Abstract
The pedunculopontine nucleus (PPN) is part of the cholinergic arm of the reticular activating system, which is mostly active during waking and REM sleep. GABAergic modulation of this area appears to regulate sleep-wake cycles. Eszopiclone (ESZ), a nonbenzodiazepine hypnotic agent, appears to modulate GABAergic receptors. However, the action site of ESZ in the brain is still unresolved. We tested the hypothesis that ESZ acts by potentiating GABAA receptors on PPN neurons. Whole-cell voltage clamp recordings were performed on PPN neurons in 7-15 day rat brainstem slices, and the potentiating effects of ESZ on the responses to the GABAA receptor agonist isoguvacine (IGV), and on GABAA receptor-mediated inhibitory post-synaptic currents (IPSCs), were determined. In the presence of tetrodotoxin, ESZ (1) increased the amplitude of the outward current induced by IGV, (2) increased its duration, and (3) enhanced the IGV-induced decrease in input resistance (Rin). The GABAA receptor antagonist gabazine (GBZ) blocked these effects. ESZ alone did not induce detectable currents or change Rin at a holding potential of −60 mV, but when held at 0 mV, ESZ induced an outward current in 13/21 PPN cells, an effect blocked by GBZ. ESZ also increased the amplitude (n = 18/21), duration (n = 17/21), and frequency (n = 13/15) of IPSCs. ESZ may potentiate GABAA inhibition in the PPN via pre- and post-synaptic modulation, which may underlie the hypnotic effects of ESZ. The differential effects of ESZ on both pre- and post-synaptic sites may partially explain why it has less significant side effects compared to other hypnotic agents.
Citation:
Ye Y; Garcia E. Potentiating effect of eszopiclone on GABAA receptor-mediated responses in pedunculopontine neurons. SLEEP 2009;32(7):879-887.
Keywords: GABAA receptor, pedunculopontine nucleus, sleep, waking
GABA RECEPTORS ARE INVOLVED IN SLEEP-WAKE PROCESSES, WITH AGENTS ACTING ON GABAA, GABAB, AND EVEN GABAC RECEPTORS EXHIBITING important clinical properties. For example, the GABAB agonist baclofen prevents REM sleep when injected into the PPN, and a role for GABAC receptors in sleep-wake control has been postulated.1–3 Eszopiclone (ESZ; Lunesta) is the active stereoisomer of zopiclone, a nonbenzodiazepine hypnotic belonging to a class of drugs known as cyclopyrrolones. ESZ is a short acting sedative hypnotic used in the treatment of primary insomnia.4–6 The nonbenzodiazepine hypnotics have been developed because of the deleterious side effects of benzodiazepines, including tolerance, dependence, and withdrawal.7 These agents bind to the benzodiazepine-GABA receptor complex at different sites than the benzodiazepines, having affinity for the GABA receptor α1 subunit, like the benzodiazepines, but also binding to the α3 and α5 subunits.8–10 Two of these subunits, α1 and α3, appear to be associated with sleep-wake control systems.11 The hypnotic effects of zopiclone have been described in experimental animals,12–14 including a recent thorough comparison of 2 hypnotics on the power spectra of the EEG in the guinea pig.15 This study showed that ESZ significantly increased NREM sleep, decreased the latency to NREM sleep, and increased the latency to REM sleep. ESZ specifically increased delta band and decreased theta band activity,15 suggesting that sleep intensity was increased compared to another nonbenzodiazepine hypnotic, zolpidem.16
The pedunculopontine nucleus (PPN) is part of the cholinergic arm of the reticular activating system (RAS), and its cells increase activity in relation to waking and REM sleep.17 PPN neurons are modulated by GABA receptors,18 and the PPN contains groups of GABAergic neurons,19 some of them are electrically coupled.20 However, the effects of ESZ on the membrane properties of PPN cells have never been determined. The present study sought to determine the effects of ESZ on the responses of PPN cells to the GABAA receptor agonist isoguvacine (IGV), 21 and on GABAA receptor mediated inhibitory post-synaptic currents (IPSCs). The purpose of these studies was to reveal how this nonbenzodiazepine hypnotic agent may affect the firing of neurons in part of the cholinergic arm of the RAS that are known to modulate sleep-wake cycles.
METHODS
Slice Preparation
Pups aged 7-15 days from adult timed-pregnant Sprague-Dawley rats (280-350g) were anesthetized with ketamine (70 mg/kg, i.m.) until tail pinch and corneal reflexes were absent. They were decapitated and the brain rapidly removed and blocked in cooled oxygenated (95% O2,5% CO2) sucrose artificial cerebrospinal fluid (sucrose-aCSF). The block of tissue was glued onto a stage and 400 μm parasagittal brainstem slices were cut with a Vibratome 1000 plus equipped with a 900R refrigeration system (Vibratome Instruments, Ted Pella, Redding, CA) under cooled oxygenated sucrose-aCSF, and then allowed to equilibrate at room temperature in oxygenated normal aCSF for at least 1 hour before recording. All animal use procedures were approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee and comply with the ethical standards described in the NIH guide. The normal aCSF consisted of (in mM): NaCl 117, KCl 4.7, MgSO4 1.2, CaCl2 2.5, NaH2PO4 2.8, NaHCO3 24.9, and glucose 11.5. The sucrose-aCSF was composed of (in mM): sucrose 233.7, NaHCO3 26, MgCl2 8, CaCl2 0.5, glucose 20, and ascorbic acid 0.4. Slices were recorded at 30°C while superfused (∼1.5mL/min) with oxygenated aCSF.
