Abstract
Myocardial bridging constitutes a congenital, usually benign, coronary abnormality defined as a segment of a major epicardial coronary artery that follows an intramural course through the myocardium. On the basis of clinical and histopathological data, myocardial bridges appear to be spared from atherosclerosis. Although the mechanisms involved are largely unknown, the surrounding myocardium appears to be a key factor by generating a unique atheroprotective hemodynamic microenvironment within bridges. The main components of this environment include low tensile stress and high shear stress. Reduced coronary wall motion due to external support of the surrounding myocardium may also play a role. Better investigation of these mechanisms in appropriate animal models is anticipated to advance our understanding of the pathophysiology of atherosclerosis, providing a framework for the development of new atheroprotective strategies.
Keywords: Atherosclerosis, Coronary artery, Myocardial bridge, Shear stress, Tensile stress, Wall motion
Abstract
Le pont myocardique constitue une anomalie coronarienne congénitale généralement bénigne, définie comme un important segment d’artère coronaire épicardique qui suit un parcours intramural dans le myocarde. Selon les données cliniques et histopathologiques, les ponts myocardiques semblent être épargnés par l’athérosclérose. Bien que les mécanismes en cause demeurent en grande partie inconnus, le myocarde avoisinant semble être un facteur clé car il produit un micro-environnement hémodynamique athéroprotecteur unique dans les ponts. Les principaux éléments de cet environnement incluent une faible contrainte de tension et une forte contrainte de cisaillement. Le mouvement réduit de la paroi coronaire découlant du soutien externe du myocarde avoisinant pourrait aussi être en cause. Une meilleure exploration de ces mécanismes dans des modèles animaux pertinents devrait faire progresser les connaissances sur la physiopathologie de l’athérosclérose et fournir un cadre pour mettre au point de nouvelles stratégies athéroprotectrices.
Myocardial bridging constitutes a congenital, usually benign, coronary abnormality defined as a segment of a major epicardial coronary artery or branch, a so-called ‘tunnelled’ artery, that follows an intramural course through the myocardium (1–3) (Figure 1). On the basis of clinical and autopsy data, myocardial bridges appear to be spared from atherosclerosis (3). Although the clinical and histopathology characteristics of myocardial bridges have been extensively described elsewhere, the sparing effect of bridges on atherosclerosis remains unknown (1–3). The present review summarizes the existing literature regarding the underlying atheroprotective mechanisms responsible for this effect. These mechanisms could potentially advance our understanding of the pathophysiology of atherosclerosis, providing a framework for the development of new atheroprotective strategies.
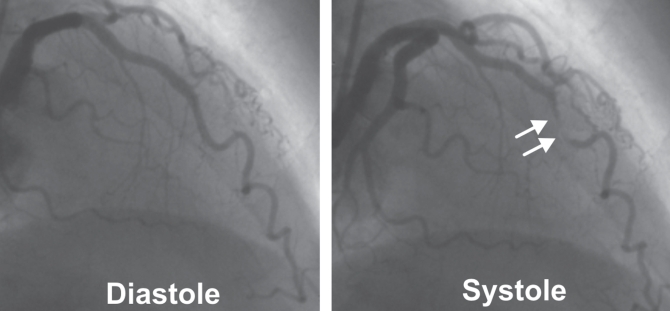
Figure 1).
Angiogram of a left anterior descending artery in diastole (left panel) and in systole (right panel), showing a myocardial bridge at the middle portion of the artery. The systolic compression of the coronary lumen is evident (arrows)
EPIDEMIOLOGY, CLINICAL MANIFESTATION AND DIAGNOSIS OF MYOCARDIAL BRIDGES
The most frequent location of myocardial bridges is the left anterior descending artery, especially at its middle one-third (3) (Figure 1). Overall, the frequency of myocardial bridges in adults varies substantially, with higher prevalence (15% to 80%) reported in histopathology studies than in angiographic studies (0.5% to 2.5%) (1,3,4). The length of bridges varies from 4 mm to 40 mm, and the depth from 1 mm to 10 mm (1,3). Although bridges are usually clinically silent, some, such as the longer and deeper ones, manifest with myocardial ischemia, rhythm conduction disturbances, acute coronary syndromes or even sudden cardiac death (3). Usually, bridges are incidentally identified during diagnostic angiography or autopsy studies. In recent years, new invasive ultrasound-based modalities, such as intravascular ultrasound and intracoronary Doppler imaging (4,5), as well as noninvasive methods (eg, computed tomography, magnetic resonance imaging) (6) have been widely used in the diagnosis of myocardial bridges.
