Abstract
Purpose
Before AIDS, endemic (African) Kaposi sarcoma (KS) was noted to occur in volcanic areas and was postulated to result from dirt chronically embedded in the skin of the lower extremities. The primary cause of all KS types is KS-associated herpesvirus (KSHV) infection, but co-factors contribute to the neoplasia. We investigated whether residential exposure volcanic or related soils was associated with the risk of classic Kaposi sarcoma (cKS) in Sicily.
Methods
Risk of incident cKS (n=141) compared to population-based KSHV seropositive controls (n=123) was estimated for residential exposure to four types of soil, categorized with maps from the European Soil Database and direct surveying. Questionnaire data provided covariates.
Results
Residents in communities high in luvisols were approximately 2.7-times more likely to have cKS than those in communities with no luvisols. Risk was not specific for cKS on the limbs, but it was elevated approximately 4–5-fold with frequent bathing or tap water drinking in high luvisols communities. Risk was unrelated to communities high in andosols, tephra, or clay soils.
Conclusions
Iron and alumino-silicate clay, major components of luvisols, may increase cKS risk, but formal investigation and consideration of other soil types and exposures are needed.
Keywords: Herpesviridae, Kaposi sarcoma, Italy, soils
INTRODUCTION
The primary cause of Kaposi sarcoma (KS), an angiogenic soft tissue sarcoma, is infection with Kaposi sarcoma-associated herpesvirus (KSHV, also known as human herpesvirus-8) (1). Cofactors for KS, particularly non-smoking and use of corticosteroid medications (2,3), are emerging but cannot explain the rarity and unusual geography of this disease. Among KSHV-seropositive adults in Sicily, who have no overt immunosuppression such as AIDS or allogeneic transplant, the annual incidence rate of classic KS (cKS) is only 6.2/100,000 and 2.5/100,000 for men and women, respectively (4).
In Africa prior to the AIDS epidemic, Ziegler noted an overlap between KS and podoconiosis, which is edema of the legs that develops in high-altitude, barefoot subsistence farmers whose feet are chronically embedded with moist, red, clay-type soil (5). Podoconiosis is a geochemical disease caused by obstruction of the lymphatics by soil particulates (6). Ziegler postulated that KS may likewise arise with chronic exposure of the skin to alumino-silicate or ferrous particulates, leading to lymphatic obstruction, microtrauma and local inflammation, or alteration of dermal immunity (7). In support of the latter mechanism, he reported that African non-AIDS KS cases had reduced delayed-type hypersensitivity skin test responses in legs as compared to the arms (8). In vitro studies suggest that iron may have even more direct effects, by blocking apoptosis and increasing proliferation of KS cells (9.10).
No association between cKS risk and silicate exposure was observed in Sardinia (11), whereas cKS risk was nearly 2-fold higher with residence in a basaltic lava area near Mount Vesuvius on the Italian mainland (12). When KSHV serology was developed, proximity to Mount Vesuvius was no longer associated with cKS risk in a sub-study of 29 cKS cases and 15 KSHV-seropositive controls (13).
We investigated the soil hypothesis using two maps of the land characteristics of Sicily where we recently completed a population-based case-control study of cKS.
METHODS
Details of the case-control study have been reported elsewhere (3). Briefly, confirmed incident cKS cases occurring from July 2002 through June 2006 were identified through active surveillance, and population-based controls were selected by sampling general practice physician’s offices from the National Health System Patients Roster, which includes every resident of Italy. Controls were frequency matched to cases by sex and age in 5-year strata. Following Institutional Review Board approval and informed consent, participants completed a standardized questionnaire and provided plasma samples, from which KSHV serostatus was determined with four assays and a conservative algorithm (3).
Area of residence (comune, henceforth community) from the questionnaire was combined with map data to estimate exposures to four types of soilscapes hypothesized a priori to be associated with risk cKS. Tephra (literally “volcanic rocks”) and clay soils (clay soilscapes near areas of volcanic rocks) were obtained from the European Soil Database (http://eusoils.jrc.it/data.html; scale 1:1,000,000), which categorizes dominant parent material for the major geographic features. Andosols (volcanic soils) and luvisols (leached soils), were from the Soil Map of Sicily by the University of Palermo (scale 1:250,000) (14).
Tephra areas are those with very irregular terrain and volcanic rock-outcrops intermingled with young volcanic soils. Andosols represent typical volcanic soil. In Sicily, both tephra and andosols are characterized by “vitric” materials, iron (as Fe2O3) around 11 wt %, and medium to very low concentration of clay (less than 35% of alumino-silicate particles ≤ 2 μ) (15,16).
