Abstract
Objectives
We have developed an image-guided surgery system based on invisible near-infrared (NIR) fluorescent light. Presently, the only clinically-available NIR fluorophore is indocyanine green (ICG), which fluoresces at ≈ 800 nm and is used for coronary angiography. Our objective was to determine if methylene blue (MB), already FDA-approved for other indications, has useful NIR fluorescence properties for image-guided cardiac surgery.
Methods
The optical properties of MB were measured after dissolution in 100% serum. Biodistribution and clearance were quantified in organs and tissues after intravenous bolus injection of 2 mg/kg MB in N = 3 rats. Coronary arteriography and cardiac perfusion were imaged in real-time after intravenous bolus injection of 1 mg/kg MB in N = 5 pigs with coronary obstructions. Coronary angiography and acute thrombi were assessed using 800 nm fluorophores, ICG and IR-786-labeled platelets, respectively.
Results
The peak absorbance and emission of MB as a NIR fluorophore occur at 667 nm and 686 nm, respectively. After intravenous injection, MB provides highly sensitive coronary angiography. A lipophilic cation, MB is extracted rapidly into tissue, with myocardium displaying unusually high uptake. MB permits real-time visualization and quantitative assessment of myocardial perfusion. Because of absent spectral overlap, use of two independent fluorophores on our imaging system permits simultaneous quantification of perfusion, venous drainage and/or intravascular thrombi.
Conclusions
MB is an effective NIR fluorophore that provides direct visualization of coronary arteriography and cardiac perfusion. In conjunction with ≈ 800 nm NIR fluorophores, important functional assessments during cardiac surgery are also possible.
Keywords: Image-Guided Surgery, Near-Infrared Fluorescence, Coronary Artery Bypass Grafting, Methylene Blue, Indocyanine Green
INTRODUCTION
Immediate and long-term outcomes of coronary artery bypass grafting (CABG) are typically dependent on the quality of the anastomosis, the conduit, and the distal myocardial run-off. In current practice, there is typically no standardized approach for the intraoperative assessment of the anastomosis or the distal run-off. Although postoperative aspirin administration and increased use of arterial grafting have improved early and late graft patency, perioperative graft occlusions are still reported at a rate between 4% and 12%.1,2 If such graft failures can be recognized intraoperatively, they may be correctable. Because graft patency and completeness of revascularization are two important prognostic factors for long-term survival after coronary surgery, a reliable and well-validated method to assess anastomoses and perfusion would be helpful.
NIR fluorescent light (700 to 900 nm), invisible to the human eye, provides an extremely high signal to background ratio (SBR) without changing the look of the tissue being imaged (reviewed in 3). Our laboratory has previously developed a one-channel intraoperative NIR fluorescence imaging system capable of visualizing otherwise invisible relevant surgical anatomy using a color video camera and NIR fluorescence from a NIR camera.4 Using a variety of large animal model systems, we have shown that NIR fluorescence can be used for effective image-guided sentinel lymph node mapping,5 cardioplegia delivery,6 ureter identification,7 and extrahepatic bile duct identification.8 Our group has also demonstrated that autologous washed platelets can be rendered NIR fluorescent at 800 nm, and when injected intravenously, will home to sites of intravascular thrombi to provide high sensitivity detection.9
There are presently two fundamental limitations to the widespread clinical use of NIR fluorescence technology: the availability of FDA-approved NIR fluorophores and the availability of imaging systems capable of visualizing more than one NIR fluorophore simultaneously. Indocyanine green (ICG) is a small molecule blood pool agent that fluoresces at 800 nm, and has previously been used for coronary angiography during CABG surgery.10–12 Methylene blue (MB) is another FDA approved fluorophore that heretofore has not been used for intraoperative imaging. In this study, we hypothesized that MB has independent and non-overlapping optical and pharmacokinetic properties compared to ICG, and that it can therefore be imaged concomitantly with ICG and other 800 nm fluorophores to provide simultaneous and unique image-guidance during cardiac surgery.
MATERIALS AND METHODS
Dual-Channel NIR Fluorescence Imaging System
Our large animal, single channel intraoperative NIR fluorescence imaging system has been described in detail previously.4,13 We recently developed a dual-NIR version that includes a light emitting diode (LED)-based light source that generates 1 mW/cm2 of 400–650 nm “white” light, 2.5 mW/cm2 of 670 nm excitation light for NIR fluorescence channel #1, and 5 mW/cm2 of 760 nm NIR excitation light for NIR fluorescence channel #2. Photon collection was achieved with footswitch-controlled zoom optics that maintain separation of the white light and two NIR fluorescence emission (700 nm and 800 nm) channels. The system has a spatial resolution of 625 µm at a field-of-view of 20 × 15 cm, and 125 µm at a field-of-view of 4 × 3 cm. Color video images were acquired on an Imitech IMC-80F camera. NIR fluorescence images were acquired on two 12-bit Orca-AG (Hamamatsu, Bridgewater, NJ) cameras used within their linear range. After computer-controlled camera acquisition, standard (white light) and two functional (NIR fluorescent light) video images can be displayed separately and merged. The imaging system was suspended over the surgical field with an articulating arm, with a working distance of 18" above the subject. Data were acquired and quantified on a Dell computer using custom LabVIEW 7.1 (National Instruments, Austin, TX) software.
NIR Fluorophore Preparation
10 mg/ml (1%) sterile vials of MB in water, and 25 mg sterile vials of ICG (a.k.a. CardioGreen™) powder, were purchased from Akorn, Inc. (Decatur, IL). The perchlorate salt of IR-786 (CAS #102185-03-5) was purchased from Sigma-Aldrich (St. Louis, MO) and bioactive, autologous NIR fluorescent pig platelets were prepared as described previously.9 Briefly, platelet-rich plasma (PRP) was isolated by centrifugation of 200 ml of whole blood at 200 × g for 20 min. Platelets were isolated from PRP by centrifugation at 1400 × g for 10 min in the presence of 50 ng/mL PGE1 and 10% (v/v) acid citrate/dextrose, pH 4.6, and resuspended at a concentration of 4 × 108 platelets/mL in Tyrode’s-HEPES buffer. The washed platelets (3.6 × 1010 total) in Tyrode's-HEPES were labeled with 2 µM IR-786 for 30 min at room temperature as described previously.9
Measurement of MB Optical Properties
All measurements were performed in 100% fetal bovine serum (FBS). Absorbance and fluorescence were measured using fiber-optic HR2000 absorbance (200–1100 nm) and USB2000FL fluorescence (350–1000 nm) spectrometers (Ocean Optics, Dunedin, FL). NIR excitation was provided by a 5 mW, 654 nm laser diode. Fluorescence quantum yield (QY) of MB in FBS was calculated using oxazine 725 in ethylene glycol (QY = 19%14) as a calibration standard, under conditions of matched absorbance (Abs = 0.08) at 654 nm.
