Abstract
Study Objectives:
The electrocardiogram (ECG)-based sleep spectrogram generates a map of cardiopulmonary coupling based on heart rate variability and respiration derived from QRS amplitude variations. A distinct spectrographic phenotype, designated as narrow-band elevated low frequency coupling (e-LFCNB), has been associated with central apneas and periodic breathing and predicts sleep laboratory failure of continuous positive airway pressure therapy. This study assesses, at a population level, the associations of this spectrographic biomarker with prevalent cardiovascular disease using the Sleep Heart Health Study (SHHS)-I dataset.
Design:
Retrospective analysis of the Sleep Heart Health Study-I dataset.
Setting:
Laboratory for complex physiologic signals analysis.
Measurements and Results:
The fully-automated ECG-derived sleep spectrogram technique was applied to 5247 (of the original 6441) polysomnograms from the SHHS-I. Associations were estimated with use of various drugs and pathologies including prevalent hypertension and cardiovascular and cerebrovascular disease. Increasing with age and more common in males, e-LFCNB is also associated with greater severity of sleep apnea and fragmented sleep. After adjustment for potential confounders, an independent association with prevalent hypertension and stroke was found.
Conclusions:
An ECG-derived spectrographic marker related to low frequency cardiopulmonary coupling is associated with greater sleep apnea severity. Whether this biomarker is solely a sign of more severe disease or whether it reflects primary alterations in sleep apnea pathophysiology (which may either cause or result from sleep apnea) is unknown. This ECG-based spectral marker is associated with a higher prevalence of hypertension and stroke.
Citation:
Thomas RJ; Weiss MD; Mietus JE; Peng CK; Goldberger AL; Gottlieb DJ. Prevalent hypertension and stroke in the sleep heart health study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling. SLEEP 2009;32(7):897-904.
Keywords: ECG, cardiopulmonary coupling, Sleep Heart Health Study, sleep spectrogram, hypertension stroke
CONVENTIONAL POLYSOMNOGRAPHY CATEGORIZES SLEEP IN STAGES N1, N2, N3, AND REM. THIS SCORING IS BASED ON PREDOMINANT ELECTROENCEPHALOGRAPHIC (EEG) frequency, amplitude, and the occurrence of characteristic wave complexes, along with eye movements and chin muscle tone, that predominate within consecutive 30-sec epochs. Scoring of respiratory abnormality during sleep identifies and counts discrete episodes of reductions in flow, which are further characterized as central or obstructive, based on measures of respiratory effort or related flow signal characteristics. While these scoring approaches yield useful information, they provide summary measures that may fail to capture other key aspects of sleep quality and respiratory disturbance during sleep. For example, conventional scoring does not take into account the sequential dynamics of respiratory fluctuations or of cardiopulmonary coupling.
Polysomnographic methods that complement standard approaches have been described. One such approach to characterize sleep uses the stability domain.1,2 A proposed EEG marker of unstable NREM sleep is termed cyclic alternating pattern (CAP),1 in which repetitive phasic events (slow wave bursts, α and β frequency intrusions into ongoing NREM sleep) alternate with a background with lower EEG amplitude. When phasic events are absent or do not reach amplitude criteria, sleep is designated as stable, or non-CAP. This approach is purely EEG-based and is limited by dependence on EEG amplitude changes.
