Abstract
KATP channels are generally cardioprotective under conditions of metabolic impairment, consisting of pore-forming (Kir6.1 and/or Kir6.2) and sulphonylurea-binding, modulatory subunits (SUR1, SUR2A or SUR2B). Cardiovascular KATP channels are generally thought to consist of Kir6.2/SUR2A subunits (in the case of heart muscle) or Kir6.1/SUR2B subunits (smooth muscle), whereas SUR1-containing channels have well-documented roles in pancreatic insulin release. Recent data, however, demonstrated the presence of SUR1 subunits in mouse cardiac tissue (particularly in atria) and a surprising protection from myocardial ischemia/reperfusion in SUR1-null mice. Here we review some of the extra-pancreatic roles assigned to SUR1 subunits and consider whether these might be involved in the sequelae of ischemia/reperfusion.
KATP channels
Amongst the various classes of ion channels described to date in the cardiovascular system, KATP channels are unique in that they serve as transducers between intracellular energy metabolism and electrical excitability. Overall, they mediate responses to stress adaptation (Zingman et al., 2002) and they have clearly defined roles in many cell types, such as triggering insulin release from pancreatic β-cells, thus precisely controlling blood glucose levels (Koster et al., 2005). KATP channels are abundantly expressed in the cardiovascular system, where they participate in the regulation of coronary blood flow under normal basal conditions (Brayden, 2002) and in the pathophysiological sequelae of myocardial ischemia/reperfusion injury (Grover, 1997).
Molecular Components of KATP channels
Molecular cloning studies have provided insights into the differences in biophysical and pharmacological properties of different types of KATP channels found in various tissues and cell types (Ashcroft, 1988; Seino and Miki, 2003; Nichols, 2006). Functional KATP channels are formed by pore-forming Kir6 subunits in combination with regulatory SUR subunits. There are two types of Kir6 subunits (Kir6.1 and Kir6.2) and two different types of SUR subunits; SUR1 and SUR2; the latter exists as two major functionally relevant isoforms (SUR2A and SUR2B) as a result of alternative splicing. Kir6.x subunits largely determine the biophysical properties and ATP inhibition whereas SURx subunits confer MgADP activation and bestow unique pharmacological specificities.
In heterologous expression systems, specific combinations of Kir6.x and SURx subunits give rise to functional channels that resemble the various subtypes of KATP channels found in different tissues and cell types. For example, co-expression of Kir6.2 and SUR1 subunits results in channels that resemble those in the pancreatic β-cell in terms of single channel conductance, gating properties, nucleotide sensitivity and pharmacological properties (Nichols, 2006). The role of SUR1 subunits has been the subject of many studies and the reader is referred to an excellent recent review (Aittoniemi et al., 2009). The purpose of this treatise is to provide an overview of the possible roles of SUR1 subunits in the cardiovascular system and how they may contribute to cardiovascular function; particularly following myocardial ischemia and reperfusion.
Cardiovascular KATP Channels: The Canonical View
When attempting to correlate molecular candidates with a particular native current expressed in a specific tissue, several criteria may be used (Coetzee et al., 1999). First, a possible relationship is signified by a close resemblance between the biophysical and pharmacological properties of native channels and those of channels expressed in heterologous expression systems. Second, there should be a correspondence between the tissue expression of mRNA and protein of the candidate subunits and the functional expression of the native current. Third, a causal relationship between native and candidate cloned K+ channel subunits can be obtained from experiments involving deletion or overexpression of the target channel subunits. Based on these criteria, there is strong evidence for the involvement of Kir6.2 and SUR2A subunits in the molecular composition of ‘cardiac’ KATP channels. For example, the co-expression of Kir6.2/SUR2A subunits in heterologous expression systems gives rise to channels that strongly resemble the native KATP channels of enzymatically isolated cardiac ventricular myocytes (Babenko et al., 1998). These subunits are both expressed in the heart. Finally, loss of ventricular KATP channels occurs either with transgenic expression of dominant negative Kir6 subunits in the heart (Tong et al., 2006) or with deletion of the Kcnj11 locus [Kir6.2 (-/-) mice (Seino and Miki, 2003)]a. Similar arguments have been used to define vascular smooth muscle KNDP channels as consisting of Kir6.1 subunits in combination with SUR2B subunits (Seino and Miki, 2003). It is important to note, however, that these criteria do not necessarily exclusively define a relationship between cloned and native proteins. Unanticipated events may occur at the molecular level, such as interactions of K+ channel subunits with other cytosolic or membrane-bound proteins or their regulation by unknown endogenous compounds and peptides or poorly understood post-translational modifications.