Surgical Preparation
All animals were used under the supervision of an approved institutional protocol. 300-g Sprague-Dawley rats from Charles River Laboratories (Wilmington, MA) were anesthetized with 65 mg/kg intraperitoneal (IP) pentobarbital, and were ventilated by tracheostomy using an MRI-1 ventilator (CWE, Inc. Ardmore, PA). Bilateral thoracotomy and laparotomy were performed, and the lungs, heart, liver, kidney, inferior vena cava (IVC), and skeletal muscles at the abdominal wall were exposed.
Adult female Yorkshire pigs (mean weight 30 kg) were purchased from E.M. Parsons and Sons (Hadley, MA). General anesthesia was induced with 4.4 mg/kg of intramuscular Telazol (Fort Dodge Labs, Fort Dodge, IA). Once sedated, animals were intubated with a cuffed endotracheal tube, and anesthesia was maintained with 2% isoflurane/balance O2. Continuous oxygen saturation, body temperature in the oral cavity, and 3-lead electrocardiography were monitored throughout the experiment. After a median sternotomy was performed, the heart was suspended in a pericardial cradle. To prevent ventricular fibrillation, animals received intravenous lidocaine (50 mg) through a left external jugular vein catheter prior to clamping a diagonal branch of the left anterior descending (LAD) coronary artery.
MB Biodistribution and Clearance
2 mg/kg MB was injected intravenously as a bolus over 30 sec. Under conditions of constant fluence rate and field-of-view, 700 nm NIR fluorescence images of the lung, heart, liver, kidney, IVC, and skeletal muscle at the abdominal wall were acquired every 500 msec until 1 min, then at 2 min, 5 min, and every 5 min thereafter for a total of 60 min.
Coronary Arteriography and Myocardial Perfusion Imaging with MB
1 mg/kg MB was injected intravenously as a bolus over 30 sec. Images were acquired every 500 msec until 1 min, then at 2 min, 5 min, and every 5 min thereafter for a total of 60 min. For some experiments, a diagonal branch of the LAD was clamped prior to MB injection. Fifteen min after interrupting arterial flow, the clamp was removed and ICG (0.06 mg/kg) was injected intravenously. Fifteen min following ICG injection, the animal was euthanized, the heart was removed, and the cut surface imaged.
Induction of Intravascular Thrombi in Coronary Arteries
Up to two hours prior to thrombus induction, 3.6 × 1010 NIR fluorescent platelets were infused intravenously. To induce thrombi, a 1 cm by 1 cm piece of filter paper was dipped in 50% (w/w) aqueous FeCl3 and the placed over a diagonal branch of the LAD. Thrombi could be detected using 800 nm NIR fluorescence within 30–45 min of FeCl3 application, after which time 1 mg/kg MB was injected intravenously as described above.
Quantitation and Statistical Analysis
NIR fluorescence was quantified using a constant-sized region-of-interest (ROI) over reproducible areas of the heart and coronary arteries: mid-anterior right ventricular free wall, mid-anterior left ventricular free wall, bifurcation of second diagonal branch, and pericardium. A Mann-Whitney U test was used for statistical comparison of parameters, with a p value of ≤ 0.05 considered statistically significant.
RESULTS
Real-Time, Dual-Channel Intraoperative NIR Fluorescence Imaging System
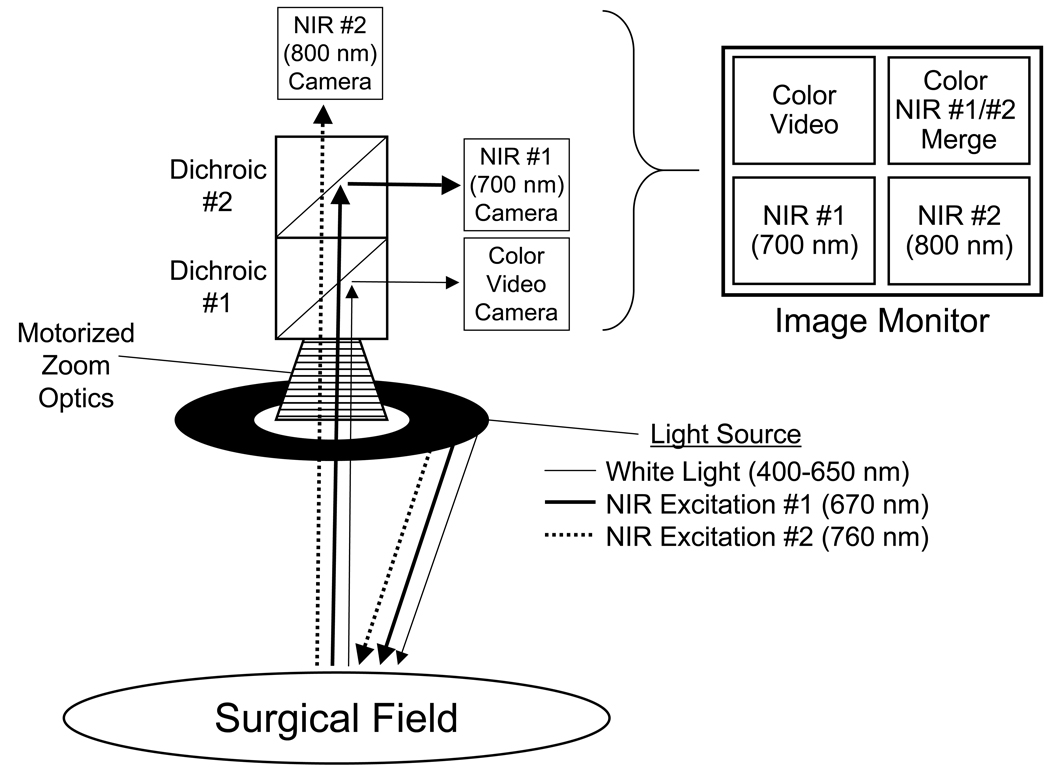
A schematic of the imaging system used in this study is shown in Figure 1. Its 18" working distance and articulated arm ensured unobtrusive imaging during cardiac surgery, and its articulated arm permitted precise positioning. By suppressing motion artifacts, cardiac gating technology preserved high-resolution imaging of the beating heart (see below). In order to display anatomy and function together, the color video, 700 nm (NIR fluorescence channel #1), and 800 nm (NIR fluorescence channel #2) images were acquired simultaneously and NIR fluorescence channels were pseudo-colored and overlaid on top of the color video image to create a separate merged image. All images were refreshed at rates up to 15 times per sec. Because NIR excitation and emission light are invisible to the human eye, the surgical field appears completely normal.