We developed a method to characterize sleep stability, using a continuous single-channel ECG, by mathematically combining QRS (R) wave amplitude fluctuations that are related to the mechanical effects of respiration (ECG-derived respiration signal) with heart rate variability changes that are associated with neuroautonomic tone modulation.3 The output of this method, called the sleep spectrogram, provides both graphical and numerical data that are measures of cardiopulmonary coupling interactions. Low frequency coupling predominates in unstable NREM sleep and correlates with periods of CAP, while high frequency coupling predominates in stable NREM sleep and correlates with non-CAP. A subset of low frequency coupling, designated as elevated-low frequency coupling (e-LFC), was found to correlate with visually scored apneas and hypopneas.4 Further, analysis of the spectral dispersion of e-LFC showed 2 distinctive patterns, a narrow spectral band e-LFC (e-LFCNB) and a broad spectral band elevated-low frequency coupling.4 We have shown that e-LFCNB is associated with central apneas and periodic breathing and is a biomarker that predicts the induction of central apneas during positive airway pressure titration.4
To further explore the pathophysiological implications and potential utility of this ECG-derived measure, we examined the clinical and polysomnographic correlates of e-LFCNB in the large, community-based cohort of middle-aged and older adults participating in the Sleep Heart Health Study. Specifically, we explored the relation of e-LFCNB to sleep quality after adjusting for sleep apnea severity, age, body mass index, and associations with hypertension or vascular disease. Our hypothesis was that e-LFCNB would be associated with greater severity of sleep apnea and an increased adjusted risk of hypertension, secondary to excessive activation of the respiratory chemoreflex.
MATERIALS AND METHODS
Subjects and Study Design
This study is cross-sectional, utilizing polysomnographic and clinical data from the baseline examination of the Sleep Heart Health Study (SHHS). The SHHS is a multicenter longitudinal study of 6,441 participants drawn from several ongoing cohort studies, aged ≥ 40 yr, designed to determine the cardiovascular consequences of sleep apnea.5 The design and objectives of the Sleep Heart Health Study and detailed descriptions of its member cohorts, protocols, and quality-control procedures, have been published.6 The baseline examination, including an overnight polysomnogram, was conducted between December 1995 and January 1998. All patients underwent conventional scoring using defined rules. For sleep spectrographic analysis, subjects were excluded for excessive ECG signal dropout (< 80% of signal available for analysis), atrial fibrillation, ventricular bigeminy, and demand ventricular or biventricular pacing, as these conditions would interfere with single-lead ECG analysis. A total of 5247 subjects underwent ECG-based sleep spectrographic analysis.
All data were received and de-identified, and Institutional Review Board exemption category IV was obtained for “research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.”
Sleep Data Collection and Scoring
Twelve-lead unattended overnight polysomnography was accomplished with Compumedics PS (Melbourne, Australia) equipment. The ECG was in lead II configuration. Sleep data were scored at a central coordinating center. Manual stage scoring used the standard method to first identify NREM stages 1-4, REM sleep, and wakefulness. Respiratory-event scoring rules were as follows: (1) Obstructive apnea was defined as a reduction in the oral-nasal thermistor signal to < 10% of baseline with continued respiratory effort. Central apneas were scored when there was no evidence of respiratory effort. (2) Hypopnea was defined as a 30% reduction in thermistor or thoracic or abdominal plethysmography signals. The frequency (per hour of sleep) of apneas plus hypopneas associated with ≥ 4% oxygen desaturation is referred to as the apnea-hypopnea index (AHI). The respiratory disturbance index (RDI) included respiratory events scored without the requirement for a 4% oxygen desaturation. Periodic breathing detection required 10 continuous minutes of concordant waxing and waning flow and respiratory effort in a crescendo-decrescendo pattern. The ECG configuration for the Sleep Heart Health Study was lead II.
Sleep Spectrogram
Details of the method have been published,3,4 and are available in the online Appendix at www.journalsleep.org. In brief, using a continuous single lead ECG, we combine information from heart rate variability and ECG-derived respiration (EDR).7,8 The latter reflects amplitude variations in the QRS complex that result from shifts in the cardiac electrical axis relative to the electrodes during respiration, and changes in thoracic impedance as the lungs fill and empty. After filtering for outliers and cubic spline resampling at 2 Hz, the cross-spectral power and coherence of these 2 signals are calculated over a 1024-sample (8.5-min) window using the Fast Fourier Transform applied to 3 overlapping 512-sample subwindows within the 1024-sample coherence window. The window is then advanced by 256 samples (2.1 min) and the computations repeated until the entire NN interval/EDR series is analyzed. For each 1024-sample window the product of the coherence and cross-spectral power is used to calculate the ratio of coherent cross power in the low frequency (0.01–0.1 Hz) band to that in the high frequency (0.1–0.4 Hz) band. This ratio is used to classify each successive sampling window as high frequency coupling (stable state) and low frequency coupling (unstable state). Very low frequency coupling (wake or REM sleep) is calculated using the ratio of coherent cross power in the 0–0.01 Hz band to the power in the 0.01–0.4 Hz band.