Cardiovascular KATP Channels: Controversies
Despite the concept of Kir6.2 and SUR2A subunits as the major molecular components of ‘cardiac’ KATP channels, there are several unresolved and emerging issues suggestive of a more complex scenario. First, it should be recognized that the cardiovascular system (even the heart itself) is composed of different cell types and that the roles and characteristics of KATP channels in these diverse cells are now only beginning to be realized. There have been several reports, for example, of Kir6.1 mRNA and protein expression in the hearts of human, guinea pig, chick, rat and mouse (Inagaki et al., 1995; Akao et al., 1997; Lu and Halvorsen, 1997; Erginel-Unaltuna et al., 1998; Pountney et al., 2001; Sun et al., 2004; Morrissey et al., 2005a; Morrissey et al., 2005b; Isidoro et al., 2007). Some studies suggested high Kir6.1 expression in the atrium (Baron et al., 1999) and there is also evidence for the expression of Kir6.1 in the vascular endothelium (Yoshida et al., 2004; Schnitzler et al., 2000b). Both mRNA and/or protein expression has also been noted for SUR1 subunits in human, guinea pig, mouse and rat heart (Hernandez-Sanchez et al., 1999; Schnitzler et al., 2000b; Morrissey et al., 2005a; Morrissey et al., 2005b). Furthermore, several SUR1 splice variants exist (Hambrock et al., 2002) with strong expression of SUR1C in the heart; but their contribution to cardiovascular function has not been investigated. Interestingly, studies with antisense oligonucleotides against KATP channel subunits demonstrated a role for SUR1 in the KATP channel current of rat ventricular myocytes (Yokoshiki et al., 1999). Furthermore, transgenic expression of SUR1 in cardiac myocytes affects native KATP channel activity (Flagg et al., 2005). Thus, there may be important diversity of SUR1 expression and function within the cardiovascular system. For example, KATP channels in the atrium and ventricle have different functional and pharmacological properties and the suggestion has been made that these channels are fundamentally different at the molecular level (Poitry et al., 2003). Indeed, we recently described that SUR1 subunits are essential components of mouse atrial and not ventricular KATP channels (Flagg et al., 2008), with the consequence that there is a strong sensitivity to the KATP channel opener diazoxide and the first-generation sulphonylurea, tolbutamide (which do not strongly affect ventricular KATP channels). It is also important to consider that adjacent or neighboring cell types such as endothelial cells, smooth muscle cells, and cardiac myoctyes can exhibit differential expression and function of KATP channels which determines coronary blood flow and cardiac function under various conditions.
A Role for SUR1 Subunits in Cardiac Ischemia/Reperfusion
In contrast to Kir6.1 (-/-) and SUR2 (-/-) mice, which exhibit cardiovascular complications such as hypertension and coronary vasospasm (Miki et al., 2002; Chutkow et al., 2002), there have been no reported adverse cardiovascular phenotypes in SUR1 (-/-) mice. However, as has been the case for many other knockout models, it is possible that such phenotypes may manifest only after stress conditions. Given the presence of SUR1 subunits of KATP channels in the cardiovascular system, we performed in-vivo ischemia/reperfusion studies to determine its function under pathophysiological conditions. We obtained mice deficient of the ABCC8 gene (SUR1-null mice) from Dr Mark Magnuson (Vanderbilt University School of Medicine, Nashville) and performed in-vivo ischemia/reperfusion studies to examine the possible role of SUR1 subunits in cardiac function after ischemia. The left coronary artery was ligated for a period of 30 min, followed by a reperfusion period of 24h or 7 days. Infarct size was determined and expressed relative to the area at risk (Elrod et al., 2008). Interestingly, SUR1 (-/-) mice had a reduced infarct size at both of these reperfusion periods (Elrod et al., 2008). Furthermore, hearts from SUR1 (-/-) mice had less fibrosis occurring post-ischemia and a reduced degree of myocardial neutrophilic infiltrate, necrosis, hemorrhage, and spindle-shaped interstitial cells. Post-ischemic cardiac dysfunction (as assessed by echocardiography) was also improved in the SUR1 (-/-) mice. These surprising findings are not consistent in any simple way with the findings of enhanced ischemic damage and loss of ischemic preconditioning in the absence of KATP channel subunits due to the genetic deletion of Kir6.2 subunits (Suzuki et al., 2002; Gumina et al., 2003; Gumina et al., 2007). To the contrary, since SUR1 (-/-) mice are protected from ischemia/reperfusion, one interpretation of our data is that SUR1 subunits might actually contribute to ischemic or post-ischemic damage.
How do SUR1 Subunits Participate in Ischemic Events?
The mechanisms by which SUR1 subunits may contribute to events that manifest during ischemia and reperfusion are unclear at present and apart from a few recent studies (Elrod et al., 2008; Flagg et al., 2008), the roles of SUR1 subunits to cardiovascular function and myocardial protection are largely unexplored. Since cardiovascular function and the cardiac response to ischemia/reperfusion can be determined at many levels (e.g. effects in different tissues and cell types, metabolic states, hormones, neurotransmitters, etc) it is worthy to review the available evidence for extra-pancreatic roles of SUR1 subunits and how cardiovascular outcome might be affected. Whilst speculative, we hope that this synopsis will stimulate debate and further research into the roles of SUR1 subunits in the cardiovascular system.
Is there a Role for Ventricular KATP channels?
