Abstract
Study Objectives:
Examine the distribution of symptoms and risk factors, and estimate the prevalence of obstructive sleep apnea (OSA) among Māori and non-Māori New Zealanders.
Design:
Mail-out survey to a stratified random sample from the electoral roll of 10,000 people aged 30-59 y, and overnight MESAM IV monitoring during sleep of a similarly aged stratified random sample of 364 people from the Wellington electoral roll.
Setting:
Nationwide survey of OSA symptoms (71% response rate) and regional home-based measurement of respiratory disturbance index (RDI, 4% oxygen desaturations/h of sleep, plus bursts of snoring or ≥ 10/min increase in heart rate).
Participants:
Sample designs aimed for equal numbers of Māori and non-Māori participants, men and women, and participants in each decade of age.
Interventions:
N/A
Measurements and results:
Māori were more likely than non-Māori to report OSAS risk factors and symptoms. After controlling for sex and age, Māori were 4.3 times more likely to have RDI ≥ 15 (95% CI = 1.3–13.9). Ethnicity was not an independent risk factor after controlling for body mass index (BMI) and neck circumference. The prevalence of OSAS (RDI ≥ 5 and ESS > 10) was conservatively estimated to be 4.4% for Māori men, 4.1% for non-Māori men, 2.0% for Māori women, and 0.7% for non-Māori women.
Conclusions:
The national survey and the regional monitoring study indicate a higher prevalence of OSA among Māori and among men. The higher prevalence among Māori appears to be attributable to recognized risk factors, notably body habitus. In addition to increased prevention and treatment services, strategies are needed to reduce ethnic disparities in OSAS prevalence.
Citation:
Mihaere KM; Harris R; Gander PH; Reid PM; Purdie G; Robson B; Neill A. Obstructive sleep apnea in New Zealand adults: prevalence and risk factors among māori and non-māori. SLEEP 2009;32(7):949-956.
Keywords: Obstructive sleep apnea syndrome, gender, ethnicity, Māori, socioeconomic deprivation
OBSTRUCTIVE SLEEP APNEA (OSA) IS PART OF A SPECTRUM OF SLEEP RELATED BREATHING DISORDERS. IT IS CHARACTERIZED BY THE OCCURRENCE OF REPEATED upper airway obstruction during sleep. OSA accompanied by excessive daytime sleepiness is referred to as obstructive sleep apnea syndrome (OSAS).1 Symptoms typically include loud snoring interrupted by breathing pauses; risk factors include age, obesity, male gender, smoking, and alcohol consumption.2
OSAS is associated with a number of adverse outcomes including hypertension, cardiovascular disease, stroke, diabetes, and motor vehicle accidents.2,3 In addition, adults with undiagnosed OSAS have been found to be high users of health care services. Research indicates that early recognition of OSAS will not only reduce morbidity but will also lead to a significant reduction in costs to health care for other conditions.4
OSAS is one of the most commonly diagnosed sleep disorders internationally and is estimated to affect 2% of women and 4% of men.5 The prevalence of OSAS has been extensively researched, but most studies include populations with a limited socioeconomic range and reflect majority ethnic groups, and are limited in their ability to examine ethnic inequalities.6 Differences in prevalence of OSAS by ethnicity are important for furthering the understanding of this syndrome, and to inform the prevention and delivery of appropriate diagnostic and treatment services to address any inequalities.
In New Zealand, Māori comprise approximately 15% of the population. Although improvements have been seen in Māori health status over the decades, compared to other New Zealanders, major disparities still remain in almost all health indicators including those which are commonly comorbid with OSAS, such as hypertension, heart disease, and stroke.7–9 Evidence from sleep clinics in New Zealand indicates that Māori patients often present with more severe OSAS.10,11 This raises concerns about accessibility of services and possible differences in prevalence between ethnic groups, which were the focus of the 2 studies reported in this paper. The first study surveyed the distribution of OSAS symptoms and risk factors in a representative national sample of Māori and non-Māori aged 30-59 years, and the second measured pathophysiological indices of OSA during sleep in a random sample of Māori and non-Māori aged 30-59 years in the Wellington region.
METHODS
Sampling Strategy
Both studies included a random sample of adults stratified by Māori or non-Māori descent and age (in decades from 30-59 years) selected from the electoral roll. The samples were designed to achieve “equal explanatory power” for Māori and non-Māori populations, enabling analysis of data for Māori and non-Māori with the same level of precision while also allowing Māori and non-Māori comparisons to be made.12 The samples could not be stratified by sex, as this information is not available on the electoral roll. It was expected, however, that random sampling would produce approximately equal numbers of men and women. Comparison of the eligible voting population of New Zealand to enrolled electors in 2001 indicated that the electoral roll captured approximately 95% of eligible voters.13
Questionnaire
Participants in each study completed a one-page double-sided sleep questionnaire that was developed through an iterative process of pre-testing and piloting.13,14 The 17 questions included information on demographics (sex, date of birth, ethnicity); sleep habits (usual sleep in 24 h, and how often participants got enough sleep, woke feeling refreshed, and snored – choices “never”, “rarely” “often”, or “always”); the Epworth Sleepiness Scale (ESS)15; whether participants had ever been told that they stop breathing sometimes during sleep (witnessed apneas); current treatment for common comorbidities of obstructive sleep apnea; and eligibility for a community services card, which provides income-tested special subsidies for access to healthcare. Questions were also included on smoking and alcohol consumption as risk factors for sleep disordered breathing.6,16,17
The 1996 NZ Census question on ethnicity was used, which allows people to self-identify with multiple ethnic groups. Those who ticked Māori, with or without another ethnic identification, were coded as Māori. Ethnicity (rather than descent based on the electoral roll) was used in the analysis of results.
