Abstract
Objectives:
Performance on many cognitive tasks varies with time awake and with circadian phase, and the forced desynchrony (FD) protocol can be used to separate these influences on performance. Some performance tasks show practice effects, whereas the Psychomotor Vigilance Task (PVT) has been reported not to show such effects. We aimed to compare performance on the PVT and on an addition test (ADD) across a 6-week FD study, to determine whether practice effects were present and to analyze the circadian and wake-dependent modulation of the 2 measures.
Design and Setting:
A 47-day FD study conducted at the Brigham and Women's Hospital General Clinical Research Center.
Participants:
Eleven healthy adults (mean age: 24.4 years, 2 women).
Measurements and Results:
For 2 baseline days and across 6 weeks of FD, we gave a test battery (ADD, PVT, self-rating of effort and performance) every 2 hours. During FD, there was a significant (P < 0.0001) improvement in ADD performance (more correct calculations completed), whereas PVT performance (mean reaction time, fastest 10% reaction times, lapses) significantly (P < 0.0001) declined week by week. Subjective ratings of PVT performance indicated that subjects felt their performance improved across the study (P < 0.0001), but their rating of whether they could have performed better with greater effort did not change across the study (P > 0.05).
Conclusions:
The decline in PVT performance suggests a cumulative effect of sleep loss across the 6-week study. Subjects did not accurately detect their declining PVT performance, and a motivational factor could not explain this decline.
Citation:
Lee JH; Wang W; Silva EJ; Chang AM; Scheuermaier KD; Cain SW; Duffy JF. Neurobehavioral performance in young adults living on a 28-h day for 6 weeks. SLEEP 2009;32(7):905-913.
Keywords: Alertness, PVT, cognitive throughput, forced desynchrony
IN MAMMALS, THE SUPRACHIASMATIC NUCLEUS IS A CENTRAL NEURAL PACEMAKER THAT GENERATES CIRCADIAN RHYTHMS IN PHYSIOLOGIC AND BEHAV-IORAL measures, including the timing of sleep, hormone production, body temperature, and waking neurobehavioral performance.1–4 Studies of humans isolated from daily changes in the environment have led to the realization that the timing and organization of sleep, alertness, and performance vary with the circadian phase of the underlying, near 24-hour oscillation.5–7 Neurobehavioral performance in many sleep-deprivation studies has also been reported to be closely linked to homeostatic factors influenced by the duration of prior wakefulness.8
Previous studies have suggested that it is the precise balance between sleep-wake homeostatic factors and the circadian rhythm of alertness and sleep propensity that allow for stable alertness and performance throughout the waking day during entrainment.9–11 A recent study in which chronic misalignment between internal biologic time and the imposed sleep-wake schedule was imposed across 4 weeks showed that cognitive performance (i.e., learning) was significantly impaired, whereas subjects in the same study who remained entrained improved their performance.12
Using forced desynchrony (FD) studies, the circadian modulation of performance and alertness has been revealed.9,13–15 A 28-hour FD protocol has shown that, even within the range of 0 to 18 hours of wakefulness, the contribution of the circadian system to variations of alertness and performance is approximately equal to the contribution of the sleep-wake homeostat in young subjects.10 A study using a 20-hour FD protocol (with a 13.7-hour wake episode) also found circadian modulation of alertness and several aspects of cognitive performance.14 Previous 28-hour FD studies have found that, when scheduled to sleep at an adverse circadian phase, healthy young adults cannot take advantage of the 9.33-hour sleep opportunity on every night.16,17 In fact, when the end of the scheduled sleep episode is at an adverse circadian phase (beginning in the biological daytime between about 110° and 290°), the latter quarter of the scheduled sleep episode is severely disrupted, with sleep efficiency averaging below 70%.17 However, how that lost sleep then impacts subsequent sleep and wake episodes and daytime performance was not explored in those studies.
A widely used performance test in studies of circadian rhythms or sleep deprivation is the Psychomotor Vigilance Task (PVT).18 This task has been reported to show no long-lasting training effects.8,18 In contrast, many other performance tests do have learning or practice effects, whereby, with repeated practice, performance on the task gradually improves.12,19,20 Therefore, consideration of those learning or practice effects should be made in analyzing data from long-duration studies. In a preliminary report, we found that a calculation performance task, a test of throughput, showed learning effects across a 4-week FD study, in which a saturating exponential function provided the best fit to the data for all subjects.21 Most prior studies in which both circadian and wake-dependent influences on performance have been assessed were of a 1- to 4-week duration.
In the study we report here, subjects participated in a 6-week 28-hour FD study designed to assess circadian period estimates. Several performance measures were given to the subjects throughout the protocol. To examine the practice effects on a calculation performance test, we included a similar addition task that had been used in our prior preliminary report.21 We also included the PVT, given the prior reports suggesting that it has no such practice effects. We aimed to examine whether performance on the addition and PVT would show significant changes across the 6-week FD study, as well as to analyze the circadian and wake-dependent homeostatic modulation on both types of performance, taking into account interindividual differences.
METHODS
Subjects
Eleven healthy volunteers, 9 men and 2 women (age 20–30 years), were studied. The data from these subjects were collected in a study on the effect of Vitamin B12 on the human circadian pacemaker. Subjects were healthy based on medical history, physical examination, blood and urine analysis, and electrocardiogram. Subjects were also psychologically healthy, based on questionnaires (Minnesota Multiphasic Personality Inventory II and Beck Depression Inventory) and on an interview with a clinical psychologist. None reported regular night work or rotating shift work within the past 3 years or crossing more than 1 time zone in the previous 3 months. The Partners Health Care Human Research Committee approved the protocol, and each subject gave written informed consent. The investigation was conducted according to the principles expressed in the Declaration of Helsinki.
