Abstract
Background
Optimal positive end-expiratory pressure (PEEP) is an important component of adequate mechanical ventilation in acute lung injury and acute respiratory distress syndrome (ARDS). In the present study we tested the effect on gastric intramucosal pH of incremental increases in PEEP level (i.e. PEEP titration) to improve oxygenation in ARDS. Seventeen consecutive patients with ARDS, as defined by consensus criteria, were included in this clinical, prospective study. All patients were haemodynamically stable, and were not receiving vasopressors. From an initial level of 5 cmH2O, PEEP was titrated at 2 cmH2O increments until the partial arterial oxygen tension was 300 mmHg or greater, peak airway pressure was 45 cmH2O or greater, or mean arterial blood pressure decreased by 20% or more of the baseline value. Optimal PEEP was defined as the level of PEEP that achieved the best oxygenation. The maximum PEEP was the highest PEEP level reached during titration in each patient.
Results
Gastric mucosal pH was measured using gastric tonometry at all levels of PEEP. The thermodilution technique was used for measurement of cardiac index. Gastric mucosal pH was similar at baseline and at optimal PEEP levels, but it was slightly reduced at maximum PEEP. Cardiac index and oxygen delivery remained stable at all PEEP levels.
Conclusion
Incremental titration of PEEP based on improvement in oxygenation does not decrease gastric intramucosal perfusion when cardiac output is preserved in patients with ARDS.
Keywords: acute lung injury, acute respiratory distress syndrome, mechanical ventilation, positive end-expiratory pressure, splanchnic perfusion
Introduction
Positive end-expiratory pressure (PEEP) is an important component of the ventilatory management of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). PEEP improves oxygenation by redistributing the alveolar fluid and restores functional residual capacity by keeping the alveoli open. However, PEEP can be detrimental because it may, particularly at high levels, decrease cardiac output by decreasing the venous return as a result of diminished pressure gradient between the systemic veins and right atrium [1], and consequently it may lead to hypoperfusion of vital organs. Ultimately, despite improving arterial oxygen content, PEEP may decrease oxygen delivery to various organs, among which the splanchnic vascular bed appears to be particularly at risk because of its predisposing features and the influence of PEEP on regional blood flow distribution.
Maintenance of splanchnic blood flow is important because splanchnic hypoperfusion may play a critical role in the pathogenesis of multiorgan dysfunction syndrome [2,3]. Mechanical ventilation has been suggested to potentiate the adverse effects of underlying critical illness on splanchnic vasculature and contribute to the development of multiorgan dysfunction syndrome, particularly when 'injurious' ventilatory strategies that produce high end-inspiratory lung volumes are employed [3]. Experimental studies suggested that mechanical ventilation with considerably high levels of PEEP can lead to splanchnic hypoperfusion and marked reduction in hepatic blood flow [4-6]. Furthermore, PEEP may decrease splanchnic blood flow in patients with no underlying lung disease [7,8]. Most available evidence regarding the effects of PEEP from animal studies has been extrapolated to humans based on the assumption that the effects of mechanical ventilation on humans and animals are similar. However, a recent study conducted in humans explored the effect of PEEP in patients with ALI [9] and did not find a consistent effect on splanchnic blood flow.
Because of the difficulties associated with measurement of pressure–volume curves, incremental titration of PEEP in an attempt to find the 'best' PEEP, based on improvement in oxygenation, is common practice in the management of hypoxaemic respiratory failure. However, it is unknown whether this strategy has an adverse effect on splanchnic perfusion. The aim of the present study was to investigate the impact of PEEP titration (based on improvement in oxygenation) on gastric mucosal perfusion in patients with ARDS, as assessed by measurement of gastric mucosal pH (pHi).
Method
Patients
The study protocol was approved by the Institutional Ethics Committee of Istanbul University Hospital. Written informed consent was obtained from each patient or the patient's next of kin. We consecutively enrolled 17 patients with ARDS admitted to the multidisciplinary intensive care unit at Istanbul University Hospital. The criteria for eligibility were a diagnosis of ARDS (based on a consensus report [10]), age older than 18 years and mean arterial pressure (MAP) greater than 60 mmHg with no haemodynamic support. All patients were enrolled within the first 24 hours following the diagnosis of ARDS. Patients with known cardiac dysfunction or pre-existing liver disease were not included in the trial.