A socioeconomic deprivation rating was assigned to each participant based on their home address, using a validated small-area index (NZDep96).18 This is a composite index of 8 variables taken from the 1996 New Zealand census data, that provides a deprivation score for each mesh-block (geographical units containing a median of 90 people) in New Zealand, ranging from 1 (least deprived) to 10 (most deprived).
Data Collection: National Survey
The project was approved by the Wellington Regional Ethics Committee. Based on responses in a pilot survey (600 participants from the Wellington region), study packages were mailed to an age-stratified sample of registered electors (5,500 of Māori descent and 4,500 non-Māori), in April 1999. The study package included an information letter (tailored for Māori or non-Māori participants), the questionnaire, a paper tape measure (for measuring neck circumference), and a return envelope. Participants could also call a toll-free number to ask questions, and to respond by telephone if they so wished. A subset of 137 participants in the pilot study answered the questionnaire by both mail and telephone (at an interval of about 5 weeks). This confirmed that there were no systematic differences between telephone and mail responses.
At approximately 2-week intervals, remaining non-responders received a postcard reminder, then a complete new study package, and then telephone follow-up was undertaken for those for whom telephone numbers could be found. As an incentive, all participants were offered the opportunity to go into a draw for a mystery holiday weekend.
Data Collection: Regional Monitoring Study
The project was approved by the Wellington Regional Ethics Committee. Between August 1999 and May 2001, study packs containing a covering letter, an information sheet (tailored for Māori or non-Māori participants), and a consent form, were progressively sent out to a sample of 1200 people in the Wellington Region. People in the sample were then contacted by telephone to elicit their participation in an unattended overnight monitoring study in their own home. Those who chose not to participate in the monitoring study were asked to complete the questionnaire over the phone.
Participants who volunteered for overnight monitoring were requested to adhere as closely as possible to their normal daily routine for the study night. At a time convenient to them, a researcher visited their home and fitted the MESAM IV ambulatory monitoring device (MAP; Martinsried, Germany), which monitors 4 variables: snoring, heart rate (HR), arterial oxygen saturation (SpO2), and body position.19–26 In addition, participants completed the questionnaire and their height, weight, and neck circumference were measured by the researcher. For non-Māori, obesity was defined as a BMI > 30 kg/m2. For Māori, obesity was defined as a BMI > 32 kg/m2.27 Neck circumference was measured by the researcher to the nearest 0.5 cm at the level of the cricothyroid membrane, and this was subsequently compared to the participant's self-measurement. Thirty-five percent of participants accurately measured their neck circumference (i.e., no difference between participant and researchers measurements), with the majority (76%) of participants within ± 1 cm of the researchers' measurement. These findings suggest that the method used to collect neck circumference in the national survey was valid and replicable.
Data Management and Analysis
All questionnaire data were double entered and checked for anomalous responses and outliers, according to a set of rules to ensure consistency. MESAM IV data were printed and scored manually in 5-min epochs by a single blinded scorer. Epochs were excluded from analyses if more than half the epoch contained artifact in the snoring, oxygen saturation, or heart rate signals. An apneic event was scored if there was an episode of oxygen desaturation of ≥ 4% from the preceding baseline in conjunction with (1) an increase in heart rate (HR) of ≥ 10 beats/min or (2) a burst of snoring associated with a desaturation episode.21
As sleep per se is not measured by the MESAM IV, sleep timing was determined either from participants pushing the event marker on the recorder or from their subjective reports of sleep and wake times, together with examination of changes in baseline heart rate within each epoch. The respiratory disturbance index (RDI) was defined as the total number of apneic events as defined above, divided by the total estimated sleep time in hours. OSA was defined at 3 thresholds of RDI ( ≥ 5, ≥ 10, ≥ 15), and OSAS was defined as OSA (RDI ≥ 5, ≥ 10, ≥ 15) with the addition of daytime hypersomnolence. Daytime hypersomnolence was defined as an ESS score > 10.
All analyses were performed using SAS (SAS, Institute; NC, USA). Response rates were calculated for each sample (Māori and non-Māori descent) and then compared with and without controlling for socioeconomic deprivation, using logistic regression models.
In both studies, univariate analyses considered the participants in 4 groups: Māori men and women, and non-Māori men and women. For categorical variables, the χ2 test was used to examine differences between groups. For continuous variables, the Wilcoxon signed ranks test was used to examine differences between group medians (when data were not normally distributed) and the t-test was used to examine differences between group means when data were normally distributed. A p-value < 0.05 was considered significant.