Whole-Cell Patch Clamp Recordings
Whole-cell patch clamp recordings were acquired using borosilicate glass pipettes (with filament) with resistance of 8–12 MΩ, which were pulled on a Sutter P-87 puller (Sutter Instruments, Novato, CA). For the experiments to determine the direct post-synaptic responses, the membrane potential was held at −60 mV, and pipettes were filled with a solution containing (in mM): K-gluconate 124, phosphocreatine di tris salt 10, HEPES 10, EGTA 0.2, Mg2ATP 3, Na2GTP 0.3, 0.02% Lucifer yellow, and 0.5% neurobiotin. Osmolarity was adjusted to ∼ 270–290 mOsm and pH to 7.4. To determine the intrinsic membrane properties of cells, a series of depolarizing and hyperpolarizing steps were applied at resting membrane potential (RMP) in current-clamp mode (−100 pA to 60 pA, 20 pA increment, 500 ms) and at −60 mV in voltage-clamp mode (−110 mV to 20 mV, 15 mV increment, 500 ms). Every 20 s, the membrane potential was held at a hyperpolarized level (holding potential [HP] = −100 mV) for 500 ms to determine the input resistance (Rin) change, then a 1000 ms voltage ramp from −100 mV to −30 mV was applied to test the current-voltage relationship of the activated currents. To identify inhibitory post-synaptic currents (IPSCs, including spontaneous IPSCs, miniature IPSCs and evoked IPSCs), the membrane potential was held at 0 mV and pipettes were filled with a solution consisting of (in mM): CsMeSO3 125, HEPES 10, phosphocreatine di-tris 10, EGTA 0.2, Mg2ATP 4, Na2GTP 0.3, K BAPTA 10, QX314 10, and 0.5% neurobiotin. pH was adjusted to 7.4 and osmolarity to ∼270–290 mOsm. Neurons were visualized using an upright microscope (Nikon FN1 with 40X water immersion objective, 1-2X magnifying turret, and Gibraltar platform) equipped for epifluorescence and near-infrared differential interference contrast optics. Bath-applied drugs were administered to the slice via a peristaltic pump (Masterflex, Cole-Parmer, Vernon Hills, IL) and a 3-way valve system.
Electrical Stimulation
For evoked IPSCs experiments, electrical stimulation was applied by a bipolar tungsten microelectrode driven by a Grass S88 stimulator (Grass Instruments) connected to an SIU5 stimulus isolation unit (Grass Instruments, West Warwick, RI). The stimulating electrode (200 KΩ resistance) was placed in the posterior PPN, 50–200 μm away from the recorded neurons. The membrane potential of the recorded neurons was held at 0 mV. Paired-pulse stimuli were delivered at 50 ms intervals every 15 s. Pulse duration was 0.1 ms and voltage was adjusted from 5-30 V to evoke a consistent response with low failure rate (1.5-1.7 × threshold).
Data Analysis
Analog signals were low-pass filtered at 2 kHz (Multiclamp 700B), and digitized at 5 kHz using a Digidata-1440A and pClamp 10 software (Molecular Devices, Sunnyvale, CA). Off-line analyses were performed using Clampfit 10 software (Molecular Devices). For direct post-synaptic experiments, amplitude and duration of inward/outward currents were determined, as well as Rin change and current-voltage relationship of the activated current. Comparisons between mean values in different conditions were statistically analyzed using paired student t-test (Origin 7.0, Microcal Software, Northampton, MA). For spontaneous and miniature IPSCs studies, amplitude, half-width duration and inter-IPSC interval were analyzed using Mini Analysis software (Synaptosoft Inc., Decatur, GA). A Kolmogorov-Smirnov test (K-S test, Clampfit 10) was used to statistically compare the above parameters in different conditions for individual cells. For comparison between mean values, paired student t-tests were used. Evoked IPSC data were imported into Mini Analysis (Synaptosoft), and amplitude and half-width duration of the evoked IPSCs were determined, which, along with paired-pulse ratio, were further statistically analyzed using paired t-tests using Origin 7.0. All data were expressed as mean ± SE (standard error).
Drugs
All neuroactive agents were applied by bath superfusion. Drugs used in this study included eszopiclone (ESZ, provided by Sepracor Inc., Marlborough, MA), selective GABAA receptor agonist isoguvacine (IGV, 10 μM),21 specific GABAA receptor antagonist gabazine (GBZ, 10 μM), selective NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV, 10 μM), competitive AMPA/kainate glutamate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), and the voltage-gated sodium channel blocker tetrodotoxin (TTX, 1 μM). Drugs were purchased from Sigma (St. Louis, MO), except for TTX, which was purchased from Tocris Bioscience (Ellisville, MO).