Protocol
All patients were ventilated using a Servo 300 Siemens ventilator (Siemens Elema, Uppsala, Sweden) using the pressure-regulated volume control mode with a tidal volume of 8–10 ml/kg (based on ideal body weight), frequency of 12 breaths/min, fraction of inspired oxygen of 1.0, and inspiratory:expiratory ratio of 1:2. Patients were sedated with midazolam (Dormicum; Hoffmann LaRoche, Basel, Switzerland) at 4 mg/hour and paralyzed with 0.1 mg/kg vecuronium (Norcuron; Organon, Oss, The Netherlands) infusion during the study. In addition to employing a radial arterial catheter for blood pressure measurement, a pulmonary artery catheter (Abbot labs, North Chicago, IL, USA) was placed in all patients for haemodynamic monitoring. No patients received any therapeutic intervention to improve haemodynamics (i.e. fluid resuscitation or catecholamine infusion) throughout the study.
Baseline PEEP (PEEPbaseline) was set at 5 cmH2O and titrated at 2 cmH2O increments until the partial arterial oxygen tension (PaO2) reached at least 300 mmHg, peak airway pressure was 45 cmH2O or greater, or MAP dropped by 20% or more from the baseline value. Criteria for overinflation of lung (and therefore for discontinuation of further titration of PEEP) were reduction in PaO2 of 10% or more and an increase in arterial carbon dioxide tension of 10% or more. Optimal PEEP (PEEPopt) was defined as the PEEP that achieved the best oxygenation, whereas maximum PEEP (PEEPmax) was the greatest level of PEEP achieved during titration in each patient.
A nasogastric catheter (TRIP Catheter; Tonometrics Division, Instrumentarium Corp., Helsinki, Finland) was inserted into the stomach to measure pHi. Correct placement of the TRIP catheter was confirmed by radiography. Enteral nutrition was withheld throughout the study, and all patients received ranitidine 50 mg intravenously. In order to allow for equilibration, pHi was measured 45 min after injection of 2.5 ml isotonic saline into the semipermeable balloon of the TRIP catheter. Partial pressure of carbon dioxide in saline solution and bicarbonate level in arterial blood were measured simultaneously using a blood gas analyzer (ABL-500; Radiometer, Copenhagen, Denmark) immediately after sampling [11] and were corrected for the equilibration time [12]. The pHi was calculated using the Henderson–Hassel-bach equation.
All measurements, including respiratory, haemodynamic parameters, arterial and mixed venous blood gas analyses, and gastric pHi, were taken at baseline and following ventilation for 45 min at each level of PEEP. Haemodynamic parameters were monitored continuously using an Horizon XL monitor (Mennen Medical Inc., New York, NY, USA). Cardiac output was measured in triplicate by thermodilution technique using 10 ml saline solution at room temperature. Cardiac index, shunt fraction, oxygen delivery (DO2) and oxygen consumption were calculated at baseline and at all PEEP levels.
Statistical analysis
Paired analysis of variance tests were used to analyze the differences between measurements. P < 0.05 was considered statistically significant. All values are presented as mean ± standard deviation.
Results
A total of 17 patients were enrolled in the present study (11 male and 6 female). The characteristics of the individual patients are shown in Table 1. The mean age of the study population was 47.2 ± 19.8, the mean Acute Physiology and Chronic Health Evaluation II score was 19.7 ± 3.5, and the mean Sequential Organ Failure Assessment score was 6.3 ± 1.8. By titrating PEEP, we were able to achieve a mean PEEPopt of 10.4 ± 3.9 cmH2O and a PEEPmax of 13.3 ± 2.9 cmH2O (P = 0.0001). The highest PEEP value applied was 17 cmH2O. Static compliance improved slightly at PEEPopt, but this did not achieve statistical significance (P = 0.84; Table 2). Changes in peak airway and mean airway pressures at PEEPbaseline, PEEPopt and PEEPmax were statistically significant (P < 0.001; Table 2). Reasons for stopping the titration of PEEP were reduction in PaO2 (from 20% to 40%; n = 6), reduction in MAP (from 25% to 60%; n = 4), adequate oxygenation (PaO2350–450 mmHg; n = 4) and excessive peak upper ariway pressure (n = 3).
Table 1.