Risk factors for OSAS symptoms (National Survey) and for OSA (Regional Monitoring Study) were identified by univariate (unadjusted) and logistic multiple regression analyses (adjusted for potential confounders). All risk factors that were associated with the outcome at the univariate level (P-value ≤ 0.10) were considered for inclusion in the multivariate models. For both studies, population prevalence statistics were weighted according to the actual age structure of each group in the population.
RESULTS
Sample Description: National Survey
The national survey study achieved a response rate of 71.4% (n = 7,048), excluding those deceased or no longer resident in New Zealand. After excluding questionnaires where ethnicity or sex data were missing, or where age did not match the electoral roll or was outside the required range, a total of 6928 questionnaires were included in the analyses, including 1463 Māori men (21%), 1732 Māori women (25%), 1714 non-Māori men (25%), and 2019 non-Māori women (29%).
For both the Māori and non-Māori samples, there was a significant trend for increasing response rates for each decade increase in age (Cochran-Armitage test for trend, P < 0.0001), and a significant trend for decreasing response rates with increasing level of socioeconomic deprivation (P < 0.0001). Consistent with national distributions,18 Māori were over-represented in the most deprived socioeconomic deciles and non-Māori participants were over-represented in the least deprived deciles (P < 0.001).
OSAS Symptoms and Risk Factors: National Survey
Table 1 summarizes the estimated population prevalence of OSAS symptoms and risk factors. All differences by ethnicity and sex were significant, except that Māori men and women were equally likely to score as excessively sleepy on the Epworth Sleepiness Scale (ESS > 10), and the groups did not differ in the proportion of people who reported never/rarely getting enough sleep or never/rarely waking refreshed. There is a clear pattern of higher prevalence of symptoms and risk factors among Māori.
Table 1.
Estimated Population Prevalence of OSAS Symptoms and Risk Factors (National Survey)
| Māori | Non-Māori | RR (95% CI) | P | |
|---|---|---|---|---|
| Men | ||||
| Risk factors | ||||
| Mean neck (cm)* | 41.98 (41.73–42.23) | 40.15 (39.99–40.31) | 1.83 (1.53–2.13) | <0.0001 |
| Current smoker (%) | 42.36 (39.54–45.17) | 24.19 (14.47–31.28) | 1.75 (1.57–1.95) | <0.0001 |
| Symptoms | ||||
| Witnessed apneas (%) | 30.25 (27.67–32.84) | 18.30 (16.44–20.15) | 1.65 (1.45–1.89) | <0.0001 |
| Snore always (%) | 16.22 (14.15–18.29) | 10.14 (8.70–11.58) | 1.60 (1.32–1.94) | <0.0001 |
| Wake refreshed never/rarely (%) | 46.61 (43.79–49.43) | 47.30 (44.86–49.75) | 0.99 (0.91–1.07) | 0.72 |
| Enough sleep never/rarely (%) | 39.84 (37.06–42.63) | 37.82 (35.43–40.21) | 1.05 (0.96–1.16) | 0.28 |
| ESS > 10 (%) | 24.60 (22.17–27.03) | 15.58 (13.83–17.33) | 1.58 (1.36–1.83) | <0.0001 |
| Women | ||||
| Risk factors | ||||
| Mean neck (cm)* | 36.16 (35.95–36.37) | 34.34 (34.20–34.48) | 1.82 (1.57–2.07) | <0.0001 |
| Current smoker (%) | 38.84 (25.37–52.31) | 13.16 (6.56–19.76) | 2.3 (2.04–2.60) | <0.0001 |
| Symptoms | ||||
| Witnessed apneas (%) | 11.47 (9.88–13.05) | 6.21 (5.13–7.28) | 1.85 (1.48–2.30) | <0.0001 |
| Snore always (%) | 6.90 (5.66–8.14) | 4.05 (3.17–4.93) | 1.70 (1.29–2.26) | 0.0002 |
| Wake refreshed never/rarely (%) | 47.85 (45.31–50.40) | 44.80 (42.60–47.00) | 1.07 (0.99–1.15) | 0.08 |
| Enough sleep never/rarely (%) | 38.16 (35.65–40.66) | 36.33 (34.19–38.47) | 1.05 (0.96–1.15) | 0.28 |
| ESS >10 (%) | 22.15 (20.00–24.29) | 12.06 (10.59–13.52) | 1.84 (1.57–2.15) | <0.0001 |
Test for difference between means rather than ratios.
Independent Risk Factors for OSA Symptoms: National Survey
Table 2 summarizes logistic multiple regression models examining the independent risk factors for reporting witnessed apneas and for reporting always snoring. Interaction terms between ethnicity and other predictive variables were tested. The only significant interaction found was between ethnicity and smoking status as risk factors for reporting witnessed apneas.
Table 2.
