Abstract
Exercise induced rhabdomyolysis is a complication of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) and mitochondrial trifunctional protein (TFP) deficiency that frequently leads to exercise avoidance. Dietary therapy for most subjects includes medium-chain triglyceride (MCT) supplementation but analysis of diet records indicates that the majority of patients consume oral MCT only with breakfast and at bedtime. We hypothesized that MCT immediately prior to exercise would provide an alternative fuel source during that bout of exercise and improve exercise tolerance in children with LCHAD deficiency. Nine subjects completed two 45 min moderate intensity (60–70% predicted maximum heart rate (HR)) treadmill exercise tests. Subjects were given 4 oz of orange juice alone or orange juice and 0.5 g MCT per kg lean body mass, 20 min prior to exercise in a randomized cross-over design. ECG and respiratory gas exchange including respiratory quotient (RQ) were monitored. Blood levels of acylcarnitines, creatine kinase, lactate, and β-hydroxybutyrate were measured prior to and immediately after exercise, and again following 20min rest. Creatine kinase and lactate levels did not change with exercise. There was no significant difference in RQ between the two exercise tests but there was a decrease in steady-state HR following MCT supplementation. Cumulative long-chain 3-hydroxyacylcarnitines were 30% lower and β-hydroxybutyrate was three-fold higher after the MCT-pretreated exercise test compared to the test with orange juice alone. Coordinating MCT supplementation with periods of increased activity may improve the metabolic control of children with LCHAD and TFP deficiency following exercise.
Keywords: Long-chain 3-hydroxyacyl-coA dehydrogenase, Long-chain 3-hydroxyacyl-coA dehydrogenase deficiency, Trifunctional protein, Trifunctional protein deficiency, Fatty-acid oxidation, Exercise, Rhabdomyolysis, Medium-chain triglyceride, β-Oxidation
Introduction
Rhabdomyolysis is a frequent complication of long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) and trifunctional protein (TFP) deficiency, the etiology of which is incompletely understood. Possible contributing factors to rhabdomyolysis include an overall energy deficit in the muscle or the effects of toxic by-products of partial fatty acid oxidation, such as hydroxyacylcarnitines or their free hydroxy fatty acids. Skeletal muscle preferentially oxidizes fatty acids for energy and may have a limited ability to rely completely on glucose for energy production during periods of stress [1].
Animal studies have demonstrated that oral medium-chain triglyceride (MCT) is rapidly absorbed into the circulatory system (<20min) and preferentially oxidized by muscle [2,3]. The majority of MCT is not stored in the body but is rapidly oxidized within hours of its consumption [2].
Children with LCHAD or TFP deficiency are frequently supplemented with MCT to provide an energy source that is not dependent on long-chain fatty acid (LCFA) oxidation. For toddlers and infants this is most often accomplished using an MCT containing infant formula. In older children and adolescents, MCT is used in cooking or taken in liquid form as a daily supplement. Upon review of the dietary intake patterns of a cohort of LCHAD and TFP deficient subjects, we observed that most subjects consumed their MCT supplement with breakfast, at dinner, and/or at bedtime. We also noted that episodes of rhabdomyolysis most frequently occurred in the mid-afternoon with periods of activity. The goal of this study was to test the hypothesis that MCT given immediately prior to exercise would improve exercise tolerance and decrease the risk of rhabdomyolysis.
Materials and methods
Subjects
Subjects were recruited from a previous study for enrollment in this project [4]. The inclusion criteria were a confirmed diagnosis of LCHAD or TFP deficiency and seven years of age or older. Nine subjects were enrolled in a randomized cross-over study of the ability to tolerate exercise with and without MCT supplementation. Six had published mutation analyses [5–8]. The subjects ranged in age from 7 to 14 yr (Table 1). All subjects were fed a controlled diet consisting of 10% of total energy from LCFA and 11% of total energy from MCT with varying amounts of carbohydrate and protein. The Institutional Review Board at OHSU approved the study protocol, and each subject’s legal guardian gave written informed consent. Subjects gave assent to participate.
Table 1.