EVIDENCE OF ABSENCE OF ATHEROSCLEROSIS WITHIN MYOCARDIAL BRIDGES
Using intravascular ultrasound in 69 patients with myocardial bridges, Ge et al (4) found that there was a high incidence of atherosclerosis at the segments proximal to the bridges, but no plaque was found within and distally to the bridges. Similar data were demonstrated in an angiographic study of 29 patients (7). In addition, Robicsek and Thubrikar (8) showed that in 24 of 26 patients with intramyocardial bridges, there was no apparent atherosclerosis in the intramyocardial segments.
These clinical findings concerning the atheroprotection of myocardial bridges are further supported by histopathology data (9). It was reported that the intima of a mural coronary segment is significantly thinner (66.3 μm) than the proximal portion (406.6 μm) of the artery (10). Endothelial cell permeability was found to be increased in atherosclerotic epicardial segments of hypercholesterolemic rabbits, but not within myocardial bridges (11). Other studies indicated that the intimal layer of a bridge is spared from foam cells and synthetic-type vascular smooth muscle cells, which precipitate plaque formation (12). At the molecular level, the association among the expression of genes encoding various atherogenic molecules, such as endothelin-1 (ET-1) and angiotensin-converting enzyme, and the occurrence of atherosclerosis in patients with myocardial bridges was studied (13). Intriguingly, both the expression of these genes and the extent of atherosclerosis were found to be reduced within myocardial bridges compared with the proximal epicardial segments.
MECHANISMS RESPONSIBLE FOR THE ABSENCE OF ATHEROSCLEROSIS WITHIN BRIDGES
The clinical and experimental observations mentioned above raise the question of why myocardial bridges are spared from atherosclerosis, while at the same time, the adjacent proximal epicardial segments are atherosclerosis-prone regions. Although the mechanisms of this discrepancy are largely unknown, the surrounding myocardium may play a key role by generating a unique atheroprotective hemodynamic microenvironment beneath bridges and a highly atherogenic milieu at their proximal edges.
Role of tensile stress
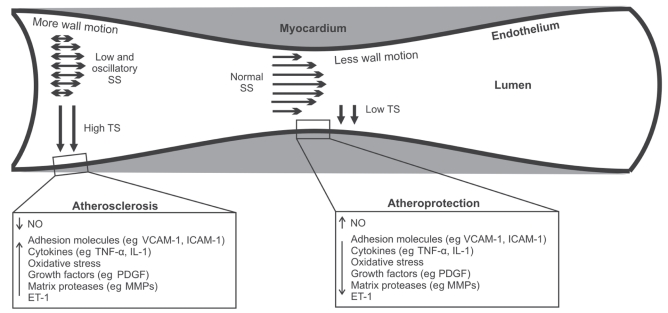
Tensile stress (TS), also known as circumferential stress, constitutes the blood pressure-derived force imposed circumferentially on the arterial wall. Its magnitude can be approximated by an equation related to Laplace’s law (T=P[r/t]), where P is the blood pressure, r is the lumen radius and t is the wall thickness. According to this formula, arterial regions with reduced blood pressure or reduced lumen size have low TS. The distribution of TS within the arterial wall of a bridge appears to play a major role in the modulation of a local atheroprotective environment. Normally, coronary flow in major epicardial segments mainly occurs in diastole, whereas in systole blood is stored in the dilated arterial wall (‘reservoir’ effect) (14–16). However, in the myocardial bridges, at the time that peak systolic pulse tends to expand the wall, the ‘tunnelled’ coronary segment undergoes remarkable compression and lumen radius reduction due to contraction of the surrounding myocardium. Although the blood pressure exhibits a significant increase within the bridged segments compared with the adjacent proximal regions (17), the net effect of these counteracting forces (ie, myocardial compression and blood pressure) is a reduced pressure gradient across the wall of the bridge and subsequently, a decreased TS (8,18), which maintains a normal endothelial function, thereby preventing the development of atherosclerosis (19) (Figure 2). Supporting the atheroprotective effect of low TS within the bridge is the observation that portions of vertebral arteries that are passing through the bone canal are free from atherosclerotic lesions compared with those outside the canal (18). Like myocardium, the surrounding bones may act as support and reduce the expansion of the vertebral arteries, thereby reducing the local TS and protecting the arteries from atherosclerosis.
