Abstract
Introduction
Metabolic acidosis is the most frequent acid–base disorder in the intensive care unit. The optimal analysis of the underlying mechanisms is unknown.
Aim
To compare the conventional approach with the physicochemical approach in quantifying complicated metabolic acidosis in patients in the intensive care unit
Patients and methods
We included 50 consecutive patients with a metabolic acidosis (standard base excess ≤ -5). We measured sodium, potassium, calcium, magnesium, chloride, lactate, creatinine, urea, phosphate, albumin, pH, and arterial carbon dioxide and oxygen tensions in every patient. We then calculated HCO3-, the base excess, the anion gap, the albumin-corrected anion gap, the apparent strong ion difference, the effective strong ion difference and the strong ion gap.
Results
Most patients had multiple underlying mechanisms explaining the metabolic acidosis. Unmeasured strong anions were present in 98%, hyperchloremia was present in 80% and elevated lactate levels were present in 62% of patients. Calculation of the anion gap was not useful for the detection of hyperlactatemia. There was an excellent relation between the strong ion gap and the albumin-corrected and lactate-corrected anion gap (r2 = 0.934), with a bias of 1.86 and a precision of 0.96.
Conclusion
Multiple underlying mechanisms are present in most intensive care unit patients with a metabolic acidosis. These mechanisms are reliably determined by measuring the lactate-corrected and albumin-corrected anion gap. Calculation of the more time-consuming strong ion gap according to Stewart is therefore unnecessary.
Keywords: acid–base disorder, hyperchloremia, metabolic acidosis, strong ion difference, strong ion gap
Introduction
Metabolic acidosis is one of the most frequent acid–base disorders occurring in the intensive care unit (ICU) [1]. It may contribute to the morbidity and mortality associated with shock, although it may also have some protective effects.
Traditional approaches are often inadequate to explain the complexity of acid–base derangements in critically ill patients. The physicochemical approach described by Stewart is based on two major principles: electroneutrality and conservation of mass [2,3]. According to this theory, there are three variables that independently determine the hydrogen ion concentration. These variables are the strong ion difference, the total concentration of nonvolatile weak acid (primarily serum proteins and phosphate), and the carbon dioxide tension (pCO2) [4,5]. Although the Stewart approach may give a better understanding of the mechanisms that underlie an acid–base disorder, it is more time consuming than conventional methods and is therefore less convenient in daily practice [6].
The purpose of the present study was to compare two different methods of quantifying metabolic acidosis in patients admitted to an ICU. We were especially interested in whether acid–base analysis according to the physicochemical approach could result in important changes in diagnosis, and therefore in therapy. We hypothesised that a less time-consuming method such as the lactate-corrected and albumin-corrected anion gap would be as efficient as the calculations according to the physicochemical approach in identifying the major causes of metabolic acidosis: hyperchloremia, hyperlac-tatemia and the presence of other unmeasured strong anions.
Methods
The study was conducted in a single, mixed medical and surgical ICU of the Jeroen Bosch Hospital, 's-Hertogenbosch, The Netherlands from August 2001 until February 2002. The local medical ethical committee waived informed consent.
We studied 50 consecutive patients who were either admitted with a metabolic acidosis or who developed a metabolic acidosis during their stay in the ICU. Metabolic acidosis was defined as a standard base excess (SBE) ≤ -5. In all patients we measured pH, arterial oxygen tension, arterial carbon dioxide tension (PaCO2), sodium, potassium, chloride, magnesium, calcium, lactate, creatinine, urea, phosphate and albumin in a single arterial blood sample. Bicarbonate was calculated using the Henderson–Hasselbach equation (pH = 6.1 + log ([HCO3-]/0.0301 PaCO2) and the SBE using the Siggaard-Andersen formulae. The urine was screened for the presence of ketones in every patient.
