Abstract
Objective:
To examine the relationships between sleep and inflammatory markers because these may be important in the development of cardiovascular disease.
Methods and Results:
The relationship between self-reported sleep duration and interleukin-6 (IL-6) (n = 4642) and high-sensitivity C-reactive protein (hs-CRP) (n = 4677) was examined in individuals from the Whitehall II study. Following multiple adjustments, there were no overall linear or nonlinear trends between sleep duration and IL-6. However, in women but not men (interaction P < 0.05), levels of IL-6 tended to be lower in individuals who slept 8 hours (11% [95% confidence interval 4 to 17]) as compared to 7 hours. With hs-CRP, in the adjusted model, there was no association between hs-CRP and sleep duration in men. However, there was a significant nonlinear association in women, the level of hs-CRP being significantly higher in women short sleepers (5 hours or less) after multiple adjustments (P = 0.04) (interaction P < 0.05).
Conclusions:
No significant variation in inflammatory markers with sleep duration was observed in men. By contrast, both IL-6 and hs-CRP levels varied with sleep duration in women. The observed pattern of variation was different according to the inflammatory marker observed. Further longitudinal studies are required to fully investigate possible temporal relationships between short sleep and markers of inflammation.
Citation:
Miller MA; Kandala NB; Kivimaki M; Kumari M; Brunner EJ; Lowe GDO; Marmot MG; Cappuccio FP. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. SLEEP 2009;32(7):857-864.
Keywords: Inflammation, Sleep, Cardiovascular disease
SLEEP DEPRIVATION ARISING THROUGH SLEEP DISTURBANCES OR THROUGH VOLUNTARY SHORTENED SLEEP IS ASSOCIATED WITH A NUMBER OF HEALTH outcomes, including cardiovascular disease.1–13 A number of studies in humans have indicated possible pathophysiologic mechanisms to support the biologic plausibility of an association between sleep deprivation and cardiovascular risk.14–17 Acute curtailments of sleep may induce an overactivity of the sympathetic nervous system, leading to increased blood pressure.18–20 Other contributing mechanisms may include overactivity of the renin-angiotensin-aldosterone system, proinflammatory responses, endothelial dysfunction, and renal impairment.14,21 However, it is also plausible that sleep habits may represent a marker of health status and quality of life rather than being a causal factor for many health outcomes.
Inflammatory mechanisms are important in the development of cardiovascular disease. Furthermore, short-term sleep-deprivation studies have shown that inflammatory markers are elevated in sleep-deprived individuals, suggesting that inflammatory mechanisms may play a role in the cardiovascular risk associated with sleep deprivation.14–16 The concentration of high-sensitivity C-reactive protein (hs-CRP), a nonspecific marker of acute-phase inflammatory response, is predictive of future cardiovascular morbidity,22 and the relationship of interleukin-6 (IL-6) to coronary heart disease is similar to that of CRP and coronary heart disease.23 Moreover, biologic variability of IL-6 is twice as high as that of hs-CRP, and, after correction for this, the association with coronary heart disease is stronger.23 However, although hs-CRP is elevated following both acute total and short-term partial sleep deprivation,24 a recent study failed to demonstrate any significant association between hs-CRP levels and sleep duration in a combined study of men and women.25
In the present analysis, we sought to examine the relationship between both hs-CRP and IL-6 and sleep duration in the Whitehall II Study. We have conducted gender-specific analyses with the inclusion of a number of potential confounding variables. This is because studies indicate that durations of sleep might be associated with detrimental effects on cardiovascular outcomes among women1–4: moreover hs-CRP levels are elevated in women,26 and gender differences in other inflammatory markers have been reported.27
METHODS
Study Population
The Whitehall II cohort was recruited in 1985-1988 (Phase 1) from 20 London-based civil service departments (participants were aged 35-55 years). The rationale, design, and methods of the study have been described in detail elsewhere.28 In this report, we use data from Phase 3, at which phase sleep data were available. At this phase, sleep duration was determined from the question “On an average weekday how many hours do you spend on the following activities; (a) Work, (b) Time with family, (c) Sleep?” Response categories were 1 to 12 hours. These categories were collapsed to form categories of 5 hours or less, 6 hours, 7 hours, 8 hours, and 9 hours or more). The participation rate of the original cohort (n = 10,308) was 83% at Phase 3. We examined only white individuals with complete data on sleep (n = 5100) and IL-6 (n = 4642), of whom 73% were men, and sleep data and hs-CRP (n = 4677), of whom 73% were men (see Tables 1a and 1b).
Table 1a.
