Abstract
Background
In a prospective observational study, we examined the temporal relationships between serum erythropoietin (EPO) levels, haemoglobin concentration and the inflammatory response in critically ill patients with and without acute renal failure (ARF).
Patients and method
Twnety-five critically ill patients, from general and cardiac intensive care units (ICUs) in a university hospital, were studied. Eight had ARF and 17 had normal or mildly impaired renal function. The comparator group included 82 nonhospitalized patients with normal renal function and varying haemoglobin concentrations. In the patients, levels of haemoglobin, serum EPO, C-reactive protein, IL-1β, IL-6, serum iron, ferritin, vitamin B12 and folate were measured, and Coombs test was performed from ICU admission until discharge or death. Concurrent EPO and haemoglobin levels were measured in the comparator group.
Results
EPO levels were initially high in patients with ARF, falling to normal or low levels by day 3. Thereafter, almost all ICU patients demonstrated normal or low EPO levels despite progressive anaemia. IL-6 exhibited a similar initial pattern, but levels remained elevated during the chronic phase of critical illness. IL-1β was undetectable. Critically ill patients could not be distinguished from nonhospitalized anaemic patients on the basis of EPO levels.
Conclusion
EPO levels are markedly elevated in the initial phase of critical illness with ARF. In the chronic phase of critical illness, EPO levels are the same for patients with and those without ARF, and cannot be distinguished from noncritically ill patients with varying haemoglobin concentrations. Exogenous EPO therapy is unlikely to be effective in the first few days of critical illness.
Keywords: acute renal failure, anemia, erythropoietin, haemoglobin, intensive care
Introduction
Progressive anaemia is common in critical illness and often requires treatment with repeated blood transfusions, which are costly and not without risk. The anaemia is usually multifactorial; causes include repeated venesection for diagnostic tests, nutritional depletion of haemopoietic factors, haemolysis, blood loss from the gastrointestinal tract or extracorporeal circuits, or depression of haemopoiesis related to the inflammatory response, referred to as the anaemia of chronic disease. In this latter category, a relative deficiency in erythropoietin (EPO) or resistance to the action of EPO has been identified in several studies [1-3]. Because recombinant human EPO is widely used as replacement therapy to treat the anaemia of chronic renal failure, and in pharmacological doses as a substitute for blood transfusion in Jehovah's witnesses [4,5], it is now being investigated as a treatment for the anaemia of critical illness [6,7]. EPO therapy has been shown to result in a reduction in blood transfusion requirements in one such study [6], although the cost-efficacy of this approach is not certain.
EPO is an essential growth factor for erythropoiesis; the stimulus for induction of EPO gene expression is a reduction in blood oxygen availability [8] from hypoxaemia or anaemia. EPO is produced mainly by renal interstitial fibroblasts and to a lesser extent by the liver [8]. Suppression of EPO production or effect may be mediated by the inflammatory response. In animal models IL-1, IL-6 and tumour necrosis factor can all suppress erythropoiesis, and IL-1 and TNF can inhibit EPO production [9-11], the effects being reversed by exogenous EPO [12].
In chronically anaemic humans, there is an inverse log/linear relationship between serum EPO levels and haemoglobin concentration [13]. In patients with acute renal failure (ARF), EPO production may be impaired from loss of EPO-producing renal interstitial fibroblasts [14,15]. In critically ill children without renal insufficiency a blunted EPO response to acute anaemia and acute hypoxaemia was seen, when compared with similar stimuli in noncritically ill patients [16]. Most studies in critically ill adults focus on the longer stay patients and report an impaired EPO response to anaemia, based on comparison either with normal reference ranges [2] or with a control group of patients with nonrenal anaemia [1,3,15]. In contrast, one study of 10 patients with sepsis or septic shock in the first 4 days following admission demonstrated marked increases in EPO levels in those patients who subsequently died, paralleling changes in the acute phase response [17]. If exogenous EPO therapy is to have a cost-effective role in the treatment of the anaemia of critical illness, then these patients should be excluded. We therefore chose to examine temporal changes in EPO concentrations during both the acute and chronic phases of critical illness, in patients with and without ARF. We also attempted to relate these findings to haemoglobin concentrations and markers of the inflammatory response.
Patients and method
Design and setting
This was a prospective observational study. The study received local ethics committee approval. Informed assent was obtained from relatives. Patients were recruited from the general and cardiac intensive care units (ICUs) of the university hospital.