Ambulatory and In-laboratory Conditions
Subjects were instructed to maintain a regular, self-selected, sleep-wake schedule of 8 hours scheduled bed rest per night at home for at least 7 nights prior to admission to the laboratory. Compliance with this was verified by sleep logs, a time-stamped sleep-wake call-in telephone system, and wrist actigraphy (Actiwatch-L; Mini-Mitter, a Respironics Co., Bend, OR). For the week prior to admission, subjects were instructed to abstain from all medications, caffeine, alcohol, and nicotine; urine toxicology screens were conducted during the screening and upon admission to the laboratory to verify compliance with this.
The inpatient portion of the studies was carried out in the Intensive Physiological Monitoring Unit of the General Clinical Research Center at Brigham and Women's Hospital. Each subject lived in a private study room for the duration of his or her 47-day inpatient study. The study room was designed to be a time-free environment without windows, clocks, or other information about time of day. Subjects maintained frequent contact with laboratory personnel and could communicate with friends and family via mail and email sent through laboratory personnel.
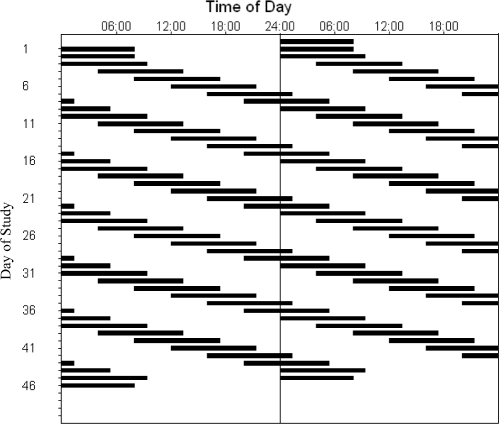
After 3 baseline days scheduled at their habitual times, subjects were scheduled on an FD protocol for 6 weeks (see Figure 1). During the FD protocol, the sleep-wake cycle was scheduled to a period of 28 hours, with a scheduled bed-rest opportunity of 9.33 hours and a waking episode of 18.67 hours. This resulted in each FD “day” beginning 4 hours later than the previous day, and subjects experienced 6 FD days per calendar week. Ambient light intensity was less than 15 lux during waking episodes (dim indoor light, ∼0.0087 W/m2, ∼3.3 lux at 137 cm from the floor facing toward the walls, with a maximum of 0.048 W/m2 anywhere in the room at 187 cm from the floor facing toward the ceiling-mounted light fixtures), and all lights were turned off during scheduled sleep episodes. Core body temperature (CBT) was measured every minute by means of a rectal thermistor (Measurement Specialties TPG, Dayton, OH), except during showers and bowel movements, and room temperature was maintained at 23° ± 2°C.
Figure 1.
Double raster plot of the 47-day study protocol. The horizontal axis represents time of day and is 48 hours in length. Black bars represent the scheduled bed-rest episodes. Subsequent days of the experiment are plotted both to the right of and below the previous day. After 3 baseline 24-hour days with 8 hours of scheduled bed rest, subjects began the forced desynchrony segment of the study, during which they were scheduled to live on a 28-hour day, with 9.33-hour bed-rest episodes beginning 4 hours later each day. This continued for 36 forced desynchrony days (equivalent to 42 calendar days).
During the 6 weeks of FD, subjects received 2 capsules approximately every 9 hours (just after wake time, in the middle of their wake episode, and just prior to bed time). For the first 2 FD weeks, these capsules were placebo for all subjects. For the remaining 4 FD weeks, half of the subjects continued to receive placebo and half of the subjects were randomly assigned to receive Vitamin B12 (3 mg/day) in a double-blind manner. For the purposes of the present analysis, subjects were considered as 1 group and were not distinguished by treatment condition.
Performance Tests
Subjects performed an approximately 20-minute computer-administered battery of neurobehavioral tests every 2 hours, beginning 2 hours after scheduled wake time. Subjects were instructed that speed and accuracy were equally important. Performance tests included an Addition Test22 (ADD) to assess cognitive throughput; the PVT,18 a 10-minute test of visual reaction time (RT); a Stroop Color-Word Test23 (results will be reported elsewhere); and a Performance Evaluation and Effort Rating Scale (PEERS).
The ADD presents the subject with a series of randomly generated pairs of 2-digit numbers on the screen (e.g., 34 + 29) and requires the subject to add the numbers in their head and type in the sum using the keyboard. When the enter key is pressed, the next pair of numbers is presented on the screen, and the task is continued for 4 minutes. We chose this task because performance on it has been shown to vary with both time awake and circadian phase.9,14,15,24 Performance on this task has also been reported to show a practice effect.12,21,25 We scored the test according to the number of correct calculations completed.
The PVT is a measure of sustained attention performance. This involves a 10-minute visual RT performance test, with an interstimulus interval randomly varying between 2 and 10 seconds. Subjects must monitor a task window on the screen and respond by pressing a response box button. Each task administration involves approximately 100 stimulus presentations. This task was chosen because of prior reports that it shows no practice effect8,18 and because performance on it has been shown to depend on both time awake and circadian phase.14,15,19,24 Mean RT, number of lapses of attention (defined as RTs > 500 milliseconds), and the fastest 10% of RTs for each test administration were used as measures of performance.