Baseline Characteristics (Phase 3, 1991-93) in 3382 Men Across Categories of Sleep Duration. The Whitehall II Study
| Sleep duration, h |
P valuea | |||||
|---|---|---|---|---|---|---|
| ≤5 | 6 | 7 | 8 | 9+ | ||
| Subjects, no. | 103 | 698 | 1650 | 845 | 86 | |
| Age, yb | 48.7 ± 5.8 | 48.7 ± 5.7 | 49.0 ± 5.9 | 49.5 ± 6.2 | 50.7 ± 6.5 | 0.031 |
| Lowest level of employmentc | 9 (8.7) | 46 (6.6) | 38 (2.3) | 27 (3.2) | 1 (1.2) | < 0.001 |
| Not married | 31 (30.1) | 136 (19.5) | 240 (14.6) | 129 (15.3) | 15 (17.4) | < 0.001 |
| Current smoking | 16 (15.5) | 91 (13.1) | 156 (9.5) | 75 (8.9) | 7 (8.1) | 0.014 |
| Weekly alcohol consumption, units | 12.6 ± 16.0 | 13.3 ± 14.4 | 12.6 ± 13.5 | 12.4 (13.2) | 14.3 (15.7) | 0.67 |
| SF-36 score | ||||||
| Mental | 49.1 ± 11.6 | 50.7 ± 8.3 | 51.8 ± 7.7 | 51.9 ± 7.9 | 50.3 ± 9.9 | 0.003 |
| Physical | 52.6 ± 7.2 | 53.3 ± 5.8 | 53.9 ± 5.2 | 53.0 ± 5.8 | 51.4 ± 7.2 | < 0.001 |
| BMI, kg/m2 | 25.7 ± 3.5 | 25.4 ± 3.3 | 24.9 ± 2.9 | 24.8 ± 3.0 | 25.9 ± 4.0 | < 0.001 |
| Waist circumference, cm | 89.2 ± 10.1 | 88.0 ± 9.8 | 86.7 ± 8.7 | 86.9 ± 9.0 | 89.6 ± 10.5 | < 0.001 |
| Blood pressure, mmHg | ||||||
| Diastolic | 82.1 ± 8.8 | 80.5 ± 8.9 | 80.1 ± 9.0 | 81.3 ± 8.9 | 83.2 ± 8.5 | < 0.001 |
| Systolic | 123.5 ± 13.6 | 121.1 ± 13.1 | 120.9 ± 12.4 | 121.9 ± 13.0 | 125.1 ± 13.1 | 0.010 |
| HDL-cholesterol, mmol/Ld | 1.29 (1.23-1.36) | 1.26 (1.23-1.30) | 1.27 (1.25-1.29) | 1.27 (1.23-1.30) | 1.16 (1.03-1.32) | 0.51 |
| Triglycerides, mmol/Ld | 1.22 (1.10-1.35) | 1.30 (1.25-1.36) | 1.28 (1.25-1.32) | 1.33 (1.28-1.38) | 1.58 (1.38-1.82) | 0.06 |
| Fasting glucose, mmol/Ld | 5.3 (5.2-5.4) | 5.3 (5.2-5.3) | 5.2 (5.2-5.3) | 5.3 (5.2-5.3) | 5.3 (5.2-5.5) | 0.56 |
| Fasting Insulin, mmol/Ld | 5.8 (5.1-6.7) | 5.4 (5.1-5.7) | 5.2 (5.0-5.4) | 5.2 (4.9-5.4) | 5.8 (4.9-6.9) | 0.14 |
| IL-6, pg/mLd | 1.52 (1.36-1.70) | 1.46 (1.39-1.52) | 1.35 (1.32-1.39) | 1.39 (1.34-1.44) | 1.47 (1.30-1.67) | 0.002 |
| hs-CRP, mg/Ld | 0.85 (0.67-1.08) | 0.86 (0.79-0.94) | 0.78 (0.74-0.83) | 0.77 (0.72-0.83) | 0.89 (0.70-1.14) | 0.19 |
Data are expressed as the mean ± SD or as number (percentage) unless otherwise indicated.
BMI refers to body mass index; HDL, high-density lipoprotein; IL-6, interleukin-6; hs-CRP, high-sensitivity C reactive protein.
P Value for comparison across sleep duration groups using the χ2 analysis for categorical variables and Kruskal-Wallis test for continuous variables.
Age ranges from 39-62 years (median: 48).
Clerical/support.
Data are presented as geometric mean (95% confidence interval).
Table 1b.
Baseline Characteristics (Phase 3, 1991-93) in 1260 Women Across Categories of Sleep Duration. The Whitehall II Study
| Sleep duration, h |
P valuesa | |||||
|---|---|---|---|---|---|---|
| ≤ 5 | 6 | 7 | 8 | 9+ | ||
| Subjects, no. | 56 | 274 | 583 | 304 | 43 | |
| Age, yb | 51.5 ± 6.0 | 49.9 ± 6.0 | 49.5 ± 5.9 | 49.1 ± 6.3 | 48.9 ± 6.2 | 0.05 |
| Lowest level of employmentc | 19 (33.9) | 83 (30.3) | 162 (27.8) | 77 (25.3) | 15 (34.9) | 0.68 |
| Not married | 30 (53.6) | 128 (46.9) | 222 (38.1) | 93 (30.7) | 13 (30.2) | < 0.001 |
| Current smoking | 12 (21.4) | 50 (18.3) | 83 (14.2) | 33 (10.9) | 2 (4.7) | 0.017 |
| Weekly alcohol consumption, units | 5.7 (7.4) | 5.9 (7.1) | 6.1 (7.2) | 6.7 (7.6) | 5.4 (9.0) | 0.11 |
| SF-36 scores | ||||||
| Mental | 49.9 ± 8.8 | 48.9 ± 10.0 | 50.5 ± 8.7 | 50.6 ± 8.9 | 48.8 ± 10.9 | 0.20 |
| Physical | 47.0 ± 10.6 | 51.1 ± 7.7 | 51.5 ± 7.6 | 50.9 ± 8.0 | 46.3 ± 11.3 | < 0.001 |
| BMI, kg/m2 | 26.0 ± 6.2 | 25.6 ± 4.6 | 24.9 ± 4.4 | 25.3 ± 4.3 | 26.0 ± 5.5 | 0.19 |
| Waist circumference, cm | 76.2 ± 14.3 | 74.9 ± 11.7 | 73.7 ± 11.1 | 74.5 ± 10.6 | 77.6 ± 12.6 | 0.20 |