Logistic Multiple Regression of Risk Factors for Reporting Witnessed Apneas and Always Snoring (National Survey)
| Variable | Witnessed Apneas (n = 6450) |
Always Snore (n = 6345) |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Ethnicity (Māori vs. non-Māori) | - | - | 0.98 (0.80–1.20) | 0.84 |
| Ethnicity (Māori vs. non-Māori non smokers) | 1.68 (1.39–2.04) | <0.0001 | - | |
| Ethnicity (Māori vs. non-Māori smokers) | 0.90 (0.71–1.14) | 0.38 | - | |
| Sex (ref. women) | 2.09 (1.75–2.49) | <0.0001 | 1.17 (0.93–1.46) | 0.17 |
| Age (yearly increase) | 1.02 (1.01–1.03) | <0.0001 | 1.02 (1.01–1.03) | 0.0014 |
| NZDep96 (increasing deciles) | 1.05 (1.02–1.07) | 0.0015 | 1.03 (0.99–1.06) | 0.15 |
| aCommunity Services Card (ref. ineligible) | 1.13 (0.96–1.32) | 0.14 | 1.04 (0.85–1.27) | 0.74 |
| Neck (cm increase) | 1.09 (1.07–1.11) | <0.0001 | 1.16 (1.13–1.18) | <0.0001 |
| Current smoker (ref. no) | - | - | 1.69 (1.40–2.05) | <0.0001 |
| Current smoker, Māori (ref. no) | 2.20 (1.76–2.76) | <0.0001 | - | |
| Current smoker, non-Māori (ref. no) | 1.18 (0.97–1.43) | 0.10 | - | |
| bAlcohol (moderate vs. non-drinker) | 0.94 (0.77–1.16) | 0.57 | 0.73 (0.56–0.93) | 0.0125 |
| bAlcohol (daily vs. non-drinker) | 1.15 (0.81–1.62) | 0.44 | 1.05 (0.69–1.61) | 0.82 |
| bAlcohol (exceed recommended vs. non-drinker) | 1.35 (1.08–1.68) | 0.0079 | 1.07 (0.82–1.40) | 0.62 |
Community Services Card can be accessed by individuals over 18 years of age and on low to middle incomes, adjusted for family size. The primary benefit of the card is subsidized visits to the doctors and prescriptions.
Categories defined according to the criteria of the Alcohol Advisory Council of New Zealand on upper limits for responsible drinking.19 Moderate–drink alcohol, but not daily and not to excess. Daily–drink alcohol daily, but not more than the recommended upper limit on an occasion or per week. Excessive–drink alcohol more than the recommended upper limit at a typical session (≥5 drinks for women; ≥7 drinks for men) or per week (>14 drinks for women, >21 drinks for men).
Ethnicity was not a significant independent risk factor except that Māori non-smokers were at greater risk of reporting witnessed apneas than non-Māori non-smokers. Men were more likely to report witnessed apneas, as were people in more deprived socioeconomic deciles. Increasing age increased the risk of reporting both witnessed apneas and always snoring. Excessive alcohol consumption increased the risk of reporting witnessed apneas, while moderate alcohol consumption reduced the risk of reporting always snoring, compared to no alcohol consumption. In addition, increasing neck size was a significant risk factor for reporting witnessed apneas and always snoring.
Sample Description: Regional Monitoring Study
Telephone contact was made with 786/1200 (66%) of the sample, of whom 364 (Māori = 169, non-Māori = 195) agreed to overnight MESAM IV monitoring in their homes. A further 341 (Māori = 137, non-Māori = 204) declined overnight monitoring but answered the questionnaire over the phone, while 81 people (Māori = 42, non-Māori = 39) declined any form of participation.
Māori were less likely than non-Māori to be contacted (OR = 0.51, 95% CI 0.39–0.66, P < 0.0001), but this difference was no longer significant after controlling for socioeconomic deprivation (OR = 0.77, 95% CI 0.59–1.02, P = 0.064). Among those who were contacted, participation rates in overnight monitoring were similar for Māori and non-Māori.
Participants who completed an overnight study did not differ in age or Māori descent from people who declined or could not be contacted. However, the likelihood of declining to participate in overnight monitoring increased with each decile increase in socioeconomic deprivation (OR = 1.22, 95% CI 1.17–1.27, P < 0.001). Māori were overrepresented in the most deprived socioeconomic deciles, and non-Māori participants were overrepresented in the least deprived deciles (P < 0.001). However, both Māori and non-Māori participants in the regional monitoring study were less socioeconomically deprived than the national averages for each group.
Participants who completed an overnight study did not differ in age, sex, ethnicity, or socioeconomic deprivation from those who only answered the questionnaire. Further, there were no significant differences between these 2 groups in reporting always snoring, witnessed apneas, or ESS > 10.
OSA Symptoms and Risk Factors: Regional Monitoring Study
Table 3 summarizes the prevalence of OSA risk factors and symptoms among the 358 participants, by ethnicity and sex (6 participants were excluded due to insufficient monitoring data). The 4 groups did not differ in age (χ2 = 2.47, df = 3, P = 0.48).