The anion gap (AG) was calculated with the formula AG= [Na+] + [K+] - [Cl-] - [HCO3-]. The anion gap corrected for albumin and lactate (AGcorr) was calculated with the formula AGcorr =AG + 0.25 (40-[albumin])-lactate [7]. The apparent strong ion difference (SIDapp) was calculated using the formula SIDapp = [Na+] + [K+] + [Ca2+] + [Mg2+] - [Cl-] - [lactate-]. The effective strong ion difference (SIDeff) was calculated using the formula SIDeff = 12.2 × pCO2 / (10-pH) + 10 × [albumin] × (0.123 × pH - 0.631) + [PO4-] × (0.309 × pH - 0.469). The strong ion gap (SIG) was calculated by subtracting the effective strong ion difference from the apparent strong ion difference: SIG = SIDapp - SIDeff.
The serum reference range for a normal AG in our laboratory is 4–12 mmol/l (Aeroset 2002; Abbott, Hoofddorf, the Netherlands). AG > 12 mmol/l was considered elevated. SIG > 0 points to the presence of unmeasured strong anions and was considered abnormal [8]. Fluid resuscitation was performed with isotonic 0.9% NaCl or short acting starch products (chloride concentration, 154 mmol/l). Polygeline colloidal fluids were not used because they not only increase serum chloride levels, but probably also increase the SIG [9]. Acute Physiology and Chronic Health Evaluation II data were collected for each patient for the first 24 hours after admission. A decrease in renal function was defined as a creatinine concentration > 110 μmol/l for males and > 100 μmol/l for females. All patients were followed up to determine the 28-day survival.
Results are reported as the mean ± standard deviation or the median (25th percentile, 75th percentile) depending on the distribution of the data. We performed linear regression analysis to compare the SIG with the AGcorr. We calculated the bias (the mean difference between the two methods) after subtracting 12 from the AGcorr and the precision (the standard deviation of the bias). The limits of agreement were defined by ± 2 standard deviations [10].
Results
Fifty patients were enrolled in the study. Patient characteristics are presented in Table 1, and acid–base and electrolyte data for the study population are presented in Table 2. Twenty-nine patients had evidence of a decreased renal function. Urine samples were positive for ketones in six patients. Hyperchloremia (serum chloride ≥ 110 mmol/l) was present in 40 patients (80%), and hyperlactatemia (serum lactate ≥ 2 mmol/l) was present in 31 patients (62%). The contributions of the three main causes of metabolic acidosis (hyperchloremia, hyperlactatemia and increased levels of other unmeasured strong anions) are presented in Table 3. Of the 29 patients with renal failure, 14 had elevated lactate levels, 20 had hyperchloremia and all 29 had an elevated SIG.
Table 1.
Patient characteristics
| Age (years) | 65 (26–89) |
| Sex (male/female) | 26/24 |
| Acute Physiology and Chronic Health Evaluation II | 22 (9–43) |
| Mechanical ventilation (%) | 92 |
| Standardised mortality ratio | 0.90 |
| Hospital mortality (%) | 38 |
| Diagnosis | |
| Septic shock | 22 |
| Hypovolemic shock | 15 |
| Cardiogenic shock | 9 |
| Other | 4 |
Table 2.
Acid–base and electrolyte data
| pH | 7.295 (7.213, 7.33) |
| Arterial carbon dioxide tension (mmHg) | 36.5 (29.25, 42) |
| Standard base excess | -8.5 (-11, -7) |
| Sodium (mmol/l) | 138 (135, 141) |
| Potassium (mmol/l) | 4 (3.52, 4.37) |
| Chloride (mmol/l) | 114 (110, 117) |
| Lactate (mmol/l) | 2.25 (1.42, 3.07) |
| Albumin (g/l) | 16 (13, 19) |
| Strong ion gap (mEq/l) | 3.61 (1.99, 6.07) |
Data presented as median (interquartile range).
Table 3.