| Blood pressure, mm Hg | ||||||
| Diastolic | 79.0 ± 8.7 | 75.6 ± 9.0 | 75.9 ± 9.2 | 75.8 ± 8.7 | 77.2 ± 9.5 | 0.08 |
| Systolic | 123.1 ± 12.7 | 115.7 ± 13.0 | 116.4 ± 13.8 | 115.7 ± 12.9 | 117.3 ± 13.8 | 0.002 |
| HDL-cholesterol, mmol/Ld | 1.66 (1.55-1.79) | 1.69 (1.64-1.74) | 1.66 (1.63-1.70) | 1.63 (1.56-1.70) | 1.59 (1.48-1.71) | 0.65 |
| Triglycerides, mmol/Ld | 1.09 (0.96-1.24) | 0.99 (0.94-1.05) | 0.98 (0.94-1.02) | 0.99 (0.93-1.04) | 1.05 (0.93-1.20) | 0.48 |
| Fasting glucose, mmol/Ld | 5.2 (5.0-5.5) | 5.0 (5.0-5.1) | 5.0 (5.0-5.1) | 5.0 (5.0-5.1) | 5.0 (4.9-5.2) | 0.17 |
| Fasting Insulin, mmol/Ld | 5.9 (4.7-7.4) | 4.4 (4.1-4.9) | 4.6 (4.4-4.9) | 4.8 (4.4-5.3) | 5.4 (4.3-6.8) | 0.31 |
| IL-6, pg/mLd | 1.79 (1.43-2.20) | 1.62 (1.51-1.74) | 1.62 (1.54-1.71) | 1.48 (1.39-1.58) | 2.02 (1.62-2.54) | 0.08 |
| hs-CRP, mg/Ld | 1.46 (1.03-2.05) | 0.97 (0.84-1.12) | 0.88 (0.80-0.97) | 0.96 (0.83-1.10) | 1.37 (0.96-1.95) | 0.020 |
Data are expressed as the mean ± SD or as number (percentage) unless otherwise indicated.
BMI refers to body mass index; HDL, high-density lipoprotein; IL-6, interleukin-6; hs-CRP, high-sensitivity C reactive protein.
P Value for comparison across sleep duration groups using the χ2 analysis for categorical variables and Kruskal-Wallis test for continuous variables.
Age ranges from 39-61 years (median: 49).
Clerical/support.
Data are presented as geometric mean (95% confidence interval).
Covariates
For the present analyses, age and other covariates were derived from the questionnaires at Phase 3. Employment grade was determined from the participant's civil service grade title, and the results were divided into 3 categories in order of decreasing salary: administrative, professional/executive, and clerical/support.
Participants were allocated to 1 of 2 smoking categories: never/ exsmoker, or current smoker. Alcohol consumption in the previous week was recorded (units per week).
General health status was assessed using the physical and mental health component summaries of the Short Form-36 (SF-36) health survey questionnaire28: low scores indicate low functioning. At the screening examination, anthropometric measures were recorded, including height, weight, and waist circumference; body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured 3 times using a standard mercury sphygmomanometer by trained and certified technicians, and the average of the last two readings was taken. Further details of these methods have been published previously.28
Individuals were asked to fast overnight if their appointment was before 11:30, and those with appointments between 12:30 and 14:30 were advised that they could have a light fat-free breakfast of unsweetened tea or coffee and plain bread or toast. Participants sat quietly without reading before having their blood pressure recorded, and, afterward, venipuncture of the left antecubital vein was performed with tourniquet. Blood was collected into plain, citrate or fluoride Sarstedt monovettes. After centrifugation, samples were immediately frozen at −80°C and stored until assay. Serum for lipid analyses was refrigerated at −4°C and assayed within 72 hours. Glucose was determined in fluoride plasma by an electrochemical glucose oxidase method. Insulin was measured by radioimmunoassay. Total cholesterol and triglyceride levels were measured in a centrifugal analyzer by enzymic colorimetric methods. High-density lipoprotein (HDL)-cholesterol was determined after dextran sulphate-magnesium chloride precipitation of non-HDL cholesterol. Low-density lipoprotein (LDL)-cholesterol concentration was calculated using the Friedewald formula.