Clay soils have iron (as Fe2O3) around 4–7 wt % and a medium to very high level of clay (35–60%). Such soils also can receive vitric volcanic ash from eruptions of Mount Etna on the east coast of Sicily. Luvisols have a medium to high level of clay (40–50 %) and iron (as Fe2O3) around 6–7 wt %. Iron linked to the alumino-silicates gives luvisols a reddish color.
By co-projecting the soil maps onto a map of Sicily, each community was assigned a score for the percentage of its area that was tephra, andosols, clay soils, or luvisols. Soil scores were assigned to individual participants based on community of residence during childhood (up to age 12) or adulthood (10 years prior to enrollment).
Using the distribution of the non-zero soil scores, the median or (for coarsely distributed data) the natural breakpoint was used to categorize each community’s soil type as none (zero), low (below median/breakpoint), or high. Associations between cKS and soil in the community (childhood or adult) were tested in logistic regression using SUDAAN to adjust for the complex sampling of controls using weighting previously described,3 and controlling for sex and age group (<68, 68–74, 75–80, ≥81). Possible interaction with soil and water data reported in the questionnaire, as well as possible confounding by previously noted cKS risk factors, were considered. The cKS cases (n=141) were compared to KSHV-seropositive controls (n=123), except as noted for a sensitivity analysis. Case-case analyses assessed whether soil exposure was specifically associated with number of cKS lesions or with a primary lesion on the limbs versus other body sites.
RESULTS
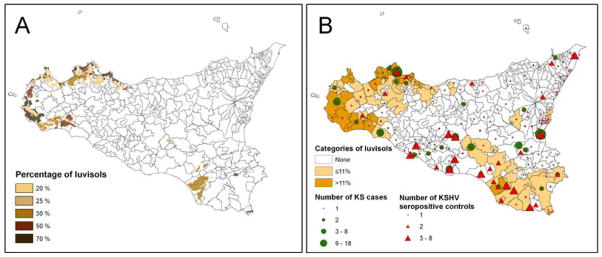
Participants who resided during adulthood in a community with “volcanic soil” - tephra or andosols - or clay soils were not at increased risk of cKS (Table 1). In contrast, residents of communities with >11% luvisols were at approximately 2.7-fold higher odds of cKS compared to residents of communities with no luvisols. Those in communities with ≤11% but >0% luvisols were at approximately 1.4-fold higher odds of cKS, and the trend was statistically significant (Ptrend=0.02). Those who lived in areas of tephra, andosols, or clay soils during childhood were not at increased odds of cKS, whereas those whose childhood residences were in areas with >11% luvisols had approximately 3-fold odds of cKS (OR 3.06, 95% CI 1.22–7.67). None of the previously noted risk factors for cKS (education, cigarette smoking, diabetes, or oral cortisone use) were found to be confounders of the association with the four types of soil (Table 1). The geographic distributions of cases, controls, and luvisols are shown in Figure 1.
Table 1.
Association of classic KS (cKS) with demographics, known risk factors and types of soil.
| Group or exposure | cKS Cases N | KSHV+ Controls | Odds Ratio* | 95% Confidence interval | P-value |
|---|---|---|---|---|---|
| Matching factors | |||||
| Sex | |||||
| Males | 89 | 86 | 1.12 | 0.64–1.97 | 0.69 |
| Females | 53 | 37 | Reference | ||
| Age (years) | |||||
| 81+ | 32 | 29 | 0.63 | 0.29–1.35 | 0.43‡ |
| 75 to 80 | 47 | 32 | 0.97 | 0.48–1.97 | |
| 68 to 74 | 34 | 32 | 0.68 | 0.31–1.48 | |
| <68 | 29 | 30 | Reference | ||
| Soil types | |||||
| Tephra in adulthood community | |||||
| >21% of land area | 6 | 7 | 0.63 | 0.18–2.19 | 0.24‡ |
| ≤21% of land area | 4 | 8 | 0.43 | 0.11–1.69 | |
| None | 131 | 106 | Reference | ||
| Andosols in adulthood community | |||||
| >5% of land area | 15 | 18 | 0.48 | 0.20–1.13 | 0.19‡ |
| ≤5% of land area | 21 | 13 | 1.47 | 0.63–3.43 | |
| None | 105 | 90 | Reference | ||
| Clay soils in adulthood community | |||||
| >11% of land area | 29 | 21 | 1.38 | 0.66–2.90 | 0.34‡ |
| ≤11% of land area | 26 | 26 | 1.27 | 0.64–2.54 | |
| None | 86 | 74 | Reference | ||
| Luvisols in adulthood community | |||||
| >11% of land area | 39 | 13 | 2.72 | 1.12–6.60 | 0.02‡ |