Figure 1. Image-Guided Surgery System Equipped with Two NIR Fluorescence Channels.
Schematic of the imaging system showing light paths for white light, NIR channel #1 excitation (670 nm) and emission (700 nm), and NIR channel #2 excitation (760 nm) and emission (800 nm). All images are acquired and displayed simultaneously, and in real-time.
Chemical and Optical Properties of MB
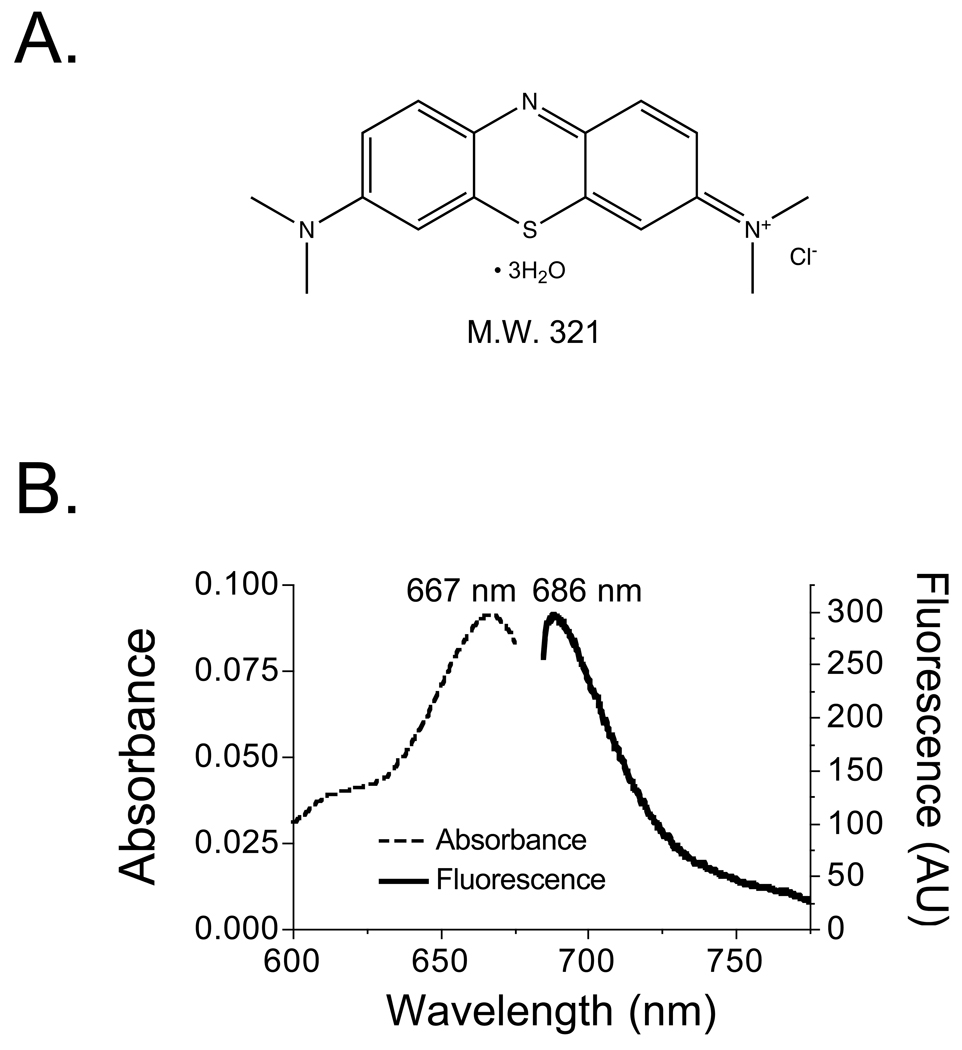
MB (methylthioninium chloride) is a lipophilic, cationic (charge = +1), 321 Da small molecule with a distributed electron resonance (Figure 2A). In 100% serum, peak absorbance and emission were found to be 667 nm and 686 nm, respectively (Figure 2B). Its extinction coefficient at 667 nm (ε667nm) was found to be 46,000 M−1cm−1. QY in 100% serum was 2.5%. The product of ε667nm and QY indicated low-moderate total fluorescence compared to other NIR fluorophores such as ICG (εPeak Absorbance = 166,000 M−1cm−1 and QY = 9% in serum5).
Figure 2. Chemical and Optical Properties of Methylene Blue.