Interleukin-6
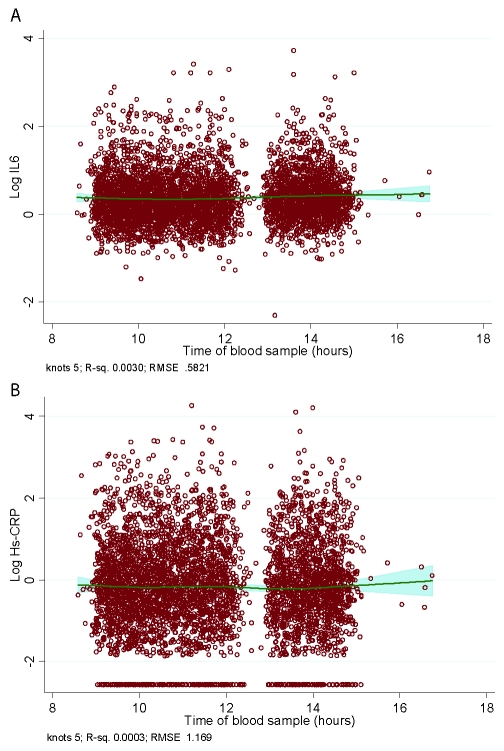
The median time for fasting blood collection to prepare serum was 11:40 (interquartile range [IQR] 10:10-13:58). IL-6 was measured in serum stored at −80°C until analysis using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) (R & D systems, Oxford, UK). Values lower than the detection limit (0.08 pg/mL) were assigned a value equal to half the detection limit. Intraassay and interassay coefficients of variation were 7.5% and 8.9%, respectively. To measure short-term biologic variation and laboratory error, a repeated sample was taken from a subset of 241 participants at Phase 3. Samples were not taken at the same time for repeat measures, and the median time for sample 1 was 11:42 (10:15-13:50) and, for the second sample, 10:09 (IQR 9:50-13:15). The average elapsed time between samples was 32 (SD = 10.5) days. Reliability between samples was assessed with interclass correlation: r = 0.62; P < 0.0001. Spline regression analysis was performed to take into account the effect of “time of blood sample,” using a restricted cubic splines. The results for IL-6 plotted against sampling time are shown in the Appendix (Panal A). A spline fit was applied to these results to determine the effect of time on the mean level. There is no major effect of time on these levels over this time period. The proportion of the variability due to “time of sampling” for IL-6 is 0.3%.
High-Sensitivity-C Reactive Protein
The median time for fasting blood collection to prepare serum was 11:40am (IQR 10:10-13:58). Hs-CRP was measured in serum stored at −80°C using a high-sensitivity immunonephelometric assay in a BN ProSpec nephelometer (Dade Behring, Milton Keynes, UK). Values below the detection limit (0.154 mg/L) were assigned a value of 0.077 mg/L. Intraassay and interassay coefficients of variation were 4.7% and 8.3%, respectively. As for IL6, a repeat sample was taken from a subset of participants at Phase 3 (n = 150). The median time for sample 1 was 11:42 (10:15-13:50) and, for the second sample, 10:09 (IQR 9:50-3.15). The average elapsed time between samples was 32 (SD = 10.5) days. Reliability between samples was assessed with interclass correlation: r = 0.83; P < 0.0001. Spline regression analysis demonstrated that there was no major effect of time on hs-CRP levels over the sampling time (Appendix (Panel B)). The proportion of the variability due to “time of sampling” for hs-CRP is 0.03%.
Ethics Committee Approval
Ethics committee approval for the Whitehall II study was obtained from the University College London Medical School committee on the ethics of human research. Subjects gave written informed consent.
Statistical Analysis
In the univariate analysis, for continuous and categorical variables, respectively, Kruskal-Wallis and χ2 tests were used to determine the statistical significance of any difference in the distribution of baseline variables at Phase 3 across categories of sleep duration.
Skewed variables were natural-log transformed so as to approach a normal distribution, and results are presented as geometric means and 95% confidence intervals (CI); these include triglyceride, HDL cholesterol, and fasting glucose levels. In addition, hs-CRP and IL-6 were positively skewed; therefore, analyses were performed on log-transformed data.
The statistical significance between inflammation at Phase 3 and categories of sleep duration, adjusted for other baseline variables, was tested in multivariate linear regression models. Results are expressed as geometric means ratios separately for IL-6 and hs-CRP by category of sleep duration, with 7 hours of sleep as the reference category (geometric means ratio = 1.00). All analyses were stratified by gender. All analyses were conducted in STATA 9.2. A spline regression analysis was performed to investigate the effect of time (using restricted cubic spline) of blood sampling on the mean levels of IL-6 and hs-CRP. Likewise, models 1 and 4 (Tables 2 and 3) were run with a spline time adjustment as well. A P value of less than or equal to 0.05 was considered statistically significant.
Table 2.
Cross-Sectional Relationships Between IL-6 and Duration of Sleepa at Phase 3 (1991-1993).The Whitehall II Study
| COEF (95% CI) | COEF (95% CI) | Reference | COEF (95% CI) | COEF (95% CI) | P valueb |
||
|---|---|---|---|---|---|---|---|
| Hours of sleep | ≤ 5 | 6 | 7 | 8 | 9+ | Linear | Nonlinear |
| Men, no. | 103 | 698 | 1650 | 845 | 86 | ||
| Model 1 | 1.11 (0.99, 1.24) | 1.06c(1.01, 1.12) | 1 | 1.01 (0.97, 1.06) | 1.06 (0.94, 1.21) | 0.05 | 0.04 |
| Model 2 | 1.09 (0.98, 1.22) | 1.05 (1.00, 1.11) | 1 | 1.01 (0.96, 1.05) | 1.06 (0.93, 1.20) | 0.15 | 0.23 |
| Model 3 | 1.07 (0.95, 1.21) | 1.04 (0.99, 1.10) | 1 | 1.02 (0.97, 1.06) | 1.05 (0.92, 1.19) | 0.27 | 0.27 |
| Model 4 | 1.08 (0.96, 1.22) | 1.05 (1.00, 1.10) | 1 | 1.02 (0.97, 1.06) | 1.03 (0.91, 1.17) | 0.17 | 0.33 |
| Women, no. | 56 | 274 | 583 | 304 | 43 | ||
| Model 1 | 1.05 (0.85, 1.31) | 0.98 (0.90, 1.07) | 1 | 0.90c (0.83, 0.97) | 1.24 (1.00, 1.53) | 0.58 | 0.04 |
| Model 2 | 1.03 (0.84, 1.27) | 0.94 (0.86, 1.02) | 1 | 0.87c (0.80, 0.94) | 1.16 (0.94, 1.45) | 0.66 | 0.11 |
| Model 3 | 1.00 (0.82, 1.22) | 0.94 (0.86, 1.02) | 1 | 0.89c (0.83, 0.96) | 1.22 (0.99, 1.51) | 0.92 | 0.10 |
| Model 4 | 1.00 (0.82, 1.22) | 0.94 (0.87, 1.02) | 1 | 0.89c (0.83, 0.96) | 1.23 (0.99, 1.53) | 0.98 | 0.06 |
Geometric mean ratio for interleukin-6 (IL-6) by category of sleep duration ( ≤ 5, 6, 7, 8, 9+) with 7 hours of sleep as the reference. COEF refers to coefficient; CI, confidence intervals.