Participants
Thirty patients were recruited to the study. The admission criteria were as follows: age 16 years or more; acute failure of at least one organ system based on the definitions of Knaus and coworkers [18]; at least 12 hours since ICU admission; the expectation of ICU care for at least 3 more days; and the presence of an indwelling arterial cannula for blood sampling. The exclusion criteria included pre-existing chronic renal failure, clinically evident active blood loss or coagulopathy, a primary haematological condition actually or potentially leading to anaemia, treatment with cytotoxic drugs or immunosuppressants, a past history of total gastrectomy or over 50% small bowel resection, or an history of alcoholism. Demographic data, clinical diagnosis, and subsequent progress and complications of the patients were recorded. Severity of illness was recorded using Acute Physiology and Chronic Health Evaluation (APACHE) II scoring based on worst values during the first 24 hours.
Study patients were subsequently classified as having either ARF (group A) or normal/transiently impaired renal function (group B), on the basis of their serum creatinine and urine output during the study period. ARF was defined using Knaus's criteria of a serum creatinine in excess of 300 μmol/l (upper limit of normal 130 μmol/l) and a urine output of less than 0.5 ml/kg per hour. Patients with an elevated serum creatinine on admission to the study were included in the normal renal function group if the creatinine concentration reverted to normal (<130 μmol/l) within 48 hours in the presence of a urine output of more than 1 ml/kg per hour.
To ensure adequate iron intake, all patients received 200 mg ferrous sulphate (or equivalent) daily. Other than this, the study was observational and did not affect patient management. Blood transfusions were given on the instruction of ICU medical staff according to the policy in place at the time, which was to transfuse if the patient was evidently bleeding or if the haemoglobin concentration was below 9 g/dl. All patients who were not being fed enterally received stress ulcer prophylaxis with nasogastric sucralfate, or intravenous ranitidine where this was not possible.
In order to obtain EPO values for anaemia unrelated to renal failure, we also analyzed outpatient laboratory samples from 82 nonuraemic patients with varying haemoglobin levels, excluding those with rheumatoid arthritis, sickle cell anaemia and solid tumours. These are referred to in the text as the comparator group.
Measurements
Daily blood samples were taken for serum EPO, electrolytes, creatinine, urea, C-reactive protein, full blood count and arterial blood gases. At recruitment and then three times per week, samples were taken for reticulocyte count, serum iron, ferritin and transferrin, IL-1β and IL-6. At recruitment and then once a week, serum vitamin B12, and folate and red cell folate were measured, and a Coombs test was performed. Sampling was continued until death or discharge from the ICU. While in the ICU, daily blood loss from sampling and other sources (including arterial line dead space) was also measured and the frequency of blood transfusions was recorded. All concurrent drug therapy was documented.
Blood samples for cytokine assays were spun within 2 hours and plasma stored at -70°C, and samples for EPO were stored at -20°C for later analysis. Serum EPO values were measured by chemiluminescence immunometric assay (Nichols Institute Diagnostics, Heston, Middlesex, UK) and values obtained ranged from under 5 to 772 mIU/ml. The normal upper limit of EPO for a heamoglobin above 10 g/dl is 35 mIU/ml. Serum IL-1β and IL-6 were measured using enzyme-linked immunosorbent assay ('Quantikine', R & D Systems, Minneapolis, USA). The assay coefficient of variation for IL-6 and IL-1β was under 10%.
Patient characteristics in the two groups were compared using the Kruskal–Wallis, χ2, Mann–Whitney U and Fisher exact tests as appropriate.
Results
Patient characteristics
Thirty patients were recruited. Five were withdrawn because of early (<3 days) death or discharge from the ICU. Of the remaining 25 patients, eight had ARF (group A) and 17 had normal or mildly impaired renal function (group B). The demographic and clinical characteristics of the two groups are shown in Tables 1 and 2.
Table 1.
Demographic data
| Group A (ARF) | Group B (non-ARF) | P | |
| Number of patients | 8 | 17 | - |
| Age (years; median [range]) | 69 (64–77) | 65 (30–86) | NS |
| Sex (male:female) | 6:2 | 12:5 | NS |
| ICU stay (days; median [range]) | 9 (5–22) | 6 (3–30) | NS |
| Admission APACHE II (median [range]) | 22.5 (12–33) | 15 (6–26) | 0.01 |
| ICU mortality (n [%]) | 5 (63) | 3 (18) | 0.035 |
| Hospital mortality (n [%]) | 5 (63) | 5 (29) | NS |
| Patients transfused (n [%]) | 6 (75) | 5 (29) | 0.043 |
| Total venesection (ml/day) | 59 ± 5 | 57 ± 9 | NS |
| Study venesection (ml/day) | 14 ± 1 | 15 ± 2 | NS |
| CAVHD filter blood loss (ml/day; n = 4) | 67 ± 53 | - | - |
| Admission serum creatinine(μmol/l; median [range]) | 366 (78–836) | 98 (54–230) < 0.001 |
Values are expressed as mean ± SD unless indicated otherwise. APACHE, Acute Physiology and Chronic Health Evaluation; ARF, acute renal failure; CAVHD, continuous arteriovenous haemodiafiltration; ICU, intensive care unit.