A. Chemical structure and molecular weight (Da) of methylthioninium chloride (methylene blue).
B. Absorbance (left axis) and fluorescence (right axis; 654 nm excitation) for 2 µM methylene blue in 100% FBS.
In Vivo Biodistribution and Clearance
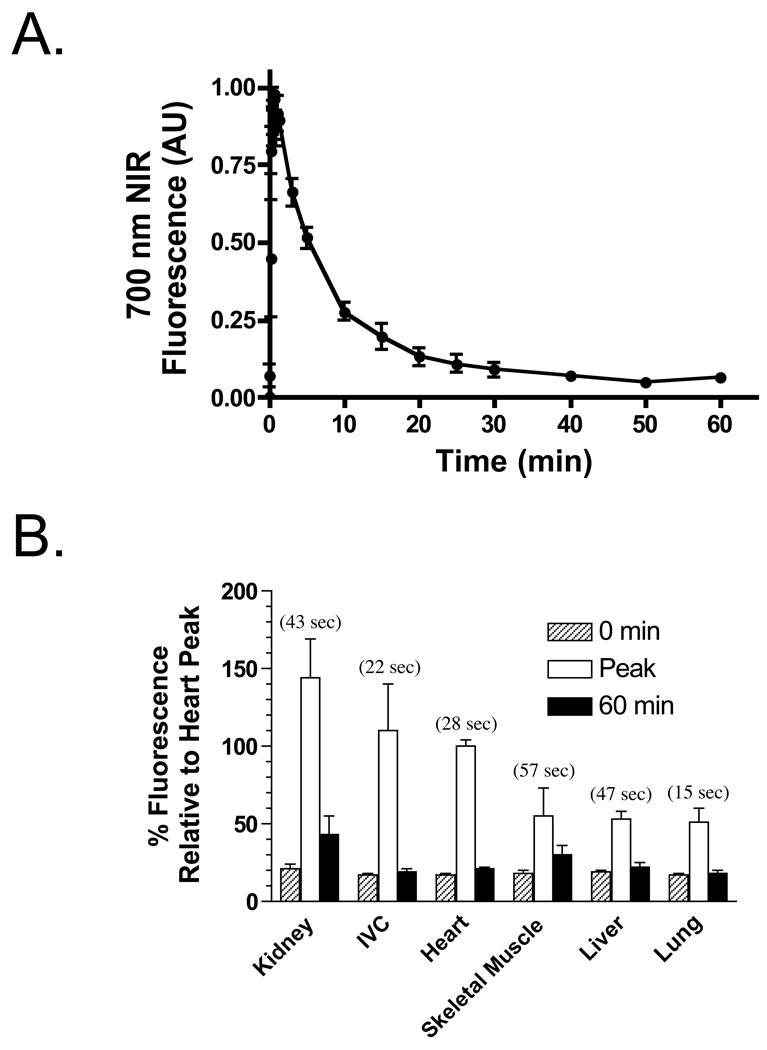
MB displays no protein binding after intravenous injection.15 After intravenous bolus injection of 2 mg/kg into rat, ROI analysis over the myocardium revealed high uptake of MB, peaking at 28 sec, and slow washout, with the NIR fluorescence signal returning to background by 40–60 min (Figure 3A). Other vital organs and tissues also exhibited uptake of MB, with peak fluorescence occurring between 15 sec and 57 sec after injection (Figure 3B). The NIR fluorescence signal from all, except kidney, returned to baseline by 60 min. Excretion of MB was found to be both renal and hepatic (data not shown).
Figure 3. In Vivo Biodistribution and Clearance of Methylene Blue.
A. Kinetics of 700 nm MB NIR fluorescence in the myocardium of rat. Shown are mean ± SEM signal intensity from N = 3 independent animals.
B. Ratio of NIR fluorescence intensity of the myocardium relative to various vital organs and tissues, after intravenous injection of 2 mg/kg MB in rats (N = 3). Shown are values at 0 min, at the time (parentheses) of peak fluorescence in the organ/tissue under study, and at 60 min.
Coronary Arteriography and Myocardial Perfusion Imaging using MB
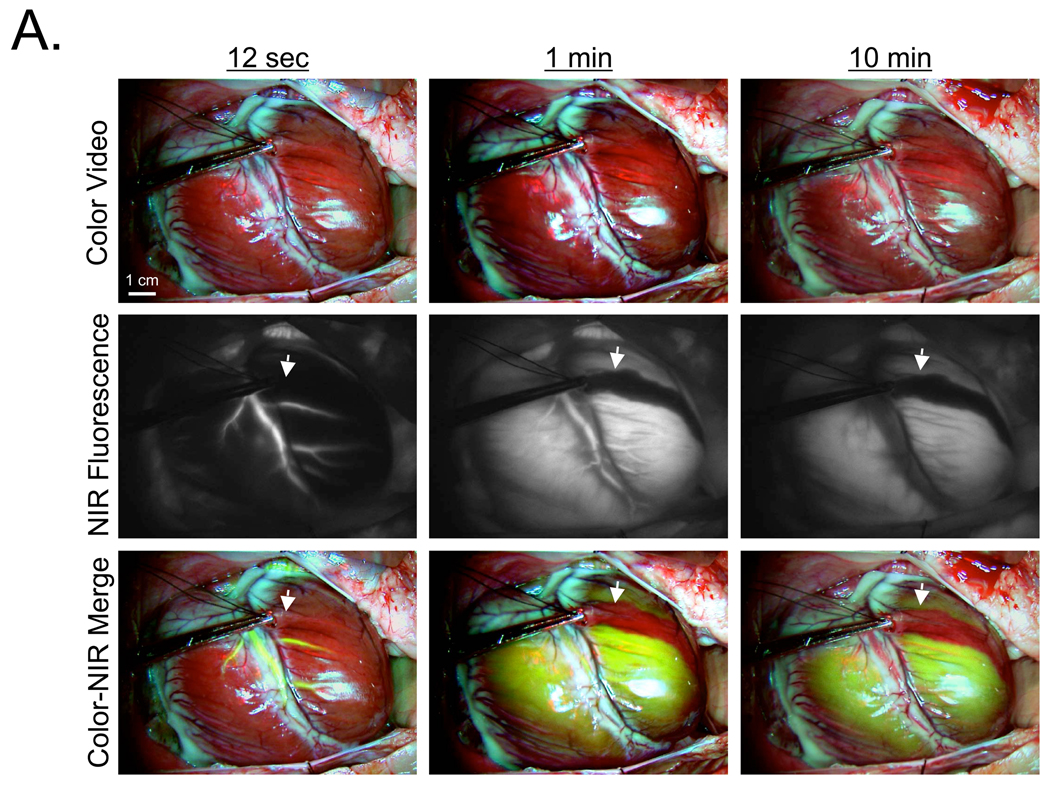
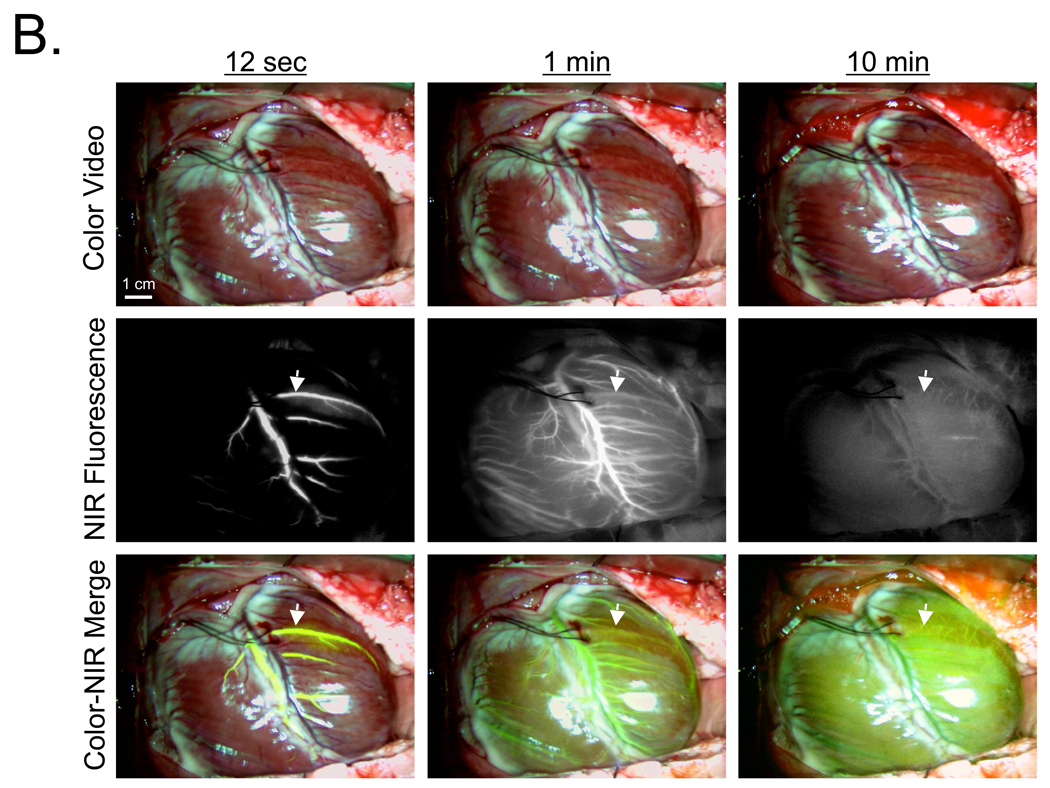
In a human-size pig, 700 nm MB fluorescence provided sensitive coronary arteriography, with the arterial phase peaking 12 sec after intravenous bolus injection (Figure 4A). Unlike blood pool agents such as ICG, and like other lipophilic cations,16 MB was extracted completely by the myocardium. This eliminates the venous phase, but also provides direct visualization of the myocardial perfusion or arterial