Distribution of the three main underlying mechanisms of metabolic acidosis
| Underlying mechanism | Number of patients (%) |
| Increased strong ion gap | 49 (98) |
| Increased lactate | 31 (62) |
| Increased chloride | 40 (80) |
| Increased strong ion gap + lactate | 31 (62) |
| Increased strong ion gap + chloride | 39 (78) |
| Increased lactate + chloride | 25 (50) |
| Increased strong ion gap + lactate + chloride | 25 (50) |
Calculation of the uncorrected AG was not useful for the detection of hyperlactatemia: sensitivity, 45%; specificity, 16%; positive predictive value, 47%; negative predictive value, 15%. Calculation of the albumin AGcorr increased the sensitivity to 100%, but the specificity decreased to 11%. The positive and negative predictive values were 65% and 100%, respectively. The mean SIDapp was 27.8 ± 4.3 mEq/l (normal, 38–42 mEq/l). In all but one patient the SIG was increased (median, 3.61 mEq [1.99, 6.07]). There was a weak but significant correlation between the lactate levels and the SIG (r2 = 0.149, P = 0.005). There was a very strong correlation between the AGcorr and the SIG (r2 = 0.934, P < 0.001; Fig. 1). The bias was 1.86 and the precision was 0.96. The limits of agreement were therefore -0.06 and 3.78 (Fig. 2)
Figure 1.
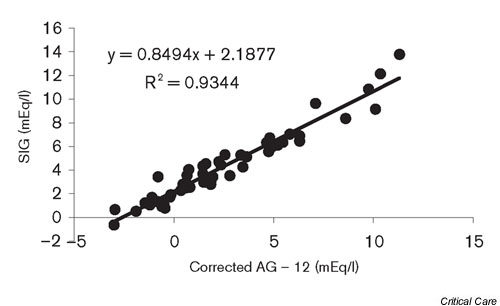
Correlation between the albumin-corrected anion gap (AG) minus lactate and the strong ion gap (SIG).
Figure 2.
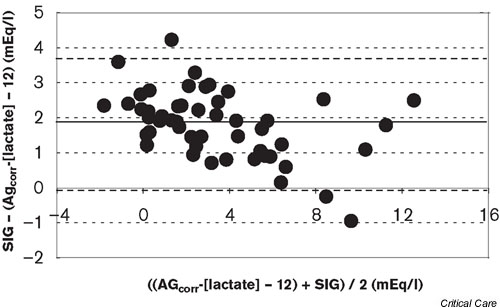
Bland-Altman analysis of the albumin-corrected anion gap minus lactate (AGcorr) and the strong ion gap (SIG) (bias, 1.81 and precision, 0.96).
Discussion
The main finding of the present study was the excellent relationship between the AGcorr and the SIG in patients with a metabolic acidosis admitted to the ICU (r2 = 0.934). Furthermore, unmeasured strong anions excluding lactate were almost universally present in this unselected patient group, as was hyperchloremia.
A positive SIG indicating the presence of unmeasured strong anions was reliably detected by the AGcorr. Durward and colleagues studied 540 children, of whom 240 developed a metabolic acidosis [6]. In their study, unmeasured strong anions were also the main component of tissue acids. In accordance with the present study, the AGcorr had the best discriminatory ability (area under curve, 0.95) and the tightest determination coefficient for the detection of tissue acids (r2 = 0.86). Durward and colleagues also found a weak but significant inverse correlation between the total amount of tissue acids and the chloride:sodium ratio. A chloride:sodium ratio > 0.79 was able to exclude a raised tissue acid level with a positive predictive value of 81% and a likelihood ratio of 4.5. The upper normal range for the chloride:sodium ratio in our hospital is 0.79. Thirty-eight (76%) patients had a chloride:sodium ratio > 0.79 in our study. This is in agreement with our definition of hyperchloremia using an absolute level of 110 mmol/l (80% hyperchloremia). We also found a significant negative correlation between the amount of unmeasured strong anions and the chloride:sodium ratio (r2 = 0.54, P < 0.001).