P Values for test of linear and nonlinear trends.
P Values at 0.05 significance level for contrast between sleep categories and the reference (7 hours).
Model 1: adjusted for age and marital status
Model 2: adjusted for age and marital status + body mass index (BMI)
Model 3: adjusted for age and marital status + BMI + smoking
Model 4: adjusted for age and marital status + BMI + smoking + systolic blood pressure and triglycerides
Table 3.
Cross-sectional relationship between hs-CRP and duration of sleep in hoursa at Phase 3 (1991-1993).The Whitehall II Study
| COEF (95% CI) | COEF (95% CI) | reference COEF (95% CI) | COEF (95% CI) | COEF (95% CI) | P valueb |
||
|---|---|---|---|---|---|---|---|
| Hours of sleep | ≤5 | 6 | 7 | 8 | 9+ | Linear | Nonlinear |
| Men, no. | 105 | 708 | 1671 | 857 | 87 | ||
| Model 1 | 1.06 (0.84, 1.34) | 1.08 (0.97, 1.19) | 1 | 0.96 (0.87, 1.05) | 1.13 (0.89, 1.45) | 0.10 | 0.17 |
| Model 2 | 0.98 (0.78, 1.24) | 1.01 (0.91, 1.12) | 1 | 0.94 (0.86, 1.03) | 1.09 (0.87, 1.37) | 0.56 | 0.76 |
| Model 3 | 0.93 (0.74, 1.17) | 0.99 (0.90, 1.10) | 1 | 0.96 (0.88, 1.05) | 1.06 (0.86, 1.32) | 0.80 | 0.73 |
| Model 4 | 0.97 (0.77, 1.22) | 1.00 (0.91, 1.10) | 1 | 0.96 (0.88, 1.04) | 1.00 (0.80, 1.15) | 0.43 | 0.70 |
| Women, no. | 56 | 269 | 578 | 303 | 43 | ||
| Model 1 | 1.55c (1.09, 2.21) | 1.03 (0.87, 1.23) | 1 | 1.01 (0.85, 1.19) | 1.45c (1.01, 2.07) | 0.97 | 0.004 |
| Model 2 | 1.49c (1.07, 2.09) | 0.95 (0.81, 1.12) | 1 | 0.93 (0.79, 1.09) | 1.27 (0.91, 1.77) | 0.82 | 0.03 |
| Model 3 | 1.38 (1.00, 1.92) | 0.94 (0.80, 1.10) | 1 | 1.00 (0.86, 1.17) | 1.43c (1.03, 1.97) | 0.57 | 0.02 |
| Model 4 | 1.42c (1.02, 1.96) | 0.95 (0.82, 1.11) | 1 | 1.00 (0.86, 1.16) | 1.35 (0.99, 1.85) | 0.71 | 0.04 |
Geometric mean ratio for high-sensitivity C reactive protein (hs-CRP) by category of sleep duration ( ≤5, 6, 7, 8, 9+) with 7 hours of sleep as the reference.
P Values for test of linear and nonlinear trends.
P Values at 0.05 significance level for contrast between sleep categories and the reference (7 hours).
Model 1: adjusted for age and marital status.
Model 2: adjusted for age and marital status + body mass index (BMI).
Model 3: adjusted for age and marital status + BMI + smoking.
Model 4: adjusted for age and marital status + BMI + smoking + systolic blood pressure and triglyceride level
RESULTS
Characteristics of the Population by Categories of Sleep Duration
The interaction between sleep duration and gender on both inflammatory markers was significant (P < 0.05); therefore, all analyses were stratified by gender. Characteristics for the participants at Phase 3 in men and women are reported in Table 1a and 1b by categories of sleep duration.
In general, participants of both sexes sleeping 5 hours or less had a poorer health status and lifestyle profile. This was particularly evident in men. Men sleeping 5 hours or less were more likely to be in the lowest employment grade, to be unmarried, to have a higher BMI and waist circumference, to have lower mental and physical health scores, to smoke, and to have a higher diastolic blood pressure. Women were more likely to be unmarried, have lower physical health scores, to smoke, and to have higher systolic blood pressure.
Men sleeping 9 hours or more were also more likely to have an increased BMI and waist circumference and to have a reduced physical health score. Likewise, women sleeping 9 hours or more were more likely to have a decreased physical health score and increased blood pressure.
No significant effect of time of blood sampling was observed on mean IL-6 or hs-CRP levels.
In men, a significant variation in IL-6 with sleep categories was observed (P = 0.002), with the IL-6 levels being highest in short and long sleepers (Table 1a). No difference in hs-CRP was observed (P = 0.19) (Table 1a). By contrast, in women, there was no difference in IL-6 levels with sleep categories (P = 0.08; Table 1b), but there was a significant difference in hs-CRP (P = 0.02; Table 1b).