RESULTS
Potentiating Effect of ESZ on Post-synaptic GABAA Responses in the PPN
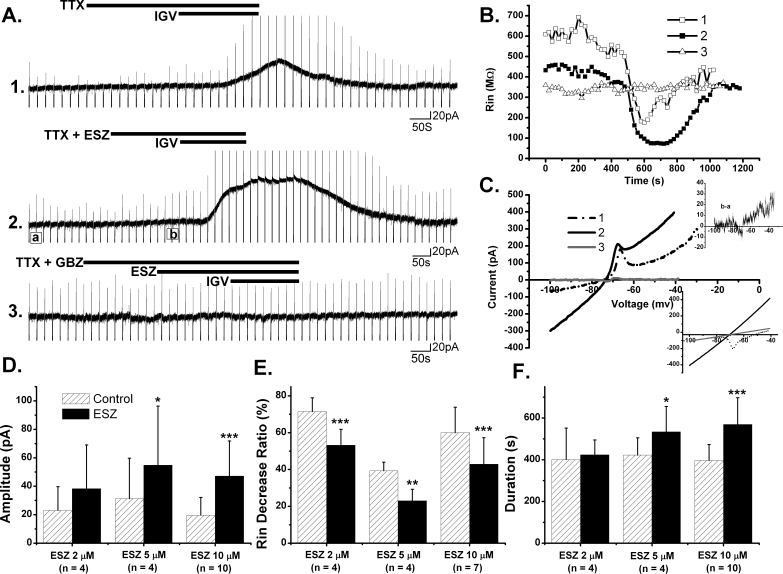
The direct post-synaptic GABAA inhibitory response was assessed by the prolonged outward current which was produced at a HP = −60 mV in PPN neurons by the selective GABAA receptor agonist IGV (10 μM). The potentiating effects of ESZ on post-synaptic GABAA receptors were tested at different concentrations (2 μM, 5 μM, and 10 μM) by superfusing ESZ for 3-5 min before additional application of IGV (∼3 min) (Figure 1A). All recordings were conducted in the presence of the sodium channel blocker TTX (1 μM) to block action potential-dependent network activity, thus presumably assessing direct responses on the membrane of recorded neurons. The amplitude, duration, and reversal potential of the IGV-activated outward currents were determined, and the Rin change was analyzed, in both the control condition (IGV alone) and in the presence of ESZ (Figure 1B and C). We found that 10 μM ESZ increased the mean amplitude of the IGV-induced outward current from 19.7 ± 3.9 pA to 47.1 ± 7.8 pA (n = 10, P < 0.0005), and increased duration from 396.5 ± 24.0 s to 568.8 ± 40.5 s (n = 10, P < 0.0005). On 7 of these PPN neurons, a voltage ramp protocol (see Methods) was applied, which revealed that IGV alone decreased Rin to 60% ± 5% of baseline, and in the presence of 10 μM ESZ the Rin decrease by IGV was 43% ± 5% (n = 7, P < 0.001). However, the reversal potential of the IGV-induced outward currents was not significantly changed by 10 μM ESZ (−67 ± 1 mV in control, −68 ± 1 mV with ESZ; n = 7, p = 0.1393), indicating that ESZ did not change the type of current activated, which is most probably mediated by chloride (Cl−) ions. In 4 of these cells, additional application of GBZ (10 μM) blocked the effect of IGV, as well as the potentiating effect of ESZ, indicating that ESZ potentiated GABAA receptor-mediated responses.
Figure 1.
The Potentiating Effect of Eszopiclone (ESZ) on Post-synaptic GABAa Receptors in the PPN. A. Amplitudes and durations of IGV induced outward current was enhanced by pretreatment with ESZ (5 μM) in the presence of TTX in this PPN neuron. ESZ alone did not induce any detectable currents (recording 2). Black bars indicate the period of drug application. B. Input resistance (Rin) change during recordings in (A). C. Reversal potential of the activated current during recordings in (A). Subtraction of the current ramp at the peak IGV effect minus that immediately before the application of IGV indicated that the current induced by IGV reversed at ∼ −74 mV with (solid black line) or without (dotted line) pretreatment with ESZ. In contrast, after prior application of GBZ, the current-voltage (I-V) curve revealed no current activated (gray line). Upper inset: subtraction of the current ramp at position (b) minus that at position (a) in recording (2) revealed a small outward current induced by ESZ at a HP more positive than −60 mV. Bottom inset: the ramp currents before (dotted line) and after (solid line) IGV application in recording (2) crossed at ∼ −74 mV, compatible with the subtraction record. In order to estimate the deflection that low-threshold spikes (LTS) can cause in the I-V relationship, a calculated I-V plot (gray) before IGV application was obtained according to the slope of the actual I-V relationship between −100 mV and −85 mV, which revealed a reversal potential ∼ −71 mV. D, E, F. Mean amplitude, Rin ratio and duration of outward currents induced by IGV with or without (control) pretreatment with ESZ at different concentrations. The Rin ratio was calculated using the average Rin of 5 records before IGV application divided by the average Rin of 5 records at the peak effect of IGV. (*P < 0.05; **P < 0.01; ***P < 0.001, paired t-test).