Figure 2).
Mechanisms responsible for the absence of atherosclerosis from myocardial bridges and the development of lesions at the adjacent proximal epicardial segments. The surrounding myocardium creates a unique atheroprotective hemodynamic microenvironment within bridges characterized by low tensile stress (TS) and normal or high shear stress (SS). Reduced wall motion of mural segments may also play a role. Conversely, a highly atherogenic milieu is created at the edge proximal to the bridge with higher TS and low, oscillatory SS. ET-1 Endothelin-1; ICAM-1 Intercellular adhesion molecule-1; IL-1 Interleukin-1; MMP Matrix metalloproteinase; NO Nitric oxide; PDGF Platelet-derived growth factor; TNF-α Tumour necrosis factor-alpha; VCAM-1 Vascular cell adhesion molecule-1
On the other hand, at the segment proximal to the bridge, the local TS is higher than the tunnelled segment and may cause structural and functional changes of vascular cells (endothelial cells and primarily smooth muscle cells), ultimately resulting in the formation of atherosclerotic lesions (19) (Figure 2). At the molecular level, the higher TS is sensed by several endothelial mechanoreceptors, such as integrins, stretch-sensitive ion channels, tyrosine kinase receptors and G-proteins, which trigger a complex network of downstream signalling cascades of serine kinases (mitogen-activated protein kinases), eventually leading to activation of transcription factors (eg, nuclear factor kappa B or activator protein-1) (19). These transcription factors are thereby associated with certain strain-sensitive elements located at promoter regions of proatherogenic genes and upregulate their expression. Such genes encode several proatherogenic molecules, such as vasoconstrictive substances (ET-1), chemoattractants (monocyte chemoattractant protein-1), adhesion molecules (vascular cell adhesion molecule-1, intercellular adhesion molecule-1), cytokines (tumour necrosis factor-alpha, interleukin-1), growth-promoting factors (platelet-derived growth factor) and matrix-degrading enzymes (matrix metalloproteinases). All of these molecules drive the atherosclerotic process by enhancing lipid accumulation, inflammation and migration of smooth muscle cells to the intima, where they acquire a synthetic phenotype and produce interstitial collagen and matrix metalloproteinases. Also, elevated TS has been proposed to induce direct endothelial injury, thereby impairing the integrity of the endothelial barrier and increasing its permeability to lipoproteins and circulating monocytes (18).
Role of shear stress and blood flow
Besides TS, shear stress (SS), another major component of the local hemodynamic environment, may contribute to the atheroprotection of mural segments, as well as the formation of atherosclerotic plaques at the entrance of intramyocardial segments (Figure 2). SS is the frictional force exerted by the circulating blood onto the endothelial surface (20). It is defined as the product of the velocity gradient near the wall and the blood viscosity; this definition suggests that SS varies proportionally with flow. Low and oscillatory SS are characterized by low time-averaged values (less than 1.5 N/m2) and significant variations in direction and magnitude over short distances, and constitute major determinants of the localization, development and progression of atherosclerosis (20–24). Through complex mechanotransduction processes similar to those initiated by TS, coronary endothelium responds to low and oscillatory SS by adopting a vasoconstrictive (ET-1), proinflammatory (adhesion molecules and cytokines), pro-oxidative, growth-promoting and prothrombotic phenotype, ultimately acquiring a predisposition to atherosclerosis (16,20,24). On the other hand, the normal pulsatile SS, with a positive time-average ranging between 1.5 N/m2 and 7.0 N/m2, increases the production of nitric oxide and downregulates the expression of proatherogenic molecules, thereby conferring atheroprotection (16,20,24).
Flow analyses proximal, within and distal to the ‘tunnelled’ artery revealed that within a bridge, there is no anterograde flow during systole, possibly due to myocardial compression (‘milking effect’), followed by an accelerated forward flow in diastole (4,17). Conversely, the collapsed bridge generates a retrograde systolic flow at the epicardial segment proximal to the bridge, accompanied by an accelerated forward flow in diastole (4,17). Thus, at the proximal segment, a time-varying bidirectional flow occurs, generating an atherogenic low and oscillatory SS microenvironment, whereas within the bridge, the SS remains unidirectional and normal, or even high, maintaining the endothelium in an atheroprotective state (Figure 2). The absence of atherosclerosis within bridges and the increased susceptibility of the adjacent proximal segments can be further explained mechanistically (25). In atherosclerosis-prone regions proximal to a bridge, where low and oscillatory SS occur, the residence time of proatherogenic blood particles (eg, lipids, inflammatory cells) and their subsequent subendothelial accumulation increase, facilitating the atherosclerotic process, whereas the normal or high flow occurring within a bridge prevents blood stagnation, thereby protecting the endothelium from atherosclerosis (26).