We further identified a subset of low frequency coupling, called elevated-low frequency coupling (e-LFC),3 that coincided with periods of scored apnea/hypopnea in the open access PhysioNet Sleep Apnea Database (http://www.physionet.org/physiobank/database/apnea-ecg/). Optimal detection thresholds (defined as those providing maximum combined sensitivity and specificity for apnea/hypopnea detection) required that the minimum low frequency power be > 0.05 normalized units and that the low to high frequency ratio be > 30 to define periods of e-LFC. When this algorithm was applied to the open-access PhysioNet BIDMC Congestive Heart Failure database (http://www.physionet.org/physiobank/database/chfdb/), LFC, the coupling frequency typically remained constant over several contiguous measurements. Because central sleep apnea is especially prevalent in those with congestive heart failure and is known to have a relatively constant cycle length, we hypothesized that by measuring the temporal dispersion of consecutive spectral peaks of apnea detection, it may be possible to distinguish central from obstructive physiology. To automate the identification of this pattern of narrow spectral band e-LFC (e-LFCNB, putative central sleep disordered breathing), we required a minimum power in this band of 0.3 normalized units and that the coupling frequency of each pair of consecutive measurements remain within 0.0059 Hz of each other over 5 consecutive overlapping coherence windows (16.9 continuous min).4 Periods of e-LFC not meeting these criteria were defined as broad spectral band e-LFC, and designated as e-LFCBB).
The sleep spectrogram technique uses 8.5-min windows that increment every 2.1 min across the data set. To detect any e-LFCNB requires 5 consecutive spectral peaks that “line-up”. To designate a polysomnogram as e-LFCNB present, a minimum detection threshold, requires nearly 17 min of metronomic, nearly identical cycle length respiratory events typical of periodic breathing. Every 1% increase in e-LFCNB is calculated as a % of sampled windows that are within 0.0059 Hz of each other, across the whole sleep period. For example, a total sleep time of 360 min (6 h) will have 171 sampling windows, each of 8.5 min, incremented every 2.1 min. A 1% increase would be approximately equal to 1.71 × 2.1 = 3.6 min of additional central sleep apnea/ periodic breathing-like oscillations. Conventional scoring may designate these respiratory events as obstructive or central. Nigh-to-night variability of spectrographic biomarkers is not significant (unpublished data).
Polysomnographic data were exported in European Data Format for sleep spectrogram analysis. Sleep staging was used to constrain automated spectrographic analysis to the sleep period. Of the 6441 SHHS-I polysomnograms, 1183 did not meet the following inclusion criteria: a minimum of 4 h of available ECG data, no dropout of ECG signal for contiguous periods > 4 min, and ≥ 80% of R waves annotated by the software as normal R waves. Thus, studies with high rates of ectopy, atrial fibrillation, and excessively tall T waves that interfered with accurate QRS detection were excluded. Demographic, clinical, and polysomnographic comparisons of the included and excluded subjects are provided in Table 1.
Table 1.