run-off (Figure 4A). As seen in rat, peak NIR fluorescence in the myocardium occurred at 1 min post MB injection, persisted for over 10 min (Figure 4A), and returned to baseline by 40–60 min. Importantly, MB NIR fluorescence contrast was so high that myocardium with an induced perfusion defect could be detected with high sensitivity (Figure 4A).
Figure 4. Real-Time, Simultaneous Assessment of Myocardial Perfusion and Coronary Angiography using MB and ICG.
A. Arterial phase (12 sec), venous phase (1 min), and late phase (10 min) after intravenous bolus injection of 1 mg/kg MB into pigs. A diagonal branch of the LAD was clamped immediately prior to MB injection, with the perfusion defect easily detected (arrows). Shown are color video (top row), NIR fluorescence (middle row), and a pseudo-colored (lime green) merge of the two (bottom row). NIR fluorescence images were acquired with a 100 msec exposure time and are displayed with identical normalizations. Images are representative of N = 5 independent animals.
B. Arterial phase (12 sec), venous phase (1 min), and late phase (10 min) after de-clamping of the diagonal branch and intravenous bolus injection of 0.06 mg/kg ICG into the pig from (A). Arrows mark the location of the previous perfusion defect seen with MB. Shown are color video (top row), NIR fluorescence (middle row), and a pseudo-colored (lime green) merge of the two (bottom row). NIR fluorescence images were acquired with a 100 msec exposure time and are displayed with identical normalizations. Images are representative of N = 5 independent animals.
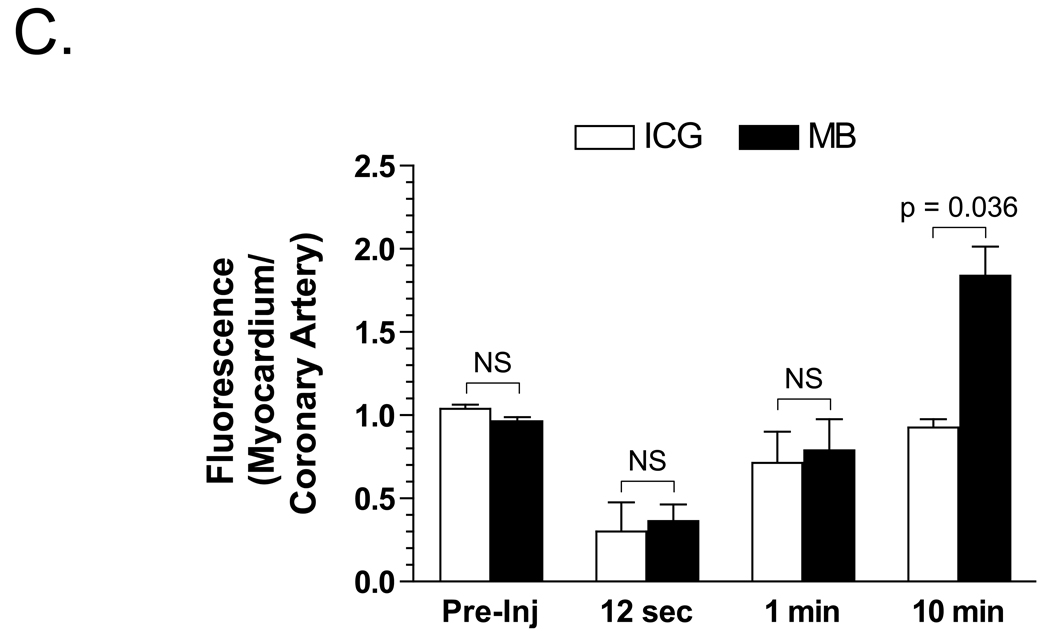
C. Quantitative analysis of the ratio of myocardial NIR fluorescence to coronary artery NIR fluorescence, 10 min after intravenous bolus injection of either ICG or MB. Shown are the mean ± S.E.M. for N = 5 independent animals, along with the statistical comparison of the two NIR fluorophores at each time point.