The PEERS is a 3-item series of questions that asks the subject to rate their performance on the preceding task (in this case, the PVT). They are asked to evaluate their overall level of performance, their level of exerted effort, and whether they could have performed better if they had increased their effort.
Data Analysis
We eliminated from our analysis any test in which the subject could have been distracted by interruption (by equipment malfunction or staff members), any PVT test that did not last for at least 8 minutes, and any test that was administered less than 1 hour following the previous test. According to these criteria, we eliminated approximately 0.4% of ADD and 0.8% of PVT tests from our final analysis. When a PVT test was excluded, we also excluded the accompanying PEERS test.
We analyzed the baseline data and FD data separately. For analysis of the baseline data, we first assigned a TIME AWAKE to each performance test based on the elapsed time since scheduled wake time that day. These data were binned into 2-hour TIME AWAKE bins (from 2–14 hours), for a total of seven 7 TIME AWAKE bins for the baseline days. We also assigned the CIRCADIAN PHASE bins on the baseline data using the same procedure described below for the FD data, applying the backward projection from the first measurement of minimum CBT on the first day of FD.
For analysis of the FD data, we assigned a TIME AWAKE to each performance test as we had done for the baseline days and binned these data into 2-hour TIME AWAKE bins (from 2–16 hours), for a total of 8 TIME AWAKE bins. We also assigned a CIRCADIAN PHASE to each performance test using the CBT data collected throughout the FD. To do this, we used a nonorthogonal spectral analysis (NOSA) technique with an exact maximum likelihood fitting procedure to estimate the intrinsic circadian period from the FD CBT data.26 Using the period and the projection of the CBT minimum on the first day of FD (assigned circadian phase 0°), we could then assign a circadian phase from 0° to 359° to each minute of the FD segment of the study. These data were then binned into 60° circadian phase bins (each approximately 4 circadian hours), for a total of 6 CIRCADIAN PHASE bins. We also assigned an FD CYCLE to each FD performance test based on the elapsed time within the 6-week FD segment, resulting in 6 FD CYCLE bins.
The SAS software package version 9.1 (SAS Institute Inc, Cary, NC) was used for all statistical analyses. All neurobehavioral performance data were analyzed using mixed-model analysis (PROC MIXED in SAS), incorporating into the model a random intercept statement allowing for means to vary between subjects.27 The mean RT and fastest 10% RT from the PVT were transformed (reciprocal transformation) to better approximate a normal distribution, with results presented graphically on the original scales to improve interpretability. A generalized linear mixed-model analysis (PROC GLIMMIX in SAS) was used for the analyses of PVT lapses and PEERS data because this procedure allows the user to specify the type of distribution. We specified a Poisson distribution for PVT lapses and a multinomial distribution for PEERS data.
All performance measures and PEERS were first assessed by testing for the main effects of TIME AWAKE, CIRCADIAN PHASE, and FD CYCLE, all of which were tested as categorical variables. We next tested all possible 2-way interactions in which the main effects were significant. When there were significant 2-way interactions, we also tested 3-way interactions. Modified Bonferroni correction factors were used for determining a significance of comparison when there was a significant effect. For all statistical tests, the significance level was defined as P value less than 0.05.
RESULTS
From 11 subjects who began the study, our final analysis was done on data from only 9 subjects (mean age: 24.4 years, seven men and two women). We excluded 1 subject from analysis because he completed only 15 of the 47 days of the study. We excluded the PVT data from another subject because examination of his PVT data suggested that he did not fully comply with the directions in performing this test and, consequently, his ADD data were also excluded from our analysis.
Baseline Condition versus FD Condition
In comparing the neurobehavioral performance data from the baseline condition with those from the entire 6-week FD condition (Table 1), there were significant effects of condition on both ADD and PVT performance while controlling for time awake. Specifically, the number of correct responses on the ADD was greater during FD than during baseline, and, on the PVT, the mean RT, fastest 10% RT, and number of lapses were greater during FD than during baseline
Table 1.
Comparison of Performance Measures from Forced Desynchrony Versus Baseline Condition
| FD (all) | FD (similar phase to BL) | |
|---|---|---|
| ADD, # correct | ||
| df | 1,2672 | 1,328 |
| F value | 611.78 | 308.38 |
| P value | < 0.0001 | < 0.0001 |
| PVT mean RT | ||
| df | 1,2665 | 1,328 |
| F value | 137.9 | 100.38 |
| P value | < 0.0001 | < 0.0001 |
| PVT, fastest 10% RT | ||
| df | 1,2665 | 1,328 |
| F value | 40.88 | 24.14 |
| P value | < 0.0001 | < 0.0001 |
| PVT Lapse | ||
| df | 1,2665 | 1,328 |
| F value | 503.56 | 403.49 |
| P value | < 0.0001 | < 0.0001 |
In the left column, the entire forced desynchrony (FD) condition is compared with the baseline (BL) condition, whereas, in the right column, only those FD days that began at a similar circadian phase as the BL days were included. ADD refers to Addition Calculation Test; PVT, Psychomotor Vigilance Task; RT, Reaction Time; df, degrees of freedom.