Table 3.
Prevalence of OSAS Symptoms and Risk Factors (Regional Monitoring Study)
| Men | Māori | non-Māori | Risk Ratio (95% CI) | P |
|---|---|---|---|---|
| Symptoms | ||||
| Witnessed apneasa | 24.83 (12.23–37.44) | 16.02 (7.65–24.40) | 1.55 (0.75–3.21) | 0.2538 |
| Always snorea | 17.00 (8.00–26.00) | 12.72 (5.08–20.36) | 1.33 (0.60–3.00) | 0.4789 |
| Never/rarely wake refresheda | 45.13 (28.7–61.59) | 42.05 (27.71–56.39) | 1.07 (0.65–1.77) | 0.7827 |
| ESS >10a | 11.87 (4.08–19.65) | 17.68 (8.29–27.07) | 0.67 (0.29–1.56) | 0.3506 |
| Risk factors | ||||
| BMIb | 29.2 (27.5–31.8) | 27.7 (24.9–33.0) | - | 0.0023 |
| Obesea | 18.17 (8.58–27.76) | 23.48 (13.10–33.88) | 0.77 (0.39–1.54) | 0.462 |
| Neck in cmb | 41 (38.0–42.0) | 40 (39.0–42.0) | - | 0.0279 |
| Current smokera | 23.2 (11.3–35.1) | 16.0 (7.5–24.5) | 1.4 (0.7–3.0) | 0.4789 |
| Women | ||||
| Symptoms | ||||
| Witnessed apneasa | 5.83 (0.00–11.70) | 3.42 (0.10–6.79) | 1.71 (0.42–6.97) | 0.4847 |
| Always snorea | 6.93 (0.00–14.01) | 1.91 (0.00–4.56) | 3.63 (0.65–20.29) | 0.1929 |
| Never/rarely wake refresheda | 42.12 (24.68–59.57) | 43.10 (28.77–57.40) | 0.98 (0.57–1.66) | 0.9334 |
| ESS >10a | 11.78 (4.51–19.05) | 10.99 (3.43–18.54) | 1.07 (0.43–2.7) | 0.8819 |
| Risk factors | ||||
| BMIb | 29.1 (24.9–33.0) | 26.1 (23.8–28.6) | - | 0.0001 |
| Obesea | 22.70 (11.95 33.46) | 15.71 (7.89–23.52) | 1.45 (0.73–2.87) | 0.302 |
| Neck in cmb | 35.5 (34.0–37.50) | 33.5 (32.5–35.5) | - | <0.0001 |
| Current smokera | 45.2 (26.4–64.0) | 12.7 (4.4–20.9) | 3.6 (1.7–7.7) | 0.0019 |
%, 95% CI;
bmedian, interquartile range.
For both men and women, Māori had higher mean BMI than non-Māori, and Māori had significantly larger median neck circumferences than non-Māori. Among women (but not men), Māori were more likely than non-Māori to report being a current smoker. Within ethnic groups, men were more likely to report witnessed apneas than women (Māori χ2 = 8.3, P = 0.02; non-Māori χ2 = 5.2, P = 0.003), but there were no other significant differences by sex.
Prevalence of OSA and OSAS: Regional Monitoring Study
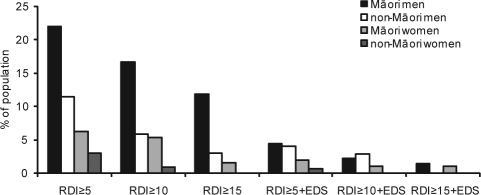
Figure 1 summarizes the estimated population prevalence of OSA and OSAS, weighted according to the actual age structure of each group in the general population.
Figure 1.
Estimated population prevalence of OSA and OSAS. Estimated population prevalences for Māori and non-Māori men and women. Respiratory disturbance (RD): an episode of oxygen desaturation ≥ 4% accompanied by an increase in heart rate ≥ 10 beats per minute and/or a burst of snoring. Excessive daytime sleepiness (EDS): ESS score > 10.
Not all differences in prevalence between Māori men and non-Māori men, and between Māori women and non-Māori women, reached statistical significance at the P < 0.05 level. However, the small numbers of individuals in each group limited the ability to detect differences. Māori were significantly more likely than non-Māori to have RDI ≥ 10 (10.9% vs. 3.3%, P = 0.02) and RDI ≥ 15 (6.5% vs. 1.5%, P = 0.03). Overall, men were more likely than women to have RDI ≥ 5 (12.5% vs. 3.4%, P = 0.01), and RDI ≥ 10 (7.0% vs. 1.4%, P = 0.03), and RDI ≥ 15 (3.9% vs. 0.2%, P = 0.01).
Independent Risk Factors for OSA: Regional Monitoring Study
Table 4 summarizes univariate relationships with OSA (RDI ≥ 15), and multiple logistic regression models that identify significant independent risk factors for OSA. Separate models were run to examine interactions between ethnicity and the other independent variables in Models 1 and 2, but no significant interactions were found.
Table 4.