RQ, and ventilation of subjects following exercise
| Sub | Age (yr) | Gender | Mutations | First testt | With MCT | No MCT | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean RQ | Steady-state VO2 | Mean RQ | Steady-state VO2 | ||||||
| 1 [5] | 7.4 | F | c.1528G > C/c.274_278del | LCHAD def. | MCT | NA | NA | NA | NA |
| 2 [5] | 8.4 | F | c.1528G > C/c.274-278del | LCHAD def. | No MCT | 1.05 | 7.5 | 0.87 | 11.6 |
| 3* | 8.7 | F | c.901G> A β-subunit/? | TFP def. | MCT | 0.97 | 18.2 | 0.86 | 15.2 |
| 4 | 10.3 | M | c.1528G > C/c.1528G > C | LCHAD def. | No MCT | 1.06 | 13.2 | 1.01 | 23.4 |
| 5 | 12.6 | M | c.1528G > C/? | LCHAD def. | MCT | 1.01 | 24.9 | 1.05 | 26.2 |
| 6 [7] | 12.7 | F | c.1528G > C/c.1528G > C | LCHAD def. | No MCT | 0.99 | 14.2 | 1.07 | 13.5 |
| 7 [6] | 13.6 | M | c.1528G > C/c.1678C > T | TFP def. | MCT | 0.93 | 14.4 | 1.01 | 15.2 |
| 8 [5] | 14.1 | F | c.1528G > C/c.479–482TAGC > AATA | LCHAD def. | No MCT | 0.89 | 19.4 | 0.94 | 16.8 |
| 9* [8] | 14.4 | F | c.901G> A β-subunit/? | TFP def. | MCT | 0.93 | 16.3 | 0.99 | 17.1 |
| Mean | 0.95 | 17.8 | 1.01 | 17.8 | |||||
Data is given for exercise tests performed following 4 oz of orange juice (No MCT) and following 4 oz orange juice and 0.5 gm/kg lean mass MCT (with MCT). Mutations are given as the change in the cDNA (c.) of the α-subunit of the trifunctional protein unless and otherwise noted. References indicate previous published mutations and
indicates siblings. Mean RQ = mean RQ during last 5 min of exercise; steady-state VO2 = median VO2 (ml/min/kg) during last 5 min of exercise.
MCT supplementation
Each subject’s lean body mass was determined by DEXA scan five days prior to the first exercise test. The morning of exercise testing, subjects were given a breakfast with a known macronutrient content that contained 11% of total energy from MCT. They rested in the Clinical Research Center and were allowed to drink only water for 3 h following breakfast. 20min prior to exercise, they were given 4 oz of orange juice and 0.5 g MCT/kg lean body mass or orange juice alone. The second exercise test was performed seven days after the first test. The order in which exercise tolerance was tested relative to MCT consumption was randomly assigned.
Exercise protocol
Exercise testing was on a treadmill with monitoring of expired gases using a metabolic cart as an independent measure of effort (Sensormedics Corp. model 29n, Yorba Lunda, CA). An intravenous (IV) catheter was placed to allow peripheral access for repeated blood draws. Resting respiratory rate, ECG, blood pressure (BP) and temperature were measured prior to exercise testing.
The exercise protocol began with a 3 min warm-up at 1.7 mph and 0% grade. Speed and grade were increased every 2 min until the subject’s heart rate (HR) was 60–70% of predicted HR maximum (HRmax, 220–age) [9–11]. This exercise protocol is designed to keep subjects below anaerobic threshold and to maximize the consumption of lipid as fuel for exercising muscle. HR and ECG were recorded for the complete duration of the test. Subjects achieved their target HR range within the first 10 min and continued at the same intensity for an additional 30 min. Expired gases (VO2 and VCO2) were collected during 1–5, 18–22, and 35–40 min of the exercise test. BP was measured at the end of each 5 min collection period. Total exercise time was 45 min. Following exercise HR, ECG, and BP were monitored until values returned to resting base-line.
Blood samples
A blood sample was obtained 10min after ingestion of the supplement but prior to the onset of exercise (time 0). Blood was sampled after exercise, and again after 20 min of rest. Blood specimens were analyzed for plasma acylcarnitines, lactate, creatine kinase, β-hydroxybutyrate, glucose, and insulin.
Analyses of plasma acylcarnitines were completed by the Biochemical Genetics Laboratory at the Mayo Clinic, Rochester, MN. Acylcarnitines were quantified by tandem mass spectroscopy and expressed as µmol/L [12]. The sum of the long-chain hydroxylated metabolites was calculated for each sample. Metabolites included in the sum were the following carnitine esters: C14:0–OH, C14:1–OH, C16:0–OH, C16:1–OH, C18:0–OH, C18:1–OH, and C18:2–OH. Serum β-hydroxybutyrate levels were determined by stable isotope dilution GC–MS (Laboratory of James Shoemaker, MD, Ph.D, Saint Louis University, St. Louis, MO).
