All patients admitted to a hospital in the province of Nova Scotia with a discharge diagnosis of an acute MI between April 15, 1998 and December 31, 2001 with long-term follow-up until December 31, 2003 were identified using the ICONS database. Patients who had been admitted with an acute MI within six months before their ‘index’ admission were excluded, as was anyone who was not a resident of Nova Scotia. The remaining patients formed the final study population.
Socioeconomic information
Because the ICONS database does not capture self-reported income data on all patients, 2001 Canadian census data were used to determine the median individual income level of the neighbourhood or dissemination area best corresponding to a patient’s place of residence. This was done by linking a patient’s six-digit postal code to a census dissemination area using the Postal Code Conversion File (16). As a result, each patient was assigned a median individual income as an estimate of their actual income. This method of employing ecologic-level measures of income where access to individual-level income measures is lacking has been validated previously (17,18). Income quintiles were generated using dissemination area-level median individual incomes from across the province and are shown here (as expressed in Canadian dollars) – lowest: less than $14,865; second: $14,865 to $17,096; middle: $17,097 to $19,985; fourth: $19,986 to $24,674; and highest: more than $24,674. Patients were placed into one of these quintiles on the basis of their estimated income.
Geographical information
The first three digits of six-digit postal codes, referred to as forward sortation areas, were used to determine the patient’s place of residence (Figure 1). Forward sortation areas with ‘0’ as the second digit, which is indicative of areas with fewer than 1000 persons, were deemed rural areas (RAs), while those with a second digit other than ‘0’ were considered urban areas (19). Among neighbourhoods defined as urban areas, those located within or directly adjacent to the metropolitan area of Halifax, Nova Scotia were designated metropolitan areas (MAs), and those located outside of greater Halifax were designated nonmetropolitan urban areas (UAs). Halifax is the site of the sole tertiary cardiac care centre, the Queen Elizabeth II (QEII) Health Sciences Centre, providing cardiac catheterization, PCI and CABG services for the entire province of Nova Scotia.
Figure 1).
Geographical groupings in Nova Scotia based on forward sortation area. Data from DMTI Spatial, Canada. Map produced by the Map and Geospatial Information Collection (MAGIC) at Dalhousie University (Halifax, Nova Scotia)
Statistical analysis
Comparisons across the three geographical groupings using χ2 tests and two-sided t tests were made based on several demographic, clinical, socioeconomic and geographical variables. These included age, sex, comorbid illness, history of coronary intervention and type of acute MI (ST segment elevation versus non-ST segment elevation). Rates of acute intervention, including thrombolysis and primary PCI within the first 24 h following admission, were considered. Because not all patients can be accommodated during their index hospitalization, rates of cardiac catheterization within the first six months after admission were also examined and compared, as well as rates of revascularization by either PCI or CABG in the first year following admission in those patients having undergone a cardiac catheterization within the first six months. Additional variables compared across strata included rates of noninvasive investigations performed during the same admission (including exercise stress testing, echocardiography, nuclear scintigraphy and wall motion studies). Rates of discharge drug prescriptions (including beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors [‘statins’] and anti-platelet agents including acetylsalicylic acid, clopidogrel and ticlopidine) in those patients discharged from the hospital were compared. Finally, differential income distribution, distance from the index hospital of admission to the QEII Health Sciences Centre, level of the admitting facility (community, regional or tertiary) and specialty of the admitting physician (cardiologist, general internist, general practitioner or other) were examined. Waiting times from the time of admission to the time of catheterization, as well as from the time of cardiac catheterization to the time of revascularization, were evaluated across geographical groupings using two-sided t tests, cumulative survival plots and log-rank tests. Unadjusted rates of all-cause mortality, readmission to the hospital for any cardiac cause and readmission to the hospital for either acute MI, unstable angina or CHF at one year and over the long term were also calculated.
The risk-adjusted impact of place of residence on rates of cardiac catheterization was determined using Cox proportional-hazard models that were fully adjusted for age, sex, comorbid illness, type of acute MI, whether the patient received thrombolytic therapy following acute MI and income level. The risk-adjusted impact of place of residence on long-term rates of all-cause mortality and readmission to the hospital was determined through the development of separate Cox proportional hazard models that were fully adjusted for age, sex, comorbid illness and income level.