Risk Factors for RDI ≥15 (Regional Monitoring Study)
| Variable | Unadjusted OR |
Adjusted OR Model 1 |
Adjusted OR Model 2 |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Ethnicity (ref. non-Māori) | 3.66 (1.16–11.58) | 0.0271 | 4.26 (1.31–13.90) | 0.0163 | 2.32 (0.65–8.30) | 0.1977 |
| Sex (ref. women) | 7.78 (1.74–34.74) | 0.0072 | 8.8 (1.94–40.17) | 0.0048 | 11.04 (1.43–85.27) | 0.0213 |
| Age (yearly increase) | 1.06 (0.99–1.13) | 0.0929 | 1.07 (1.00–1.14) | 0.0534 | 1.06 (1.00–1.14) | 0.0914 |
| BMI (kg/m2) | 1.23 (1.12–1.35) | <0.0001 | 1.23 (1.07–1.41) | 0.0028 | ||
| Neck (cm increase) | 1.42 (1.21–1.67) | <0.0001 | 1.05 (0.84–1.30) | 0.6933 | ||
n = 358. Model 1: fixed factors ethnicity, sex, age; no covariates. Model 2: fixed factors ethnicity, sex, age, BMI, neck circumference; no covariates. After removing BMI from Model 2: OR for neck circumference = 1.22, 95% CI = 1.09–1.62, P = 0.0043.
Although the prevalence of RDI ≥ 15 was higher among Māori than non-Māori, ethnicity was not an independent risk factor after controlling for BMI and neck circumference. Similarly, age was no longer a significant independent risk factor after controlling for BMI and neck circumference. In a version of Model 2 that included neck size but not BMI, neck circumference was a significant independent risk factor for RDI ≥ 15 (OR = 1.22, 95% CI = 1.09–1.62, P = 0.0043). Socioeconomic deprivation had a significant univariate relationship with RDI ≥ 15 (for each decile increase in deprivation OR = 1.21, 95% CI = 1.04–1.42, P = 0.0017), but was no longer significant in multivariate models that included ethnicity, gender, age, and either BMI or neck circumference.
DISCUSSION
The two studies presented here provide the first estimates of the prevalence of OSAS symptoms and risk factors among New Zealanders aged 30-59 years. The studies have the particular strength that the influence of ethnicity and socioeconomic deprivation could be considered simultaneously.
Prevalence Estimates
The national survey (n = 6928) found a higher prevalence of risk factors (larger neck size, higher prevalence of smoking, greater socioeconomic deprivation) among Māori than non-Māori, and among men. There was also higher reporting of OSAS symptoms (witnessed apneas, always snoring, and excessive sleepiness) among Māori.
The prevalence estimates for OSA and OSAS from the objective monitoring study (n = 358) confirm the pattern of higher prevalence among Māori and among men. Using the criterion of RDI ≥ 5, the estimated prevalence of OSA is 22.0% for Māori men, 11.4% for non-Māori men, 6.3% for Māori women, and 3.0% for non-Māori women. At a threshold of RDI ≥ 15, Māori were more likely than non-Māori to have OSA (RR = 4.36, 95% CI 2.3–8.3), but the numbers were too small to break this down by sex within ethnic groups. Using the criteria of RDI ≥ 5 plus ESS > 10, the estimated population prevalence of OSAS is 4.4% for Māori men, 4.1% for non-Māori men, 2.0% for Māori women, and 0.7% for non-Māori women. For the criteria of RDI ≥ 15 and ESS > 10, the estimated population prevalence of OSAS is 1.3% for Māori and 0% for non-Māori. These results support clinical observations that suggest a higher prevalence of OSAS amongst Māori.10,11 Ethnic inequalities in sleep disordered breathing have previously been reported.28,30–32 In the present study, OSA prevalence estimates for Māori were closer to the estimates for the Wisconsin Cohort5 and the Australian population study,21 whereas prevalence estimates for non-Māori were significantly lower.
Several factors could have contributed to conservative prevalence estimates in the regional monitoring study. Firstly, less socioeconomically deprived participants were more likely to be contacted. This would have tended to reduce the estimated prevalence of OSA and OSAS. Increasing deprivation was associated with higher prevalence of OSAS symptoms and risk factors in the national survey, and has been shown in other studies to be associated with increased prevalence of obesity and smoking.18,33 Because Māori are overrepresented in the more deprived socioeconomic deciles, the response bias by deprivation may also have lead to an underestimation of the difference in OSAS prevalence between Māori and non-Māori.
Another possible factor relates to using a conservative measure of RDI in prevalence estimates. Only 4% desaturation events with evidence of snoring bursts and a change in heart rate were counted in the respiratory disturbance index. This would not, however, explain the difference between our prevalence estimates and those of the Australian study, which used the same scoring criteria for respiratory events.21 In contrast, the Wisconsin Cohort study included both apneas and hypopneas (measured by polysomnography) in the definition of respiratory events.5
A third possible factor affecting our prevalence estimates is the limitation that the MESAM IV recording system does not measure sleep per se.19,22,34–36 A systematic overestimate of sleep duration, based on subjective reporting and/or use of the MESAM IV event marker, could have led to an underestimate of the RDI, which is averaged per hour of sleep.