Data analysis
Differences between the exercise tests with and without MCT were analyzed with a mixed model in which the fixed effects included with and without MCT, order in which tests occurred and diet consumed. Change in measured parameters was expressed as area under the curve (AUC) and was calculated with the trapezoidal method. AUC for blood parameters was considered a good representation of the effect of exercise and was used as the dependent variable in the model. P ≤ 0.05 was considered statistical significant and analysis was performed with the PROC MIXED procedure of SAS (SAS Institute Inc, Cary, North Carolina). Results are presented as the mean ± standard deviation.
Results
All subjects completed the two treadmill tests with no adverse events or post-exercise muscle pain. One subject (1) was not able to tolerate the mouth-piece so respiratory gases were not measured. There was a problem with IV access for two subjects (two and four), and some data points were not collected. For all subjects, plasma lactate levels were within the normal range prior to and following exercise, and there was no significant change in lactate with the exercise test (data not shown). Previous studies in boys also observed no rise in plasma lactate with submaximal exercise [13]. Plasma CK levels were significantly elevated prior to the exercise test when the subjects were given orange juice only in two subjects (seven and eight; 9436 and 20,152 mmol/L, respectively). One subject complained of mild muscle aches and lethargy the day prior to the exercise test but not on the day of the test; the other subject was asymptomatic. The mean (±standard deviation) pre-exercise CK levels in the other subjects were 140 ± 93 mmol/L prior to the exercise test pretreated with orange juice alone and 281 ± 263 mmol/L in all subjects prior to the exercise test pretreated with orange juice and MCT. The results of the plasma CK prior to exercise were not known by the investigators until after the exercise test was complete. However, the exercise test did not result in a significant rise