Role of coronary wall motion
Another potential mechanism for the sparing of bridges from atherosclerosis involves coronary motion (Figure 2). As long as the epicardial coronary segments are closely attached to the beating heart, they sustain a periodic motion during the cardiac cycle. Pulsatile coronary motion affects coronary geometry and this, in turn, influences the local hemodynamic environment, initiating a self-perpetuating vicious cycle, which drives atherosclerosis (16,27). Lyon et al (28) studied the effect of arterial wall motion on the development of atherosclerosis in cynomolgus monkeys fed an atherosclerotic diet, and concluded that the inhibition of wall motion may confer protection against atherosclerosis. This observation could potentially provide a mechanistic explanation for the void of atherosclerosis at the bridges because, on contraction of ventricular muscle, the ‘tunnelled’ coronary segment undergoes remarkable systolic compression, thereby limiting its motion over the cardiac cycle. Conversely, the absence of such an external support around the adjacent epicardial coronary portions makes their phasic motion more prominent, and thereby may increase their susceptibility to plaque development.
CONCLUSION AND RESEARCH OPPORTUNITIES
Myocardial bridges constitute a well-defined coronary abnormality that is spared from atherosclerosis. Although the precise mechanisms responsible for the absence of atherosclerosis are not clear, it appears that the contractile myocardium that encompasses the mural artery constitutes a key modulator by generating a local hemodynamic environment of low TS, high SS and reduced phasic coronary motion, ultimately conferring atheroprotection. However, in some cases, the myocardial bridges lead to an acute coronary syndrome, possibly due to an exaggerated coronary constriction at systole, and subsequent intermittent flow cessation. In fact, myocardial bridging has been reported as a potential cause of acute myocardial infarction in the absence of atherothrombosis, especially in young, healthy individuals (3,29). Despite the ischemic aspect of bridges, their freedom from atherosclerosis constitutes an interesting observation that it is worth studying in animal models. Gorillas and gibbons have an epicardial coronary artery network, which is in contrast to chimpanzees and orangutans, whose coronary arteries tend to follow a mural course (25). The comparative investigation of coronary atherosclerosis in these species may provide further insight into the local hemodynamic environment (eg, SS and TS patterns over the cardiac cylce) that prevents the development of atherosclerotic lesions within bridges. A better understanding of this environment may enable us to design new local intervention strategies that may be applied in atherosclerosis-prone coronary regions with minimal lesions (eg, inner curvatures, bifurcations) to alter the local hemodynamic environment toward a more atheroprotective state, thereby preventing the evolution of these early lesions to more advanced stages.
Footnotes
GRANTS: The authors received a grant from the Greek State Scholarships Foundation, the Aristotle University of Thessaloniki Research Committee and the Hellenic Harvard Foundation.