Table 2.
Primary reason for intensive care unit admission
| Group A (ARF; n) | Group B (non-ARF; n) | |
| Post-cardiac surgery organ failure | 4 | 5 |
| Major surgery | 0 | 2 |
| Acute respiratory failure | 2 | 5 |
| Severe sepsis | 1 | 1 |
| Post-cardiac arrest | 1 | 3 |
| Guillain–Barré syndrome | 0 | 1 |
ARF, acute renal failure.
Of the patients in group A, four received renal replacement therapy with continuous arteriovenous haemodiafiltration; one received peritoneal dialysis and three required supportive treatment only. The median (range) serum creatinine concentrations on admission to the study are given in Table 1. Serum creatinine concentrations exceeded 300 μmol/l during the study period in all group A patients, and rapidly fell to or remained within the normal range in the group B patients. Patients in group A had a significantly higher APACHE II score at recruitment, a higher ICU mortality rate and a greater incidence of blood transfusion than did those in group B.
Changes in haemoglobin, erythropoietin and interleukin-6
Figures 1, 2 and 3 summarize the changes over time in EPO, haemoglobin and IL-6 concentrations, respectively, during the ICU stay for both groups of patients.
Figure 1.
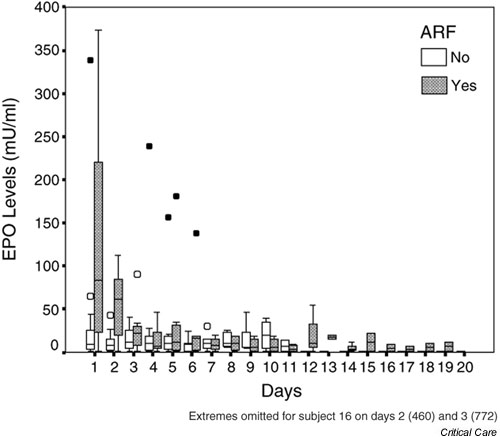
Box and whisker plot of erythropoietin (EPO) concentrations against time for all patients. Hollow circles indicate outliers (cases with values of the variable between 1.5 and 3 times the length of the corresponding box for that day and group); filled circles indicate extreme values (cases with values greater than 3 times the corresponding box for that day and group). ARF, acute renal failure.
Figure 2.
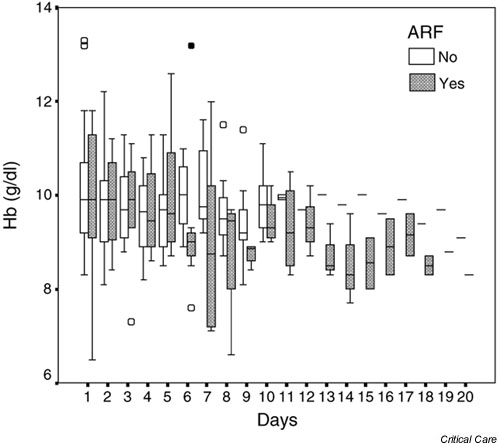
Box and whisker plot of haemoglobin concentrations against time for all patients. Hollow circles indicate outliers (cases with values of the variable between 1.5 and 3 times the length of the corresponding box for that day and group); filled circles indicate extreme values (cases with values greater than 3 times the corresponding box for that day and group). ARF, acute renal failure.
Figure 3.
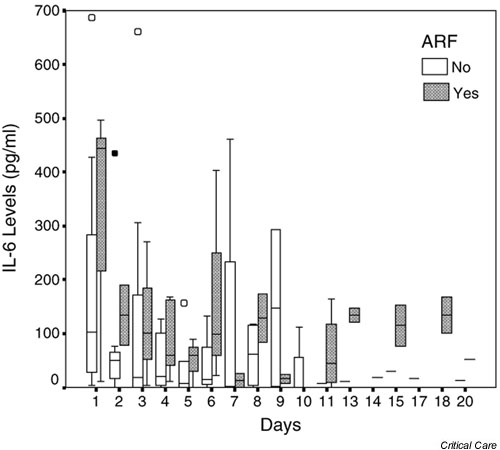
Box and whisker plot of IL-6 concentrations against time for all patients. Hollow circles indicate outliers (cases with values of the variable between 1.5 and 3 times the length of the corresponding box for that day and group); filled circles indicate extreme values (cases with values greater than 3 times the corresponding box for that day and group). ARF, acute renal failure.