Statistical significance was indicated by P<0.05 in the analyses, all of which were performed using the SAS software package version 8.2 (SAS, USA).
RESULTS
Between April 15, 1998, and December 31, 2001, 7351 patients were admitted to hospitals across Nova Scotia with a discharge diagnosis of acute MI. Of these, 2113 resided in MAs (age- and sex-adjusted rate 247.2 per 100,000 persons per year), 2114 resided in UAs (242.0 per 100,000 persons per year) and 3124 resided in RAs (226.2 per 100,000 persons per year). Residents of MAs were most likely to be admitted to the QEII Health Sciences Centre, while residents of UAs and RAs were most likely to be admitted to a regional health care facility (Table 1). The majority of patients in Nova Scotia were admitted under the care of either a general internist or general practitioner, regardless of place of residence. However, a greater percentage of patients from MAs were cared for primarily by a cardiologist than those from either UAs or RAs (Table 1).
TABLE 1.
Comparison of baseline patient, physician and hospital characteristics across geographical groupings
| Variable | MA, % (n=2113) | UA, % (n=2114) | RA, % (n=3124) | P |
|---|---|---|---|---|
| Age, years | 0.46 | |||
| >70 | 46.1 | 47.7 | 46.6 | |
| 61–70 | 23.3 | 21.8 | 22.3 | |
| 51–60 | 18.7 | 19.7 | 18.6 | |
| ≤50 | 12.0 | 10.8 | 12.5 | |
| Female sex | 39.8 | 37.8 | 35.6 | 0.009 |
| Smoking history | 63.0 | 62.6 | 59.5 | 0.01 |
| Hypercholesterolemia | 37.2 | 34.8 | 33.1 | 0.01 |
| Diabetes | 27.2 | 27.9 | 26.3 | 0.45 |
| Hypertension | 54.5 | 51.6 | 50.0 | 0.006 |
| Congestive heart failure | 12.6 | 11.2 | 10.2 | 0.02 |
| Previous MI/unstable angina | 27.7 | 29.1 | 27.3 | 0.34 |
| COPD/asthma | 19.2 | 16.1 | 16.1 | 0.006 |
| Renal failure | 7.1 | 3.8 | 3.5 | <0.0001 |
| Cerebrovascular disease | 11.8 | 10.8 | 9.9 | 0.09 |
| Prior CABG/PCI | 11.5 | 10.2 | 9.2 | 0.03 |
| ST elevation MI | 42.0 | 45.6 | 46.3 | 0.006 |
| Income quintile | <0.0001 | |||
| Lowest | 8.9 | 30.9 | 16.4 | |
| Second | 6.9 | 27.6 | 31.4 | |
| Middle | 14.3 | 22.7 | 39.1 | |
| Fourth | 27.5 | 15.7 | 10.4 | |
| Highest | 42.5 | 3.2 | 2.7 | |
| Distance from tertiary care centre, km | <0.0001 | |||
| 0 to 70 | 97.3 | 3.1 | 19.4 | |
| 71 to 163 | 1.7 | 31.4 | 42.8 | |
| >163 | 1.1 | 65.6 | 37.8 | |
| Admitting hospital type | <0.0001 | |||
| Community | 0.6 | 5.0 | 18.6 | |
| Regional | 29.1 | 92.4 | 71.3 | |
| Tertiary | 70.4 | 2.6 | 10.1 | |
| Attending physician | <0.0001 | |||
| Cardiologist | 19.2 | 4.8 | 3.6 | |
| General internist | 51.3 | 58.7 | 52.0 | |
| General practitioner | 24.3 | 32.7 | 41.1 | |
| Other | 5.2 | 3.8 | 3.3 | |
CABG Coronary artery bypass grafting; COPD Chronic obstructive pulmonary disease; MA Metropolitan area; MI Myocardial infarction; PCI Percutaneous coronary intervention; RA Rural area; UA Nonmetropolitan urban area
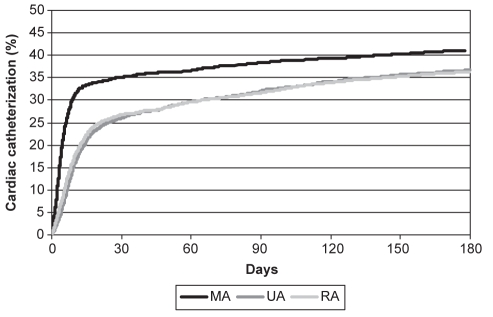