Characteristics of the 17 patients studied
| Patient number | Diagnosis at admission | Age (years) | Sex | APACHE II score | SOFA score | Additional organ failure |
| 1 | Multiple trauma | 74 | M | 15 | 5 | R, N |
| 2 | Hepatic coma | 26 | F | 23 | 10 | R, H, N, L |
| 3 | Cerebral ischaemia | 70 | M | 22 | 6 | H, N |
| 4 | Sepsis | 55 | M | 21 | 6 | H, L |
| 5 | Intracranial haemorrhage | 58 | M | 15 | 7 | H, N |
| 6 | Acute pancreatitis | 50 | M | 23 | 9 | R, H, L |
| 7 | Multiple trauma | 24 | M | 19 | 6 | R, H |
| 8 | Pneumonia | 18 | F | 12 | 5 | R |
| 9 | Postoperative sepsis | 28 | F | 21 | 7 | R, H, N, L |
| 10 | Acute pancreatitis | 74 | M | 23 | 7 | R, H, L |
| 11 | Tetanus | 62 | M | 22 | 4 | R, H |
| 12 | Pneumonia | 62 | M | 21 | 3 | H |
| 13 | Bronchopneumonia | 37 | F | 16 | 4 | R, |
| 14 | Multiple trauma | 40 | M | 21 | 5 | R, H |
| 15 | Postoperative scoliosis | 19 | F | 24 | 7 | R, H, L |
| 16 | Aortoduodenal fistula | 69 | M | 17 | 6 | R, H, L |
| 17 | Intra-abdominal sepsis | 37 | F | 20 | 7 | R, H, L |
APACHE, Acute Physiology and Chronic Health Evaluation; F, female; H, haematological system; L, hepatic system; M, male; N, neurological system; R, renal system; SOFA, Sequential Organ Failure Assessment.
Table 2.
Parameters measured during titration of positive end-expiratory pressure
| Parameter | PEEPbaseline | PEEPopt | PEEPmax | P value |
| PEEP (cmH2O) | 5 | 10.4 ± 3.9 | 13.3 ± 2.9 | 0.0001 |
| Ppeak (cmH2O) | 27.2 ± 5 | 31.5 ± 6.2 | 35 ± 5 | 0.0001 |
| Pmean (cmH2O) | 11.4 ± 1.9 | 15.9 ± 4.9 | 19.3 ± 2.8 | 0.0001 |
| PaO2 (mmHg) | 136.6 ± 48.7 | 231 ± 86.1 | 226 ± 99.8 | 0.001 |
| PaCO2 (mmHg) | 38.4 ± 7.02 | 37 ± 7.63 | 37.4 ± 8.19 | 0.70 |
| pHi | 7.31 ± 0.13 | 7.32 ± 0.12 | 7.30 ± 0.12 | 0.84 |
| P(t-a)CO2 (mmHg) | 3.74 ± 8.31 | 5.27 ± 5.49 | 7.42 ± 7.39 | 0.353 |
| CI (l/min per m2) | 4.03 ± 1.47 | 3.72 ± 1.4 | 3.62 ± 1.21 | 0.79 |
| CO (l/min) | 7.02 ± 2.46 | 6.61 ± 2.41 | 6.5 ± 2.15 | 0.13 |
| MAP (mmHg) | 89 ± 17.7 | 88.4 ± 15.3 | 83 ± 15.9 | 0.49 |
| CVP (mmHg) | 13 ± 2.9 | 12 ± 3.4 | 11.5 ± 2.8 | 0.45 |
| PCWP (mmHg) | 15 ± 3.3 | 12.7 ± 3.07 | 12.5 ± 3.1 | 0.10 |
| DO2 (ml/min per m2) | 689 ± 232.9 | 659.9 ± 221.7 | 638.5 ± 197.4 | 0.14 |
| VO2 (ml/min per m2) | 244.2 ± 73.4 | 233 ± 42.08 | 251.2 ± 49.7 | 0.84 |
| Qs/Qt (%) | 34.41 ± 6.23 | 23.1 ± 9.18 | 27.4 ± 6.65 | 0.03 |
| O2ext (%) | 21.88 ± 4.65 | 27.03 ± 3.22 | 28.51 ± 8.53 | 0.57 |
| Cst (ml/cmH2O) | 32.3 ± 9.7 | 33 ± 8.35 | 32.9 ± 8.5 | 0.84 |
CI, cardiac index; CO, cardiac output; Cst, static compliance; CVP, central venous pressure; DO2, oxygen delivery; MAP, mean arterial pressure; O2ext, oxygen extraction ratio; PaO2, partial arterial oxygen tension; PCWP, pulmonary capillary wedge pressure; PEEP, positive end-expiratory pressure; pHi, gastric mucosal pH; Pmean, mean airway pressure; P(t-a)CO2, gap between partial tissue and arterial carbon dioxide tension; Ppeak, peak airway pressure; Qs/Qt, shunt fraction; VO2, oxygen consumption.