Characteristics of Included vs. Excluded Sleep Heart Health Study Data
| Measure | Included Data | Excluded Data | P |
|---|---|---|---|
| Age (years) | 62.2 ± 10.9 | 60 ± 9.9 | < 0.001 |
| Sex (M/F) | 51.6 / 49.4 | 48 / 52 | 0.4* |
| Epworth Sleepiness Scale score | 7.8 ± 4.4 | 7.5 ± 4.5 | 0.06 |
| Body mass index | 28.7 ± 5.3 | 29.4 ± 6.1 | < 0.001 |
| Total sleep time (TST) | 354.9 ± 63.4 | 350.1 ± 68.6 | 0.05 |
| Sleep efficiency | 81.5 ± 10.6 | 79.9 ± 11.9 | 0.001 |
| Stage 1 NREM (% TST) | 5.6 ± 4.1 | 5.9 ± 4.6 | 0.03 |
| Stage 2 NEEM (% TST) | 57.2 ± 11.7 | 59.7 ± 11.9 | < 0.001 |
| Stage 3+4 NREM (% TST) | 17.4 ± 11.8 | 15 ± 11.6 | < 0.001 |
| Stage REM | 19.8 ± 6.3 | 19.4 ± 6.5 | 0.06 |
| Arousal index (/h of sleep) | 19.2 ± 10.5 | 19.6 ± 10.6 | 0.2 |
| Apnea-hypopnea index (/h of sleep) | 9.1 ± 12.7 | 9.4 ± 12.4 | 0.5 |
| Respiratory disturbance index (/h of sleep) | 33.7 ± 19.7 | 34.3 ± 20 | 0.3 |
| Central apnea index (/h of sleep) | 0.5 ± 2.2 | 0.5 ± 2.3 | 0.8 |
| Periodic breathing (+/−) | 2.5 / 97.5 | 2.8 / 97.2 | 0.5* |
| Diabetes (+/−) | 10.6 / 89.6 | 23.8 / 76.2 | < 0.001* |
| Hypertension medication use (+/−) | 39.1 / 60.9 | 38.6 / 61.4 | 0.8* |
| Myocardial infarction (+/−) | 7 / 93 | 9.7 / 90.3 | 0.001* |
| Angina (+/−) | 7.6 / 92.4 | 8.5 / 91.5 | 0.07* |
| CABG (+/−) | 3.4 / 96.6 | 3.2 / 96.8 | 0.1* |
| Pacemaker (+/−) | 0.8 / 99.2 | 0.7 / 99.3 | 0.09* |
| Stroke (+/−) | 3.5 / 96.5 | 4.3 / 95.7 | 0.3* |
| Heart failure (+/−) | 1.8 / 98.2 | 3.1 / 96.9 | < 0.001 |
= χ2; +/ − = proportions present/ absent. Race differences were prominent. The % of polysomnograms (included vs. excluded categories) for Caucasian, African American, Native American /Alaskan, Asian/ Pacific Islander, and Hispanic / Mexican American were 75.5% vs. 46.1%, 8.3% vs. 1.7%, 9.7% vs. 49.6%, 1.5 vs. 0.7 and 5% vs. 1.6%, respectively. Nearly half of the excluded studies were Native-American /Alaskan, χ2, 1000, P < 0.001.
The ECG-derived sleep spectrogram software has been licensed to Embla, Inc, by the Beth Israel Deaconess Medical Center (BIDMC) and is available as an add-in to the RemLogic suite of polysomnographic software. The analysis and figures in this study were generated by the original code at the BIDMC.
Clinical Data
Clinical data at the time of the baseline polysomnogram provided by the SHHS Coordinating Center included demographic variables: age, sex, body mass index, race/ethnicity; drug use; standard polysomnographic variables of sleep stages and respiratory abnormality including recognition of periodic breathing; treated hypertension (here defined as the use of antihypertensive medications), total hypertension (treated hypertension, or systolic blood pressure > 140, or diastolic blood pressure > 90 mm Hg measured at the time of the polysomnogram), and self-report of a doctor diagnosis of cardiovascular disease (history of angina, myocardial infarction, coronary angioplasty, or coronary artery bypass grafting) or stroke. Presence of diabetes was defined as the use of insulin or oral hypoglycemic drugs.