KATP channels are strongly implicated in the pathophysiological sequelae of myocardial ischemia and the consensus is that their opening protects the heart against periods of ischemia. Ventricular sarcolemmal KATP channels are thought to induce action potential shortening and (to some extent) K+ loss in the heart, which may preserve energy consumption and attenuate Ca2+ accumulation during the ischemic period. The strongest evidence arguing for a protective role of sarcolemmal KATP channels come from genetic models; for example the cardioprotective effect of diazoxide is abolished in Kir6.2 (-/-) mice (Suzuki et al., 2003). It seems unlikely (although not impossible) that ventricular KATP channels are involved in the protection that we observe with the SUR1 (-/-) mice since we observed essentially normal KATP channel activity in ventricular myocytes from the SUR1 (-/-) mice (Elrod et al., 2008; Flagg et al., 2008) and we did not observe significant remodeling of RNA expression of the other KATP channel subunits (Kir6.1, Kir6.2, SUR2A and SUR2B) in the hearts of the SUR1 (-/-) mice (Elrod et al., 2008).
Is there a Role for Mitochondrial KATP channels?
In addition to the role of sarcolemmal KATP channels in cardioprotection following ischemia as described above, there is also support for a role of mitochondrial KATP (mito-KATP) channels (Gross and Peart, 2003). The arguments for an involvement of mito-KATP channels hinge in part on reports of a) functional KATP channels in cardiac mitochondrial inner membranes (Inoue et al., 1991), b) modulation of such channels when they are incorporated into lipid bilayers by certain KATP channel modulating compounds such as diazoxide and 5-hydroxydecanoate (Garlid et al., 1993) and c) that these latter compounds influence the outcome of ischemic preconditioning (Gross and Peart, 2003). A caveat with this line of reasoning is that some of these compounds (such as diazoxide and 5-hydroxydecanoate) actually have strong effects on sarcolemmal ventricular KATP channels under metabolically impaired conditions (Notsu et al., 1992) or when cytosolic ADP levels are elevated (D'hahan et al., 1999), as would be expected to occur under ischemic conditions. Unfortunately, the molecular basis of mito-KATP channels remains unclear at present. Importantly, studies to date have neither identified SUR1 subunits in mitochondrial preparations nor have SUR1 subunits been localized in mitochondria using various techniques (Ardehali et al., 2004; Cuong et al., 2005). In addition to these arguments, from first principles it is unlikely that mito-KATP channels are involved in the gain in protection that we observe in the SUR1 (-/-) mice since this observation is contrary to the protective role assigned to mito-KATP channels. Nevertheless, our data suggesting a role for SUR1 subunits in the consequences of cardiac ischemia has important implications for the interpretation of much of the literature on cardioprotection, since the compounds that are often implicitly or explicitly used as specific openers and blockers of mito-KATP channels may also mediate effects through SUR1-based sarcolemmal channels, which are highly sensitive to some of these compounds (such as diazoxide and tolbutamide).
Are Atrial KATP Channels Involved?
Our recent finding of high expression levels of SUR1 mRNA and protein in the atrium and the demonstration that these subunits are requisite for atrial KATP channel activity (Flagg et al., 2008) raises the significant possibility that such atrial KATP channels might be involved in events following myocardial ischemia. Certainly, the unusually high sensitivity of atrial KATP channels to drugs such as diazoxide (Poitry et al., 2003; Flagg et al., 2008) suggests that these channels may have been unintentionally targeted in prior studies when modulation of mito-KATP channels was intended with certain pharmacological approaches. We cannot rule an involvement of SUR1-based KATP channels in the ischemic response on the basis of our data and it is possible that atrial KATP channels may be preferentially activated during and after ischemia since these channels have been described to be unusually sensitive to metabolic inhibition (Poitry et al., 2003) and to be activated by events such as cell stretch (Van Wagoner, 1993; Baron et al., 1999). Although pharmacological activation of atrial KATP channels with nicorandil has been documented to be proarrhythmic in isolated rabbit atria (Le et al., 1992), the consequences of atrial KATP channels during ischemia are not known. One possibility is that they regulate the release of substances such as ANP (Xu et al., 1996; Saegusa et al., 2005), which in turn contributes to ischemic damage (Houng et al., 2009).
Is There a Role for SUR1 Subunits in the Vasculature?