At 5 μM and 2 μM concentrations, ESZ produced lower potentiating effects on post-synaptic GABAA responses compared to 10 μM ESZ (Figure 1D, E and F). The mean amplitude of IGV-induced outward currents was increased from 31 ± 14 pA to 55 ± 21 pA following 5 μM ESZ (n = 4, P < 0.05). The mean duration was increased from 422 ± 41 s to 534 ± 61 s (n = 4, P < 0.05). The Rin ratio was changed from 39% ± 3% of baseline to 23% ± 4% (n = 3, P < 0.01). Following 2 μM ESZ, the IGV-induced current amplitude increased from 23 ± 8 pA to 38 ± 15 pA (n = 4, p = 0.1254), its duration increased from 401 ± 75 s to 423 ± 35 s (n = 4, p = 0.7416), and the Rin ratio decreased from 71% ± 4% to 53% ± 4% (n = 4, P < 0.0005). These experiments indicated that ESZ produced concentration-dependent potentiating effects on GABAA receptor-mediated responses in the PPN starting at concentration as low as 2 μM.
In all of the above recordings, no detectable current or Rin change was induced by ESZ alone at HP = −60 mV. However, the current-voltage (I-V) curve obtained from the subtraction of the current ramp during ESZ superfusion minus that in the control condition (Figure 1C upper inset) revealed a small outward current when the membrane potential was held more positive than −60 mV. This would indicate that ESZ might be acting as a partial agonist on GABAA receptors or it might be potentiating GABAA-receptors that are activated by endogenously-released GABA.
In our direct post-synaptic experiments, about one-third of PPN cells showed a low-threshold spike (LTS) calcium current, which was blocked by IGV. The most likely interpretation is that the large decrease in Rin induced by activation of GABAA receptors might have induced a shunting effect on this voltage-dependent current. Previous studies described the presence of three types, I, II and III, of PPN neurons based on their intrinsic properties (type I with LTS current, type II with Ia current, and type III with both) 22–24 We should note that 12.5% of cells in the post-synaptic experiments had only LTS, which is known as a type I PPN cell and reported to be present in non-cholinergic neurons.25 18.7% of cells showed both LTS and Ia currents, which can be either cholinergic or not.
Effects of ESZ on the Electrically Evoked Inhibitory Post-synaptic Currents (IPSCs) in the PPN
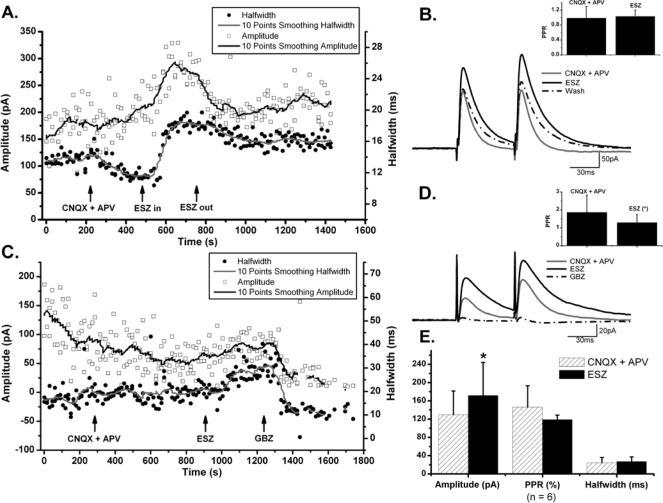
In the previous results, we investigated the potentiating effects of ESZ on IGV-induced currents, which probably were mediated mostly by GABAA extrasynaptic receptors that were activated by superfusion of IGV. However, synaptic GABAA receptors that are directly opposed to GABAergic synapses might have different subunit configurations, and as a consequence may have different sensitivity to ESZ. In order to examine whether ESZ potentiates synaptic GABAA receptors, we electrically stimulated putative presynaptic GABAergic neurons and recorded the evoked GABAA receptor-mediated current induced by endogenously released GABA. For this purpose, a bipolar stimulating electrode was placed in the dorsal posterior PPN, where a group of small GABAergic neurons have been identified.19 Paired-pulse evoked IPSCs were recorded in PPN neurons 50–200 μm away at HP = 0 mV (n = 6) (Figure 2). The amplitude and half-width duration and the paired-pulse ratio (or PPR, calculated as the amplitude of the second evoked IPSC divided by the first one26) were analyzed in the control condition (during superfusion of CNQX and APV to block AMPA/kainate and NMDA receptors, respectively), and during additional ESZ (10 μM) superfusion. In the presence of CNQX and APV, the mean amplitude of the evoked IPSCs was 130 ± 21 pA, and the addition of ESZ increased IPSC amplitude to 172 ± 30 pA (n = 6, P < 0.05). Comparison of evoked IPSC half-width duration before and during application of ESZ on individual cells, using 20 events in each condition, revealed that 3 of 6 cells showed a significant increase in IPSC duration (independent t-test, n = 20, P < 0.001), but the change in the other 3 neurons was less than 1 ms and non-significant (independent t-test, n = 20, P > 0.05). The increase in the mean half-width duration was from 17.4 ± 2.2 ms to 23.2 ± 3.3 ms (n = 43, P = 0.0895). These data indicate that ESZ increased evoked IPSC duration only in a subset of PPN neurons. Ten pairs of evoked IPSCs recorded with or without ESZ were selected for each cell to examine whether ESZ induced a change in PPR (Figure 2). In 6 PPN neurons, 3 cells showed a decrease in PPR, but only 2 were significant (independent t-test, P < 0.05); 2 cells had an increase in PPR, 1 significant (independent t-test, P < 0.05); and 1 cell showed no change.
Figure 2.