Although PEEP significantly improved shunt fraction, and consequently PaO2, its greater effect on cardiac output led to a reduction in DO2 both at PEEPopt and PEEPmax. However, none of the changes in haemodynamic parameters, including those in central venous pressure, pulmonary artery occlusion pressure, cardiac output, cardiac index and DO2, achieved statistical significance (Table 2 and Fig. 1). PaO2 values remained stable at each level of PEEP. The mean pHi was 7.31 ± 0.13 at baseline and 7.32 ± 0.12 at PEEPopt; it decreased to 7.29 ± 0.12 at PEEPmax, but this reduction was not statistically significant (P = 0.84). Similar to pHi, alterations in the gap between partial tissue and arterial carbon dioxide tension (P(t-a)CO2) were not significant (P = 0.353).
Figure 1.
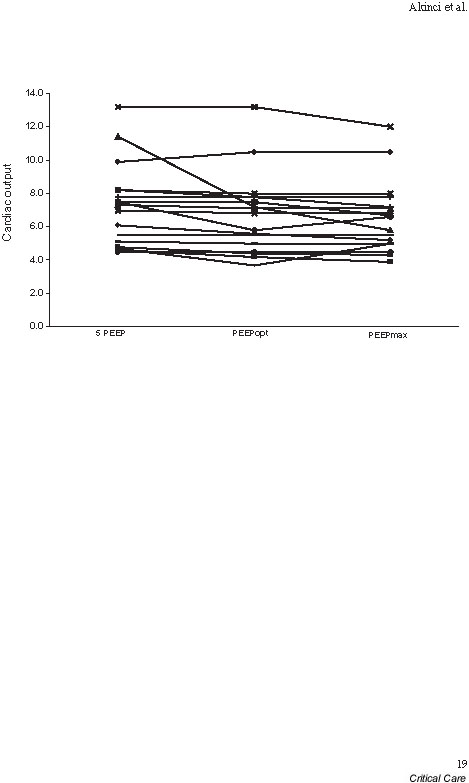
Cardiac output changes at baseline positive end-expiratory pressure (PEEPbaseline; 5 cmH2O), PEEPopt and PEEPmax.
Although the increase in PEEP had no impact on the group as a whole, changes in pHi and P(t-a)CO2 during PEEP titration differed between individual patients (Table 3). The pHi decreased in eight patients (47%), it increased in five (29.4%) and it was unchanged in four (23.5%) at PEEPopt as compared with PEEPbaseline. The pHi at PEEPmax was lower in 12 (70.6%) and higher in five (29.4%) patients as compared with baseline values. The P(t-a)CO2 values increased in nine (52.3%) patients at PEEPopt and in 10 (58.3%) patients at PEEPmax as compared with PEEPbaseline (Table 3). However, there were no statistically significant differences in P(t-a)CO2 values between PEEPbaseline, PEEPopt and PEEPmax (P = 0.353; Table 2). Interestingly, DO2 in those patients who exhibited a rise in pHi did not increase. Rather, DO2 in these patients also decreased (although this was not statistically significant) at PEEPopt and PEEPmax, to a degree similar to that in patients who exhibited a drop in pHi.
Table 3.