Multiple Regression Analysis
A correlation matrix between inflammatory markers and risk factors helped to identify confounders, which were used in the multiple regression analysis. The level of the inflammatory markers at 7 hours of sleep was used as a reference. Results are shown in Table 2 for IL-6 and Table 3 for hs-CRP. Age and marital status, which vary significantly with categories of sleep (Table 1), were used as covariates in the basic Model (Model 1) to which other variables were added.
Interleukin-6
In men, linear and nonlinear trends, when adjusted for age and marital status, were significant (P = 0.05 and P = 0.04). This was not significant when employment status was used instead of marital status (results not shown). The associations were no longer statistically significant when adjusted for BMI, smoking, serum lipid levels, and blood pressure. Substitution of employment status instead of marital status into Model 4 had no appreciable difference (P value for nonlinear trend 0.40 and 0.33). Likewise, substitution of waist measurement for BMI (0.36 and 0.33) or fasting insulin level for serum triglyceride level (0.21 and 0.33) had no appreciable effect. The adjustment for time of blood sampling had no effect on the outcome of Model 1 (P = 0.05 and P = 0.05 [linear]) and (P = 0.04 and P = 0.03 [nonlinear]) or Model 4 (P = 0.17 and P = 0.20 [linear]) and (P = 0.33 and P = 0.028 [nonlinear]: Results shown as Model and Model with time adjustment).
In women, the relationship between IL-6 and sleep was not linear following adjustment for age and marital status (P < 0.04), but the nonlinear trend was not significant following other model adjustments (Table 2). However, a consistent observation was that those individuals who slept 8 hours per night, as compared with those who slept 7 hours, had significantly lower levels of IL-6 (P < 0.05). Similar results were again observed when substituting employment status for marital status (0.06 and 0.06) or waist circumference for BMI (0.08 and 0.06). However, if adjustment for serum fasting insulin level instead of triglyceride level was made, the result was just significant (0.04 and 0.06).
The adjustment for time of blood sampling had no effect on the outcome of Model 1 (P = 058 and P = 0.61 [linear]) and (P = 0.04and P = 0.08 [nonlinear]) or of Model 4 (P = 0.98 and P = 0.92 [linear]) and (P = 0.06 and P = 0.11 [nonlinear]): Results shown as Model and Model with time adjustment).
The level of IL-6 was higher in postmenopausal women (1.81 [1.72-1.90]) compared with premenopausal women (1.44 [1.38-1.51]), irrespective of use of hormone replacement therapy among postmenopausal women or use of oral contraceptive agents in premenopausal women.
High-Sensitivity-C Reactive Protein
In men, there was no effect of category of duration of sleep on hs-CRP values (Table 3). This was also the case when the substitutions outlined in the previous section were made. The adjustment for time of blood sampling had no effect on the outcome of Model 1 (P = 0.10 and P = 0.10 [linear]) and (P = 0.17 and P = 0.14 [nonlinear]) or Model 4 (P = 0.43 and P = 0.47 [linear]) and (P = 0.70 and P = 0.72 [nonlinear]): Results shown as Model and Model with time adjustment).
In women, there was a significant nonlinear relationship between hs-CRP and duration of sleep (P < 0.004; Model 1). Levels of hs-CRP were significantly higher in women who slept less than 5 hours, compared with women who slept 7 hours, and these differences were maintained following the multiple adjustments for measures of obesity, smoking, and blood pressure. The levels of hs-CRP were also higher in women who slept more than 9 hours, but this difference was no longer significant after the adjustment for measures of obesity in the final fully adjusted model. Moreover, adjustment for employment status had no appreciable difference (P = 0.05 and 0.04) on the nonlinear trend, and, likewise, adjustment for fasting insulin (P = 0.05) adjustment for waist measurement instead of BMI attenuated the nonlinear effect (P = 0.06 and P = 0.04). The adjustment for time of blood sampling had no effect on the outcome of Model 1 (P = 0.97 and P = 0.90 [linear]) and (P = 0.004 and P = 0.005 [nonlinear]) or Model 4 (P = 0.71 and P = 0.77 [linear]) and (P = 0.04 and P = 0.05 [nonlinear]): Results shown as Model and Model with time adjustment).
The level of hs-CRP was higher in postmenopausal women (1.21 [1.10-1.33]), compared with premenopausal women (0.77 [0.71-0.85]). Although use of hormone replacement therapy among postmenopausal women did not affect the level of hs-CRP, although lower values were detected in premenopausal women not taking oral contraceptive agents, compared with those taking oral contraceptive agents (results not shown).
DISCUSSION
Our findings from more than 4600 British white-collar civil servants suggest that the associations of sleep duration with IL-6 and hs-CRP are different and that they are dependent on gender. Unadjusted observations suggest that IL-6 levels may vary with sleep in men and not women, but that the opposite is true for hs-CRP. However, following multiple adjustments for a number of confounding factors, no significant variation in inflammatory markers with sleep duration was observed in men. By contrast, hs-CRP levels varied significantly with sleep duration in women, and there was a trend for IL-6 to vary with sleep, but the observed pattern of variation was different according to the inflammatory marker observed.