A summary of the distribution of EPO levels during the study is presented in Fig. 1. First day values were higher in the patients with ARF (median value 83.5 mIU/ml) than in those without (median value 9 mIU/ml; Mann–Whitney U = 36, P = 0.061). EPO levels declined rapidly in the ARF patients, and by ICU day 3 the two groups were indistinguishable. EPO values remained low in most patients throughout their ICU stay. Haemoglobin concentrations fell to between 8 and 11 g/dl in most patients (Fig. 2). In the 23 patients who developed moderate or severe anaemia (haemoglobin <10 g/dl), the reticulocyte count exceeded 100 × 109/l in only nine out of 78 samples.
Markers of the inflammatory response
IL-6 concentrations ranged from 0 to 686 pg/ml (Fig. 3; normal values in health <12.5 pg/ml). Levels were high initially in both groups of patients, presumably reflecting disease activity, but gradually decreased over time. Higher levels were seen throughout the study, but particularly at recruitment, in group A (ARF) than in group B. Concentrations of C-reactive protein were elevated in all patients on recruitment, and remained elevated throughout the study in all except two patients, both with preserved renal function. IL-1β could not be detected in the serum of any of the patients.
Haematological variables
Indices of red cell volume and haemoglobin content were normal in all patients at recruitment. Serum vitamin B12 concentrations were normal or slightly high in all patients throughout the study. Serum and red cell folate concentrations were normal throughout the study, except for two patients with a slightly low serum folate at recruitment; in both patients this variable had normalized by the end of the first week. Serum iron levels were low on recruitment in all except one patient, ranging from below 1.0 to 12.6 μmol/l (normal range 10–32 μmol/l [males] and 5–30 μmol/l [females]). Serum transferrin levels were also low on recruitment in all but three patients, ranging from 0.57 to 2.46 g/l (normal range 2.0–3.6 g/l). Serum ferritin concentrations were more variable (37–2376 μg/l) but were above the normal range (18–300 μg/l) in 16 (64%) patients and were markedly elevated (>1000 μg/l) in three patients. This pattern is typical of acute illness, not iron deficiency [7,19,20]. Seven patients had a positive Coombs test result; one of these patients had received intravenous immunoglobulin for Guillain-Barré syndrome, three had received penicillin-type drugs, and in the remaining three no obvious cause was found.
Blood transfusion
No attempt was made to influence ICU transfusion practice, which at the time of this study was to give blood to physiologically stable patients if the haemoglobin concentration was less than 9 g/dl. The requirement for blood transfusion was greater in those patients with ARF (Table 3). Significant external bleeding was not seen in any patient during the study period, with the exception of one patient in group A. This patient developed bleeding from an aortoduodenal fistula 20 days after recruitment and received a large transfusion over the following 2 days before she died; this accounted for the highest transfusion requirement over the study period of 28 units (Table 3).
Table 3.
Blood transfusion
| Group | n | Patients transfused (n [%]) | Number of units of blood transfused (median [range]) |
| A (ARF) | 8 | 6 (75) | 5 (2–28) |
| B (Non-ARF) | 17 | 5 (29) | 2 (2–3) |
ARF, acute renal failure.
Partial arterial oxygen tension
Arterial blood gases were measured frequently in all patients while in the ICU. The mean daily arterial partial arterial oxygen tension ranged from 12–17 kPa.
Comparison with the nonhospitalized patients
Figure 4 shows single paired results of EPO and haemoglobin for the 82 nonhospitalized ambulant patients in the comparator group. Haemoglobin concentrations ranged from 5.0 to 15.0 g/dl. As expected, there was a negative log-linear correlation between the two variables, represented by the line of best fit on the graph. The figure also shows median paired values of EPO and haemoglobin for each of the ICU patients. The ICU patients are not distinguishable from the noncritically ill patients on the basis of EPO levels, indicating that there is no obvious failure of EPO production in this group. The range of haemoglobin concentrations is narrower in the ICU patients, influenced by disease and by blood transfusion. EPO was below the lower limit of detection in six (24%) of the critically ill patients and 27 (32.9%) of the comparator group patients.