Alterations coronary blood flow might be expected to determine the outcome of ischemic episodes. Indeed, coronary KATP (or KNDP) channels have been implicated in pathogenesis of the coronary vasculature as well as in redistribution of blood flow within the heart during ischemia (reactive hyperemia) and hypoxic-dilation of coronary arteries (Kanatsuka et al., 1992; Daut et al., 1990; Von Beckerath et al., 1991). As such, KATP/KNDP channels in the vasculature may contribute to the sequelae of myocardial ischemia. Endothelial KATP channels add another dimension to the regulation of coronary blood flow. For example, vasodilation of coronary arteries by adenosine and hyperosmolarity may be mediated in part by activating endothelial KATP channels (Kuo and Chancellor, 1995; Ishizaka and Kuo, 1997). Endothelial KATP channels may also contribute to shear stress-induced endothelial release of the vasodilator nitric oxide in rabbit aorta (Hutcheson and Griffith, 1994) and we demonstrated that block of endothelial KATP channels enhances the release of the vasoconstrictor peptide, endothelin-1 (Malester et al., 2007). The weight of the evidence suggests that smooth muscle KNDP channels are comprised of Kir6.1/SUR2B subunits (Seino and Miki, 2003) and the available evidence suggests that endothelial KATP channels may be composed of heteromultimers of Kir6.1/Kir6.2/SUR2B subunits (Schnitzler et al., 2000a; Yoshida et al., 2004). A role for SUR1 subunits in the vasculature under physiological conditions has not been described although it has been reported that SUR1 expression is upregulated after ischemic events, even in tissues where it is not normally expressed, such as the endothelium (Simard et al., 2006). There is emerging evidence for a detrimental role for SUR1 subunits under certain pathophysiological conditions. SUR1 levels are upregulated after ischemic events; even in tissues where it is not normally expressed, such as the endothelium (Simard et al., 2006). SUR1 subunits are reported to form non-selective sulphonylurea-sensitive channels. Recent data suggest that SUR1 subunits associate with TRPM4 subunits (Gerzanich et al., 2009), which may modulate the membrane potential and ionic gradients, thus contributing to post-ischemic injury [such as necrosis following spinal chord injury (Simard et al., 2007)]. At present, it is unknown whether any such pathophysiological response involving SUR1 subunits may occur in the heart after myocardial ischemia and this should be examined in future studies. If indeed SUR1 subunits contribute to ischemic injury in this manner, this may present new opportunities for the treatment of myocardial injury with sulphonylureas (particularly with low doses or classes of compounds that do not affect SUR2-based KATP channels). Studies of the pathogenesis of myocardial ischemia/reperfusion injury have predominantly been focused on the role of loss of function or gain of function of the myocardial cell KATP channels. Given the important regulatory effects of the coronary KATP channels on vasodilation it is important to consider the role of vascular KATP channels on myocardial blood flow and cardiac contractility following ischemia and reperfusion.
Is There a Role for SUR1 Subunits in the Peripheral Nervous System?
It may be possible for SUR1-based KATP channels to alter the local cardiac release of neurotransmitters such as norepinephrine (NE) and acetylcholine, which are known to affect the ischemic outcome (Karlsberg et al., 1979; Przyklenk and Kloner, 1995). There is evidence for a KATP channel involvement in the release events in both sympathetic and parasympathetic nerves. Studies of the pathogenesis of myocardial ischemia/reperfusion injury have predominantly been focused on the role of loss of function or gain of function of the myocardial cell KATP channels. Given the important regulatory effects of the coronary KATP channels on vasodilation it is important to consider the role of vascular KATP channels on myocardial blood flow and cardiac contractility following ischemia and reperfusion.
KATP channels regulate neurotransmitter release from sympathetic nerves
A novel role for KATP channels has recently been described in the sympathetic nervous system, where norepinephrine release was found to be inhibited by active KATP channels (Oe et al., 1999). Supporting this conclusion, KATP channel openers inhibit NE release as well as the increase in atrial rate induced by electrical stimulation of the sympathetic ganglion (Oe et al., 1999; Mohan and Paterson, 2000). In contrast, KATP channel blockers had opposite effects. Interestingly, NE release (as well as changes in atrial rate by sympathetic nerve stimulation) was exquisitely sensitive to diazoxide (Mohan and Paterson, 2000), which activates SUR1-containing KATP channels much more effectively than SUR2-containg KATP channels. However, the molecular basis of this effect on NE release (as well as the signaling pathways responsible for KATP channel-regulated exocytosis) still needs to be investigated. The high sensitivity of these channels to sulphonylureas raises the potential problem that sulphonylurea treatment during diabetes may have undesired consequences on the response of the heart to sympathetic stimulation and to the generation of arrhythmias. These issues need to be addressed further using appropriate tools, such as animals that selectively lack KATP channels in specific tissues.
KATP channels regulate neurotransmitter release from parasympathetic nerves
Acetylcholine (Ach) is synthesized and released in the central nervous system as well as from autonomic ganglia in the peripheral nervous system and in postganglionic parasympathetic neurons. In the heart, vagal nerve stimulation causes Ach release, which slows the heart rate by G-protein-mediated activation of Kir3.x channels. It was found that Ach-release evoked by electrical stimulation of isolated guinea pig atria was stimulated by KATP channel blockers, suggesting a role for negative feedback of KATP channels in exocytotic processes in these neurons (Kilbinger et al., 2002). Interestingly, Ach release from neurons in the ileum is affected similarly by KATP channel activity (Zini et al., 1991), whereas mesenteric neurons are unaffected by KATP channel modulation (Schworer and Kilbinger, 1989; Kilbinger et al., 2002). This suggests a diversity of function of KATP channels in various neuronal compartments, which remains to be studied. The KATP channels in dorsal vagal neurons have been suggested to be composed of Kir6.2/SUR1 channels (Karschin et al., 1998) (as in the pancreas and the atrium), which again raises issues of potential cardiovascular effects of sulphonylurea treatment in the setting of diabetes. As stated above, the study of the molecular composition of the channels in these neurons, the molecular mechanisms responsible for coupling KATP channel activity to exocytotic release and the pathophysiological consequences of these findings await more refined tools (such as mice lacking KATP channels in specific tissue compartments).
Other roles for KATP channel subunits?