The perfusion defect was reversed, and 0.06 mg/kg ICG was injected intravenously as a bolus. Using the dual channel capability of the imaging system, the opened artery could be assessed in real-time, with peak fluorescence occurring at 12 sec post injection (Figure 4B). Because ICG uptake into myocardium is negligible, venous filling may be assessed (Figure 4B), but not perfusion. Quantitative comparison of the ratio between myocardial fluorescence and coronary artery fluorescence, at various time points after intravenous injection of ICG and MB, is shown in Figure 4C, and confirms the qualitative results. Taken together, MB and ICG, in conjunction with the dual channel imaging system, permitted simultaneous, real-time, quantitative assessment of arterial filling, venous filling, and myocardial perfusion during cardiac surgery in the beating heart.
Simultaneous Detection of Coronary Arterial Flow, Myocardial Perfusion, and Acute Thrombi
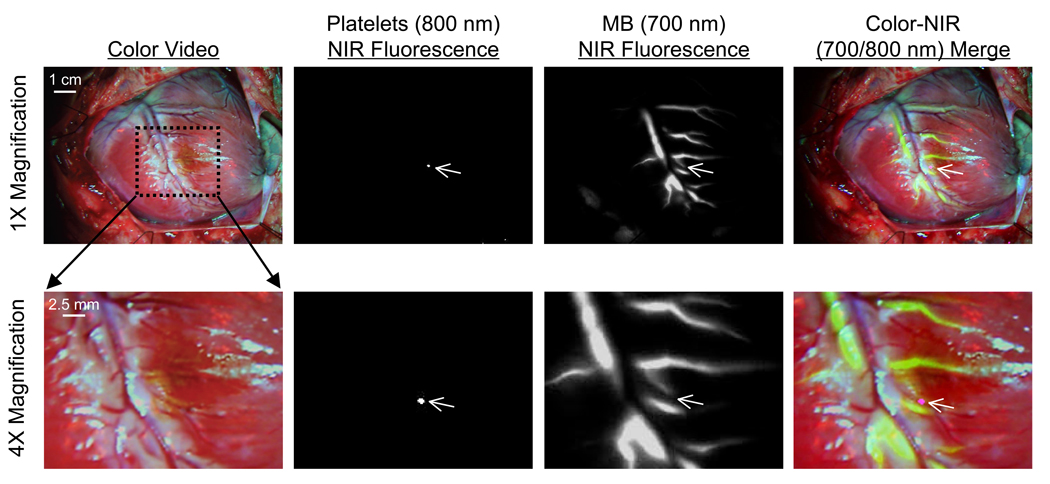
Presently, there is no method available for the sensitive detection of intravascular thrombi during cardiac surgery. We have previously demonstrated that intravenously injected 800 nm NIR fluorescent platelets will home to sites of intravascular thrombi to provide high sensitivity detection.9 The present study demonstrates that the combination of 800 nm NIR fluorescent platelets and 700 nm MB, in conjunction with the dual channel imaging system, can be used to detect thrombi forming in even small branches of the coronary arteries, and importantly, can provide functional information about vessel patency. The diagonal branch of the LAD harboring a thrombus is completely occluded, as assessed using MB (Figure 5). Indeed, the thrombus shown in Figure 5 is the smallest one seen in 4 animals, measuring sub-millimeter in size, which highlights the sensitivity of the technology and the motion-suppression, high-resolution capabilities of the imaging system.
Figure 5. Real-Time, Simultaneous Assessment of Acute Coronary Artery Thrombi and Coronary Artery Patency.
A coronary artery thrombus was induced in a diagonal branch of the LAD by treatment with FeCl3 (center of dotted rectangle). Thrombus size and location was quantified by intravenous injection of IR-786-labeled autologous platelets (800 nm fluorescence; pseudo-colored in magenta in merged image). Coronary artery patency (and tissue perfusion) was quantified by intravenous bolus injection of 1 mg/kg MB (700 nm fluorescence; pseudo-colored in lime-green in merged image). Shown are color video (1st column), 800 nm NIR fluorescence (2nd column), 700 nm NIR fluorescence (3rd column) and a pseudo-colored merge of the three (4th column) at 12 sec post MB injection. The area within the dotted rectangle in the top row is shown magnified 4-fold in the bottom row. NIR fluorescence images were acquired with a 100 msec exposure time and are displayed with identical normalizations. Images are representative of N = 4 independent animals.
DISCUSSION
In coronary artery bypass grafting, there is still no widely accepted or broadly used intraoperative technique to assess the quality of the anastomosis or the bypass graft itself. The clinical consequences of early graft failure are devastating. In a recent report, the 30-day mortality among patients with early postoperative graft failure was more than 9%.17 Since graft patency is the predominant factor in long-term survival and avoidance of re-intervention after CABG surgery, an intraoperative means of assessing revascularization would be extremely helpful. This need to assess intraoperative patency and myocardial perfusion has been emphasized in recent studies,18 which demonstrate significant surgeon-dependent variations in graft patency in off-pump CABG procedures.
Among graft assessment techniques currently available, iodine-based coronary angiography remains the gold standard for postoperative assessment. However, catheterization and fluoroscopy equipment is typically not available in the OR. Although the assessment of graft patency using thermal angiography,19 electromagnetic flowmeter,20 or Doppler flow measurement,21 have been attempted clinically, such techniques generally do not provide images and have not been routinely implemented in surgery thus far.22 Transit-time ultrasonic flow measurement (TTF measurement) has also become a popular method to assess graft patency.23,24 Although TTF is rapid and simple to use, the technique does not produce an image, and interpretation of flow data is often difficult and less intuitive than an angiographic presentation of the graft status.