Because the baseline days occurred at a fixed range of circadian phases, to determine whether these observed differences between baseline condition and FD condition were due to the broader range of phases during FD or due to some other factor or factors associated with the FD condition, we conducted a secondary analysis restricting our comparison to those FD days that occurred at the same circadian phases as the baseline days. To do this, we first assessed the baseline circadian phase of wake time for each of the 9 subjects. The baseline wake time phases ranged from 33.75° to 66.75° with 1 exception (subject 26N8, the subject with the shortest circadian period, whose wake time phase was 87.8°). We conducted our analyses both with and without this latter subject to determine whether including him would affect the overall results of this analysis. Because the results were similar whether or not his data were included, we chose to include his data in the results reported here. For this analysis, we selected only those FD days in which wake time phase was in the 33.75° to 66.75° range; when we did this, 1 subject (25K2) had no FD wake times in that phase range, and, therefore, our comparison of baseline days with FD days beginning at the same circadian phases was conducted on only 8 subjects (a total of 16 baseline days and 30 FD days from those 8 subjects). This reanalysis of baseline versus FD condition performance also showed significant effects of condition on both ADD and PVT performance (Table 1).
ADD Test Performance
Although our outcome measure on the ADD test was number correct, we first examined the percentage of correct responses on the ADD test to determine whether that factor varied within or between the subjects. For most of the subjects, the percentage correct was above 85% on all trials (range 86.8% – 100%). However, 1 subject's (2605) percentage of correct responses began at a lower level (69.8%) and then increased significantly over the experiment (R2 = 0.33, P < 0.0001). We therefore conducted our analysis of ADD performance both with and without this subject's data to ensure that the overall results were not biased by his increased performance accuracy across the study. The overall results did not differ whether this subject's data were included or excluded, so we therefore report results with his data included.
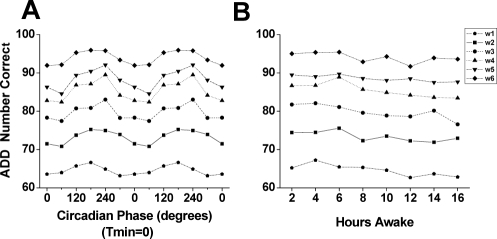
We found a significant effect of time awake (F7,2554 = 3.04, P = 0.0034) on ADD performance, with stable performance until about 6 hours awake and a very gradual decline after that (see Panel A in Figure 2). We also observed a significant effect of circadian phase (F5,2556 = 10.25, P < 0.0001) on ADD performance, with ADD performance lowest near the phase of the CBT minimum (0°) and highest around 240° (see Panel A in Figure 2).
Figure 2.
Main effects of circadian phase (left) and time awake (right) on performance across the 6-week forced desynchrony segment. Data in the left (circadian phase) panels are double-plotted with respect to the circadian phase of the core body temperature rhythm (with the fitted temperature nadir (Tmin) = 0°). Data in the right (time awake) panel are plotted with respect to the time since scheduled wake time. Panel A: Performance on an addition calculation test (ADD), showing number of correct calculations per 4-minute test. Panel B: Psychomotor Vigilance Task (PVT) mean reaction time (RT), in milliseconds (msec). Panel C: PVT fastest 10% RT. Panel D: Number of PVT lapses (RT > 500 milliseconds). Note that the y axes for Panels B-D are inverted to better visualize how performance on all 4 measures change in parallel.
When we examined performance on the ADD test across the 6 weeks of FD, there was a significant effect of FD week on the number of correct responses (F5,2556 = 588.65, P < 0.0001), with a continuous gradual increase in ADD performance week by week (see Figure 3). There was no significant interaction effect among FD week, circadian phase, and time awake (P > 0.05).
Figure 3.
Impact of forced desynchrony week on performance on the addition calculation test (ADD) with respect to circadian phase (left panel) and with respect to time awake (right panel). For each panel, the data from each of the 6 forced desynchrony weeks are plotted separately so that the change across the 6 weeks can be visualized. Data in the left panel are double plotted with respect to the circadian phase of the core body temperature rhythm (with the fitted temperature nadir (Tmin) = 0°).
PVT Performance
We observed significant effects of time awake (F7,2547 = 14.84, P < 0.0001) and circadian phase (F5,2549 = 72.45, P < 0.0001) on mean RT. As Panel B of Figure 2 illustrates, mean RT gradually declined across the wake episode (right panel) and mean RT was slowest around the nadir of the CBT rhythm (left panel).
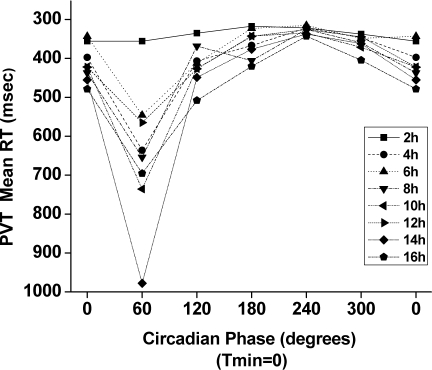
There were also significant effects of FD week on mean RT (F5,2549 = 44.05, P < 0.0001). There was also a significant interaction between time awake and circadian phase (F35,2502) = 1.83, P = 0.0021). As Figure 4 suggests, this interaction was most prominent at 60° and was not significant at 180° and 240° (P > 0.05).
Figure 4.
Interaction of time awake with circadian phase on mean reaction time (RT). In this plot, Psychomotor Vigilance Test (PVT) mean RT data from each 2-hour time awake bin are shown single plotted with respect to the circadian phase of the core body temperature rhythm (with the fitted temperature nadir (Tmin) = 0°). Note that the y axis is inverted to show that better performance is up and worse performance is down.