in plasma CK levels, even in subjects with an elevated CK prior to the beginning of the test. 20 min following exercise CK concentrations were 11,000 and 22,000 in subjects seven and eight, respectively and were 159 ± 102 in the other seven subjects following the exercise test pretreated with orange juice alone. Mean CK concentrations were 288 ± 260 in all subjects following the exercise test pretreated with orange juice and MCT.
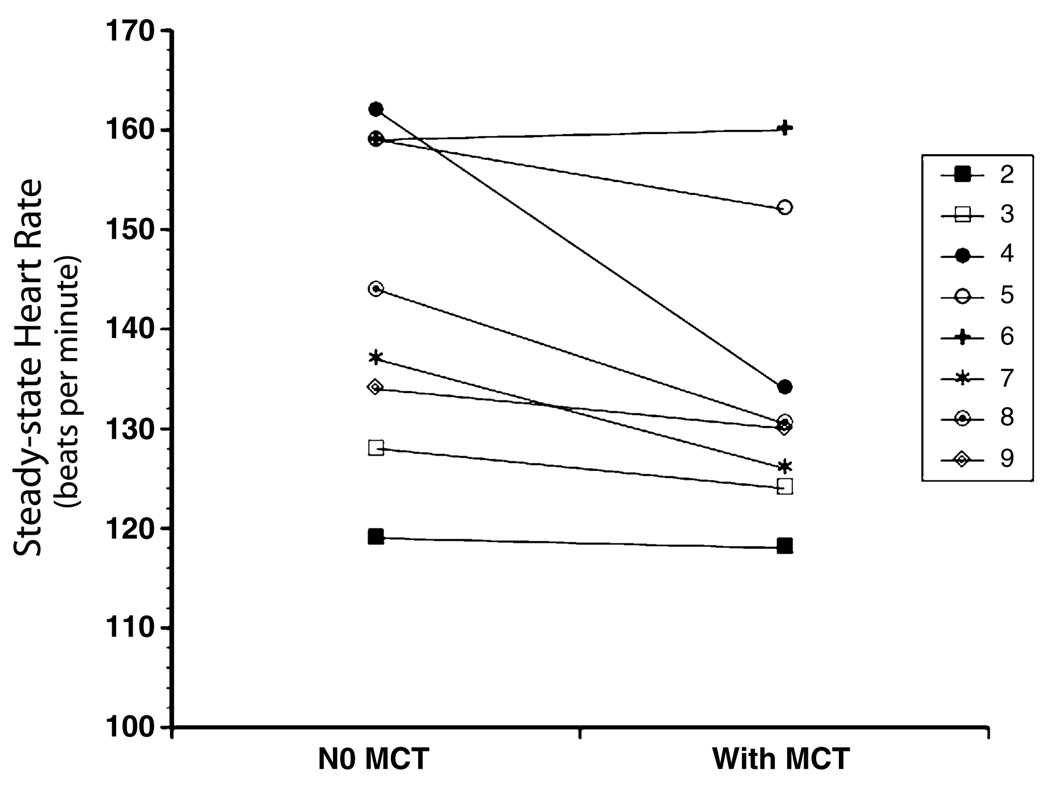
There was no difference in the work performed (kilojoules, KJ) between the two exercise tests (data not shown). Mean RQ during 35–40min of all the exercise tests was 0.96 suggesting carbohydrate was the primary energy substrate. There was no difference in RQ between the two exercise tests with and without MCT pretreatment (Table 1). Importantly, steady-state HR was lower during the exercise test pretreated with MCT (Fig. 1). The largest decrease in HR with MCT supplementation was observed in one subject (subject four) who also displayed significantly lower minute ventilation (VO2). Steady-state VO2is an important driver of HR; and may be the reason for the lower HR observed for this subject. However, the mean steady state VO2 measured of all subjects did not differ between the two exercise tests whether this subject was included or excluded from the data analysis, and the decrease in HR observed among the all other subjects following MCT supplementation remained significantly different even when the data was analyzed without subject four. This suggests that MCT pretreatment was associated with a lower steady-state HR for the same VO2 and amount of work performed.
Fig. 1.
Change in steady-state HR between two moderate intensity exercise tests in children with LCHAD or TFP deficiency. Mean HR during 35–40min of the exercise test pretreated with MCT (124 ± 14) was significantly lower than the mean HR of the exercise test pretreated with orange juice alone (132 ± 16) at P ≤ 0.05.
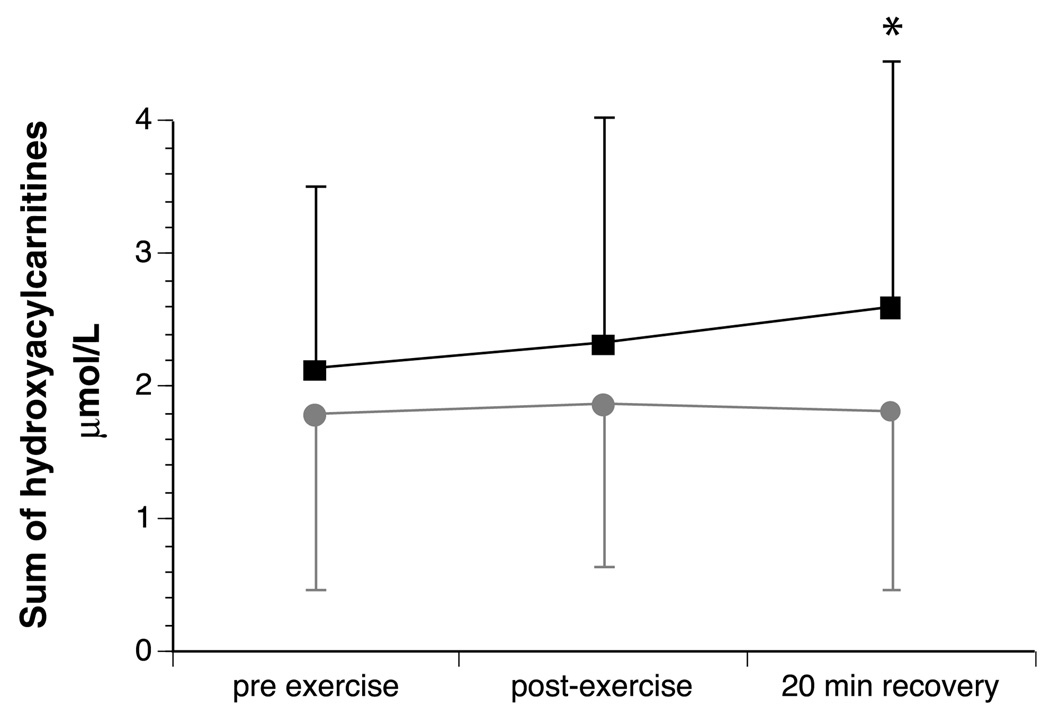
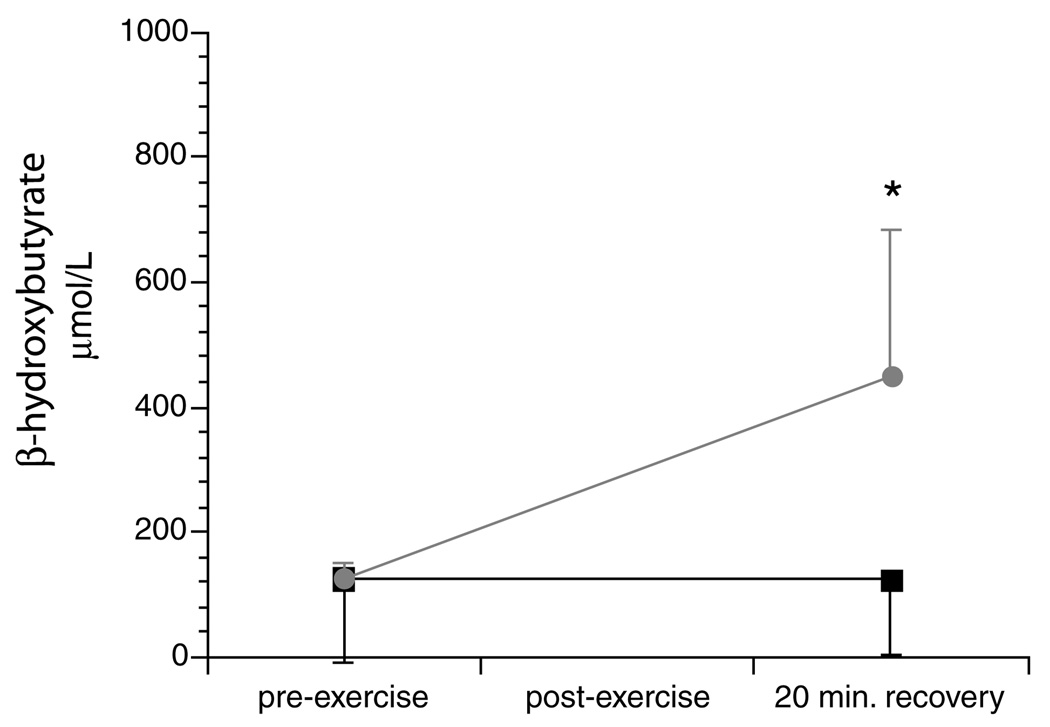