To assess the association of e-LFCNB with sleep quality using regression analysis, the following thresholds were used: ≤ 20% slow wave sleep (stage 3 + 4), arousal index ≥ 25 per hour of sleep, ≥ 10% stage 1 sleep, ≤ 20% REM sleep, ≤ 360 min total sleep time, and ≤ 85% sleep efficiency. The distribution of these variables in the full SHHS sample is (mean and standard deviation): slow wave sleep (stage 3 + 4) 17.4% ± 11.8%, total arousal index 19.2 ± 10.5 per h of sleep, stage 1 5.6% ± 4.1%, REM sleep 19.8% ± 6.3%, total sleep time 354.9 ± 63.4 min, and sleep efficiency 81.4% ± 10.6%.
To assess the association of e-LFCNB with sleep apnea severity adjusted for age, sex, and body mass index, categories of apnea hypopnea index were created using thresholds of 5, 15, and 30 events per hour.
To assess the association of the presence of e-LFCNB with stable sleep state as detected by the total sleep time percentage of high frequency coupling, a threshold of 50% was used, based on our own normative data and the mean for the entire SHHS dataset (44.2% ± 21.9%).
Statistical Methods
Summary statistics included means and standard deviations or percentages for demographic, clinical, and polysomnographic variables. Unpaired t-tests or χ2 analysis was used to assess differences between spectrographically determined groups (with and without e-LFCNB). Logistic regression was employed to determine odds ratios and confidence intervals for associations between e-LFCNB and variables of interest. Multiple regression was used to adjust for age, sex, body mass index, and apnea-hypopnea index in the context of medication use, using e-LFCNB as a continuous variable. STATA SE9 was used for analysis. Values are reported as mean ± SD, unless otherwise specified.
RESULTS
Narrow Band Elevated Cardiopulmonary Coupling Demographics
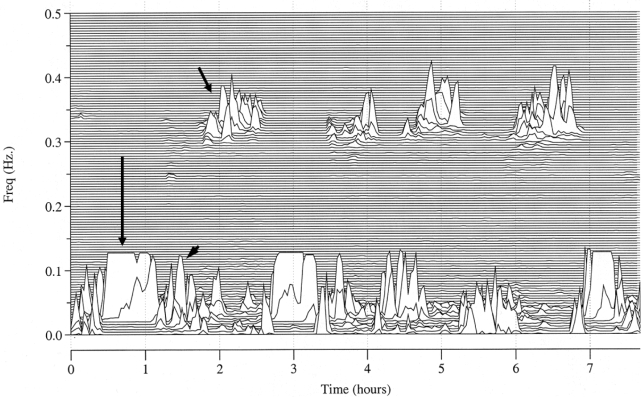
Elevated-LFCNB (Figure 1) was detected in 1233 of 5247 subjects (23.5%). There were statistically significant differences between those with and without this spectral biomarker. Those in whom e-LFCNB was detected were older (64.7 ± 10.9 years vs. 61.4 ± 10.8 years), more likely to be men (63.3% vs. 45.1%), slightly heavier (body mass index 29.3 ± 5.2 vs. 28.6 ± 5.3 kg/m2), and sleepier (Epworth score 8.8 ± 8 vs. 7.7 ± 7.5; see Table 2). Increasing sleep apnea severity was associated with an increasing prevalence of e-LFCNB (Table 3). Across 10-year age categories (< 50 years [678 subjects], 51-60 years [1739 subjects], 61-70 years [1369 subjects], 71-80 years [1240 subjects], and > 80 years [218 subjects]), there was a progressive increase in the presence of e-LFCNB: 14.9%, 20.1%, 24.7%, 29.5%, and 35.3% respectively. Moreover, there was an increase in the amount of e-LFCNB: 1% ± 3.4%, 1.5% ± 4.7%, 2.1% ± 6.2%, 3% ± 7.2% and 4.5% ± 10.6%, respectively, ANOVA F4, 5239 = 27.7, P < 0.001, all differences significant by Tukey test.