This is the first large-scale study to describe the associations between measures of inflammation and sleep duration in both men and women. Although our findings are restricted to a Caucasian population, the study has the benefit that the population has been extensively characterized and, thus, allows for the adjustment of well-known confounders, including measures of obesity, serum lipid levels, and measures of social position. Although only self-reported measures of sleep duration were used, which did not explicitly ask participants to differentiate between time asleep and time in bed, it is accepted that it is not feasible to obtain more detailed assessment of sleep duration in such large epidemiologic studies. An association between self-reported duration of sleep and diary, actigraphy, or polysomnography estimates has been previously reported.3,29
Cross-sectional findings from this cohort show that unadjusted levels of IL-6 varied with sleep duration in men but not women but that these findings were not significant following adjustment for multiple risk factors, including measures of adiposity. A previous study demonstrated that sleep deprivation is associated with slowed glucose metabolism,30 and, although this could lead to an increase in central adiposity, our previous longitudinal study did not show any associations between sleep duration and the development of obesity.12 The level of IL-6 appeared to show more variability in women than in men (Figure 1). Although in women, as in men, there was no significant nonlinear relationship between sleep duration and IL-6 (P = 0.06), in each model, the level of IL-6 was significantly lower in those who slept 8 hours compared with the 7-hour reference group. However, the reason for this is unclear.
Previously, in a small adolescent cohort (n = 143; 50% female; 36% black), a negative correlation between sleep duration and hs-CRP was reported, although this was attenuated on adjustment for apnea-hypopnea index.31 However, in adults, no association between hs-CRP and sleep duration has been reported.25 This is in contrast with the results of short-term sleep studies, which have shown that hs-CRP is elevated following both acute total and short-term partial sleep deprivation.24 In this study, there was no relationship between hs-CRP and sleep duration in men. However, there was a significant nonlinear relationship in women. In particular, women who slept 5 hours or less had significantly higher hs-CRP levels than those sleeping 7 hours, even after multiple adjustments. Short sleep has previously been associated with an increase in all-cause mortality,11 and elevated hs-CRP is a nonspecific early marker of cardiovascular disease risk.22 It is possible that the observed elevation in hs-CRP in the short-sleeping women may reflect residual confounding due to preexisting morbidity, which may have been either undetected or poorly captured by our survey measures.
Low employment grade is associated with increased mortality,28 higher levels of a number of stressors such as work stress, low control at home, job and financial insecurity, and economic difficulties, and these may be associated with increased markers of inflammation.32–34 We did not find any major effect of employment grade on the observed sleep-related effects, and, given that marital status appeared to have a stronger association with sleep duration, the models used an adjustment for marital status instead of employment grade. Moreover, in the final model, substitution of marital status for employment grade had no effect. Furthermore, it is possible that these adjustments may not completely account for all social and economic factors likely to be patterned by these measures. This may be of particular importance in women, for whom a large percentage of individuals are in the lowest employment grade, and these factors may, therefore, contribute to observed gender differences.
This study adds to the growing body of evidence that suggests that there is a nonlinear relationship between cardiovascular risk factors and duration of sleep.35 Furthermore, it supports the idea that short sleep is associated with an increase in cardiovascular risk and that the association between sleep duration and cardiovascular risk is markedly different in men and women.10,36 Recently we reported a cross-sectional U-shaped relationship between sleep duration and mortality in individuals from the Whitehall II study,11 a cross-sectional relation between short sleep and obesity12 and between short sleep and hypertension,10 though the latter was only evident in women. This observation is supported by the recent study by Suarez et al. that demonstrated that indexes of sleep disturbance are associated with greater psychological distress, higher levels fasting insulin, fibrinogen, and inflammatory biomarkers but only in women.37 It is possible that some of the previous studies have not observed this gender difference because either they had an insufficient number of women in their cohorts or because they adjusted for gender as opposed to using gender-stratified analyses.25
Constant-routine studies in young adults, which have examined the inflammatory response to total sleep deprivation, suggest that sleep deprivation may trigger a stress response that includes stimulation of both proinflammatory and antiinflammatory cytokines.38 Furthermore, inflammatory cytokines display marked circadian rhythms, and, although sleep deprivation resulted in significantly decreased hs-CRP levels in the morning and evening in these studies, IL-6 levels were decreased during sleep deprivation across most of the day. Our samples were taken at only 1 time point, and this, as well as the fact that hs-CRP may have more downstream effects than IL-6, may, in part, account for the observed difference in response between hs-CRP and IL-6. Sleep induces a 24-hour oscillation between predominant type 1 (defense mediated, proinflammatory) and type 2 (antiinflammatory) cytokines and, in this way, acts to globally increase the efficacy of adaptive immune responses.39 Furthermore, the balance between type 1 and type 2 cytokines is gender dependent, with men showing a greater type 2 response.40 This may also contribute to the observed gender differences.