Effects of ESZ on Electrically Evoked Inhibitory Post-synaptic Currents (IPSCs) in the PPN. A. In the presence of CNQX + APV, ESZ (10 μM) increased the amplitude and half-width duration of evoked IPSCs. The amplitude of evoked IPSCs recovered after washing in CNQX + APV, whereas, the half-width duration only partially recovered. B. Average of 5 evoked IPSCs in control (CNQZ + APV), with ESZ (10 μM) and washing in CNQX + APV for the cell shown in (A). Note the change in both amplitude and half-width duration. Inset shows the change of paired-pulse ratio (PPR) before and after ESZ application, which slightly increased (independent t-test, P > 0.05). C. In another PPN cell, ESZ increased both the amplitude and half-width duration of evoked IPSCs, effects which were completely blocked by GBZ. D. Average of 5 evoked IPSCs in CNQX + APV, after addition of ESZ (10 μM) and in GBZ for the cell in (C). Inset shows that PPR significantly decreased (*P < 0.05, independent t-test) after ESZ addition in this cell.
In order to confirm that the changes in amplitude, duration and PPR of evoked IPSCs were due to ESZ, and not to the addition of CNQX + APV, 3 of 6 cells were washed with CNQX + APV after addition of ESZ. Full recovery of amplitude and PPR, and partial recovery of duration were observed. For the other 3 cells, GBZ was added after ESZ, which completely blocked the evoked IPSCs, suggesting that the evoked IPSCs were due to activation of GABAA receptors.
ESZ Modulation of Spontaneous IPSCs in PPN Neurons
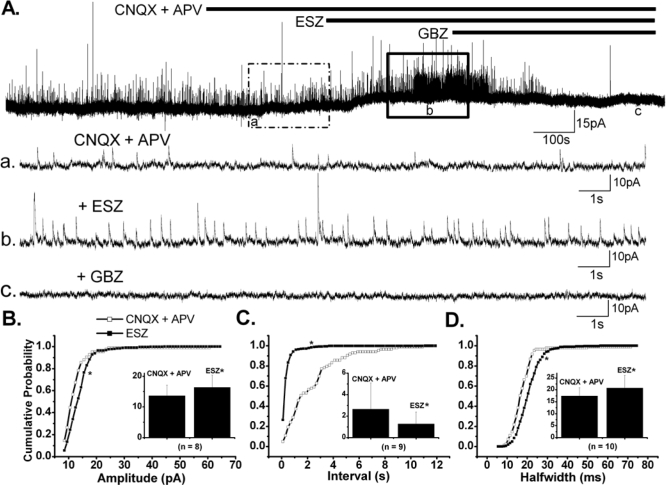
Spontaneous IPSCs (sIPSCs) were recorded in PPN neurons at HP = 0 mV in the presence of CNQX + APV, and the effects of ESZ (10 μM) on the amplitude, half-width duration and inter-IPSCs interval were assessed (n = 10). A significant increase in mean sIPSC amplitude (13.6 ± 1.2 pA in control, 16.4 ± 1.4 pA with ESZ; paired t-test: P < 0.05) was observed in the majority of the neurons recorded (n = 8/10). The cumulative probability distribution of amplitude collected from these 8 neurons was significantly altered (P < 0.00001, K-S test), manifesting as a rightward shift in the curve, correlating with the amplitude increase (Figure 3B). However, 2 of 10 PPN neurons showed a significant decrease in the amplitude of cumulative distribution plots (P < 0.00001, K-S test). Unlike amplitude, the mean half-width duration of sIPSCs was consistently increased by ESZ in all 10 neurons tested (17.5 ± 1.1 ms in control, 20.8 ± 1.7 ms with ESZ; paired t-test, P < 0.005). The cumulative distribution of sIPSC duration of these neurons showed a rightward shift, confirming the increasing effect (P < 0.00001, K-S test; Fig 3D). On 9 of 10 neurons, we observed that the mean inter-IPSCs interval was significantly decreased from 2.7 ± 0.8 s to 1.3 ± 0.4 s (paired t-test, n = 9, P < 0.05). For the other cell, the cumulative distribution of inter-IPSCs intervals showed a significant increase (P < 0.00001, K-S test). No direct correlation was found between neurons with decreased amplitude and increased inter-IPSCs interval. These results indicate that ESZ exerted a general potentiating effect on GABAergic transmission, which may involve modulation of postsynaptic GABAA receptors and an enhancement of GABA release, probably as a result of an increased excitability of GABAergic interneurons.
Figure 3.
Effects of ESZ on Spontaneous IPSCs in the PPN. A. A voltage clamp recorded PPN neuron, with HP = 0 mV. Note that a small outward current was induced by ESZ (10 μM), and that the amplitude and frequency of sIPSCs were increased. All effects were blocked by GBZ. B, C, D. Cumulative probability distributions of amplitude, inter-IPSC time interval and half-width duration of sIPSCs, respectively, for the PPN cell in (A); which indicated that ESZ increased amplitude and half-width duration of spontaneous IPSCs, and decreased inter-IPSC interval in this PPN cell. Insets in B, C and D show the effects of ESZ on the mean amplitude, inter-IPSC interval and half-width duration of sIPSCs respectively for the group (n = 10). 8/10 cells demonstrated an increase in the mean amplitude of spontaneous IPSCs (*P < 0.05, n = 8). 9 cells had significant decreases in the mean inter-IPSC interval (*P < 0.05 n = 9). All cells demonstrated increased half-width duration after ESZ (*P < 0.005, n = 10). (*on the cumulative probability curve means P < 0.00001, K-S test).