| ≤11% of land area | 30 | 30 | 1.39 | 0.70–2.76 | |
| None | 72 | 78 | Reference | ||
| Previously noted risk factors† | |||||
| Education | |||||
| 1st–4th grade | 44 | 62 | 0.37 | 0.20–0.68 | 0.001 |
| 5th grade or more | 97 | 60 | Reference | ||
| Cigarette smoking | |||||
| Never | 71 | 45 | 3.39 | 1.19–9.66 | 0.07‡ |
| Former | 61 | 57 | 2.01 | 0.81–5.01 | |
| Current | 10 | 21 | Reference | ||
| Diabetes | |||||
| Yes | 45 | 15 | 3.54 | 1.67–7.50 | 0.001 |
| No | 97 | 108 | Reference | ||
| Took oral cortisone in the last 10 years | |||||
| Yes | 53 | 26 | 2.70 | 1.42–5.14 | 0.003 |
| No | 89 | 97 | Reference | ||
| Stratification variables† | |||||
| Worked with plants or soil | |||||
| Yes | 77 | 72 | 1.07 | 0.60–1.91 | 0.83 |
| No | 65 | 51 | Reference | ||
| Barefoot during childhood | |||||
| No | 64 | 65 | Reference | ||
| Yes | 78 | 56 | 0.74 | 0.42–1.29 | 0.30 |
| Frequency of bathing during adulthood | |||||
| More than 3 per week | 68 | 45 | Reference | ||
| Three per week or less | 74 | 78 | 0.68 | 0.36–1.26 | 0.22 |
| Drank well or cistern water | |||||
| Sometimes/never | 35 | 30 | Reference | ||
| Always/usually | 107 | 93 | 1.04 | 0.53–2.02 | 0.91 |
| Drank community or tap water | |||||
| Sometimes/never | 59 | 53 | Reference | ||
| Always/usually | 83 | 70 | 0.90 | 0.51–1.57 | 0.71 |
Odds ratios adjusted for sex and age group.
Odds ratios also adjusted for category of chromic luvisol in adulthood community
P-value for trend
Figure 1. Geographic distributions of luvisols, cases of classic Kaposi sarcoma (KS), and matched KS-herpesvirus (KSHV) seropositive controls in the 382 communities in Sicily.
A) Concentration (proportion) of luvisols, based on island-wide surveying. Luvisols were concentrated along the western coast and, to a lesser extent, in the southeast.
B) Communities with distributions of KS cases (green circles) and KSHV seropositive controls (red triangles), and categorized by proportion of land mass with luvisols [none, low (<11%, median for non-zero communities), or high (≥11%).
Comparing those in the highest strata of luvisols to those with none, we further stratified by exposures to water and soil (Table 2). Those in high luvisols areas who bathed more than three times weekly had about 5-fold higher odds of KS than those in unexposed areas who bathed less frequently. Likewise those in high luvisols areas who usually or always drank community tap water tended to have higher odds of KS than those in unexposed areas who drank tap water infrequently, though neither of these tendencies were statistically significant (P=0.14 for both bathing and drinking community tap water). Residents of high luvisols areas were not at increased cKS risk if they worked with plants or soil, were barefoot during childhood, or usually drank well or cistern water.
Table 2.
Odds of KS stratified by chromic luvisol during adulthood and environmental exposures.
| Chromic Luvisol |
|||
|---|---|---|---|
| None | Low (≤11%) OR (95% CI)* | High (>11%) | |
| Worked with plants or soil | |||
| No | 1.00 (reference) | 1.68 (0.57–5.01) | 3.31 (0.95–11.6) |
| Yes | 1.22 (0.59–2.54) | 1.50 (0.60–3.77) | 2.69 (0.82–8.88) |
| Barefoot during childhood | |||
| No | 1.00 (reference) | 1.35 (0.54–3.38) | 3.97 (0.84–18.9) |
| Yes | 0.88 (0.42–1.84) | 0.98 (0.36–2.66) | 1.61 (0.56–4.62) |
| Frequency of bathing during adulthood | |||
| Three per week or less | 1.00 (reference) | 1.26 (0.49–3.21) | 1.51 (0.46–4.97) |
| More than three per week | 1.15 (0.51–2.59) | 1.77 (0.72–4.35) | 5.31 (1.60–17.6) |
| Drank well or cistern water | |||
| Sometimes/never | 1.00 (reference) | 1.25 (0.60–2.60) | 2.59 (1.01–6.64) |
| Always/usually | 0.41 (0.10–1.72) | 1.81 (0.37–8.82) | 2.39 (0.38–15.2) |
| Drank community tap water | |||
| Sometimes/never | 1.00 (reference) | 1.44 (0.61–3.41) | 1.70 (0.59–4.85) |
| Always/usually | 0.74 (0.36–1.52) | 0.93 (0.35–2.50) | 4.07 (0.96–17.3) |
Sex and age-group adjusted.