Patients who resided in MAs were more likely to be women and to have a history of smoking, hypercholesterolemia, hypertension, chronic obstructive pulmonary disease and/or asthma, renal failure, CHF and previous PCI or CABG (Table 1). They were less likely to have experienced an ST segment elevation acute MI (Table 1) and were subjected to lower rates of thrombolytic therapy (Table 2). However, this cohort had significantly higher rates of primary PCI, exercise stress testing, echocardiography and nuclear scintigraphy (Table 2). They also had higher rates of prescribed cardiac medications on discharge, including prescriptions for angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and antiplatelet agents (Table 2). Furthermore, they had higher rates of and shorter wait times for cardiac catheterization during the first six months following admission (Table 2 and Figure 2). Following cardiac catheterization, there were no differences among rates of coronary revascularization, by either PCI or CABG, across the geographical groupings, and there was no significant difference in wait times from cardiac catheterization to coronary revascularization (Table 2).
TABLE 2.
Invasive, noninvasive and pharmacological management of patients with acute myocardial infarction
| Variable | MA | UA | RA | P |
|---|---|---|---|---|
| Invasive management | ||||
| Thrombolysis, % | 27.7 | 33.2 | 34.2 | <0.0001 |
| Primary PCI, % | 7.3 | 1.3 | 1.7 | <0.0001 |
| Rate of cardiac catheterization, % | 45.6 | 37.3 | 37.3 | <0.0001 |
| Time to cardiac catheterization, days | 15.0 | 32.1 | 28.7 | <0.0001 |
| Rate of CABG/PCI, % | 72.2 | 75.2 | 73.5 | 0.35 |
| Time from admission to revascularization, days | 28.8 | 51.5 | 44.5 | <0.0001 |
| Time from catheterization to revascularization, days | 16.5 | 22.0 | 19.0 | 0.05 |
| Noninvasive management, % | ||||
| Exercise stress test | 47.0 | 41.1 | 41.7 | <0.0001 |
| Echocardiography | 24.9 | 24.1 | 17.6 | <0.0001 |
| Scintigraphy | 4.6 | 3.4 | 3.1 | 0.02 |
| Wall motion study | 13.4 | 11.4 | 16.4 | <0.0001 |
| Discharge medications, % | ||||
| Beta-blockers | 87.9 | 86.6 | 86.8 | 0.44 |
| ACE inhibitors/AT-II blockers | 63.1 | 60.9 | 57.7 | 0.0008 |
| HMG coenzyme A reductase inhibitors | 55.1 | 48.6 | 46.1 | <0.0001 |
| Antiplatelet agents | 91.1 | 89.2 | 88.7 | 0.03 |
ACE Angiotensin-converting enzyme; AT-II Angiotensin-II receptor; CABG Coronary artery bypass grafting; HMG 3-Hydroxy-3-methylglutaryl; MA Metropolitan area; PCI Percutaneous coronary intervention; RA Rural area; UA Nonmetropolitan urban area
Figure 2).
Cumulative rates of cardiac catheterization in the first six months following admission for acute myocardial infarction stratified by place of residence (log-rank P<0.0001). MA Metropolitan area; RA Rural area; UA Nonmetropolitan urban area
After adjusting for differences among patients in terms of age, sex, comorbid illness, type of acute MI, whether the patient received thrombolytic therapy following acute MI and income level, residence in either UAs (hazard ratio [HR] 0.77, 95% CI 0.69 to 0.87) or RAs (HR 0.75, 95% CI 0.67 to 0.84) emerged as an independent predictor of lower rates of cardiac catheterization within the first six months following admission for acute MI.