ESZ Modulation of Miniature IPSCs in the PPN
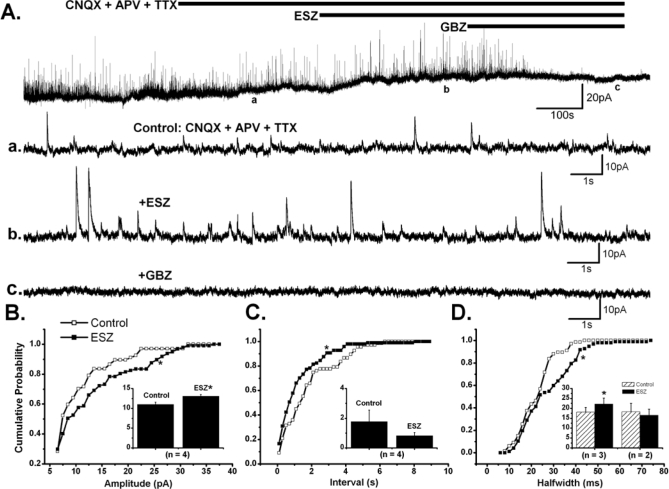
The spontaneous IPSCs correspond to the sum of 2 types of synaptic activity. One is TTX-sensitive and reflects the level of spontaneous firing in axons of GABAergic neurons that are presynaptic to the recorded cells, and the other type of activity is TTX-resistant and corresponds to the action potential-independent spontaneous release of GABA at synapses or miniature IPSCs (mIPSCs). A change in the frequency of mIPSCs is usually indicative of presynaptic modulation of the probability of transmitter release, whereas a change in their amplitude suggests a postsynaptic modulation of GABAA receptor properties such as conductance and open time. In order to further distinguish whether the potentiating effects of ESZ on GABAA currents are pre- or post-synaptic, we examined whether ESZ modulates mIPSC properties. Similar to the effects on sIPSCs, ESZ (10 μM) showed differential effects on mIPSCs in the PPN, which were recorded in the presence of CNQX + APV + TTX (Figure 4A). In 4 of 5 cells, a significant increase in the mean amplitude of mIPSCs (11 ± 0.6 pA in control, 13 ± 0.4 pA with ESZ; paired t- test, P < 0.01), and a shift to the right in the cumulative amplitude distribution of these cells (P < 0.0001, K-S test), was observed (Figure 4B). In the remaining cell, a significant decrease was identified in the cumulative distribution of amplitudes (P < 0.00001, K-S test). For half-width duration, a significant increase in the cumulative probability distribution (P < 0.00001, K-S test), as well as in the mean value (18.1 ± 2.5 ms in control, 22.2 ± 3.0 ms with ESZ; paired t-test, P < 0.05) was observed in 3 of 5 cells. The cumulative distribution of durations collected from the other 2 cells showed a significant decrease (P < 0.00001, K-S test) (Figure 4D). For the inter-IPSCs intervals, a significant decrease was observed on 4 of 5 PPN neurons studied (P < 0.0001, K-S test) (Figure 4C), whereas there was a significant increase in the other cell, based on the cumulative distribution plots (P < 0.00001, K-S test), perhaps indicating an effect of ESZ on pre-synaptic terminals. However, the decrease in the mean inter-IPSCs intervals (1.8 ± 0.8 s in control, 0.8 ± 0.2 s with ESZ) was nonsignificant (paired t-test, n = 4, P = 0.2325). All recorded mIPSCs were blocked by GBZ. These results indicate that ESZ may have both pre- and postsynaptic effects in most cells, but some cells showed only postsynaptic effects.
Figure 4.
Effects of ESZ on Miniature IPSCs in the PPN. A. Membrane current records (HP = 0 mV) show that ESZ (10 μM) induced a small outward current in the presence of CNQX, APV and TTX; meanwhile, it increased the amplitude and frequency of mIPSCs, which were blocked by GBZ. B, C, D. The cumulative probability distributions of amplitude, inter-IPSC time interval and half-width duration of mIPSCs, respectively, for the PPN cell in (A). Insets in B, C and D show the effects of ESZ on mean amplitude, inter-IPSC time interval and half-width duration for the group (n = 5). ESZ increased the amplitude of mIPSCs in 4/5 cells (*P < 0.01, n = 4). 4/5 cells showed a decrease in inter-IPSC interval (P = 0.2325, n = 4) after addition of ESZ. For the half-width duration of mIPSCs, 3 cells had a significant increase (*P < 0.05, n = 3) after ESZ application, and 2 cells showed a decrease. (*on the cumulative probability distribution plots indicates P < 0.00001, K-S test).
It should be noted that 13 of 21 PPN neurons recorded at HP = 0 mV, showed an outward current averaging 14.3 ± 2.7 pA, which was completely (n = 6) or partially (n = 4) blocked by GBZ. This observation is compatible with the I-V curve obtained in the post-synaptic effects experiment, in which a small outward current appeared when the membrane potential was more positive than −60 mV.