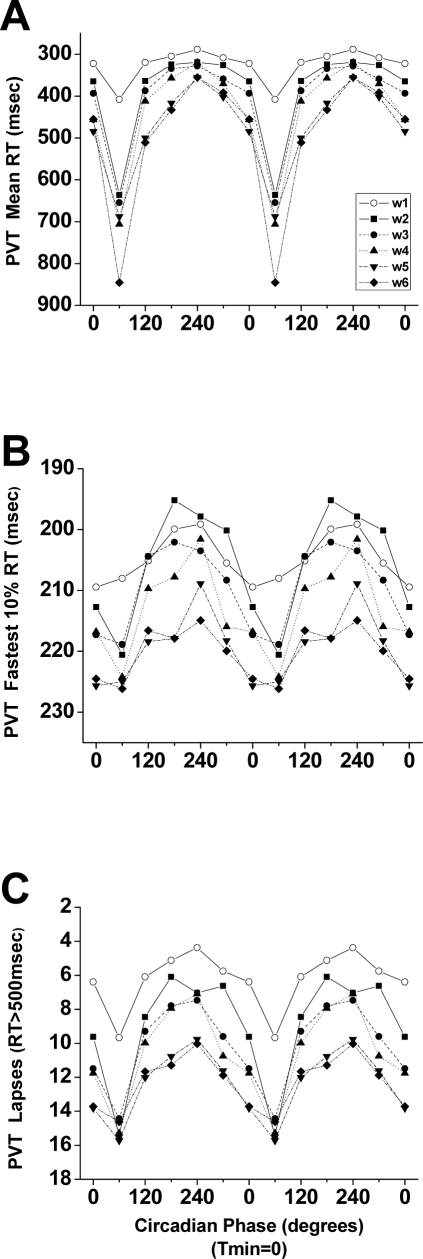
The fastest 10% RT were also significantly affected by time awake (F7,2547 = 4.69, P < 0.0001), circadian phase (F5,2549 = 38.14, P < 0.0001), and FD week (F5,2549 = 37.45, P < 0.0001). Panel C of Figure 2 shows that both the wake-dependent (right panel) and circadian (left panel) influences on fastest 10% RT paralleled overall mean RT. For the fastest 10% RT, there was a significant interaction between FD week and circadian phase (F25, 2512 = 1.95, P = 0.0033; see Panel B of Figure 5).
Figure 5.
Interaction of forced desynchrony week with circadian phase on Psychomotor Vigilance Test (PVT) reaction time (RT) data. PVT data from each forced desynchrony week are shown double plotted with respect to the circadian phase of the core body temperature rhythm (with the fitted temperature nadir (Tmin) = 0°). Panel A: mean RT. Panel B: fastest 10% RT. Panel C: lapses (RT > 500 milliseconds). Note that the y axes are inverted to show that better performance is up and worse performance is down.
For PVT lapses, we observed a significant effect of time awake (F7,2267 = 62.20, P < 0.0001), circadian phase (F5,2267 = 220.09, P < 0.0001), and FD week (F5,2267 = 181.49, P < 0.0001). Panel D of Figure 2 shows that there were progressively more lapses with longer time awake (right panel) and that lapses were most frequent around circadian phase 60° and least frequent around 240° (left panel). We also observed significant interactions between time awake and circadian phase (F35,2267 = 5.85, P < 0.0001), between time awake and FD week (F35,2267 = 3.95, P < 0.0001), and between circadian phase and FD week (F25,2267 = 6.47, P < 0.0001; see Panel C of Figure 5). In addition, the only 3-way interaction (time awake x circadian phase x FD week) we observed was seen on PVT lapses (F175,2267 = 3.86, P < 0.0001).
The results of PEERS (taken following each PVT) showed that there was a significant effect of each FD week (vs baseline) on the subjective performance level (F6,2662 = 5.05, P < 0.0001) and on the subjective level of exerted effort (F6,2665 = 5.47, P < 0.0001). Ratings on these questions showed relative improvements across the 6-week FD period from the baseline. However, there was no significant change in self-rated effort on performance across the 6 weeks.
DISCUSSION
Our present analysis focused on how performance on 2 different tasks, one a simple RT task and another a test of cognitive throughput, changed across a 6-week, inpatient, forced-desynchrony study. We found that there was a significant effect of FD week on both performance measures. In general, performance on the cognitive throughput task improved across the 6 weeks, whereas performance on the RT task worsened across the 6 weeks of the study when compared with baseline performance. These findings were the same even when we compared only those FD days that occurred at the same circadian phases as baseline.
Our finding that performance on the PVT declined over the course of the 6-week study was not expected. A previous report suggested that vigilance performance was maintained at a stable level when sleep was scheduled to 9 hours of time in bed each night.28 In our study, subjects were allowed 9.33 hours of time in bed each night, although, when their sleep was scheduled out of phase, they likely could not sleep for that entire time. However, in our analysis in which we examined only those FD days that occurred at similar phases to baseline, subjects very likely did sleep well on the nights before those days included in our analysis because, as we have reported in previous studies, young adults are able to remain asleep for 8 to 9 hours at those phases.16,17 Therefore, our finding of a decline in PVT performance across the 6 weeks of study even when only considering those days that occurred at a “normal” phase suggests that there was likely a cumulative effect of sleep loss causing the impaired performance.