Risk Factors for OSA and OSAS
The finding that ethnicity is not a significant independent risk factor for RDI ≥ 15, after controlling for BMI is consistent with a New Zealand clinical study11 and with findings in the Sleep Heart Health Study,28 However, it contradicts the findings of others37 who reported that ethnic differences in correlations of craniofacial features were associated with OSA risk, after controlling for a number of potential risk factors.
Sex, socioeconomic deprivation, and age were independent risk factors for reporting witnessed apneas among participants in the national survey. As expected, in the regional monitoring study the prevalence of OSA was higher in men for all 3 thresholds of RDI. However, sex was not an independent risk factor for RDI ≥ 15 after controlling for BMI and neck circumference. Age was not independently associated with RDI ≥ 15 in the smaller sample of the regional monitoring study, in contrast to the findings of the Wisconsin sleep cohort.28 Increasing socioeconomic deprivation (OR = 1.2, 95% CI = 1.0–1.4) was associated with higher risk for RDI ≥ 15 at the univariate level, but the relationship was no longer significant after controlling for ethnicity, sex, age, and measures of body habitus. However, the regional monitoring study had limited power to detect differences by deprivation.
In summary, this large national questionnaire survey and more in-depth MESAM IV monitoring study confirm that the prevalence of OSA and OSAS is higher among Māori than non-Māori. The distributions of known risk factors in the 2 population groups contribute to this difference. There is also evidence that Māori seen at sleep clinics have more severe OSAS than non-Māori,10,11 suggesting that there may be barriers for Māori in accessing specialist services. These findings have particular relevance for prevention and for the provision of diagnostic and treatment services that address these inequalities. The higher risk among Māori of the development of sleep problems and their negative consequences indicates Māori needs should be prioritized.
Increased funding is needed to provide adequate diagnostic and treatment services for OSAS for the New Zealand population. A nationwide sleep service infrastructure is needed to ensure that existing sleep services are utilized to their capacity, to coordinate targeted strategies to reduce ethnic disparities in OSAS prevalence, and to provide accessible, evidenced-based services that integrate primary care providers with secondary and tertiary services.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Gander has performed research for The Boeing Company and has consulted for The Boeing Company and Quantas Airways. Dr. Neill has received research support from Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We acknowledge with gratitude the participants who gave their time and support for this study. We would also like to thank Ron Grunstein for loaning us the MESAM-IV equipment and Waiwai Hla for scoring the MESAM-IV studies. Particular thanks to Naina Watene, Pori Workman, Clint Ormbsy, Vonda Taumata, and the late Vera Keefe-Ormsby of Te Rōpū Rangahau Hauora a Eru Pōmare who have helped on these projects over the years. Financial support was provided by Health Research Council of New Zealand (for the national survey, HRC Project Grant 99/185; for the regional monitoring study, HRC Project Grant 99/138, and a Māori health PhD Scholarship to Kara Mihaere), and a Postdoctoral Bridging Grant from Ngā Pae o te Māramatanga, to Kara Mihaere. Fisher and Paykel Healthcare provided funding for the incentive prize in the national survey.
No off-label or investigational use discussed.
Research Location: School of Medicine and Health Sciences, University of Otago, Wellington, Sleep/Wake Research Centre, Massey University.
REFERENCES
- 1.American Sleep Disorders Association. International classification of sleep disorders, revised: diagnostic and coding manual. Rochester, Minnesota: American Sleep Disorders Association; 1997. [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Wittmann V, Rodenstein DO. Health care costs and the sleep apnea syndrome. Sleep Med Rev. 2004;8:269–79. doi: 10.1016/j.smrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Partinen M, Hublin C. Epidemiology of sleep disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Elsevier Saunders; 2005. pp. 626–647. [Google Scholar]
- 7.Robson B, Harris R, editors. Hauora Māori standards of Health IV. Wellington: Te Ropu Rangahau Hauora a Eru Pomare. 2007 Available at http://www.hauora.Māori.nz.