Figure 4.
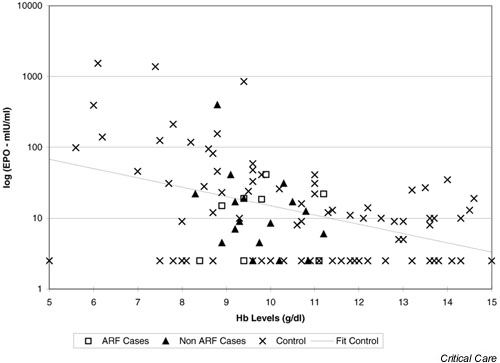
Analysis of median levels of erythropoietin (EPO) and haemoglobin (Hb) for cases (using all data from each patient), compared with comparator group subjects (single data values). (Comparator group subjects are referred to as 'controls'.) ARF, acute renal failure.
Discussion
We found that EPO levels are high in the first 48 hours of critical illness in patients with ARF, suggesting an acute renal response to injury. These patients also had the highest APACHE II scores and mortality rates. EPO levels then decline over time, together with a reduction in haemoglobin concentrations, and by day 3 in the ICU the EPO levels for almost all patients are in the low normal range. This indicates failure of EPO effect rather than failure of production as the mechanism for the anaemia of critical illness.
The optimal haemoglobin level for critically ill patients is not known. The study conducted by Hebert and coworkers [21] showed that transfusion thresholds can safely be set at a haemoglobin level of 7–9 g/dl. The clinical requirement for blood transfusion in critically ill patients inevitably obscures relationships between EPO and haemoglobin levels in this population, which makes it difficult to demonstrate inhibition of EPO-induced erythropoiesis at more extreme degrees of anaemia with certainty.
The raised concentrations of IL-6 and C-reactive protein reflect the acute inflammatory response. Abel and coworkers [17] studied serum levels of EPO, IL-1 and IL-6 in patients with sepsis and septic shock. They found that both EPO and IL-6 levels increased in a manner resembling the acute phase response, but they only studied patients for up to 4 days following ICU admission. If pharmacological doses of EPO are to be used to prevent anaemia in critically ill patients, then treatment should be deferred until the third ICU day, at least in the more severely ill patients.
Most of our patients presented with a low serum iron, high ferritin and low transferrin. This pattern is typical of the anaemia of chronic disease, not iron deficiency, which is characterized by a low ferritin. These changes occur rapidly in acute as well as chronic illness [7,19,20], as part of the systemic inflammatory response. The high C-reactive protein and IL-6 levels combined with the low transferrin and high ferritin support the view that the anaemia was not a consequence of iron deficiency. None of the patients suffered from clinically evident bleeding. However, diagnostic venesection or filter changes in patients undergoing continuous arteriovenous haemodiafiltration accounted for significant iatrogenic blood loss (Table 1).
Conclusion
In summary, we found high initial levels of EPO in critically ill patients with ARF. Levels of EPO decline rapidly, and during the chronic phase of critical illness levels are indistinguishable from those in ambulant patients with nonrenal anaemia, suggesting a failure of EPO effect rather than production. These findings support the use of pharmacological doses of EPO in the chronic, but not the acute, phase of critical illness.
Competing interests
The costs of the assays were covered by a grant from Janssen-Cilag, the manufacturers of Epoetin Alpha. None of the authors has any financial interest in this company or its products.
Key messages
• In patients with ARF, serum EPO concentrations are raised during the first 48 hours of critical illness
• After this stage, EPO concentrations for patients with and without ARF are in the low normal range
• If exogenous EPO therapy is to be used in critical illness, then it is more likely to be effective in the chronic rather than the acute phase
Abbreviations
APACHE = Acute Physiology and Chronic Health Evaluation; ARF = acute renal failure; EPO = erythropoietin; ICU = intensive care unit; IL = interleukin.
Acknowledgments
Acknowledgements
We are grateful for the assistance of our ICU nursing staff in the conduct of the study, and for financial assistance from Janssen-Cilag, the manufacturers of Epoetin Alpha.