In 2 prior studies in which the effects of different nightly durations of sleep on vigilance were tested, Belenky et al28 and Van Dongen et al8 reported nonsignificant increases in PVT lapses across 7 to 14 days in their 7- and 8-hour per night sleep-opportunity conditions. Wright et al12 also reported that PVT median RT and lapses increased across 4 weeks of a laboratory study with an 8-hour per night sleep opportunity and that the changes in performance were not explained by changes in the subjects's reported motivation to perform. In our analysis of our subjects's self-reported effort and motivation, we found no main effect of FD on motivation when compared with baseline, after controlling for circadian phase and time awake. In fact, most responses on the motivation factor scale were “No, I couldn'st have done better” or “Yes, I could have done a little better”. If results from this self-report scale can be relied upon (see below), this implies that the decline in performance on the PVT in our subjects was not the result of a decrement of motivation to perform. Further supporting the idea that lack of motivation cannot explain the decline in RT performance in our subjects is the fact that their performance on the ADD test increased across the 6 weeks of study.
Although a previous study on the influence of motivation on ADD performance indicated that high subjective ratings of motivation were associated with high performance beyond circadian and sleep-wake dependent factors,29 other reports have suggested that motivation does not always change in the same way as does performance,8,30 in line with our current finding. Unexpectedly, our data also showed that subjects's self-assessed levels of performance on the PVT got better across the FD period from the baseline. Because this was contrary to the actual impairment of PVT performance, it seems that the subjects's awareness of their own performance level was also impaired across the FD period.
Our mixed-model analysis indicated that there were main effects of time awake, circadian phase, and FD week on the number of correct ADD responses. Notably, there was a continuous improvement in ADD performance across the 6 weeks of FD, and the posthoc analysis indicated that there were significant differences in ADD performance among each FD week (Figure 3). In a previous report on ADD performance over a similar month-long FD protocol, we found an increase in performance that could be described by a saturating exponential function.21 Our present results are consistent with that finding and suggest the possibility that there would be further gradual improvement in ADD29 performance even beyond 6 weeks. In the 4-week entrainment study by Wright et al.,12 they reported that the improvement of ADD performance continued at a relatively constant rate in subjects whose circadian system remained synchronized with their sleep-wake schedule, whereas the subjects who did not remain synchronized did not show any such improvement. Although our subjects were not synchronized, they still showed week-by-week improvement in ADD performance across the 6 study weeks.
As in prior studies, we found a robust circadian modulation of ADD performance, with lowest performance levels at the phase near the nadir of the core temperature rhythm and best performance at the phases approximately opposite to that.9,13–15 We also observed a significant effect of time awake on ADD performance, with a gradual decline starting near the beginning or in the early part of the wake episode.10,14,15 As in prior studies, we also observed an interaction between time awake and circadian phase, with little circadian modulation of alertness or performance near the start of the waking day and a more pronounced circadian influence as time awake increased.
Our study also revealed that there were significant main effects of time awake and circadian phase on all 3 aspects of PVT performance. Slowest PVT performance was near the nadir of the CBT rhythm, and the fastest performance was around 240°. This is similar to what has been reported from other studies.14 Mean RT and fastest 10% RT started to increase after about 6 hours awake, whereas lapses continuously increased across the waking episode. This suggests that the number of lapses may be a more sensitive measure of the homeostatic influence on vigilance than are the RT measures.
We also saw significant interactions between time awake and circadian phase on mean RT and lapses but not on the fastest 10% RT. For mean RT, the effect of time awake at circadian phase 60°, just after the CBT nadir and near the time of awakening under entrained conditions, was different from that at 180° to 240°. It suggests a stronger circadian modulation of vigilance around the CBT nadir.
There was a significant main effect of FD week on mean RT, fastest 10% RT, and number of PVT lapses in our study. However, in contrast to the continuous week-by-week improvement that we observed in ADD performance, these performance parameters showed a worsening across the 6 weeks of FD. Our posthoc comparisons indicated that mean RT and number of lapses started to increase significantly from the first week, whereas the fastest 10% RT did not start to worsen until the third week.
A significant interaction between circadian phase and FD week was found in the fastest 10% RT and lapses but not in the mean RT. In addition, the lapses showed all significant 2-way interactions among time awake, circadian phase, and FD week, as well as a significant 3-way interaction. This finding suggests that the number of lapses of attention (here defined with an absolute threshold as RT > 500 milliseconds) is the most sensitive and vulnerable measure on the PVT in studies of this type in which the relationship between circadian phase and time awake is manipulated and assessed multiple times.
In the beginning of our discussion, we suggested that a possible explanation for the PVT performance decline was the cumulative sleep debt that results from subjects being scheduled to sleep at adverse circadian phases. When sleep is scheduled at adverse phases for multiple nights, subjects do not have a sufficient opportunity to “make up” much lost sleep in a 9.3-hour sleep opportunity. Therefore, when the FD schedule cycles back into an appropriate phase for sleep and subsequent wake, subjects's performance could remain impaired due to this cumulative sleep loss. Evidence that cumulative sleep loss is not quickly paid back comes from several studies of sleep extension. Roehrs et al31 reported that young subjects with self-reported sleep durations of 7 to 8 hours took nearly 2 weeks of 10 hours bedtime extension to level off to a total sleep time (TST) of 8.6 hours, whereas a study by Wehr's group on extended sleep in 14-hour nights for 4 weeks reported that TST eventually leveled off at 8.9 hours.32 More recently, Klerman and Dijk33 reported that the asymptotic value for TST was 9.2 hours in healthy young subjects with habitual sleep durations of 7 to 9 hours. Results from all of these studies imply that average sleep need in young adults is in the range of 8.5 to 9 hours. If this is the case, subjects in our study could have “made up” only about 20 to 45 minutes of lost sleep on the nights when they were sleeping at an ideal phase and, thus, were unlikely to have had the ability to pay off their cumulative sleep loss from prior nights, resulting in their declining PVT performance.