- 8.Ministry of Health. Reducing Inequalities in Health. Wellington: Ministry of Health, HP3521. 2002 Available at http://www.moh.govt.nz/
- 9.Ministry of Health. He Korowai Oranga: Māori Health Strategy. Wellington: Ministry of Health, HP3541. 2002 Available at http://www.moh.govt.nz/
- 10.Frith RW, Cant BR. Obstructive sleep apnoea in Auckland: diagnosis and treatment. New Zeal Med J. 1985;98:745–8. [PubMed] [Google Scholar]
- 11.Baldwin DR, Kolbe J, Troy K, et al. Comparative clinical and physiological features of Māori, Pacific Islanders and Europeans with sleep related breathing disorders. Respirology. 1998;3:253–260. doi: 10.1111/j.1440-1843.1998.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 12.Robson B. Mana Whakamarama - Equal Explanatory Power: Māori and non-Māori sample size in national surveys. Wellington: Te Ropu Rangahau Hauora a Eru Pomare; 2002. [Google Scholar]
- 13.Harris R. Obstructive Sleep Apnoea Syndrome: Symptoms and risk factors among Māori and non-Māori adults in Aotearoa. Wellington: University of Otago at Wellington School of Medicine and Health Sciences; 2003. Master of Public Health Thesis. [Google Scholar]
- 14.Gander PH, Marshall NS, Harris RB, Reid P. Sleep, sleepiness and motor vehicle accidents: a national survey. Aust N Z J Public Health. 2005;29:16–21. doi: 10.1111/j.1467-842x.2005.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–24. [PubMed] [Google Scholar]
- 17.Young T, Peppard P, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Cir Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 18.Salmond C, Crampton P, Sutton F. Deprivation and health. In: Howden-Chapman P, Tobias M, editors. Social inequalities in health: New Zealand 1999. Wellington: Ministry of Health; 2000. pp. 9–63. [Google Scholar]
- 19.Stoohs R, Guilleminault C. MESAM 4: an ambulatory device for the detection of patients at risk for obstructive sleep apnea syndrome (OSAS) Chest. 1992;101:1221–7. doi: 10.1378/chest.101.5.1221. [DOI] [PubMed] [Google Scholar]
- 20.Roos M, Althaus W, Rhiel C, Penzel T, Peter JH, von Wichert P. Comparative use of MESAM IV and polysomnography in sleep-related respiratory disorders. Pneumologie. 1993;47(Suppl 1):112–8. [PubMed] [Google Scholar]
- 21.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- Esnaola S, Duran S, Infante-Rivard C, Rubio S, Fernandez A. Diagnostic accuracy of a portable recording device (MESAM IV) in suspected obstructive sleep apnoea. Eur Respir J. 1996;9:2597–605. doi: 10.1183/09031936.96.09122597. [DOI] [PubMed] [Google Scholar]
- 23.Cirignotta F, Mondini S, Gerardi R, Mostacci B, Sancisi E. Unreliability of automatic scoring of MESAM 4 in assessing patients with complicated obstructive sleep apnea syndrome. Chest. 2001;119:1387–92. doi: 10.1378/chest.119.5.1387. [DOI] [PubMed] [Google Scholar]
- 24.Richman RM, Elliott LM, Burns CM, Bearpark HM, Steinbeck KS, Caterson ID. The prevalence of obstructive sleep apnoea in an obese female population. Int J Obes Relat Metab Disord. 1994;18:173–7. [PubMed] [Google Scholar]
- 25.Hui DS, Chan JK, Ko FW, et al. Prevalence of snoring and sleep-disordered breathing in a group of commercial bus drivers in Hong Kong. Int Med J. 2002;32:149–57. doi: 10.1046/j.1444-0903.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 26.Hui DSC, Chan JKW, Ho ASS, Choy DKL, Lai CKW, Leung RCC. Prevalence of snoring and sleep-disordered breathing in a student population. Chest. 1999;116:1530–6. doi: 10.1378/chest.116.6.1530. [DOI] [PubMed] [Google Scholar]
- 27.Swinburn BA, Ley SJ, Carmichael HE, Plank LD. Body size and composition in Polynesians. Int J Obesity. 1999;23:1178–83. doi: 10.1038/sj.ijo.0801053. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 29.Dixon JB, Schachter LM, O'Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnography. Chest. 2003;123:1134–41. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 30.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care. 1995;152:1946–9. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 31.Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalence of sleep-disordered breathing in ages 40-64 years: a population-based survey. Sleep. 1997;20:65–76. doi: 10.1093/sleep/20.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 33.Salmond C, Crampton P, Hales S, Lewis S, Pearce N. Asthma prevalence and deprivation: a small area analysis. J Epidemiol Commun Health. 1999;53:476–80. doi: 10.1136/jech.53.8.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penzel T, Amend G, Meinzer K, Peter JH, von Wichert P. MESAM: A heart rate and snoring recorder for detection of obstructive sleep apnea. Sleep. 1990;13:175–182. doi: 10.1093/sleep/13.2.175. [DOI] [PubMed] [Google Scholar]
- 35.Koziej M, Cieslicki JK, Gorzelak K, Sliwinski P, Zielinski J. Hand-scoring of MESAM 4 recordings is more accurate than automatic analysis in screening for obstructive sleep apnoea. Eur Respir J. 1994;7:1771–5. doi: 10.1183/09031936.94.07101771. [DOI] [PubMed] [Google Scholar]
- 36.Duran Cantolla J, Esnaola Sukia S, Rubio Aramendi R, Egea Santaolalla C. [Validity of a portable recording system (MESAM IV) for the diagnosis of sleep apnea syndrome] Arch Bronconeumol. 1994;30:331–8. [PubMed] [Google Scholar]
- 37.Coltman R, Taylor DR, Whyte K, Harkness M. Craniofacial form and obstructive sleep apnea in Polynesian and Caucasian men. Sleep. 2000;23:943–50. [PubMed] [Google Scholar]