SUR1 subunits may also contribute to physiological and pathophysiological events in a manner that may be unrelated to its usual role as an ion channel accessory subunit. For example, recent evidence suggests that SUR1 subunits enhance the apoptosis induced by sulfonylureas and other compounds (Hambrock et al., 2006; Hambrock et al., 2007; Ackermann et al., 2008) and it is unclear that this involves any channel activity. Future studies should be designed to investigate the role of SUR1 subunits in promoting apoptosis in the cardiovascular system; particularly following myocardial ischemia. SUR1 subunits have also been reported to directly associate with proteins that regulate the molecular process of exocytosis. For example, SUR1 may bind to Epac (Kang et al., 2008) and to RIM2 (Shibasaki et al., 2004), a Rab-effector protein that acts as a scaffold for the binding of other exocytotic proteins. Interestingly, an interaction of SUR2A with plasma membrane soluble NSF attachment receptor (SNARE) components (syntaxin 1A) has also been described (Kang et al., 2006). It may be possible that SUR subunits may have scaffolding functions and participate in exocytotic release processes (Leung et al., 2007), analogous to the role of some voltage-gated K+ channel subunits (e.g. Kv2.1) that interact with SNARE proteins to physically mediate secretion (Mohapatra et al., 2007). Finally, it should be considered that SUR1 is a member of a large family of ABC transporters. With the exception of SURs and CFTR (both members of the ABCC subfamily), many ABC proteins have established transport functions. With high specificity, different family members transport a variety of substances across membranes ranging from amino acids, sugars, inorganic ions, polysaccharides, peptides, lipids and drugs (Higgins, 1992). No such transport function or substrate has been identified for SUR1 subunits to date, but if they exist, then it may be possible that SUR1 affects the ischemic outcome through such a mechanism.
Clinical Significance
The cardiovascular risk of sulfonylureas (e.g. effects on infarct size and arrhythmias after ischemia) is controversial (Negroni et al., 2007). The University Group Diabetes Program studies have suggested an excess cardiovascular mortality in tolbutamide-treated subjects (Meinert et al., 1970), but second generation sulfonylureas may pose less of a cardiovascular risk (Schwartz and Meinert, 2004). The ‘cardiac’ KATP channel has conventionally been thought to consist of Kir6.2/SUR2A subunits and to be less sensitive to sulphonylureas than the SUR1-containing channels (such as the pancreatic β-cell KATP channel). Our demonstration of a specific cardiovascular role of SUR1 subunits (Elrod et al., 2008) and high levels of SUR1 expression in the mouse atrium (Flagg et al., 2008) may have important ramifications in the cardiovascular risk of diabetic patients treated with sulfonylureas. One might predict that sulfonylureas may be cardioprotective (reduced infarct size after ischemia/reperfusion) by blocking the cardiovascular SUR1-containing channels. Although blocking KATP channels could be considered to be pro-excitatory and potentially pro-arrhythmic, early results suggest the opposite (KATP channel opening with nicorandil is pro-arrhythmic in isolated atria (Le et al., 1992), probably due to altered refractory periods. Thus, the finding that post-ischemic events are influenced by SUR1 subunits is important and is worthy of further investigation.
Directions for Future Research
KATP channels are present in multiple tissues and cell types within the cardiovascular system and these channels differ from each other in terms of their biophysical and pharmacological properties. Overall, the nature of the physiological and pathophysiological outcome will be determined by the synchronous interaction the various KATP/KNDP channels in diverse tissue and cell types. To fully understand the function of the KATP channel in the complex biological setting of an intact animal requires tissue-specific elimination of KATP channel subunits and developing such animal models must be a high priority in future studies. It will be particularly important to investigate the cell and tissue-specific expression of SUR1 subunits across different species, before and after ischemic events. The use of genetic mouse models with tissue specific knockout of KATP channels is an ideal tool to address these important experiments. It will be important to investigate potential deleterious roles of SUR1 subunits in the cardiovascular system (and perhaps independently of their understood role in forming KATP channels) in cellular processes as diverse as apoptosis, exocytosis and the formation of non-selective cation channels.
Figure 1.
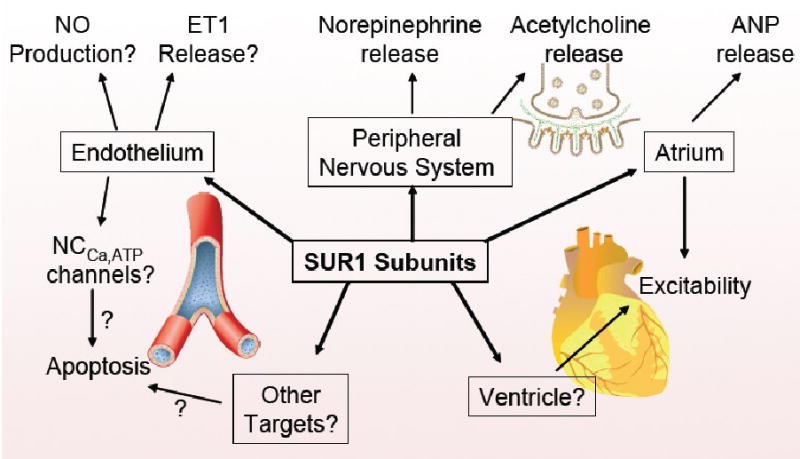
Sulfonylurea receptor type 1 (SUR1) subunits are involved in cardioprotection. Multiple targets and cellular processes may be involved and these may interact synergistically.