In a case-case analysis, those who lived in areas with luvisols were not more likely to have had a primary KS lesion on the limbs compared to other body sites. Number of lesions also was not associated with luvisols residence area. Compared to a reference group of zero current lesions, ORs (and 95% CIs) for having 1, 2–7, or 8 or more current lesions were: 0.94 (0.28–3.15), 1.12 (0.41–3.02) and 0.51 (0.18–1.45), respectively.
In sensitivity analyses that included seroindeterminate controls (n=78), residents of areas with >11% luvisols had higher odds of KS, controlled for age group and sex (adulthood OR 2.21, 95% CI 1.11–4.39, P=0.02; childhood OR 2.22, 95% CI 1.10–4.48, P=0.03). Further, in analyses that included all controls (including seronegatives, n=1031) and controlled for KSHV serostatus in three levels (positive, indeterminate, and negative), the association with the highest level of luvisols remained (adulthood OR 2.84, 95 % CI 1.22–6.63, P=0.02; childhood OR 2.93, 95% CI 1.27–6.78, P=0.01).
DISCUSSION
We found an increased risk of cKS among KSHV-seropositive people who resided during childhood or adulthood in communities of Sicily with luvisols, soils with medium to high level of clay and iron. These results support the hypothesis that environmental exposure, perhaps to alumino-silicate or iron-rich soil, may contribute to the development of KS (5,9,10,17).
Although luvisols, clay soils, and two “volcanic soils” were selected a priori, this association must be viewed with caution if not skepticism. The exposure measure was very crude. Because we lacked a precise residence location for each participant, we assigned the average soil content to the communities, some of which are quite large. Questionnaire responses indicating dermal contact with soil were not associated with cKS risk regardless of luvisols (3). The lack of a higher risk for limbs compared to other skin sites also suggests that dermal contact with luvisols does not underlie the association. Potential interaction or effect modification of soil by water exposures was postulated, but the results were paradoxical. Risk of cKS risk appeared to be increased with frequent bathing and drinking community tap water.
It should be noted that the different types of soil are also associated with varied agricultural uses and geographic morphologies such as elevation, slope, and regularity of the terrain. Luvisols and most clay soils in Sicily are generally found on useful, level or gently sloping terrain. In contrast, people have little contact with tephra and andosols, which generally are on steep slopes or irregular terrain. Future studies need to consider land features, uses, and types of contact with soil, plants and water (18). KS risk might be postulated to reflect exposure to iron or other environmental factors or to skin sensitization by bathing.
Acknowledgments
We are grateful to Dr. Giuseppe Lo Papa and Matt Airola for their assistance to this project.
Funding: This study was supported by the Intramural Research Program of the National Cancer Institute, in part under a contract with RTI International [N02-CP-91027] and in part under contract with Science Applications International Corporation [N01-CO-12400].