Figure 1.
Spectral characteristics of high frequency coupling, narrow and broadband elevated-low frequency coupling. High frequency coupling (short arrow), narrowband elevated-low frequency coupling (long arrow) and broadband elevated-low frequency coupling (arrow head), mapped across the whole night.
Table 2.
Characteristics Based on ECG Spectrogram Phenotypes
| Variable | e-LFCNB absent (n = 4014) | e-LFCNB present (n = 1233) | P |
|---|---|---|---|
| Age (years) | 61.4 ± 10.8 | 64.7 ± 10.9 | < 0.001 |
| Sex (M/F) | 1811 / 2203 | 781 / 452 | < 0.001 |
| BMI (kg/m2) | 28.6 ± 5.3 | 29.3 ± 5.2 | < 0.001 |
| ESS | 7.7 ± 7.5 | 8.3 ± 8 | < 0.001 |
| Total sleep time (TST) | 355.9 ± 63.9 | 351.8 ± 61.8 | 0.10 |
| Stage 1 (% TST) | 5.4 ± 4 | 6.2 ± 4.7 | < 0.001 |
| Stage 2 (% TST) | 56.5 ± 11.8 | 59.4 ± 11.2 | < 0.001 |
| Stage 3+4 (% TST) | 18.1 ± 11.9 | 15.1 ± 11.4 | < 0.001 |
| REM (% TST) | 20 ± 6.3 | 19.3 ± 6.3 | 0.002 |
| AHI (per h of sleep) | 6.5 ± 9 | 17.6 ± 18 | < 0.001 |
| Arousal index (per h of sleep) | 17.8 ± 9.2 | 23.8 ± 13 | < 0.001 |
| CAI (per h of sleep) | 0.1 ± 0.7 | 0.9 ± 3.1 | < 0.001 |
| Sleep efficiency (%) | 81.8 ± 10.7 | 80.4 ± 10.3 | 0.002 |
| HFC* | 47.4 ± 21.6 | 33.8 ± 19.5 | < 0.001 |
| LFC* | 33 ± 17 | 49.8 ± 18.9 | < 0.001 |
| e-LFC* | 12.8 ± 11.1 | 28.8 ± 17.9 | < 0.001 |
| VLFC* | 16.7 ± 7.3 | 14.4 ± 6.6 | < 0.001 |
Percentage of signal detection windows with the specific cardiopulmonary coupling spectral peak in the total sleep period. BMI: body mass index; ESS: Epworth sleepiness score; AHI: apnea-hypopnea index; CAI: central apnea index; HFC: High Frequency Coupling; LFC: Low frequency coupling; e-LFC: elevated-Low Frequency Coupling; NB – narrow spectral band; VLFC: Very Low Frequency Coupling
Table 3.
Severity of Sleep Apnea and Prevalence of Narrow Band Elevated-Low Frequency Coupling
| Apnea hypopnea index (AHI / h of sleep) | Number of subjects | e-LFCNB present (% prevalence) | e-LFCNB (mean % for each AHI group) |
|---|---|---|---|
| 0–4.9 | 2748 | 314 (11.4) | 0.5 (1.8) |
| 5–14.9 | 1539 | 408 (26.5) | 1.6 (3.6) |
| 15–29.9 | 619 | 274 (44.3) | 4 (7.2) |
| > 30 | 338 | 235 (69.5) | 12.8 (15.2) |
Using the following categories of body mass index (kg/m2, % of total population): < 25 (24.4%), 25–30 (41.4%), 30.1–35 (23.1%), 35.1–40 (7.7%), and > 40 (3.5%), e-LFCNB was detected in 19.5%, 23%, 27.9%, 25.8%, and 27.9% respectively.