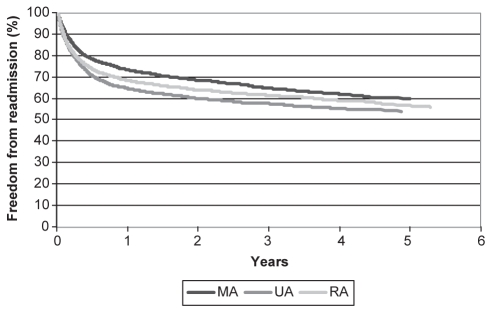
While long-term rates of all-cause mortality did not differ between the three geographical groupings (Table 3), patients who resided in either UAs or RAs experienced higher long-term rates of readmission to the hospital for any cardiac cause (Table 3 and Figure 3). After adjustment for differences between patients in terms of age, sex, comorbid illness and income quintile, residence in either UAs or RAs emerged as an independent predictor of increased long-term rates of readmission to the hospital for any cardiac cause (Table 4). When this analysis was repeated using only the composite of readmission for acute MI, unstable angina or CHF as the long-term outcome of interest, residence in UAs emerged as an independent predictor of this end point (HR 1.19, 95% CI 1.05 to 1.35), while residence in RAs did not (HR 1.03, 95% CI 0.91 to 1.17).
TABLE 3.
Unadjusted one-year and long-term rates of mortality and readmission
| Variable | MA | UA | RA | P |
|---|---|---|---|---|
| One-year rate, % | ||||
| All-cause mortality | 21.3 | 21.0 | 21.0 | 0.96 |
| Readmission (all-cause cardiac) | 26.8 | 35.3 | 31.7 | <0.0001 |
| Readmission (acute MI, unstable angina, congestive heart failure) | 19.8 | 25.3 | 21.5 | <0.0001 |
| Long-term rate, % | ||||
| All-cause mortality | 32.0 | 31.6 | 32.3 | 0.88 |
| Readmission (all-cause cardiac) | 37.0 | 43.8 | 40.5 | <0.0001 |
| Readmission (acute MI, unstable angina, congestive heart failure) | 29.2 | 34.5 | 30.5 | 0.0004 |
MA Metropolitan area; MI Myocardial infarction; RA Rural area; UA Nonmetropolitan urban area
Figure 3).
Kaplan-Meier survival curves, with readmission to the hospital for any cardiac cause stratified by place of residence as the outcome of interest (log-rank P<0.0001). MA Metropolitan area; RA Rural area; UA Nonmetropolitan urban area
TABLE 4.
Multivariate predictors of long-term readmission to the hospital for any cardiac cause
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age, years | |||
| >70 | 0.88 | 0.77–0.99 | 0.04 |
| 61–70 | 0.97 | 0.85–1.10 | 0.62 |
| 51–60 | 0.94 | 0.82–1.07 | 0.33 |
| ≤50 | 1.00 | – | – |
| Female sex | 0.94 | 0.87–1.02 | 0.13 |
| Smoking history | 1.06 | 0.98–1.15 | 0.15 |
| Hypercholesterolemia | 1.22 | 1.13–1.32 | <0.0001 |
| Diabetes | 1.21 | 1.12–1.31 | <0.0001 |
| Hypertension | 1.13 | 1.05–1.22 | 0.001 |
| Congestive heart failure | 0.99 | 0.88–1.12 | 0.93 |
| Previous MI/unstable angina | 1.27 | 1.16–1.38 | <0.0001 |
| COPD/asthma | 0.99 | 0.91–1.10 | 0.99 |
| Renal failure | 0.97 | 0.82–1.16 | 0.76 |
| Cerebrovascular disease | 0.98 | 0.87–1.10 | 0.70 |
| Prior CABG/PCI | 1.14 | 1.01–1.28 | 0.03 |
| Income | |||
| Lowest | 1.00 | – | – |
| Second | 1.04 | 0.90–1.19 | 0.61 |
| Middle | 1.15 | 1.00–1.33 | 0.05 |
| Fourth | 1.03 | 0.89–1.19 | 0.71 |
| Highest | 1.08 | 0.93–1.26 | 0.30 |
| Geography | |||
| MA | 1.00 | – | – |
| UA | 1.24 | 1.11–1.39 | 0.0001 |
| RA | 1.12 | 1.01–1.25 | 0.04 |
CABG Coronary artery bypass grafting; COPD Chronic obstructive pulmonary disease; HR Hazard ratio; MA Metropolitan area; MI Myocardial infarction; PCI Percutaneous coronary intervention; RA Rural area; UA Nonmetropolitan urban area
DISCUSSION
The present study aimed to determine the impact of a patient’s place of residence on rates of cardiac catheterization and on long-term rates of all-cause mortality and readmission to hospitals in Nova Scotia. Following adjustment for differences between patients in terms of age, sex, comorbid illness and estimated income level, place of residence in areas remote from the single tertiary cardiac care centre in Nova Scotia emerged as being independently associated with lower rates of cardiac catheterization within the first six months following admission, as well as higher long-term rates of readmission to the hospital for any cardiac cause. No differences were noted regarding long-term rates of all-cause mortality.