Acknowledgments
D.J.L. is supported by a grant from the National Institutes of Health 7R01 HL060849-09. C.G.N. is supported by a grant from the National Institutes of Health 1R01HL095010-01 and W.A.C. is supported by a grant from the National Institutes of Health 5R01HL085820-02 and partial support is provided by the 7th Masonic District.
Footnotes
The SUR2 (-/-) mice have abnormal (glibenclamide insensitive) KATP channels in ventricular myocytes, which may be the result of KATP channel isoforms that remain to be fully characterized (Pu et al., 2008).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann S, Hiller S, Osswald H, et al. 17beta -Estradiol modulates apoptosis in pancreatic beta -cells by specific involvement of the sulfonylurea receptor (SUR) isoform SUR1. J Biol Chem. 2008;284:4905–4913. doi: 10.1074/jbc.M807638200. [DOI] [PubMed] [Google Scholar]
- Aittoniemi J, Fotinou C, Craig TJ, et al. Review. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos Trans R Soc Lond B Biol Sci. 2009;364:257–267. doi: 10.1098/rstb.2008.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao M, Otani H, Horie M, et al. Myocardial ischemia induces differential regulation of KATP channel gene expression in rat hearts. J Clin Invest. 1997;100:3053–3059. doi: 10.1172/JCI119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- Baron A, van Bever L, Monnier D, Roatti A, Baertschi AJ. A novel K(ATP) current in cultured neonatal rat atrial appendage cardiomyocytes. Circ Res. 1999;85:707–715. doi: 10.1161/01.res.85.8.707. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol. 2002;29:312–316. doi: 10.1046/j.1440-1681.2002.03650.x. [DOI] [PubMed] [Google Scholar]
- Chutkow WA, Pu J, Wheeler MT, et al. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cuong DV, Kim N, Joo H, et al. Subunit composition of ATP-sensitive potassium channels in mitochondria of rat hearts. Mitochondrion. 2005;5:121–133. doi: 10.1016/j.mito.2004.12.001. [DOI] [PubMed] [Google Scholar]
- D'hahan N, Moreau C, Prost AL, et al. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc Natl Acad Sci U S A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J, Maierrudolph W, Vonbeckerath N, et al. Hypoxic dilation of coronary arteries is mediated by ATP- sensitive potassium channels. Science. 1990;247:1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Elrod JW, Harrell M, Flagg TP, et al. Role of sulfonylurea receptor type 1 subunits of ATP-sensitive potassium channels in myocardial ischemia/reperfusion injury. Circulation. 2008;117:1405–1413. doi: 10.1161/CIRCULATIONAHA.107.745539. [DOI] [PubMed] [Google Scholar]
- Erginel-Unaltuna N, Yang WP, Blanar MA. Genomic organization and expression of KCNJ8/Kir6.1, a gene encoding a subunit of an ATP-sensitive potassium channel. Gene. 1998;211:71–78. doi: 10.1016/s0378-1119(98)00086-9. [DOI] [PubMed] [Google Scholar]
- Flagg TP, Kurata HT, Masia R, et al. Differential Structure of Atrial and Ventricular KATP: Atrial KATP Channels Require SUR1. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg TP, Remedi MS, Masia R, et al. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal K(ATP) J Mol Cell Cardiol. 2005 doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Pikula S, et al. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1993;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Woo SK, Vennekens R, et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat Med. 2009;15:185–191. doi: 10.1038/nm.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003 Sep;285(3):H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- Grover GJ. Pharmacology of ATP-sensitive potassium channel (KATP) openers in models of myocardial ischemia and reperfusion. Can J Physiol Pharmacol. 1997;75:309–315. doi: 10.1139/cjpp-75-4-309. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, O'Cochlain DF, Kurtz CE, et al. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–H1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Pucar D, Bast P, et al. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Hambrock A, de Oliveira Franz CB, Hiller S, et al. Resveratrol binds to the sulfonylurea receptor (SUR) and induces apoptosis in a SUR subtype-specific manner. J Biol Chem. 2007;282:3347–3356. doi: 10.1074/jbc.M608216200. [DOI] [PubMed] [Google Scholar]
- Hambrock A, de Oliveira Franz CB, Hiller S, Osswald H. Glibenclamide-induced apoptosis is specifically enhanced by expression of the sulfonylurea receptor isoform SUR1 but not by expression of SUR2B or the mutant SUR1(M1289T) J Pharmacol Exp Ther. 2006;316:1031–1037. doi: 10.1124/jpet.105.097501. [DOI] [PubMed] [Google Scholar]
- Hambrock A, Preisig-Muller R, Russ U, et al. Four novel splice variants of sulfonylurea receptor 1. Am J Physiol Cell Physiol. 2002;283:C587–C598. doi: 10.1152/ajpcell.00083.2002. [DOI] [PubMed] [Google Scholar]