The sum of long-chain plasma hydroxyacylcarnitines measured before exercise was similar between the two tests (Table 2). However, hydroxyacylcarnitines were significantly lower 20min after exercise when subjects were given MCT compared to the exercise test with orange juice alone (Fig. 2). There was a significant reduction in total area under the curve for hydroxyacylcarnitine concentrations following the exercise test pretreated with MCT. Plasma β-hydroxybutyrate measured before exercise was similar between the two exercise tests. There was a dramatic rise in plasma β-hydroxybutyrate 20min following the exercise test pretreated with MCT; β-hydroxybutyrate did not increase following orange juice alone (Fig. 3). The increase in plasma ketones suggested ongoing oxidation of MCT oil during exercise.
Table 2.
Plasma β-hydroxybutyrate (β-Hba) and sum of the long-chain hydroxyacylcarnitine of subjects following exercise
| Sub | Age (yr) | Gender | No MCT | With MCT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Hba (µmol/L) | Hydroxyacylcarnitines (µmol/L) | β-Hba (µmol/L) | Hydroxyacylcarnitines (µmol/L) | |||||||||
| Pre | Recovery | Pre | Post | Recovery | Pre | Recovery | Pre | Post | Recovery | |||
| 1 | 7.5 | F | 5.01 | 4.99 | 5.09 | 4.43 | 3.95 | 4.14 | ||||
| 2 | 8.4 | F | 1.36 | 1.55 | 2.27 | 2.08 | 2.80 | |||||
| 3 | 8.7 | F | 37.5 | 29.2 | 0.20 | 0.20 | 0.22 | 104 | 127 | 0.23 | 0.25 | 0.25 |
| 4 | 10.3 | M | 1.95 | 2.15 | 2.15 | 1.44 | 1.71 | 1.78 | ||||
| 5 | 12.6 | M | 2.91 | 3.89 | 4.54 | 2.24 | 2.18 | 1.98 | ||||
| 6 | 12.7 | F | 360 | 300 | 3.01 | 3.46 | 3.20 | 150 | 600 | 1.89 | 2.00 | 1.88 |
| 7 | 13.6 | M | 119 | 192 | 2.22 | 2.19 | 2.66 | 150 | 602 | 2.73 | 2.75 | 3.23 |
| 8 | 14.1 | F | 73.5 | 25.6 | 2.26 | 2.18 | 2.90 | 95.2 | 268 | 0.85 | 0.85 | 0.93 |
| 9 | 14.4 | F | 37 | 71 | 0.27 | 0.35 | 0.38 | 130 | 650 | 0.23 | 0.33 | 0.35 |
| Mean | 125.4 | 123.56 | 2.13 | 2.33 | 2.60 | 125.84 | 449.4 | 1.79 | 1.87 | 1.82 | ||
Data is given for exercise tests performed following 4oz of orange juice (No MCT) and following 4 oz orange juice and 0.5 gm/kg lean mass MCT (with MCT).