The unmeasured strong anions involved in the SIG remain largely unidentified. These anions appear, for example, in the circulation during sepsis and liver failure, and may be a variety of organic and inorganic compounds [8]. The use of urea-linked polygelines, for example, as the priming fluid for the extracorporeal circuit during cardiac surgery has also been shown to increase the SIG [9]. They represent approximately 5.6 mEq anions per 500 ml fluid. Also, the (over)use of several medications such as salicylates and penicillin can be a cause of a positive SIG. The importance of a raised SIG in clinical practice, however, is unknown. Cusack and colleagues recently showed that the pH and SBE were better outcome predictors than the SIG in a group of mixed medical and surgical ICU patients [11]. Furthermore, normal levels for the SIG in critically ill patients are unknown. We defined SIG > 0 as abnormal but these data were based on measurements in healthy volunteers [8]. Cusack and colleagues found a much higher SIG in critically ill patients but they provide no separate data for the patients with a normal SBE. If we assume that normal AG ≤ 12 mEq/l, the intercept in Figure 1 suggests that the normal SIG in our critically ill patients is close to 2 mEq/l.
A significant part of the acidosis in the present patients is probably related to the resuscitation with isotonic saline and starch products. This can be deduced from the frequent occurrence of hyperchloremia in our patients in relation to the plasma sodium concentration. Both have a chloride concentration of 154 mmol/l. This results in a reduction of the strong ion difference, which in turn produces an increase in the number of hydrogen ions to preserve electrical neutrality. The term 'dilutional acidosis' used in relation to high volume resuscitation should therefore be abandoned. Hyperchloremic acidosis after fluid resuscitation is a well-known phenomenon in the ICU [12-14]. The clinical consequences, however, are unknown.
There is no proof to date that the use of a more balanced resuscitation fluid will result in a better patient outcome. Kellum showed that a balanced resuscitation fluid (Hextend®, Abbott, Chicago, IL, USA); chloride concentration, 124 mmol/l) resulted in a better short-term survival in a rat sepsis model compared with isotonic saline [15]. Waters and colleagues compared isotonic saline with lactated Ringer's solution in patients undergoing abdominal aortic aneurysm repair [16]. Patients in the normal saline group developed a more severe acidosis and received a larger volume of platelet transfusion. However, there were no differences in the duration of mechanical ventilation, the ICU stay, the hospital stay and the incidence of complications. Furthermore, the use of Ringer's lactate has been associated with postoperative hypercapnic acidosis and hyponatremia [17]. Therefore, the importance of resuscitation-induced hyperchloremic acidosis remains to be determined.
Hyperlactatemia was the third cause of metabolic acidosis in the present study. Considering the high number of patients with sepsis, this is not surprising. The importance of hyperlac-tatemia as a marker of shock and its prognostic significance are well known. We demonstrated that a normal AG does not exclude the presence of hyperlactatemia (sensitivity, 45%; negative predictive value, 15%). Although the sensitivity of the AGcorr for the detection of hyperlactatemia increased to 100%, it was not specific (11%). Therefore, determination of the (corrected) AG is not a good substitute for the direct measurement of lactate in patients with a metabolic acidosis in the ICU. As expected, there was a weak but significant correlation between lactate levels and the SIG. This weak correlation was especially pronounced in patients with normal or slightly elevated lactate levels.
Several weaknesses of the present study should be mentioned. We only studied patients with a clear metabolic acidosis (SBE ≤ -5), and the SIG and lactate levels of patients with a normal or marginally normal SBE are therefore unknown. Furthermore, although the patients were included immediately when the SBE became ≤ -5, changes over time may have influence over the type of acidosis detected.
In conclusion, the present study demonstrates that multiple underlying mechanisms are present in most ICU patients with a metabolic acidosis. These mechanisms are reliably determined by measuring the lactate-corrected and albumin-corrected anion gap. Calculation of the more time-consuming strong ion gap according to Stewart, although a gold standard, is therefore unnecessary for clinical purposes. Further studies should focus on the nature and importance of the unmeasured strong anions that are almost universally present in these patients.
Competing interests
None declared.