DISCUSSION
Briefly, our results suggest that ESZ (1) increased the amplitude of the outward current induced by IGV, (2) increased its duration, and (3) decreased input resistance (Rin), compared to levels using IGV alone. The GABAA receptor antagonist gabazine (GBZ) blocked these effects, demonstrating that ESZ potentiates GABAA receptor activation. ESZ alone did not induce detectable currents or change Rin at a holding potential of −60 mV, but when held at 0 mV, ESZ induced an outward current in most PPN cells, an effect blocked by GBZ. This suggests the possibility that a direct effect of ESZ may be present, at least in some PPN cells. ESZ also (1) increased the amplitude, (2) increased duration, and (3) decreased the interval of spontaneous and miniature IPSCs in most PPN cells. These results suggest that ESZ may potentiate GABAA inhibition in the PPN via both pre- and post-synaptic modulation, which may underlie the hypnotic effects of ESZ.
The present studies were carried out in brainstem slices, which have been used extensively in the study of PPN cell properties.18–20 Such studies can reveal the mechanism of action of neuroactive agents but the absence of sleep-wake cycle activity in vitro represents a limitation of these studies. Another limitation is the need to study developing systems, specifically while the GABAergic system is undergoing considerable change. The depolarizing influence of GABA early in development has been known for years.27 Basically, early in development the intracellular Cl− concentration is quite high, so that intracellular recordings specifically in the PPN confirmed that GABA agonists actually induced depolarization rather than hyperpolarization, before 15 days of age.18 In our conditions, however, the patch pipette solution had virtually no Cl−, therefore, the GABAA-mediated currents were always outward irrespective of age.
Our results using activation of GABAA receptors with IGV indicated that ESZ potentiated the effects of IGV, and may not change the type of current activated, which is most probably mediated by Cl− channels (Figure 1). In all cells tested, the GABAA receptor blocker GBZ blocked the effect of IGV, as well as the potentiating effect of ESZ, emphasizing that ESZ potentiated the activation of GABAA receptors. The fact that no current was induced by ESZ at HP = −60 mV, but a small outward current was present at HP = 0 mV (Figure 1C upper inset), suggests that this outward current may represent the potentiating effect of ESZ on spontaneous GABAA responses that might be due to tonic activation of extrasynaptic GABAA receptors by ambient GABA as it has been shown in cerebellar granule cells,28 the dentate gyrus,29 and thalamic neurons.30,31 Alternatively, ESZ might directly activate GABAA receptors to a small degree, at least in some cells.
Our results using electrical stimulation employed blockers of fast excitatory responses (CNQX + APV) to isolate inhibitory responses. ESZ increased the amplitude and half-width duration of evoked IPSCs, confirming its potentiating effects on endogenous GABAergic transmission. In addition, the use of paired stimuli allowed indirect assessment of pre-synaptic effects. It is generally thought that paired pulse facilitation results from the residual increase in calcium concentration in the pre-synaptic terminal after the initial stimulus.19 A change in the PPR suggests modulation of presynaptic terminals because a pure postsynaptic effect would be expected to be translated into identical changes in the responses to both pulses. Therefore, the decrease in PPR may indicate the presence of pre-synaptic potentiating effects of ESZ on GABA release.
Our data on spontaneous IPSCs suggested that ESZ had mainly enhancing effects, that appeared to occur through post-synaptic GABAA receptors, but we could not discount potentiation of GABA release via pre-synaptic effects. The increase in the frequency of miniature IPSCs confirms that ESZ acts on GABAA receptors at pre-synaptic terminals. These differential effects may be related to the apparent activation of GABA receptor α1 subunits, as well as binding to α3 subunits,10–12 and may account for its ability to perhaps increase sleep intensity.15 These differential effects may be related to the selective expression of GABAA receptor α1 and α3 subunits at pre- or post-synaptic membrane, which were reported to be bound and activated by ESZ.8–10 The selectivity of ESZ on specific GABAA receptor subunits may account for its ability to increase sleep intensity.16
The effects of ESZ in our study are similar to those reported for zolpidem, which significantly increased the duration, amplitude and frequency of mIPSCs of subthalamic neurons at concentration > 1 μM but only increased the duration of mIPSCs at lower concentrations.32 Zolpidem, like ESZ, binds with high affinity on the benzodiazepine site of GABAA receptors containing α1 subunit. It would be important in future experiments to determine whether the benzodiazepine antagonist flumazenil could block the potentiating effects induced by ESZ. We should note that the recommended dose for improving sleep onset and/or sleep maintenance is 2-3 mg in the adult.33 Assuming homogeneous plasma concentrations in a 70 kg adult, the concentration of ESZ should be ∼1-2 μM. While in this study we used mainly 10 μM ESZ to obtain detectable electrophysiological effects at the single cell level, the neurobehavioral effects of ESZ might be considerable at relatively low concentrations.