Levels of positive end-expiratory pressure achieved and corresponding levels of gastric mucosal pH, and partial tissue and arterial carbon dioxide tension gap
| pHi | P(t-a)CO2 | |||||||
| Patient number | PEEPopt (cmH2O) | PEEPmax (cmH2O) | At PEEPbaseline | At PEEPopt | At PEEPmax | At PEEPbaseline | At PEEPopt | At PEEPmax |
| 1 | 7 | 9 | 7.46 | 7.4 | 7.4 | -1.7 | -1 | -3.5 |
| 2 | 13 | 15 | 7.45 | 7.43 | 7.33 | -1.7 | 6.7 | 17.5 |
| 3 | 17 | 17 | 7.26 | 7.28 | 7.28 | 3 | -2 | -2 |
| 4 | 5 | 15 | 7.19 | 7.19 | 7.16 | 10.2 | 8 | 13 |
| 5 | 7 | 11 | 7.45 | 7.37 | 7.32 | -3.4 | 3.4 | 11 |
| 6 | 15 | 15 | 7.23 | 7.05 | 7.05 | -0.7 | 11.6 | 12.6 |
| 7 | 7 | 15 | 7.24 | 7.26 | 7.23 | 1.5 | 0.9 | 16.1 |
| 8 | 9 | 13 | 7.18 | 7.18 | 7.24 | 11.2 | 14 | 10.8 |
| 9 | 17 | 17 | 7.45 | 7.44 | 7.44 | -1 | 10 | 5 |
| 10 | 15 | 17 | 7.15 | 7.23 | 7.2 | 4.6 | 10.5 | 13.2 |
| 11 | 11 | 15 | 7.45 | 7.42 | 7.29 | -4.7 | 5 | 8.9 |
| 12 | 13 | 15 | 7.50 | 7.43 | 7.46 | -0.3 | 8 | -3.6 |
| 13 | 13 | 13 | 7.27 | 7.46 | 7.46 | -3.5 | 1 | 7.3 |
| 14 | 5 | 9 | 7.36 | 7.36 | 7.27 | 3.4 | 3.8 | 7.4 |
| 15 | 7 | 9 | 7.38 | 7.37 | 7.37 | 2.1 | 13 | 16 |
| 16 | 5 | 13 | 7.14 | 7.14 | 7.08 | 7 | -3.8 | -2.6 |
| 17 | 9 | 9 | 7.15 | 7.46 | 7.46 | 26.6 | 0.5 | -1 |
Positive end-expiratory pressure (PEEP) at baseline was 5 cmH2O. pHi, gastric mucosal pH; P(t-a)CO2, gap between partial tissue and arterial carbon dioxide tension.
Discussion
The results of the present study indicate that incremental increases in PEEP do not impact on splanchnic perfusion, as assessed by gastric tonometry, when cardiac output (and consequently DO2) are maintained.
In animals, PEEP decreases hepatosplanchnic perfusion in a dose-dependent manner, with a limited effect at PEEP levels of less than 10 cmH2O [2,4,5]. Alterations in splanchnic blood flow attributed to PEEP occur in parallel to those in cardiac output and consequently can be reversed with restoration of blood pressure [4,13]. Despite experimental evidence, concerns regarding the effects of PEEP on splanchnic perfusion remain theoretical because large studies in humans are lacking. Similarly, in humans without ALI or ARDS, PEEP reduces splanchnic oxygenation and this is accompanied by decreases in cardiac output, albeit with no change in lactate levels [14]. Recently, Kiefer and colleagues [9] reported no change in splanchnic perfusion when PEEP was titrated on the linear portion of the pressure–volume curve in patients with ALI [9].
The results presented here, which demonstrate a lack of impact on splanchnic blood flow when PEEP is not accompanied by decreased cardiac output, corroborate those from animal studies [4,13] and from the recent human study conducted by Kiefer and coworkers [9]. The lack of change in pHi at PEEPopt (11 cmH2O) is in agreement with our current understanding that PEEP at 10 cmH2O has a limited effect on splanchnic blood flow. Furthermore, the presence of ARDS limited the relative impact of increased thoracic pressure on the cardiovascular system.
Perhaps more important, these observations were valid for a wide range of PEEP levels, from 5 cmH2O to as high as 17 cmH2O. We ascribed the lack of significant changes in cardiac output and DO2 in the patients to adequate volume status and preload. Relative hypovolaemia appears to be the most likely explanation for the reductions in cardiac output and splanchnic blood flow observed in animal studies. Gastric pHi, and consequently splanchnic blood flow, remained stable at PEEPopt and PEEPmax when cardiac output and DO2 remained relatively unchanged. Preservation of splanchnic blood flow at PEEPopt and PEEPmax was attributed to an increase in oxygen extraction ratio that was sufficient to compensate for the small, insignificant drop in cardiac output and DO2 that occurred during PEEP titration [15].
It is also noteworthy that there may be individual variations in pHi in response to PEEP. Although differences in pHi response among individuals cannot explained on the basis of changes in DO2, they may be attributed to differences in the relative impact of underlying critical illness on splanchnic perfusion and variations in splanchnic vascular response (i.e. severity and/or duration of vasoconstriction, extraction ratio) to small changes in DO2 among individuals.