Our findings confirm those of studies (2–13) in other provincial jurisdictions across Canada with regard to regional variation in rates of cardiac catheterization following an acute MI. However, we were unable to attribute such regional variation to differences in socioeconomic status, an association that has been demonstrated in earlier studies from Ontario and Quebec (5,9). Rather, place of residence emerged as an independent predictor of rates of cardiac catheterization following an acute MI. This is similar to findings from the United States (20) in which decreased distance between the patient’s residence and the nearest cardiac revascularization services was found to be predictive of increased rates of invasive cardiac care.
The relationship that we documented between residence in either UAs or RAs, and reduced rates of and prolonged wait times for cardiac catheterization may reflect barriers in access to care that exist at the level of the health care provider. We have shown that patients in Nova Scotia who were admitted closest to or at the QEII Health Sciences Centre were more likely to be admitted under a cardiologist. Prior studies from the United States (21,22) have shown that patients with an acute MI who were admitted under a cardiologist were more likely to undergo coronary angiography and subsequent coronary revascularization. This appears to hold true in Nova Scotia, where rates of cardiac catheterization were highest in areas in which patients were more likely to be cared for primarily by a cardiologist. We hypothesized that the reduced rates of cardiac catheterization seen in patients from UAs or RAs would be associated with increased rates of noninvasive testing but found that this was not the case because patients with the highest rates of cardiac catheterization were also those with the highest rates of noninvasive testing.
Alternatively, patient-specific factors may have influenced whether a patient sought advanced cardiac care and, ultimately, underwent cardiac catheterization. A recent study by Alter et al (6) demonstrated that a patient’s socioeconomic status, as measured by self-reported income and education levels, influenced his/her access to cardiac services as well as his/her perception of the care received. It may be postulated that patients living in closer proximity to the tertiary cardiac care centre represented a group of people who were more motivated to seek out the highest level of care available to them, thus causing higher rates of cardiac catheterization in this group. Conversely, patients from more remote areas, particularly elderly patients, may have been less inclined to travel to the tertiary care centre, either because they were unwilling to undergo more aggressive treatment or because they were satisfied with the care available to them at their local hospital.
Following cardiac catheterization, rates of subsequent PCI or CABG and wait times from the point of catheterization to the point of revascularization did not differ by place of residence. This likely reflects the relatively uniform approach to revascularization employed by practitioners in Nova Scotia and the effectiveness of a peer-reviewed cardiovascular surgery conference system practised at the QEII Health Sciences Centre, where patients who are referred for surgery are impartially reviewed by a panel of cardiovascular specialists that determines surgical eligibility and priority based on such criteria as coronary anatomy, stress-test results and functional status, regardless of place of residence (23).
In the present study, long-term survival was found to be similar across the three geographical groups. Studies in both Canada and the United States have repeatedly shown that the impact of increased rates of cardiac catheterization on long-term survival is negligible (3,4,6,9,10,20,24,25). However, randomized clinical trials have demonstrated reduced rates of symptom recurrence, readmission to the hospital and, in some instances, rates of mortality, in patients admitted with an acute MI who were managed with an early invasive strategy, compared with those who were managed conservatively (26–28). In their meta-analysis of 23 randomized trials comparing the results of primary PCI with those of thrombolytic therapy in patients with ST segment elevation acute MI, Keeley et al (29) touted the benefits of primary PCI in reducing mortality and morbidity, both in the short term and in the long term. It is possible that the lack of a mortality benefit in patients from MAs despite their higher rates of cardiac catheterization reflects the absence of a comprehensive primary PCI program at the QEII Health Sciences Centre during the study period relative to other cardiac care centres in Canada (13).