- Hernandez-Sanchez C, Ito Y, Ferrer J, Reitman M, LeRoith D. Characterization of the mouse sulfonylurea receptor 1 promoter and its regulation. J Biol Chem. 1999;274:18261–18270. doi: 10.1074/jbc.274.26.18261. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. 67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Houng AK, McNamee RA, Kerner A, et al. Atrial natriuretic peptide increases inflammation, infarct size and mortality after experimental coronary occlusion. Am J Physiol Heart Circ Physiol. 2009 doi: 10.1152/ajpheart.00684.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson IR, Griffith TM. Heterogeneous populations of K+ channels mediate EDRF release to flow but not agonists in rabbit aorta. Am J Physiol. 1994;266:H590–H596. doi: 10.1152/ajpheart.1994.266.2.H590. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, et al. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–5694. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Ishizaka H, Kuo L. Endothelial ATP-sensitive potassium channels mediate coronary microvascular dilation to hyperosmolarity. Am J Physiol. 1997;273:H104–H112. doi: 10.1152/ajpheart.1997.273.1.H104. [DOI] [PubMed] [Google Scholar]
- Isidoro TN, Philip-Couderc P, Papageorgiou I, et al. Expression and function of ATP-dependent potassium channels in late post-infarction remodeling. J Mol Cell Cardiol. 2007;42:1016–1025. doi: 10.1016/j.yjmcc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Kanatsuka H, Sekiguchi N, Sato K, et al. Microvascular sites and mechanisms responsible for reactive hyperemia in the coronary circulation of the beating canine heart. Circ Res. 1992;71:912–922. doi: 10.1161/01.res.71.4.912. [DOI] [PubMed] [Google Scholar]
- Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J Physiol. 2008;586:1307–1319. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Ng B, Leung YM, et al. Syntaxin-1A actions on sulfonylurea receptor 2A can block acidic pH-induced cardiac K(ATP) channel activation. J Biol Chem. 2006;281:19019–19028. doi: 10.1074/jbc.M513160200. [DOI] [PubMed] [Google Scholar]
- Karlsberg RP, Penkoske PA, Cryer PE, Corr PB, Roberts R. Rapid activation of the sympathetic nervous system following coronary artery occlusion: relationship to infarct size, site, and haemodynamic impact. Cardiovasc Res. 1979;13:523–531. doi: 10.1093/cvr/13.9.523. [DOI] [PubMed] [Google Scholar]
- Karschin A, Brockhaus J, Ballanyi K. KATP channel formation by the sulphonylurea receptors SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J Physiol. 1998;509:339–346. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H, Krause A, Mang CF, Englert H, Wirth K. Effects of K(ATP) channel modulators on acetylcholine release from guinea-pig isolated atria and small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:371–377. doi: 10.1007/s00210-002-0539-9. [DOI] [PubMed] [Google Scholar]
- Koster JC, Permutt MA, Nichols CG. Diabetes and insulin secretion: the ATP-sensitive K+ channel (K ATP) connection. Diabetes. 2005;54:3065–3072. doi: 10.2337/diabetes.54.11.3065. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chancellor JD. Adenosine potentiates flow-induced dilation of coronary arterioles by activating KATP channels in endothelium. Am J Physiol. 1995;269:H541–H549. doi: 10.1152/ajpheart.1995.269.2.H541. [DOI] [PubMed] [Google Scholar]
- Le GB, Hatem S, Le Heuzey JY, et al. Pro-arrhythmic effect of nicorandil in isolated rabbit atria and its suppression by tolbutamide and quinidine. Eur J Pharmacol. 1992;229:91–96. doi: 10.1016/0014-2999(92)90290-k. [DOI] [PubMed] [Google Scholar]
- Leung YM, Kwan EP, Ng B, Kang Y, Gaisano HY. SNAREing voltage-gated K+ and ATP-sensitive K+ channels: tuning beta-cell excitability with syntaxin-1A and other exocytotic proteins. Endocr Rev. 2007;28:653–663. doi: 10.1210/er.2007-0010. [DOI] [PubMed] [Google Scholar]
- Lu CW, Halvorsen SW. Channel activators regulate ATP-sensitive potassium channel (Kir6.1) expression in chick cardiomyocytes. FEBS lett. 1997;412:121–125. doi: 10.1016/s0014-5793(97)00760-6. [DOI] [PubMed] [Google Scholar]
- Malester B, Tong X, Ghiu IA, et al. Transgenic Expression of A Dominant Negative KATP Channel Subunit in the Mouse Endothelium: Effects on Coronary Flow and Endothelin-1 Secretion. FASEB J. 2007;21:2162–2172. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830. Suppl-830. [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, et al. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Mohan RM, Paterson DJ. Activation of sulphonylurea-sensitive channels and the NO-cGMP pathway decreases the heart rate response to sympathetic nerve stimulation. Cardiovasc Res. 2000;47:81–89. doi: 10.1016/s0008-6363(00)00057-2. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Vacher H, Trimmer JS. The surprising catch of a voltage-gated potassium channel in a neuronal SNARE. Sci STKE. 2007;2007:e37. doi: 10.1126/stke.3932007pe37. [DOI] [PubMed] [Google Scholar]
- Morrissey A, Parachuru L, Leung M, et al. Expression of K(ATP) channel subunits during perinatal maturation in the mouse heart. Pediatr Res. 2005a;58:185–192. doi: 10.1203/01.PDR.0000169967.83576.CB. [DOI] [PubMed] [Google Scholar]