Fig. 2.
Mean (±standard deviation) plasma sum of long-chain hydroxyacylcarnitines prior to, and following moderate intensity exercise and after 20 min of recovery in nine children with LCHAD or TFP deficiency. ■represent levels during the exercise test pretreated with orange juice alone.  represent levels during the exercise test pretreated with orange juice and MCT. There was no significant difference between tests pre- or post-exercise. There was significantly lower plasma hydroxyacylcarnitines after 20 min of recovery when subjects were pretreated with orange juice and MCT. * indicates a signifiant difference between the two tests at P < 0.05.
represent levels during the exercise test pretreated with orange juice and MCT. There was no significant difference between tests pre- or post-exercise. There was significantly lower plasma hydroxyacylcarnitines after 20 min of recovery when subjects were pretreated with orange juice and MCT. * indicates a signifiant difference between the two tests at P < 0.05.
Fig. 3.
Mean (+standard deviation) plasma β-hydroxybutyrate prior to moderate intensity exercise and after 20 min of recovery in nine children with LCHAD or TFP deficiency.■ represent levels during the exercise test pretreated with orange juice alone.  represent levels during the exercise test pretreated with orange juice and MCT. * indicates a significant difference between the two tests at P ≤ 0.05.
represent levels during the exercise test pretreated with orange juice and MCT. * indicates a significant difference between the two tests at P ≤ 0.05.
Discussion
Rhabdomyolysis is one of the most frequent complications of LCHAD and TFP deficiency. Because of muscle pain with exertion, many patients with LCHAD or TFP deficiency are sedentary and avoid exercise. We found that MCT ingested immediately prior to exercise could be utilized as an energy source, potentially by muscle directly or in support of liver ketogenesis, and their availability significantly altered the pattern of post-exercise metabolites.
The significant decrease in post-exercise hydroxylated long chain acylcarnitine levels with MCT-pretreatment suggests that MCT supplementation decreased the overall LCFA oxidation in favor of other energy substrates such as MCT or glucose. MCT is rapidly absorbed via the portal vein and taken up by hepatocytes, where they may be oxidized in support of ketogenesis [14]. The post-exercise rise in plasma β-hydroxybutyrate we witnessed in children with TFP deficiency, who do not readily produce ketone bodies, supports the hypothesis that MCT was used as energy substrate by the liver. However, we have previously demonstrated that C8 and C10 fatty acids are present in plasma of MCT-supplemented subjects with LCHAD or TFP deficiency suggesting that these fatty acids may be available to exercising muscle for oxidation [15]. It is also possible that ketone bodies produced in the liver following MCT supplementation were then utilized by exercising muscle. Wherever the direct site of MCT utilization, we propose that MCT supplementation provided an additional energy substrate and suppressed LCFA oxidation during exercise in children with LCHAD or TFP deficiency.
MCT supplementation has previously been shown to decrease hydroxylated long chain acylcarnitine production from LCHAD or TFP-deficient cultured skin fibroblasts and to lower plasma hydroxyacylcarnitines at rest and during acute metabolic crises in children with LCHAD or TFP deficiency [15–17]. The exact relationship between elevated plasma hydroxyacylcarnitine levels and the incidence of rhabdomyolysis in this patient population has not been well defined. Elevated hydroxyacylcarnitines are observed during metabolic decompensation but whether they increase prior or during a crisis has not been determined. Prospective studies correlating cumulative plasma hydroxyacylcarnitine levels with the incidence of rhabdomyolysis are needed to determine this relationship. Our hypothesis is that suppressing LCFA oxidation as indicated by lower plasma levels of hydroxylated long chain acylcarnitines will be associated with fewer rhabdomyolysis episodes. Future studies examining the incidence of rhabdomyolysis in subjects who take MCT prior to exercise compared to those who take MCT with breakfast or dinner are needed to test this hypothesis.