In the present study, rates of readmission to the hospital for any cardiac cause over time were significantly increased in patients living outside of MAs, likely reflecting the reduced rates of and delays in access to cardiac catheterization experienced by affected patients. However, other factors may have played a pivotal role in determining whether a patient was readmitted to the hospital. First, although the use of evidence-based cardiovascular medications in Nova Scotia is rising and approaching reasonable levels for certain drug classes (30), we found significant variation in prescription rates of disease-modifying cardiovascular medications across the three geographical strata, which may, in turn, explain the differing readmission rates. Second, the level of follow-up with either a specialist or a nonspecialist after initial discharge from the hospital may have varied considerably across geographical groupings and subsequently impacted the patient’s eventual need for readmission (31). Finally, the threshold for readmission to the hospital, particularly for diagnoses other than acute coronary syndrome, may have been lower in peripheral, nontertiary care centres in which health care providers may have been less comfortable managing such conditions on an outpatient basis.
The present study is the first to employ a province-wide, population-based, disease-specific registry to examine the effect of clinical and non-clinical factors on access to invasive cardiac services and long-term outcomes following an acute MI. It provides valuable insights into the role that place of residence plays in determining the level of care that a patient with an acute MI receives and the potential deleterious effect that it may have on rates of readmission to the hospital over time. However, the present study is not without its limitations. First, there may be issues surrounding data accuracy, in particular as they pertain to the reporting of comorbid illnesses. Residents of MAs were younger and had a higher income but, surprisingly, they possessed a greater burden of comorbid illness. Patients from RAs were expected to have the highest burden of comorbid illness and patients from MAs were expected to have the lowest. While this finding may reflect the migration of individuals with advanced comorbid illnesses (eg, patients with renal failure requiring dialysis) to areas in which specialized expertise and services exist, it may also be the result of under-reporting or under-diagnosis of comorbid conditions such as hypercholesterolemia and hypertension among residents of UAs and RAs. This limitation is likely not exclusive to clinical registries. In the present study, the distribution of comorbid illnesses across geographical groupings only served to underestimate the differences in risk-adjusted rates of readmission seen between residents of UAs and RAs, and residents of MAs. Second, a clinical registry, while more detailed than an administrative dataset, is still not fully comprehensive. Therefore, one cannot ensure that all potentially important confounding variables have been adjusted for and that the differences seen in long-term outcomes are the result of residual confounding. Finally, individual-level data concerning socioeconomic status, including personal income, education and occupation level, were lacking, thereby necessitating the use of ecologic-level markers of socioeconomic status. While the use of ecologic-level measures of socioeconomic status in health outcomes research has previously been validated (17,18), a number of more recently published studies have indicated that treatment of ecologic-level measures as patient-level factors may yield erroneous conclusions and that such measures should be treated as group-level or contextual-level variables rather than patient-level variables (5,9,32–34).
CONCLUSION
In patients admitted with an acute MI in Nova Scotia, residence outside of MAs was associated with diminished rates of cardiac catheterization, longer waiting times and increased long-term readmission rates after adjusting for differences between patients in terms of age, sex, comorbid illness and income level. Despite universal health care coverage and a small geographical area, the centralization of tertiary cardiovascular services in Nova Scotia appears to be associated with significant disparities in access to invasive cardiovascular diagnostic and interventional treatment procedures according to location of residence. Importantly, patients with the greatest access barriers were those with the worst outcomes. Further studies are required to better understand the reasons underlying these apparent inequalities in the Canadian public health care system.
Footnotes
FUNDING: Dr Hassan is a PhD student funded by the Canadian Cardiovascular Outcomes Research Team and the Nova Scotia Health Research Foundation.