ICG angiography is a promising new technique for intraoperative assessment during CABG surgery.12,11 Recent publications demonstrate that ICG angiography may be an effective and reproducible method for the evaluation of graft status.10 In this study, we describe a dual-channel NIR fluorescence imaging system capable of visualizing 800 nm ICG fluorescence simultaneously with 700 nm MB fluorescence. We also demonstrate that MB, already FDA-approved for intravenous use in the treatment of methemoglobinemia at a dose of 1–2 mg/kg, has unusually high uptake into myocardium, and thus serves as a simple, yet powerful, visual tracer of myocardial perfusion and arterial runoff. ICG is a true blood pool agent, i.e., it flows into arteries then out of veins, providing approximately 3–4 seconds of imaging in each structure as it passes through. Although ICG can be used as a surrogate for cardiac perfusion,25 quantitation is difficult and perfusion is never actually visualized. MB, on the other hand, provides imaging of arteries, but is then efficiently extracted into the myocardium during first-pass through the capillaries. Importantly, because there is no spectral overlap between ICG and MB, our dual-channel imaging system permits the complementary information from both to be imaged simultaneously.
MB does have limitations, though, for clinical use. A factitious and transient drop of the pulse oximetry reading is always observed after its intravenous injection.26 MB's extinction coefficient and QY in serum are relatively low, and serious adverse reactions have been reported when used at doses of 7.5 mg/kg.27 Even at 1 mg/kg, it is usually given as a slow bolus over 15 min, rather than the 15 sec administration used in this study. Although no acute toxicity was seen in either rat or pig, this is important consideration for translation to patients. Of course, absolute fluorescence intensity is the product of 670 nm NIR excitation fluence rate, MB concentration in the myocardium, and camera exposure time. In experiments not shown, the dose could be reduced 2-fold when either fluence rate or exposure time was doubled, and 4-fold when both were doubled. Since there is a tradeoff between exposure time and resolution because of cardiac motion, we expect that fluence rate will be the key to lowering MB dose, with a maximum likely being 10 mW/cm2 (4-fold higher than used in this study, leading to a 4-fold lower MB dose) due to changes in FDA-regulated color balance that occur with higher fluence rate. Finally, opaque and semi-opaque structures overlying vessels and the myocardium can lead to false-positive filling defects, making the Color-NIR Merge images of paramount importance for interpreting results.
The dual-channel capability of the imaging system would be particularly useful with acute intravascular thrombi. Thrombus formation is a key event in the pathogenesis of acute occlusion of the coronary arteries,28 yet there is presently no method for its sensitive detection. The 700 nm MB NIR fluorescence did not interfere with 800 nm NIR fluorescence from platelets, permitting real-time and simultaneous assessment of thrombus size, arterial patency, and myocardial perfusion, and thus the opportunity for intraoperative intervention. One can envision that with the development of additional targeted 800 nm fluorophores, such as those specific for cardiac stem cells, fibrosis, or other important features, cardiac surgeons could use the 800 nm channel to tailor image-guidance during cardiac surgery.
One major limitation of the simple reflectance imaging system used in this study is depth penetration and surface weighting of fluorescence intensity. Although it can detect fluorescent lymph nodes through up to 1 cm of solid tissue29 and 5 cm of lung tissue30 against a low autofluorescent background, it is unlikely that our imaging system will be able to detect sub-endocardial or septal ischemia in the absence of epicardial ischemia. Recent advances in optical tomography suggest that depth penetration up to 2 cm may someday be possible, although no study to date has attempted to apply tomography to moving objects, such as the heart.
Finally, the decay of MB NIR fluorescence from the myocardium is a complex function of physiologic, chemical, and optical variables. MB is sensitive to redox state, and in the presence of a reducing agent is converted to the colorless, non-fluorescent chemical leucomethylene blue. Leucomethylene blue is also uncharged, which permits diffusion out of myocytes and clearance from the body. Thus, an added feature of MB imaging of the heart may be a direct visual assessment of redox state, with a highly reducing environment leading to lower peak fluorescence and a more rapid return to baseline, and a highly oxidizing environment leading to high peak fluorescence and a slower return to baseline. Studies exploring whether these simple quantitative measurements may provide the cardiac surgeon with additional information about the oxidative (i.e., ischemic) state of the heart are ongoing.
In summary, MB as a 700 nm NIR fluorophore for visualization of coronary arteriography and myocardial perfusion, independent 800 nm NIR fluorophores for visualization of other physiological process, and a dual-channel intraoperative NIR fluorescence imaging system provide unprecedented image-guidance during cardiac surgery.
ACKNOWLEDGMENTS
We thank Barbara L. Clough and Alice Gugelmann for editing, and Eugenia Trabucchi for administrative assistance. This work was supported by NIH grants #R01-CA-115296 (J.V.F.), #R01-EB-005805 (J.V.F.), #R01-HL-063250 (R.F.), and an Established Investigator Award from the American Heart Association (R.F.).
Sources of Funding: This work was supported by NIH grants #R01-CA-115296 (J.V.F.), #R01-EB-005805 (J.V.F.), #R01-HL-063250 (R.F.), and an Established Investigator Award from the American Heart Association (R.F.).