An alternative explanation for why our subjects's performance on the RT task declined across the 6 weeks is that continually asking them to perform every 2 hours every day across the 6 weeks was just too much of a cognitive load, and that if they had “weekends” or days off during which they were not asked to perform, they could have maintained their performance despite the cumulative sleep loss. However, as noted above, the subjects were able to improve their performance on the ADD task across the 6 weeks of study and that task has a higher cognitive load than does the PVT. Furthermore, subjects had approximately 100 minutes free following each approximately 20-minute test battery, so their overall workload was not excessive.
The results of ADD performance in our study showed a continuous improvement in the number of correctly completed calculations week by week throughout 6 weeks of FD. The ADD test requires cognitive operations that include attention, working memory, and arithmetic processing. The improved performance on ADD that was observed across the 6 weeks may reflect increased efficiency, speed, or both, in the above cognitive processes.12 In contrast with the ADD performance, PVT performance did not improve across the 6 weeks but, in fact, was significantly worsened. Because PVT performance includes attention34 and motor response, the systematic slowing on the PVT suggests that the improvement in ADD performance was likely due to changes in working memory, arithmetic processing, or both, rather than due to an improvement in attention. In fact, the worsening of PVT performance suggests that attention during the ADD test was also likely worsening across the 6 weeks of FD, and the overall improvement in ADD performance therefore likely would have been even greater without the impairment in attention.
Our findings have implications for workers in situations in which night work, extended work shifts, or systematic sleep loss occurs. About 30% to 40% of the general population reports getting less than 7 hours of sleep per night.35,36 If workers do this week after week, they likely have slightly impaired attention under normal circumstances. However, if their system is pushed (by working an extended shift or by working at night, for example), they will likely suffer greater impairments, and their risk of accident and error will be greater due to the interaction of their cumulative sleep debt and the circadian phase at which they are working. In such a situation, the adverse homeostatic effect of extended time awake (and/or sleep debt) on performance would not be counterbalanced by the circadian drive for wakefulness (as is the case under entrained conditions).
Our findings also have potential application to individuals who are acquiring new skills that require working memory and attention. Our findings indicate that the ability to acquire and retain skills could be improved by being well rested and by avoiding the time of greatest vulnerability near the circadian phase associated with the nadir of the CBT rhythm. Our findings also indicate that worst performance on both performance tasks occurred when extended time awake interacted with the circadian phase near the CBT nadir. Therefore, performance or learning situations in which these 2 factors interact, such as staying awake all night (to complete an assignment or to study for an exam), will likely result in serious adverse effects on learning and subsequent performance.
Our results also are consistent with prior reports that self-assessment of performance level frequently does not correspond with objective performance, with individuals reporting greater alertness or performance than their objective performance demonstrates.8 Given this mismatch, mathematical models can be an important tool in fatigue management, schedule planning, online monitoring of performance, and even countermeasure design for performance impairment.37–40
A short-term sleep deprivation study using positron emission tomography and a serial addition-subtraction task revealed a larger reduction in activity in the corticothalamic network mediating alertness, attention, and higher-order cognitive processes.41 Other cognitive neurophysiologic measures—such as digital signal-processing actigraphy; oculometrics, including saccadic eye movements and the pupillary light reflex; and high-frequency electroencephalography have been suggested42 to better understand how sleep loss affects cognitive performance. Our results on ADD and PVT performance need to be revisited with simultaneous measurement of neuroimaging or neurophysiologic correlates of sleepiness and attention. Another consideration with regard to waking performance relates to a recent report of a polymorphism in the PER3 gene.43 In that study, the decrement of cognitive performance in response to sleep loss was reported to be significantly greater in PER35/5 individuals, compared with PER34/4 individuals. Those findings were interpreted to indicate that this polymorphism predicts individual differences in the sleep loss-induced decrement in performance, and differential susceptibility was hypothesized to be mediated by effects on sleep-wake homeostasis. We were unable to obtain PER3 genotypes for every subject in our study and, therefore, could not include this as a covariate in our analysis. Future studies on genetically determined variability in susceptibility to homeostatic and circadian effects on performance (examining PER3 as well as other sleep and circadian genes) are required to better understand individual differences in performance-related changes in response to sleep loss.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors wish to thank the study participants; D. McCarthy and J. Row for subject recruitment; the Brigham and Women's Hospital General Clinical Research Center (BWH GCRC) staff and the Division of Sleep Medicine (DSM) Chronobiology Core staff for assisting with study execution and data collection; J. M. Ronda for development and support of the data collection, data management, and data-processing software; M. J. Duverne-Joseph for assistance with data processing; and C. A. Czeisler for overall support. The studies were supported by several National Institutes of Health (NIH) grants: R21 AT002571 (to JFD); M01 RR02635 (the BWH GCRC, where the studies were conducted); and data analysis support from P01 AG09975 (Project 2 to JFD, Core B to EB Klerman) and R01 HL080978 (to JFD); KDS was supported by NIH fellowships T32 HL007901 and F32 AG031690. JHL was supported by a 2007 Research Grant from Kangwon National University, Korea.