The effect of short sleep duration on cardiovascular risk may be mediated through sleep disorders and sleepiness due to inadequate sleep and sleeping difficulties.41,42 Unfortunately, these were not measured at Phase 3 of the Whitehall II study. However, the effects observed in this study were maintained after adjusting for important covariates of sleep disorders, such as obesity. In terms of cardiovascular prevention, our findings are consistent with the idea that sleeping 7 or 8 hours per night appears to be optimal for health, but prospective studies are needed to confirm this concept. Although evidence from randomized and controlled studies in healthy individuals14 have indicated that there is a temporal relationship between acute and short-term chronic sleep deprivation and inflammation, further work is required, possibly with improved assessment of long-term exposure (repeated self-reported sleep duration) to investigate these mechanisms more fully in large longitudinal epidemiologic cohorts.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. Dr. Cappuccio holds the Cephalon Chair, an endowed post at Warwick Medical School, the result of a donation from the company. The appointment to the Chair was made entirely independently of the company, and the postholder is free to devise his own program of research. Cephalon does not have any stake in the Intellectual Property (IP) associated with the postholder, and the Chair has complete academic independence from the company. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Whitehall II study has been supported by grants from the Medical Research Council; British Heart Foundation; Health and Safety Executive; Department of Health; National Heart Lung and Blood Institute (HL36310), US, NIH: National Institute on Aging (AG13196), US, NIH; Agency for Health Care Policy Research (HS06516); and the John D and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. M. Ki is supported by the Academy of Finland, and MGM by an MRC Research Professorship. We thank all participating civil service departments and their welfare, personnel, and establishment officers; the Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team. We thank Dr. A. Rumley for the hs-CRP and IL-6 measurements. This work is part of the Sleep, Health & Society Programme at the University of Warwick supported by grants from the Research Development Fund of the University of Warwick, the Wingate Foundation, and the NHS Workforce.
Appendix
Spline regression analysis. Plots show the effect of sampling time on mean (A) interleukin-6 (IL-6) and (B) high-sensitivity-C reactive protein (hs-CRP levels) (restricted cubic splines).
REFERENCES
- 1.Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Ayas NT, White DP, Al-Delaimy WK, Manson JE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 3.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 4.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamakoshi A, Ohno Y. Self-reported sleep duration as a predictor of all-cause mortality: results from the J Am Coll Cardiol study, Japan. Sleep. 2004;27:51–4. [PubMed] [Google Scholar]
- 6.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 7.Kohatsu ND, Tsai R, Young T, VanGilder R, Burmeister LF, Stromquist AM, Merchant JA. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166:1701–5. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 8.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, Stranges S, Kandala N-B, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:694–701. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrie J, Shipley MJ, Cappuccio FP, et al. Sleep duration and change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol. 2008;167:321–9. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappuccio FP, Taggart FM, Kandala N-B, Currie A, Stranges S, Miller MA. Meta-analysis of short sleep and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller M A, Cappuccio F P. Invited Review: Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 15.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–9. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 17.Shearer WT, Reuben JM, Mullington JM, et al. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–70. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- 18.Tochikubo O, Ikeda A, Miyajima E, Ishii M. Effects of insufficient sleep on blood pressure monitored by a new multibiomedical recorder. Hypertension. 1996;27:1318–24. doi: 10.1161/01.hyp.27.6.1318. [DOI] [PubMed] [Google Scholar]
- 19.Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–5. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 20.Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. Am J Hypertens. 1999;12:63–8. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 21.Wolk R, Somers VK. Sleep and the metabolic syndrome. Exp Physiol. 2007;92:67–78. doi: 10.1113/expphysiol.2006.033787. [DOI] [PubMed] [Google Scholar]
- 22.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 23.Danesh J, Mann AG, Kaptoge S, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 25.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)—no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel DA, Srinivasan SR, Xu JH, Li S, Chen W, Berenson GS. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism. 2006;55:699–705. doi: 10.1016/j.metabol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Miller MA, Sagnella GA, Kerry SM, Strazzullo P, Cook DG, Cappuccio FP. Ethnic differences in circulating soluble adhesion molecules: the Wandsworth Heart and Stroke Study. Clin Sci (Lond) 2003;104:559–60. doi: 10.1042/CS20020333. [DOI] [PubMed] [Google Scholar]
- 28.Marmot MG, Davey Smith G, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–93. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 29.Signal TL, Gale J, Gander PH. Sleep measurement in flight crew: comparing actigraphic and subjective estimates to polysomnography. Aviat Space Environ Med. 2005;76:1058–63. [PubMed] [Google Scholar]
- 30.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 31.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 32.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–9. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 33.Owen N, Poulton T, Hay FC, Mohamed-Ali V, Steptoe A. Socioeconomic status, C-reactive protein, immune factors, and responses to acute mental stress. Brain Behav Immun. 2003;17:286–95. doi: 10.1016/s0889-1591(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 34.Gimeno D, Brunner EJ, Lowe GD, Rumley A, Marmot MG, Ferrie JE. Adult socioeconomic position, C-reactive protein and interleukin-6 in the Whitehall II prospective study. Eur J Epidemiol. 2007;22:675–83. doi: 10.1007/s10654-007-9171-9. [DOI] [PubMed] [Google Scholar]
- 35.Knutson KL, Turek FW. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep. 2006;29:878–9. doi: 10.1093/sleep/29.7.878. [DOI] [PubMed] [Google Scholar]
- 36.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 37.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain Behav Immun. 2008;22:960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frey DJ, Fleshner M, Wright KP., Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21:1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 40.Matarese G, Sanna V, Fontana S, Zappacosta S. Leptin as a novel therapeutic target for immune intervention. Curr Drug Targets Inflamm Allergy. 2002;1:13–22. doi: 10.2174/1568010023344931. [DOI] [PubMed] [Google Scholar]
- 41.Koskenvuo M, Kaprio J, Telakivi T, Partinen M, Heikkila K, Sarna S. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J. 1987;294:16–9. doi: 10.1136/bmj.294.6563.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partinen M, Telakivi T. Epidemiology of obstructive sleep apnea syndrome. Sleep. 1992;15(Suppl):S1–4. doi: 10.1093/sleep/15.suppl_6.s1. [DOI] [PubMed] [Google Scholar]