REFERENCES
- 1.Canada Health Act, chapter C-6 (section 10)1984
- 2.Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med. 1999;341:1359–67. doi: 10.1056/NEJM199910283411806. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues EJ, Simpson E, Richard H, Pilote L. Regional variation in the management of acute myocardial infarction in the province of Quebec. Can J Cardiol. 2002;18:1067–76. [PubMed] [Google Scholar]
- 4.Alter DA, Naylor CD, Austin PC, Tu JV. Long-term MI outcomes at hospitals with or without on-site revascularization. JAMA. 2001;285:2101–8. doi: 10.1001/jama.285.16.2101. [DOI] [PubMed] [Google Scholar]
- 5.Alter DA, Naylor D, Austin PC, Chan BTB, Tu JV. Geography and service supply do not explain socioeconomic gradients in angiography use after acute myocardial infarction. CMAJ. 2003;168:261–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Alter DA, Iron K, Austin PC, Naylor CD, for the SESAMI Study Group Socioeconomic status, service patterns, and perceptions of care among survivors of acute myocardial infarction in Canada. JAMA. 2004;291:1100–7. doi: 10.1001/jama.291.9.1100. [DOI] [PubMed] [Google Scholar]
- 7.Alter DA, Tu JV, Austin PC, Naylor CD. Waiting times, revascularization modality, and outcomes after acute myocardial infarction at hospitals with and without on-site revascularization facilities in Canada. J Am Coll Cardiol. 2003;42:410–9. doi: 10.1016/s0735-1097(03)00640-5. [DOI] [PubMed] [Google Scholar]
- 8.Alter DA, Brandes S, Irvine J, Iron K. Impact of socioeconomic status on cardiovascular outcomes in Canada. Pharmacoeconomics Outcomes Res. 2003;3:691–702. doi: 10.1586/14737167.3.6.691. [DOI] [PubMed] [Google Scholar]
- 9.Pilote L, Joseph L, Belisle P, Penrod J. Universal health insurance coverage does not eliminate inequities in access to cardiac procedures after acute myocardial infarction. Am Heart J. 2003;146:1030–7. doi: 10.1016/S0002-8703(03)00448-4. [DOI] [PubMed] [Google Scholar]
- 10.Seidel JE, Ghali WA, Faris PD, et al. for the APPROACH Investigators Geographical location of residence and uniformity of access to cardiac revascularization services after catheterization. Can J Cardiol. 2004;20:517–23. [PubMed] [Google Scholar]
- 11.Hartford K, Roos LL, Walld R. Regional variation in angiography, coronary artery bypass surgery, and percutaneous transluminal coronary angioplasty in Manitoba, 1987 to 1992: The funnel effect. Med Care. 1998;36:1022–32. doi: 10.1097/00005650-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Johansen H, Nair C, Mao L, Wolfson M. Revascularization and heart attack outcomes. Health Reports. 2002;13:35–46. [PubMed] [Google Scholar]
- 13.Pilote L, Merrett P, Karp I, et al. Cardiac procedures after an acute myocardial infarction across nine Canadian provinces. Can J Cardiol. 2004;20:491–500. [PubMed] [Google Scholar]
- 14.Cox JL, on behalf of the ICONS Investigators Optimizing disease management at a health care system level: The rationale and methods of the Improving Cardiovascular Outcomes in Nova Scotia (ICONS) study. Can J Cardiol. 1999;15:787–96. [PubMed] [Google Scholar]
- 15.Montague T, Cox J, Kramer S, et al. Improving Cardiovascular Outcomes in Nova Scotia (ICONS): A successful public-private partnership in primary healthcare. Hosp Q. 2003;6:32–8. doi: 10.12927/hcq..16498. [DOI] [PubMed] [Google Scholar]
- 16.Statistics Canada Postal Code Conversion File<http://www.statcan.ca:8096/bsolc/english/bsolc?catno=92F0153G> (Version current at March 31, 2005).
- 17.Mustard CA, Derksen S, Berthelot J-M, Wolfson M. Assessing ecologic proxies for household income: A comparison of household and neighbourhood level income measures in the study of population health status. Health Place. 1999;5:157–71. doi: 10.1016/s1353-8292(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein MM. Ecologic proxies for household income: How well do they work for the analysis of health and health care utilization. Can J Public Health. 2004;95:90–4. doi: 10.1007/BF03405773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canada Post Postal Guide Section<http://www.canadapost.ca/tools/pg/manual/b03-e.asp> (Version current at March 10, 2005).