DISCLOSURE STATEMENT
This study was supported in part by Sepracor. Dr. Garcia-Rill has received income for speaking engagements for various companies. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by Sepracor Inc. and USPHS grants NS20246 and RR20146
REFERENCES
- 1.Datta S. Activation of pedunculopontine tegmental PKA prevents GABAB receptor activation-mediated rapid eye movement sleep suppression in the freely moving rat. J Neurophysiol. 2007;97:3841–50. doi: 10.1152/jn.00263.2007. [DOI] [PubMed] [Google Scholar]
- 2.Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–31. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- 3.Arnaud C, Gauthier P, Gottesmann C. Study of a GABAC receptor antagonist on sleep-waking behavior in rats. Psychopharmacol. 2001;154:415–9. doi: 10.1007/s002130000653. [DOI] [PubMed] [Google Scholar]
- 4.Goa KL, Heel RC. Zopiclone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as a hypnotic. Drugs. 1986;32:48–65. doi: 10.2165/00003495-198632010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hoehns JD, Perry PJ. Zolpidem: a nonbenzodiazepine hypnotic for treatment of insomnia. Clin Pharm. 1993;12:814–28. [PubMed] [Google Scholar]
- 6.Hair PI, McCormack PL, Curran MP. Eszopiclone: a review of its use in the treatment of insomnia. Drugs. 2008;68:1415–34. doi: 10.2165/00003495-200868100-00005. [DOI] [PubMed] [Google Scholar]
- 7.Morin AK. Strategies for treating chronic insomnia. Amer J Manag Care. 2006;12:230–45. [PubMed] [Google Scholar]
- 8.Blanchard JC, Boileau A, Julou L. Brain receptor and zopiclone. Int Pharmacopsychiat. 1982;17:59–69. [PubMed] [Google Scholar]
- 9.Doble A, Canton T, Malgoris C, et al. The mechanism of action of zopiclone. Eur Psychiat. 1995;10:117–28. doi: 10.1016/0924-9338(96)80093-9. [DOI] [PubMed] [Google Scholar]
- 10.Davies MJ, Newell G, Derry JM, Martin IL, Dunn SMJ. Characterization of the interaction of zopiclone with γ-aminobutyric acid type A receptors. Mol Pharmacol. 2001;58:756–62. doi: 10.1124/mol.58.4.756. [DOI] [PubMed] [Google Scholar]
- 11.Seighart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier P, Arnaud C, Stutzman JM, Gottesman C. Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat. Psychopharmacology. 1997;130:139–43. doi: 10.1007/s002130050221. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman C, Gandolfo G, Arnaud C, Gauthier P. The intermediate stage and paradoxical sleep in the rat: influence of three generations of hypnotics. Eur J Neurosci. 1998;10:409–14. doi: 10.1046/j.1460-9568.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimoto M, Higuchi H, Kamata M, Yoshida K, Shimizu T, Hishikawa Y. The effects of benzodiazepine (triazolam), cyclopyrrolone (zopiclone) and imidazopyridine (zolpidem) hypnotics on the frequency of hippocampal theta activity and sleep structure in rats. Eur Psychopharmacol. 1999;9:29–35. doi: 10.1016/s0924-977x(97)00102-8. [DOI] [PubMed] [Google Scholar]
- 15.Xi M, Chase MH. Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep. 2008;31:1043–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Tobler I, Borbely AA. Sleep EEG in the rat as a function of prior waking. Electroenceph Clin Neurophysiol. 1986;64:74–6. doi: 10.1016/0013-4694(86)90044-1. [DOI] [PubMed] [Google Scholar]
- 17.Steriade M, McCarley RW. Brainstem control of wakefulness and sleep. New York: Plenum Press; 1990. [Google Scholar]
- 18.Bay KD, Beck P, Skinner RD, Garcia-Rill E. GABAergic modulation of developing pedunculopontine nucleus (PPN) NeuroReport. 2007;18:249–53. doi: 10.1097/WNR.0b013e328011e6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye M, Hayar A, Garcia-Rill E. Electrical coupling in pedunculopontine nucleus (PPN) neurons. Neurosci Abst. 2007;30:581–10. [Google Scholar]
- 20.Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30:1405–14. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 22.Leonard CS, Llinas R. Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M, Biesold D, editors. Brain cholinergic systems. Oxford: Oxford Science; 1990. pp. 205–23. [Google Scholar]
- 23.Kamondi A, Williams J, Hutcheon B, Reiner P. Membrane properties of mesopontine cholinergic neurons studied with the whole-cell patch-clamp technique: implications for behavioral state control. J. Neurophysiol. 1992;68:1359–72. doi: 10.1152/jn.1992.68.4.1359. [DOI] [PubMed] [Google Scholar]
- 24.Takakusaki K, Kitai ST. Ionic mechanisms involved in the spontaneous firing of tegmental pedunculopontine nucleus neurons of the rat. Neuroscience. 1997;78:771–94. doi: 10.1016/s0306-4522(96)00540-4. [DOI] [PubMed] [Google Scholar]
- 25.Takakusaki K, Shiroyama T, Yamamoto T, Kitai ST. Cholinergic and noncholinergic tegmental pedunculopontine projection neurons in rats revealed by intracellular labeling. J Comp Neurol. 1996;371:345–61. doi: 10.1002/(SICI)1096-9861(19960729)371:3<345::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–76. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–9. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 28.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 29.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–8. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 30.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 31.Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med. 2006;15(2):S12–8. [PubMed] [Google Scholar]
- 32.Chen L, Xie JX, Fung KS, Yung WH. Zolpidem modulates GABA(A) receptor function in subthalamic nucleus. Neurosci Res. 2007;58:77–85. doi: 10.1016/j.neures.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Najib J. Eszopiclone, a nonbenzodiazepine sedative-hypnotic agent for the treatment of transient and chronic insomnia. Clin Ther. 2006;28:491–516. doi: 10.1016/j.clinthera.2006.04.014. [DOI] [PubMed] [Google Scholar]