Because of concerns about the reliability of pHi for assessing mucosal perfusion, we also calculated the P(t-a)CO2 because it has been proposed to be a better parameter than pHi [16]. The pHi level can sometimes be misleading, particularly in situations in which gastric tissue and arterial bicarbonate levels are not equal. In addition, unlike pHi, which can change with the degree of alveolar ventilation, P(t-a)CO2 remains a reliable parameter because both components (i.e. partial arterial and tissue carbon dioxide tension) are similarly influenced by changes in alveolar ventilation, unless they are associated with alterations in cardiac output [17]. In the present study, changes in P(t-a)CO2 were not statistically significant and correlated with changes in pHi. Consequently, we used pHi values in our discussion because we believe that pHi reliably reflects the accurate tissue pH in patients.
Our results corroborate those from the only other study that evaluated the impact of PEEP on splanchnic perfusion in patients with ALI. Similar to Kiefer and colleagues [9], we found no change either in pHi or P(t-a)CO2 during PEEP titration. However, there were several differences between two studies. Whereas Kiefer and colleagues used pressure–volume curves for PEEP titration, we titrated PEEP on the basis of improvement in oxygenation, which is a commonly used method in clinical practice because determination of pressure–volume curves can sometimes be cumbersome. Furthermore, the present study was larger and we included patients with more severe disease (ratio of fractional inspired oxygen to PaO2: 139 in the present study versus 168 in that conducted by Kiefer and coworkers).
However, the present study has several limitations. The first and perhaps most important limitation of the study is the liberal titration of PEEP in order to determine its impact on pHi, as described under Method (see above). We acknowledge that in day-to-day clinical practice, some of the patients would not have been managed with such aggressive titration of PEEP and therefore would not have received the levels of PEEP achieved in the study, rendering the clinical implications of these observations quite limited. Second, we did not directly measure splanchnic perfusion but assessed it indirectly by monitoring pHi using gastric tonometry. Although the diagnostic value of gastric tonometry has been questioned because some methodological problems, we believe that we minimized most of these limitations and improved the reproducibility of our measurements by immediate analysis of samples, use of H2 blockers [18] and lack of enteral nutrition [19], rendering it possible to use gastric pHi to evaluate splanchnic perfusion. Third, PEEPopt in the study (approximately 11 cmH2O) was lower than levels reported in other ARDS studies [20]. Higher tidal volume (10 ml/kg) leading to higher mean airway pressure, the termination criteria used in our study, and the differences in titration technique (based on oxygenation versus pressure–volume curve) may account for this difference. Finally, pHi was measured after patients had been exposed to different levels of PEEP for a short duration. Although short-term application of high PEEP did not significantly change pHi, it is conceivable that longer durations or higher numbers of patients would have led to more prominent reductions and statistically significant differences.
Collectively, the present findings indicate that determination of PEEPopt by titration of PEEP based on improvement in oxygenation is a safe strategy, with no impairment in gastric mucosal perfusion, when cardiac output is preserved. Maintenance of cardiac output during mechanical ventilation with high PEEP may be adequate to prevent its unwanted effects on organs in the splanchnic vasculature. Nonetheless, the possibility that PEEP can alter splanchnic perfusion when it is applied at high levels and for longer durations cannot be completely excluded.
Key message
• Incremental increase in PEEP to identify the optimal value does not affect splanchnic perfusion as assessed by gastric tonometry
Competing interests
None declared.
Abbreviations
ALI = acute lung injury; ARDS = acute respiratory distress syndrome; DO2 = oxygen delivery; MAP = mean arterial pressure; Pao2 = partial arterial oxygen tension; P(t-a)CO2 = gap between partial tissue and arterial carbon dioxide tension; PEEP = positive end-expiratory pressure; pHi = gastric mucosal pH.