Plasma hydroxyacylcarnitines do appear to be negatively correlated with the progression of chorioretinopathy of LCHAD and TFP deficiency [4]. Lowering post-exercise hydroxyacylcarnitine levels by coordinating MCT supplementation with periods of activity may help to prevent the progression of chorioretinopathy in children with LCHAD or TFP deficiency regardless of the effects on rhabdomyolysis.
It is interesting to note that plasma CK levels did not correlate with plasma hydroxyacylcarnitine levels. Subjects with elevated CK levels during exercise did not have correspondingly elevated hydroxyacylcarnitines. Similarly, subjects with the highest hydroxyacylcarnitine levels had normal CK levels. The cause of increased CK levels in subjects with LCHAD or TFP deficiency is unknown. It may be related to muscle cell damage from energy depletion associated with decreased food intake or increased energy expenditure. However, the rise in plasma CK levels may happen well after the period of energy depletion. Theoretically, a rise in plasma hydroxyacylcarnitines should be observed during a period of energy depletion from increases in cellular fatty acid oxidation. Changes in plasma acylcarnitines may occur early and resolve quickly compared to CK levels and, therefore, may be a better indicator of overall energy status.
We hypothesized that MCT provided prior to exercise would be fully oxidized during the exercise protocol, allow sparing of carbohydrate, and consequently would lead to a decrease in RQ. Subjects expended approximately 150 kcal during this bout of exercise; if 50% of the energy expended or 75 kcal were derived from MCT with the remainder of energy coming from carbohydrate, then we would predict an RQ of 0.9. However, during the actual trial, we did not observe any significant difference in RQ between the two exercise tests with and without MCT supplementation. This result suggests that the predominant energy source for exercising muscle continued to be carbohydrate despite the observed increase in plasma β-hydroxybutyrate following MCT supplementation. There are several possible reasons for this observation. The subjects did receive about 12.5 g of carbohydrate or 50 kcal in the orange juice prior to both tests; this may have suppressed muscle fatty acid oxidation. It may have been due to the relatively small contribution of oxidized MCT to total energy expenditure or the insensitivity of RQ to measure minor changes in whole body substrate oxidation. Still, our finding that MCT supplementation was consistently associated with a lower average HR but similar oxygen uptakes for a constant amount of work, suggests that MCT supplementation can have a positive effect on energy metabolism during exercise. Future studies are needed to accurately assess the oxidation of oral MCT and its effects on HR during exercise.
The goal of this research is to determine an effective intervention that will allow subjects with LCHAD and TFP deficiency to be active and prevent exercise-induced rhabdomyolysis. Formal exercise testing in children with LCHAD and TFP deficiency has not previously been reported. Here we demonstrate that moderate intensity exercise is safe in this group of patients. It is significant that all subjects completed two 45min moderate intensity treadmill tests with no incidence of hypoglycemia or complaints of muscle pain. Several results in this study suggest coordinating MCT supplementation with periods of activity may improve exercise tolerance in these patients. Subjects pretreated with MCT had lower heart rates during an equal workload of exercise, decreased post-exercise hydroxyacylcarnitines and increased post-exercise ketones. Further long-term studies are needed to determine whether altering MCT intake will decrease the frequency and severity of rhabdomyolysis in these children.
Acknowledgments
We thank Dr. Arnold Strauss (Vanderbilt University, Nashville, TN) for providing the mutation analysis results of subjects not available in the medical record and Dr. Dawn Peters for assistance with the statistical analysis. This research was supported by PHS Grants RR000334 and F32 DK065400.