- Morrissey A, Rosner E, Lanning J, et al. Immunolocalization of K(ATP) channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiology. 2005b;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni JA, Lascano EC, del Valle HF. Glibenclamide action on myocardial function and arrhythmia incidence in the healthy and diabetic heart. Cardiovasc Hematol Agents Med Chem. 2007;5:43–53. doi: 10.2174/187152507779315868. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Notsu T, Ohhashi K, Tanaka I, et al. 5-Hydroxydecanoate Inhibits ATP-Sensitive K+ Channel Currents in Guinea-Pig Single Ventricular Myocytes. Eur J Pharmacol. 1992;220:35–41. doi: 10.1016/0014-2999(92)90008-r. [DOI] [PubMed] [Google Scholar]
- Oe K, Sperlagh B, Santha E, et al. Modulation of norepinephrine release by ATP-dependent K(+)-channel activators and inhibitors in guinea-pig and human isolated right atrium. Cardiovasc Res. 1999;43:125–134. doi: 10.1016/s0008-6363(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Poitry S, van Bever L, Coppex F, Roatti A, Baertschi AJ. Differential sensitivity of atrial and ventricular K(ATP) channels to metabolic inhibition. Cardiovasc Res. 2003;57:468–476. doi: 10.1016/s0008-6363(02)00715-0. [DOI] [PubMed] [Google Scholar]
- Pountney DJ, Sun ZQ, Porter LM, et al. Is the molecular composition of K(ATP) channels more complex than originally thought? Biochemical and electrophysiological evidence for heteromultimeric assembly of the KATP channel subunits Kir6.1 and Kir6.2. J Mol Cell Cardiol. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Kloner RA. Low-dose i.v. acetylcholine acts as a “preconditioning-mimetic” in the canine model. J Card Surg. 1995;10:389–395. doi: 10.1111/j.1540-8191.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Pu JL, Ye B, Kroboth SL, et al. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive K(ATP) activity. J Mol Cell Cardiol. 2008;44:188–200. doi: 10.1016/j.yjmcc.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saegusa N, Sato T, Saito T, et al. Kir6.2-deficient mice are susceptible to stimulated ANP secretion: K(ATP) channel acts as a negative feedback mechanism? Cardiovasc Res. 2005;67:60–68. doi: 10.1016/j.cardiores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Schnitzler M, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol. 2000a;525(Pt 2):307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. 307-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler MM, Derst C, Daut J, Preisig-Muller R. ATP-sensitive potassium channels in capillaries isolated from guinea-pig heart. J Physiol (Lond) 2000b;525:307–317. doi: 10.1111/j.1469-7793.2000.t01-1-00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004;47:564–574. doi: 10.1353/pbm.2004.0071. [DOI] [PubMed] [Google Scholar]
- Schworer H, Kilbinger H. Effects of cromakalim on acetylcholine release and smooth muscle contraction in guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:706–708. doi: 10.1007/BF00168666. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–440. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tsymbalyuk O, Ivanov A, et al. Endothelial sulfonylurea receptor 1-regulated NC Ca-ATP channels mediate progressive hemorrhagic necrosis following spinal cord injury. J Clin Invest. 2007;117:2105–2113. doi: 10.1172/JCI32041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Cao K, Yang G, et al. Selective expression of Kir6.1 protein in different vascular and non-vascular tissues. Biochem Pharmacol. 2004;67:147–156. doi: 10.1016/j.bcp.2003.08.041. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Saito T, Sato T, et al. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Porter LM, Liu G, et al. Consequences of Cardiac Myocyte-Specific Ablation of KATP channels in Transgenic Mice expressing Dominant Negative Kir6 Subunits. Am J Physiol Heart Circ Physiol. 2006;291:H543–H551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- Von Beckerath N, Cyrys S, Dischner A, Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Jiao JH, Pence RA, Baertschi AJ. ATP-sensitive potassium channels regulate stimulated ANF secretion in isolated rat heart. Am J Physiol Heart Circ Physiol. 1996;271:H2339–H2345. doi: 10.1152/ajpheart.1996.271.6.H2339. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. Antisense oligodeoxynucleotides of sulfonylurea receptors inhibit ATP-sensitive K+ channels in cultured neonatal rat ventricular cells. Pflugers Arch. 1999;437:400–408. doi: 10.1007/s004240050794. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Feig J, Ghiu IA, Artman M, Coetzee WA. K(ATP) channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol. 2004;37:857–869. doi: 10.1016/j.yjmcc.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini S, Ben-Ari Y, Ashford ML. Characterization of sulfonylurea receptors and the action of potassium channel openers on cholinergic neurotransmission in guinea pig isolated small intestine. J Pharmacol Exp Ther. 1991;259:566–573. [PubMed] [Google Scholar]