REFERENCES
- 1.Chesebro JH, Clements IP, Fuster V, Elveback LR, Smith HC, Bardsley WT, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med. 1982;307:73–78. doi: 10.1056/NEJM198207083070201. [DOI] [PubMed] [Google Scholar]
- 2.FitzGibbon GM, Burton JR, Leach AJ. Coronary bypass graft fate: angiographic grading of 1400 consecutive grafts early after operation and of 1132 after one year. Circulation. 1978;57:1070–1074. doi: 10.1161/01.cir.57.6.1070. [DOI] [PubMed] [Google Scholar]
- 3.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka E, Choi HS, Fujii H, Bawendi MG, Frangioni JV. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006;13:1671–1681. doi: 10.1245/s10434-006-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohnishi S, Lomnes SJ, Laurence RG, Gogbashian A, Mariani G, Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 6.Soltesz EG, Laurence RG, De Grand AM, Cohn LH, Mihaljevic T, Frangioni JV. Image-guided quantification of cardioplegia delivery during cardiac surgery. Heart Surg Forum. 2007;10:E381–E386. doi: 10.1532/HSF98.20071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka E, Ohnishi S, Laurence RG, Choi HS, Humblet V, Frangioni JV. Real-time intraoperative ureteral guidance using invisible near-infrared fluorescence. J Urol. 2007;178:2197–2202. doi: 10.1016/j.juro.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka E, Choi HS, Humblet V, Ohnishi S, Laurence RG, Frangioni JV. Real-time intraoperative assessment of the extrahepatic bile ducts in rats and pigs using invisible near-infrared fluorescent light. Surgery. 2008 doi: 10.1016/j.surg.2008.03.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaumenhaft R, Tanaka E, Graham GJ, De Grand AM, Laurence RG, Hoshino K, et al. Localization and quantification of platelet-rich thrombi in large blood vessels with near-infrared fluorescence imaging. Circulation. 2007;115:84–93. doi: 10.1161/CIRCULATIONAHA.106.643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai ND, Miwa S, Kodama D, Koyama T, Cohen G, Pelletier MP, et al. A randomized comparison of intraoperative indocyanine green angiography and transit-time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg. 2006;132:585–594. doi: 10.1016/j.jtcvs.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 11.Reuthebuch O, Haussler A, Genoni M, Tavakoli R, Odavic D, Kadner A, et al. Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest. 2004;125:418–424. doi: 10.1378/chest.125.2.418. [DOI] [PubMed] [Google Scholar]
- 12.Taggart DP, Choudhary B, Anastasiadis K, Abu-Omar Y, Balacumaraswami L, Pigott DW. Preliminary experience with a novel intraoperative fluorescence imaging technique to evaluate the patency of bypass grafts in total arterial revascularization. Ann Thorac Surg. 2003;75:870–873. doi: 10.1016/s0003-4975(02)04669-6. [DOI] [PubMed] [Google Scholar]
- 13.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–562. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 14.Sens R, Drexhage KH. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Luminesc. 1981;24:709–712. [Google Scholar]
- 15.Tsopelas C, Sutton R. Why certain dyes are useful for localizing the sentinel lymph node. J Nucl Med. 2002;43:1377–1382. [PubMed] [Google Scholar]
- 16.Nakayama A, Bianco AC, Zhang CY, Lowell BB, Frangioni JV. Quantitation of brown adipose tissue perfusion in transgenic mice using near-infrared fluorescence imaging. Mol Imaging. 2003;2:37–49. doi: 10.1162/15353500200303103. [DOI] [PubMed] [Google Scholar]
- 17.Fabricius AM, Gerber W, Hanke M, Garbade J, Autschbach R, Mohr FW. Early angiographic control of perioperative ischemia after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;19:853–858. doi: 10.1016/s1010-7940(01)00692-3. [DOI] [PubMed] [Google Scholar]
- 18.Khan NE, De Souza A, Mister R, Flather M, Clague J, Davies S, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–28. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 19.Falk V, Walther T, Kitzinger H, Rauch T, Diegeler A, Autschbach R, et al. An experimental approach to quantitative thermal coronary angiography. Thorac Cardiovasc Surg. 1998;46:25–27. doi: 10.1055/s-2007-1010179. [DOI] [PubMed] [Google Scholar]
- 20.Louagie YA, Haxhe JP, Buche M, Schoevaerdts JC. Intraoperative electromagnetic flowmeter measurements in coronary artery bypass grafts. Ann Thorac Surg. 1994;57:357–364. doi: 10.1016/0003-4975(94)90997-0. [DOI] [PubMed] [Google Scholar]
- 21.Matre K, Birkeland S, Hessevik I, Segadal L. Comparison of transit-time and Doppler ultrasound methods for measurement of flow in aortocoronary bypass grafts during cardiac surgery. Thorac Cardiovasc Surg. 1994;42:170–174. doi: 10.1055/s-2007-1016481. [DOI] [PubMed] [Google Scholar]
- 22.Canver CC, Cooler SD, Murray EL, Nichols RD, Heisey DM. Clinical importance of measuring coronary graft flows in the revascularized heart. Ultrasonic or electromagnetic? J Cardiovasc Surg (Torino) 1997;38:211–215. [PubMed] [Google Scholar]
- 23.D'Ancona G, Karamanoukian HL, Ricci M, Schmid S, Bergsland J, Salerno TA. Graft revision after transit time flow measurement in off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2000;17:287–293. doi: 10.1016/s1010-7940(00)00332-8. [DOI] [PubMed] [Google Scholar]
- 24.Hol PK, Fosse E, Mork BE, Lundblad R, Rein KA, Lingaas PS, et al. Graft control by transit time flow measurement and intraoperative angiography in coronary artery bypass surgery. Heart Surg Forum. 2001;4:254–257. discussion 257–258. [PubMed] [Google Scholar]
- 25.Detter C, Wipper S, Russ D, Iffland A, Burdorf L, Thein E, et al. Fluorescent cardiac imaging: a novel intraoperative method for quantitative assessment of myocardial perfusion during graded coronary artery stenosis. Circulation. 2007;116:1007–1014. doi: 10.1161/CIRCULATIONAHA.106.655936. [DOI] [PubMed] [Google Scholar]
- 26.Pinero A, Illana J, Garcia-Palenciano C, Canizarese F, Canteras M, Canadillas V, et al. Effect on oximetry of dyes used for sentinel lymph node biopsy. Arch Surg. 2004;139:1204–1207. doi: 10.1001/archsurg.139.11.1204. [DOI] [PubMed] [Google Scholar]
- 27.Martindale SJ, Stedeford JC. Neurological sequelae following methylene blue injection for parathyroidectomy. Anaesthesia. 2003;58:1041–1042. doi: 10.1046/j.1365-2044.2003.03415_23.x. [DOI] [PubMed] [Google Scholar]
- 28.Gawaz M. Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovasc Res. 2004;61:498–511. doi: 10.1016/j.cardiores.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soltesz EG, Kim S, Laurence RG, DeGrand AM, Parungo CP, Dor DM, et al. Intraoperative sentinel lymph node mapping of the lung using near-infrared fluorescent quantum dots. Ann Thorac Surg. 2005;79:269–277. doi: 10.1016/j.athoracsur.2004.06.055. discussion 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]