References
- 1.Jeukendrup AE, Saris WH, Wagenmakers AJ. Fat metabolism during exercise: a review–part II: regulation of metabolism and the effects of training. Int. J. Sports Med. 1998;19:293–302. doi: 10.1055/s-2007-971921. [DOI] [PubMed] [Google Scholar]
- 2.Odle J. New insights into the utilization of medium-chain triglycerides by the neonate: observations from a piglet model. J. Nutr. 1997;127:1061–1067. doi: 10.1093/jn/127.6.1061. [DOI] [PubMed] [Google Scholar]
- 3.Odle J, Benevenga NJ, Crenshaw TD. Utilization of medium-chain triglycerides by neonatal piglets: II. Effects of even- and odd-chain triglyceride consumption over the Wrst 2 days of life on blood metabolites and urinary nitrogen excretion. J. Anim. Sci. 1989;67:3340–3351. doi: 10.2527/jas1989.67123340x. [DOI] [PubMed] [Google Scholar]
- 4.Gillingham MB, Weleber RG, Neuringer M, Connor WE, Mills M, van Calcar S, Ver Hoeve J, Wolff J, Harding CO. EVect of optimal dietary therapy upon visual function in children with long-chain 3-hydroxyacyl CoA dehydrogenase and trifunctional protein deficiency. Mol. Genet. Metab. 2005;86:124–133. doi: 10.1016/j.ymgme.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, Strauss AW. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N. Engl. J. Med. 1999;340:1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- 6.Isaacs JD, Jr, Sims HF, Powell CK, Bennett MJ, Hale DE, Treem WR, Strauss AW. Maternal acute fatty liver of pregnancy associated with fetal trifunctional protein deficiency: molecular characterization of a novel maternal mutant allele. Pediatr. Res. 1996;40:393–398. doi: 10.1203/00006450-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Spiekerkoetter U, Sun B, Khuchua Z, Bennett MJ, Strauss AW. Molecular and phenotypic heterogeneity in mitochondrial trifunctional protein deficiency due to beta-subunit mutations. Hum. Mutat. 2003;21:598–607. doi: 10.1002/humu.10211. [DOI] [PubMed] [Google Scholar]
- 8.Treem WR, Rinaldo P, Hale DE, Stanley CA, Millington DS, Hyams JS, Jackson S, Turnbull DM. Acute fatty liver of pregnancy and long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency. Hepat. 1994;19:339–345. [PubMed] [Google Scholar]
- 9.Anderson SD, Godfrey S. Cardio-respiratory response to treadmill exercise in normal children. Clin. Sci. 1971;40:433–442. doi: 10.1042/cs0400433. [DOI] [PubMed] [Google Scholar]
- 10.Gilliam TB, Sady S, Thorland WG, Weltman AL. Comparison of peak performance measures in children ages 6 to 8, 9 to 10, and 11 to 13 years. Res Q. 1977;48:695–702. [PubMed] [Google Scholar]
- 11.Godfrey S, Davies CT, Wozniak E, Barnes CA. Cardio-respiratory response to exercise in normal children. Clin. Sci. 1971;40:419–431. doi: 10.1042/cs0400419. [DOI] [PubMed] [Google Scholar]
- 12.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8–c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol. Genet. Metab. 2001;73:38–45. doi: 10.1006/mgme.2001.3170. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson BO, Koch G. EVect of physical training on hemodynamic response during submaximal and maximal exercise in 11–13-year old boys. Acta Physiol. Scand. 1973;87:27–39. doi: 10.1111/j.1748-1716.1973.tb05363.x. [DOI] [PubMed] [Google Scholar]
- 14.Odle J, Benevenga NJ, Crenshaw TD. Utilization of medium-chain triglycerides by neonatal piglets: chain length of even- and odd-carbon fatty acids and apparent digestion/absorption and hepatic metabolism. J. Nutr. 1991;121:605–614. doi: 10.1093/jn/121.5.605. [DOI] [PubMed] [Google Scholar]
- 15.Gillingham MB, Connor WE, Matern D, Rinaldo P, Burlingame T, Meeuws K, Harding CO. Optimal dietary therapy of long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 2003;79:114–123. doi: 10.1016/s1096-7192(03)00073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duran M, Wanders RJ, de Jager JP, Dorland L, Bruinvis L, Ketting D, Ijlst L, van Sprang FJ. 3-Hydroxydicarboxylic aciduria due to long-chain 3-hydroxyacyl-coenzyme A dehydrogenase deficiency associated with sudden neonatal death: protective eVect of medium-chain triglyceride treatment. Eur. J. Pediatr. 1991;150:190–195. doi: 10.1007/BF01963564. [DOI] [PubMed] [Google Scholar]
- 17.Shen JJ, Matern D, Millington DS, Hillman S, Feezor MD, Bennett MJ, Qumsiyeh M, Kahler SG, Chen YT, Van Hove JL. Acylcarnitines in fibroblasts of patients with long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and other fatty acid oxidation disorders. J. Inherit. Metab. Dis. 2000;23:27–44. doi: 10.1023/a:1005694712583. [DOI] [PubMed] [Google Scholar]