LIST OF ABBREVIATIONS
- AIDS
Acquired Immune Deficiency Syndrome
- KS
Kaposi sarcoma
- KSHV
Kaposi sarcoma -associated herpesvirus
- cKS
classic Kaposi sarcoma
- OR
Odds ratio
- 95% CIs
95% Confidence intervals
Footnotes
The authors declare no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore PS, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995 May 4;332:1181–5. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 2.Goedert JJ, Vitale F, Lauria C, Serraino D, Tamburini M, Montella M, Messina A, Brown EE, Rezza G, Gafa L, Romano N Classical Kaposi’s Sarcoma Working Group. Risk factors for classical Kaposi’s sarcoma. J Natl Cancer Inst. 2002;94:1712–8. doi: 10.1093/jnci/94.22.1712. [DOI] [PubMed] [Google Scholar]
- 3.Anderson LA, Lauria C, Romano N, Brown EE, Whitby D, Graubard BI, Li Y, Messina A, Gafa L, Vitale F, Goedert JJ. Risk factors for classical Kaposi sarcoma in a population-based case-control study in sicily. Cancer Epidemiol Biomarkers Prev. 2008;17:3435–43. doi: 10.1158/1055-9965.EPI-08-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vitale F, Briffa DV, Whitby D, Maida I, Grochowska A, Levin A, Romano N, Goedert JJ. Kaposi’s sarcoma herpes virus and Kaposi’s sarcoma in the elderly populations of 3 mediterranean islands. Int J Cancer. 2001;91:588–91. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1089>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler JL. Endemic Kaposi’s sarcoma in Africa and local volcanic soils. Lancet. 1993;342:1348–51. doi: 10.1016/0140-6736(93)92252-o. [DOI] [PubMed] [Google Scholar]
- 6.Fuller LC. Podoconiosis: Endemic nonfilarial elephantiasis. Curr Opin Infect Dis. 2005;18:119–22. doi: 10.1097/01.qco.0000160899.64190.15. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler JL, Simonart T, Snoeck R. Kaposi’s sarcoma, oncogenic viruses, and iron. J Clin Virol. 2001;20:127–30. doi: 10.1016/s1386-6532(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler JL, Katongole-Mbidde E. Diminished delayed hypersensitivity responses in the legs and feet of patients with endemic Kaposi’s sarcoma. Trans R Soc Trop Med Hyg. 1996;90:173–4. doi: 10.1016/s0035-9203(96)90126-1. [DOI] [PubMed] [Google Scholar]
- 9.Simonart T, Noel JC, Andrei G, Parent D, Van Vooren JP, Hermans P, Lunardi-Yskandar Y, Lambert C, Dieye T, Farber CM, Liesnard C, Snoeck R, Heenen M, Boelaert JR. Iron as a potential co-factor in the pathogenesis of Kaposi’s sarcoma? Int J Cancer. 1998;78:720–6. doi: 10.1002/(sici)1097-0215(19981209)78:6<720::aid-ijc9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Simonart T, Degraef C, Andrei G, Mosselmans R, Hermans P, Van Vooren JP, Noel JC, Boelaert JR, Snoeck R, Heenen M. Iron chelators inhibit the growth and induce the apoptosis of Kaposi’s sarcoma cells and of their putative endothelial precursors. J Invest Dermatol. 2000;15:893–900. doi: 10.1046/j.1523-1747.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 11.Montesu MA, De Marco R, Cottoni F. Soil silicates and Kaposi’s sarcoma in Sardinia. Lancet. 1995;346:1436–7. doi: 10.1016/s0140-6736(95)92457-4. [DOI] [PubMed] [Google Scholar]
- 12.Montella M, Franceschi S, Geddes M, Arniani S, Cocchiarella G. Classic Kaposi’s sarcoma and volcanic soil in southern Italy. Lancet. 1996;347:905. doi: 10.1016/s0140-6736(96)91388-4. [DOI] [PubMed] [Google Scholar]
- 13.Montella M, Serraino D, Crispo A, Rezza G, Carbone S, Tamburini M. Is volcanic soil a cofactor for classic Kaposi’s sarcoma? Eur J Epidemiol. 2000;16:1185–6. doi: 10.1023/a:1010923009048. [DOI] [PubMed] [Google Scholar]
- 14.Fierotti G, Dazzi C, Raimondi S. A report on the Soil Map of Sicily (scale 1:250.000) Regione Sicilia, Ass; Territorio Ambiente. Palermo: 1988. pp. 5–19. [Google Scholar]
- 15.Palumbo B, Angelone M, Bellanca A, Dazzi C, Hauser S, Neri R, Wilson J. Influence of inheritance and pedogenesis on heavy metal distribution in soils of Sicily, Italy. Geoderma. 2000;95:247–266. [Google Scholar]
- 16.Lo Papa G, Palermo V, Parisi S, Laudicina VA, Tusa D, Scalenghe R. Caratteristiche di una sequenza di suoli forestali nel versante nord occidentale dell’Etna (in Italian). Atti del Convegno “La conservazione della risorsa suolo”. Boll SISS. 2003;52(N 1–2):493–512. [Google Scholar]
- 17.Simonart T, De Dobbeleer G, Stallenberg B. Classic Kaposi’s sarcoma of the palm in a metallurgist: Role of iron filings in its development? Br J Dermatol. 2003;148:1061–3. doi: 10.1046/j.1365-2133.2003.05331.x. [DOI] [PubMed] [Google Scholar]
- 18.Dazzi C. Environmental features and land use of Etna (Sicily – Italy) In: Bartoli Arnalds F, Buurman P, Òskarsson H, Stoops G, Garcia-Rodeja E., editors. Soils of Volcanic Regions in Europe. Springer; 2007. pp. 629–644. [DOI] [Google Scholar]