References
- Guyton AC, Lindsey AW, Abernathy B, Richardson T. Venous return at various right atrial pressure and the normal venous return curve. Am J Physiol. 1957;189:609–615. doi: 10.1152/ajplegacy.1957.189.3.609. [DOI] [PubMed] [Google Scholar]
- Pastores SM, Katz DP, Kvetan V. Splanchnic ischemia and gut mucosal injury in sepsis and the multiple organ dysfunction syndrome. Am J Gastroenterol. 1996;91:1697–1710. [PubMed] [Google Scholar]
- Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119:1222–1241. doi: 10.1378/chest.119.4.1222. [DOI] [PubMed] [Google Scholar]
- Brienza N, Revelly JP, Ayuse T, Robotham JL. Effects of PEEP on liver arterial and venous blood flows. Am J Respir Crit Care Med. 1995;152:504–510. doi: 10.1164/ajrccm.152.2.7633699. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Effects of PEEP on splanchnic hemodynamics and blood volume. Acta Anaesthesiol Scand. 1993;37:427–431. doi: 10.1111/j.1399-6576.1993.tb03742.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson D, Almquist P, Haglund U. Effects of positive end-expiratory pressure on splanchnic circulation and function in experimental peritonitis. Arch Surg. 1991;126:631–636. doi: 10.1001/archsurg.1991.01410290109021. [DOI] [PubMed] [Google Scholar]
- Winso O, Biber B, Gustavsson B, Holm C, Milsom I, Niemand D. Portal blood flow in man during graded positive end-expiratory pressure ventilation. Intensive Care Med. 1986;12:80–85. doi: 10.1007/BF00254516. [DOI] [PubMed] [Google Scholar]
- Bonnet F, Richard C, Glaser P, Lafay M, Guesde R. Changes in hepatic flow induced by continuous positive pressure ventilation in critically ill patients. Crit Care Med. 1982;10:703–705. doi: 10.1097/00003246-198211000-00001. [DOI] [PubMed] [Google Scholar]
- Kiefer P, Nunes S, Kosonen P, Takala J. Effect of positive end-expiratory pressure on splanchnic perfusion in acute lung injury. Intensive Care Med. 2000;26:376–383. doi: 10.1007/s001340051170. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Argitas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R, and the Consensus Committee. The American-European Consensus Conference on ARDS. Definition, mechanism, relevant outcomes and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Takala J, Parviainen I, Siloaho M, Ruokonen E, Hamalainen E. Saline PCO2 is an important source of error in the assessment of gastric intramucosal pH. Crit Care Med. 1994;22:1877–1879. [PubMed] [Google Scholar]
- Fiddian-Green RG. Tonometry: theory and applications. Intensive Care World. 1992;9:60–65. [PubMed] [Google Scholar]
- Matuschak GM, Pinsky MR, Roger RM. Effects of positive end-expiratory pressure on hepatic blood flow and performance. J Appl Physiol. 1987;62:1377–1383. doi: 10.1152/jappl.1987.62.4.1377. [DOI] [PubMed] [Google Scholar]
- Berendes E, Lippert G, Loick HM, Brussel T. Effects of positive end-expiratory pressure ventilation on splanchnic oxygenation in humans. J Cardiothorac Vasc Anesth. 1996;10:598–602. doi: 10.1016/s1053-0770(96)80136-4. [DOI] [PubMed] [Google Scholar]
- Geiger K, Georgieff M, Lutz H. Side effects of positive pressure ventilation on hepatic function and splanchnic circulation. Int J Clin Monit Comput. 1986;3:103–106. doi: 10.1007/BF01880762. [DOI] [PubMed] [Google Scholar]
- Schlichtig R, Mehta N, Gayowski TJ. Tissue-arterial PCO2 difference is a better marker of ischemia than intramural pH (pHi) or arterial pH-pHi difference. J Crit Care. 1996;11:51–56. doi: 10.1016/s0883-9441(96)90020-9. [DOI] [PubMed] [Google Scholar]
- Bernardin G, Lucas P, Hyvernat H, Deloffre P, Mattei M. Influence of alveolar ventilation changes on calculated gastric intramucosal pH and gastric-arterial PCO2 difference. Intensive Care Med. 1999;25:249–251. doi: 10.1007/s001340050834. [DOI] [PubMed] [Google Scholar]
- Heard SO, Helsmoortel CM, Kent JC, Shahnarian A, Fink MP. Gastric tonometry in healthy volunteers: effect of ranitidine on calculated intramural pH. Crit Care Med. 1991;19:271–274. doi: 10.1097/00003246-199102000-00025. [DOI] [PubMed] [Google Scholar]
- Kolkman JJ, Groenevelt AB, Meuswissen SG. Effect of gastric feeding on intragastric P(CO2) tonometry in healty volunteers. J Crit Care. 1999;14:34–38. doi: 10.1016/s0883-9441(99)90006-0. [DOI] [PubMed] [Google Scholar]
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]