- 20.Gregory PM, Malka ES, Kostis JB, Wilson AC, Arora JK, Rhoads GG. Impact of geographic proximity to cardiac revascularization services on service utilization. Med Care. 2000;38:45–57. doi: 10.1097/00005650-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Jollis JG, DeLong ER, Peterson ED, et al. Outcome of acute myocardial infarction according to the specialty of the admitting physician. N Engl J Med. 1996;335:1880–7. doi: 10.1056/NEJM199612193352505. [DOI] [PubMed] [Google Scholar]
- 22.Casale PN, Jones JL, Wolf FE, Pei Y, Eby LM. Patients treated by cardiologists have a lower in-hospital mortality for acute myocardial infarction. J Am Coll Cardiol. 1998;32:885–9. doi: 10.1016/s0735-1097(98)00325-8. [DOI] [PubMed] [Google Scholar]
- 23.Cox JL, Petrie JF, Pollak PT, Johnstone DE. Managed delay for coronary artery bypass graft surgery: The experience at one Canadian center. J Am Coll Cardiol. 1996;27:1365–73. doi: 10.1016/0735-1097(96)00028-9. [DOI] [PubMed] [Google Scholar]
- 24.Every NR, Larson EB, Litwin PE, et al. for the Myocardial Infarction Triage and Intervention Project Investigators The association between on-site cardiac catheterization facilities and the use of coronary angiography after acute myocardial infarction. N Engl J Med. 1993;329:546–51. doi: 10.1056/NEJM199308193290807. [DOI] [PubMed] [Google Scholar]
- 25.Rouleau JL, Moye LA, Pfeffer MA, et al. for the SAVE Investigators A comparison of management patterns after acute myocardial infarction in Canada and the United States. N Engl J Med. 1993;338:779–84. doi: 10.1056/NEJM199303183281108. [DOI] [PubMed] [Google Scholar]
- 26.Cannon CP, Weintraub WS, Demopoulos LA, Robertson DH, Gormley GJ, Braunwald E. Invasive versus conservative strategies in unstable angina and non-Q-wave myocardial infarction following treatment with tirofiban: Rationale and study design of the international TACTICS-TIMI 18 Trial. Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy. Thrombolysis In Myocardial Infarction. Am J Cardiol. 1998;82:731–6. doi: 10.1016/s0002-9149(98)00540-2. [DOI] [PubMed] [Google Scholar]
- 27.Madsen JK, Grande P, Saunamaki K, et al. Danish multicenter randomized study of invasive versus conservative treatment in patients with inducible ischemia after thrombolysis in acute myocardial infarction (DANAMI). DANish trial in Acute Myocardial Infarction. Circulation. 1997;96:748–55. doi: 10.1161/01.cir.96.3.748. [DOI] [PubMed] [Google Scholar]
- 28.FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators Invasive compared with non-invasive treatment in unstable coronary-artery disease: FRISC II prospective randomised multicentre study. Lancet. 1999;354:708–15. [PubMed] [Google Scholar]
- 29.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 30.Putnam W, Burge FI, Lawson B, et al. Evidence-based cardiovascular care in the community: A population-based cross-sectional study. BMC Fam Pract. 2004;5:6. doi: 10.1186/1471-2296-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayanian JZ, Landrum MB, Guadagnoli E, Gaccione P. Specialty of ambulatory care physicians and mortality among elderly patients after myocardial infarction. N Engl J Med. 2002;347:1678–86. doi: 10.1056/NEJMsa020080. [DOI] [PubMed] [Google Scholar]
- 32.Austin PC, Tu JV, Alter DA. Comparing hierarchical modeling with traditional logistic regression analysis among patients hospitalized with acute myocardial infarction: Should we be analyzing cardiovascular outcomes data differently? Am Heart J. 2003;145:27–35. doi: 10.1067/mhj.2003.23. [DOI] [PubMed] [Google Scholar]
- 33.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–92. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 34.Duncan C, Jones K, Moon G. Context, composition and heterogeneity: Using multilevel models in health research. Soc Sci Med. 1998;46:97–117. doi: 10.1016/s0277-9536(97)00148-2. [DOI] [PubMed] [Google Scholar]