REFERENCES
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–6. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Menaker M, Takahashi JS, Eskin A. The physiology of circadian pacemakers. Annu Rev Physiol. 1978;40:501–26. doi: 10.1146/annurev.ph.40.030178.002441. [DOI] [PubMed] [Google Scholar]
- 3.Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- 4.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 5.Mills JN. Circadian rhythms during and after three months in solitude underground. J Physiol. 1964;174:217–31. doi: 10.1113/jphysiol.1964.sp007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 7.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflugers Arch. 1981;391:314–8. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]
- 8.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 10.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 11.Czeisler CA, Dijk DJ, Duffy JF. Entrained phase of the circadian pacemaker serves to stabilize alertness and performance throughout the habitual waking day. In: Ogilvie RD, Harsh JR, editors. Sleep Onset: Normal and Abnormal Processes. Washington, DC: American Psychological Association; 1994. pp. 89–110. [Google Scholar]
- 12.Wright Jr. KP, Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 13.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1478–87. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–81. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 16.Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505(3):851–8. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann Med. 1999;31:130–40. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 18.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods. 1985;17:652–5. [Google Scholar]
- 19.Dinges DF, Kribbs NB. Performing while sleepy: effects of experimentally-induced sleepiness. In: Monk TH, editor. Sleep, Sleepiness and Performance. Chichester, UK: John Wiley and Sons, Ltd; 1991. pp. 97–128. [Google Scholar]
- 20.Mazur JE, Hastie R. Learning as accumulation: a reexamination of the learning curve. Psychol Bull. 1978;85:1256–74. [PubMed] [Google Scholar]
- 21.Jewett ME, Wright Jr. KP, Duffy JF, Rodriguez DM, Czeisler CA. Practice effects observed over a month-long 28-hour forced desynchorony protocol in a cognitive throughput task are well described by a saturating exponential function. Sleep. 2001;24:A4–5. [Google Scholar]
- 22.Klein KE, Wegmann HM, Athanassenas G, Hohlweck H, Kuklinski P. Air operations and circadian performance rhythms. Aviat Space Environ Med. 1976;47:221–30. [PubMed] [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- 24.Wright Jr. KP, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 25.Dean II DA, Wyatt JK, Dijk DJ, Czeisler CA, Klerman EB. Quantifying practice effects within groups and individuals: examples from a month long forced deynchrony protocol. Sleep. 2008;31:A54. [Google Scholar]
- 26.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 27.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 28.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 29.Hull JT, Wright KP, Jr., Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003;18:329–38. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- 30.Daurat A, Aguirre A, Foret J, Gonnet P, Keromes A, Benoit O. Bright light affects alertness and performance rhythms during a 24-h constant routine. Physiol Behav. 1993;53:929–36. doi: 10.1016/0031-9384(93)90271-g. [DOI] [PubMed] [Google Scholar]
- 31.Roehrs T, Shore E, Papineau K, Rosenthal L, Roth T. A two-week sleep extension in sleepy normals. Sleep. 1996;19:576–82. [PubMed] [Google Scholar]
- 32.Barbato G, Barker C, Bender C, Giesen HA, Wehr TA. Extended sleep in humans in 14 hour nights (LD 10:14): relationship between REM density and spontaneous awakening. Electroencephalogr Clin Neurophysiol. 1994;90:291–7. doi: 10.1016/0013-4694(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 33.Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep—implications for insomnia. Curr Biol. 2008;18:1118–23. doi: 10.1016/j.cub.2008.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horowitz TS, Cade BE, Wolfe JM, Czeisler CA. Searching night and day: a dissociation of effects of circadian phase and time awake on visual selective attention and vigilance. Psychol Sci. 2003;14:549–57. doi: 10.1046/j.0956-7976.2003.psci_1464.x. [DOI] [PubMed] [Google Scholar]
- 35.Ohayon MM, Caulet M, Guilleminault C. How a general population perceives its sleep and how this relates to the complaint of insomnia. Sleep. 1997;20:715–23. doi: 10.1093/sleep/20.9.715. [DOI] [PubMed] [Google Scholar]
- 36.Washington DC: National Sleep Foundation; 2003. Executive summary of the 2003 Sleep in America Poll. [Google Scholar]
- 37.Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public policy: consensus report. Sleep. 1988;11:100–9. doi: 10.1093/sleep/11.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Dongen HPA, Mott CG, Huang JK, Mollicone DJ, McKenzie FD, Dinges DF. Optimization of biomathematical model predictions for cognitive performance impairment in individuals: accounting for unknown traits and uncertain states in homeostatic and circadian processes. Sleep. 2007;30:1129–43. doi: 10.1093/sleep/30.9.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dean DA, II, Fletcher A, Hursh SR, Klerman EB. Developing mathematical models of neurobehavioral performance for the real world. J Biol Rhythms. 2007;22:246–58. doi: 10.1177/0748730407301376. [DOI] [PubMed] [Google Scholar]
- 40.Klerman EB, St. Hilaire MA. On mathematical modeling of circadian rhythms, performance, and alertness. J Biol Rhythms. 2007;22:91–102. doi: 10.1177/0748730407299200. [DOI] [PubMed] [Google Scholar]
- 41.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 42.Russo MB, Stetz MC, Thomas ML. Monitoring and predicting cognitive state and performance via physiological correlates of neuronal signals. Aviat Space Environ Med. 2005;76:C59–63. [PubMed] [Google Scholar]
- 43.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–8. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]