REFERENCES
- 1.Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106:2616–22. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 2.Alegria JR, Herrmann J, Holmes DR, Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005;26:1159–68. doi: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 3.Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: Overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–9. doi: 10.1016/s0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 4.Ge J, Jeremias A, Rupp A, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–16. doi: 10.1053/euhj.1999.1661. [DOI] [PubMed] [Google Scholar]
- 5.Lovell MJ, Knight CJ. Invasive assessment of myocardial bridges. Heart. 2003;89:699–700. doi: 10.1136/heart.89.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantarci M, Duran C, Durur I, et al. Detection of myocardial bridging with ECG-gated MDCT and multiplanar reconstruction. AJR Am J Roentgenol. 2006;186:S391–4. doi: 10.2214/AJR.05.0307. [DOI] [PubMed] [Google Scholar]
- 7.Herrmann J, Higano ST, Lenon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J. 2004;25:2134–42. doi: 10.1016/j.ehj.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Robicsek F, Thubrikar MJ. The freedom from atherosclerosis of intramyocardial coronary arteries: Reduction of mural stress – a key factor. Eur J Cardiothorac Surg. 1994;8:228–35. doi: 10.1016/1010-7940(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 9.Lee SS, Wu TL. The role of the mural coronary artery in prevention of coronary atherosclerosis. Arch Pathol. 1972;93:32–5. [PubMed] [Google Scholar]
- 10.Risse M, Weiler G. [Coronary muscle bridge and its relations to local coronary sclerosis, regional myocardial ischemia and coronary spasm. A morphometric study] Z Kardiol. 1985;74:700–5. [PubMed] [Google Scholar]
- 11.Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997;430:163–71. doi: 10.1007/BF01008038. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Hosoda Y, Osaka T, et al. The significance of myocardial bridge upon atherosclerosis in the left anterior descending coronary artery. J Pathol. 1986;148:279–91. doi: 10.1002/path.1711480404. [DOI] [PubMed] [Google Scholar]
- 13.Masuda T, Ishikawa Y, Akasaka Y, Itoh K, Kiguchi H, Ishii T. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. J Pathol. 2001;193:408–14. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH792>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Berne R, Levy M. Cardiovascular physiology. St Louis: Mosby; 2001. [Google Scholar]
- 15.Toyota E, Ogasawara Y, Hiramatsu O, et al. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288:H1598–603. doi: 10.1152/ajpheart.01103.2003. [DOI] [PubMed] [Google Scholar]
- 16.Chatzizisis YS, Giannoglou GD, Parcharidis GE, Louridas GE. Is left coronary system more susceptible to atherosclerosis than right? A pathophysiological insight. Int J Cardiol. 2007;116:7–13. doi: 10.1016/j.ijcard.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Klues HG, Schwarz ER, vom Dahl J, et al. Disturbed intracoronary hemodynamics in myocardial bridging: Early normalization by intracoronary stent placement. Circulation. 1997;96:2905–13. doi: 10.1161/01.cir.96.9.2905. [DOI] [PubMed] [Google Scholar]
- 18.Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg. 1995;59:1594–603. doi: 10.1016/0003-4975(94)01037-d. [DOI] [PubMed] [Google Scholar]
- 19.Haga JH, Li YS, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–60. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 21.Stone PH, Coskun AU, Kinlay S, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: In vivo 6-month follow-up study. Circulation. 2003;108:438–44. doi: 10.1161/01.CIR.0000080882.35274.AD. [DOI] [PubMed] [Google Scholar]
- 22.Giannoglou GD, Soulis JV, Farmakis TM, Farmakis DM, Louridas GE. Haemodynamic factors and the important role of local low static pressure in coronary wall thickening. Int J Cardiol. 2002;86:27–40. doi: 10.1016/s0167-5273(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 23.Chatzizisis YS, Giannoglou GD. Pulsatile flow: A critical modulator of the natural history of atherosclerosis. Med Hypotheses. 2006;67:338–40. doi: 10.1016/j.mehy.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular and vascular behavior. J Am Coll Cardiol. 2007;49:2379–93. 25. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 25.Scher AM. Absence of atherosclerosis in human intramyocardial coronary arteries: A neglected phenomenon. Atherosclerosis. 2000;149:1–3. doi: 10.1016/s0021-9150(99)00464-5. [DOI] [PubMed] [Google Scholar]
- 26.Soulis JV, Giannoglou GD, Chatzizisis YS, et al. Spatial and phasic oscillation of non-Newtonian wall shear stress in human left coronary artery bifurcation: An insight to atherogenesis. Coron Artery Dis. 2006;17:351–8. doi: 10.1097/00019501-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Zhu H, Friedman MH. Relationship between the dynamic geometry and wall thickness of a human coronary artery. Arterioscler Thromb Vasc Biol. 2003;23:2260–5. doi: 10.1161/01.ATV.0000095976.40874.E0. [DOI] [PubMed] [Google Scholar]
- 28.Lyon RT, Runyon-Hass A, Davis HR, Glagov S, Zarins CK. Protection from atherosclerotic lesion formation by reduction of artery wall motion. J Vasc Surg. 1987;5:59–67. [PubMed] [Google Scholar]
- 29.Bauters C, Chmait A, Tricot O, Lamblin N, Van Belle E, Lablanche JM. Images in cardiovascular medicine. Coronary thrombosis and myocardial bridging. Circulation. 2002;105:130. doi: 10.1161/hc0102.100421. [DOI] [PubMed] [Google Scholar]