Key messages
• Metabolic acidosis in intensive care unit patients is usually explained by multiple underlying mechanisms
• Underlying mechanisms of metabolic acidosis are reliably determined by measuring the lactate-corrected and albumin-corrected anion gap. Calculation of the more time-consuming strong ion gap according to Stewart is therefore unnecessary
Abbreviations
AG = anion gap; AGcorr = anion gap corrected for albumin and lactate; ICU = intensive care unit; PaCO2 = arterial carbon dioxide tension; pCO2 =carbon dioxide tension; SBE = standard base excess; SIDapp = apparent strong ion difference; SIDeff = effective strong ion difference; SIG = strong ion gap.
See related Commentary http://ccforum.com/content/7/3/219
References
- Gauthier PM, Szerlip HM. Metabolic acidosis in the intensive care unit. Crit Care Clin. 2002;18:289–308. doi: 10.1016/s0749-0704(01)00012-4. [DOI] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharmacol. 1983;61:1441–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Determinants of blood pH in health and disease. Crit Care. 2000;4:6–14. doi: 10.1186/cc644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes P. Hypoproteinemia, strong-ion difference, and acid–base status in critically ill patients. J Appl Physiol. 1998;84:1740–1748. doi: 10.1152/jappl.1998.84.5.1740. [DOI] [PubMed] [Google Scholar]
- Figge J, Rossing TH, Fencl V. The role of serum proteins in acid–base equilibrium. J Lab Clin Med. 1991;117:453–467. [PubMed] [Google Scholar]
- Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA. The value of the chloride:sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001;27:828–835. doi: 10.1007/s001340100915. [DOI] [PubMed] [Google Scholar]
- Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26:1807–1810. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Strong ion gap: a methodology for exploring unexplained anions. J Crit Care. 1995;10:51–55. doi: 10.1016/0883-9441(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Hayhoe M, Bellomo R, Liu G, McNicol L, Buxton B. The aetiology and pathogenesis of cardiopulmonary bypass-associated metabolic acidosis using polygeline pump prime. Intensive Care Med. 1999;25:680–685. doi: 10.1007/s001340050930. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;i:307–310. [PubMed] [Google Scholar]
- Cusack RJ, Rhodes A, Lochhead P, Jordan B, Perry S, Ball JAS, Grounds RM, Bennett ED. The strong ion gap does not have prognostic value in critically ill patients in a mixed medical/surgical adult ICU. Intensive Care Med. 2002;28:864–869. doi: 10.1007/s00134-002-1318-2. [DOI] [PubMed] [Google Scholar]
- Prough DS, Terry White R. Acidosis associated with peri-operative saline administration. Dilution or delusion? Anesthesiology. 2000;93:1167–1169. doi: 10.1097/00000542-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Liskaser FJ, Bellomo R, Hayhoe M, Story D, Poustie S, Smith B, Letis A, Bennet M. Role of pump prime in the aetiology and pathogenesis of cardiopulmonary bypass-associated acidosis. Anesthesiology. 2000;93:1170–1173. doi: 10.1097/00000542-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Rehm M, Orth V, Scheingraber S, Kreimeier U, Brechtelsbauer H, Finsterer U. Acid–base changes caused by 5% albumin versus 6% hydroxyethyl starch solution in patients undergoing acute normovolemic hemodilution. Anesthesiology. 2000;93:1174–1183. doi: 10.1097/00000542-200011000-00007. [DOI] [PubMed] [Google Scholar]
- Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid–base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- Waters JH, Gotlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer's solution for intra-operative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg. 2001;93:817–822. doi: 10.1097/00000539-200110000-00004. [DOI] [PubMed] [Google Scholar]
- Takil A, Eit Z, Irmak P, Yilmaz GF. Early postoperative respiratory acidosis after large intravascular infusion of lactated ringer's solution during major spine surgery. Anesth Analg. 2002;95:294–298. doi: 10.1097/00000539-200208000-00006. [DOI] [PubMed] [Google Scholar]