References
- Rogiers P, Zhang H, Leeman M, Nagler J, Neels H, Melot C, Vincent J-L. Erythropoietic response is blunted in critically ill patients. Intensive Care Med. 1997;23:159–162. doi: 10.1007/s001340050310. [DOI] [PubMed] [Google Scholar]
- Von Ahsen N, Muller C, Serke S, Frei U, Eckardt K-U. Important role of nondiagnostic blood loss and blunted erythropoietic response in the anaemia of medical intensive care patients. Crit Care Med. 1999;27:2630–2639. doi: 10.1097/00003246-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Hobisch-Hagen P, Wiedermann F, Mayr A, Fries D, Jelkmann W, Fuchs D, Hasibeder W, Mutz N, Klingler A, Schobersberger W. Blunted erythropoietic response to anemia in multiply traumatized patients. Crit Care Med. 2001;29:743–737. doi: 10.1097/00003246-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Kraus P, Lipman J. Erythropoietin in a patient following multiple trauma. Anaesthesia. 1992;47:962–964. doi: 10.1111/j.1365-2044.1992.tb03199.x. [DOI] [PubMed] [Google Scholar]
- Koestner JA, Nelson LD, Morris JA, Safcsak K. Use of recombinant human erythropoietin (r-HuEPO) in a Jehovah's witness refusing transfusion of blood products. J Trauma. 1990;30:1406–1408. doi: 10.1097/00005373-199011000-00016. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Gettinger A, Rodriguez RM, Pearl RG, Gubler D, Enny C, Colton T, Corwin MJ. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350. doi: 10.1097/00003246-199911000-00004. [DOI] [PubMed] [Google Scholar]
- van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28:2773–2778. doi: 10.1097/00003246-200008000-00015. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992;72:449–489. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- Jelkmann WE, Fandrey J, Frede S, Pagel H. Inhibition of erythropoietin production by cytokines. Implications for the anaemia involved in inflammatory states. Ann N Y Acad Sci. 1994;718:300–311. [PubMed] [Google Scholar]
- Johnson CS, Keckler DJ, Topper MI, Braunschweiger PG, Furmanski P. In vivo haematopoietic effects of recombinant interleukin-1-alpha in mice: stimulation of granulocytic, monocytic, megakaryocytic and early erythroid progenitors, suppression of late-stage erythropoiesis, and reversal of erythroid suppression with erythropoietin. Blood. 1989;73:678–683. [PubMed] [Google Scholar]
- Pojda Z, Aoki Y, Sobiczewska E, Machaj E, Tsuboi A. In vivo administration of interleukin-6 delays haematopoietic regeneration in sublethally irradiated mice. Exp Haematol. 1992;20:862–867. [PubMed] [Google Scholar]
- Johnson CS, Cook CA, Furmanski P. In vivo suppression of erythropoiesis by tumour necrosis factor-alpha (TNF-alpha): reversal with exogenous erythropoietin (EPO) Exp Haematol. 1990;8:109–113. [PubMed] [Google Scholar]
- Erslev AJ. Drug therapy: erythropoietin. N Engl J Med. 1991;324:1339–1344. doi: 10.1056/NEJM199105093241907. [DOI] [PubMed] [Google Scholar]
- Nielsen OJ, Thaysen JH. Erythropoietin deficiency in acute tubular necrosis. J Intern Med. 1990;227:373–380. doi: 10.1111/j.1365-2796.1990.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Lipkin GW, Kendall RG, Russon LJ, Turney JH, Norfolk DR, Brownjohn AM. Erythropoietin deficiency in acute renal failure. Nephrol Dial Transplant. 1990;5:920–922. doi: 10.1093/ndt/5.11.920. [DOI] [PubMed] [Google Scholar]
- Krafte-Jacobs B, Levetown ML, Bray GL, Ruttimann UE, Pollack MM. Erythropoietin response to critical illness. Crit Care Med. 1994;22:821–826. doi: 10.1097/00003246-199405000-00018. [DOI] [PubMed] [Google Scholar]
- Abel J, Spannbrucker N, Fandrey J, Jelkmann W. Serum erythropoietin levels in patients with sepsis and septic shock. Eur J Haematol. 1996;57:359–363. doi: 10.1111/j.1600-0609.1996.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Prognosis in acute organ system failure. Ann Surg. 1985;202:685–692. doi: 10.1097/00000658-198512000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DA. Anaemia of chronic disease. Med Clin North Am. 1992;76:567–579. doi: 10.1016/s0025-7125(16)30340-6. [DOI] [PubMed] [Google Scholar]
- Corwin HL, Krantz SB. Anaemia of the critically ill: 'acute' anaemia of chronic disease. Crit Care Med. 2000;28:3098–3099. doi: 10.1097/00003246-200008000-00079. [DOI] [PubMed] [Google Scholar]
- Hebert P, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]