In multiple regression analysis using e-LFCNB as a continuous measure, age, male sex, body mass index, and apnea-hypopnea index were all significant predictors of e-LFCNB (F4, 5137 = 541, P < 0.001). There were no racial differences in the prevalence of e-LFCNB, either as a categorical non-Hispanic whites vs. all others (OR: 1.01, CI: 0.96–1.08, P = 0.7) or continuous variable using categories of non-Hispanic whites, Hispanic, African American, Asian/ Pacific Islander, and Native American/ Alaskan (ANOVA F4, 5240 = 0.38, P = 0.8).
B. Relation of Narrow Band Elevated Cardiopulmonary Coupling to Polysomnographic and Spectrographic Variables
There was a statistically significant association between e-LFCNB as a continuous variable and the respiratory disturbance index (linear regression F1,5245 = 698.3, P < 0.001, Spearman rho = 0.33). Compared to those without e-LFCNB, subjects with the presence of e-LFCNB had statistically significant differences in nearly all polysomnographic and ECG-derived spectrographic variables (Table 2). Some of these differences were small and unlikely to be clinically significant, such as stage 1 sleep (6.2% ± 4.7% vs. 5.4% ± 4%; P < 0.001), but the apnea hypopnea index was nearly 3 times higher in those with e-LFCNB (17.6 ± 18 vs. 6.5 ± 9; P < 0.001). The odds of having an apnea hypopnea index ≥ 5, 15, or 30 was significantly elevated by the presence of e-LFCNB after adjusting for age, sex and body mass index (OR 3.9, [CI: 3.4–4.6]; OR 4.9, [CI: 4.2–5.8]; OR 7.9, [CI: 6.1–10.2]; respectively, all P < 0.001, Table 3). After adjusting for age, sex, body mass index, and apnea-hypopnea index, the presence of e-LFCNB was associated with increased risk of an elevated arousal index (OR 1.29 [CI: 1.09–1.52], P = 0.003, Table 4). The spectrographic marker of stable sleep state (high frequency coupling) was lower in those with e-LFCNB (33.8 ± 19.5 vs. 47.4 ± 21.6, P < 0.001), and total e-LFC elevated (28.8 ± 17.9 vs. 12.8 ± 11.1; P < 0.001). The presence of e-LFCNB was associated with a significantly increased probability of having reduced high frequency coupling (using a threshold of 50%), odds ratio 2.1 [C.I. 1.8–2.4, P < 0.001] after adjustment for age, sex, body mass index, race, and apnea-hypopnea index.
Table 4.
Effect of Narrowband Elevated-Low Frequency Coupling on Sleep Quality
| Sleep Quality Measure | OR [C.I.], P |
|---|---|
| Total sleep time (TST) | 0.87 [0.75–1.01], P = 0.06 |
| Sleep efficiency | 1.00 [0.86–1.16], P = 0.98 |
| Stage 1 sleep ≥ 10% TST | 0.90 [0.74–1.10], P = 0.32 |
| Slow wave sleep ≤ 20% TST | 1.18 [1.01–1.37], P = 0.04 |
| REM sleep ≤ 20% TST | 0.87 [0.75–0.99], P = 0.05 |
| Arousal index ≥ 25/ h of sleep | 1.29 [1.09–1.52], P = 0.003 |
OR: odds ratio; C.I.: confidence intervals; Adjustments: age, sex, body mass index, apnea-hypopnea index.
C. Relationship of Visually Detected Periodic Breathing and Spectrographically Detected Narrow Band Elevated Low Frequency Cardiopulmonary Coupling
The sleep spectrogram detected e-LFCNB far more frequently (1233/ 5247, 23.5% of subjects) than visual inspection of the polysomnogram recognized periodic breathing (129/ 5247, 2.5% of subjects), χ2 96.2, P < 0.001. e-LFCNB was detected in 77 of 129 (59.7%) subjects with visually recognized periodic breathing, although visually scored periodic breathing was present in only 6.3% of those with e-LFCNB. However, the post-adjustment odds of an apnea hypopnea index ≥ 5, 15, or 30 per hour of sleep in those with visually identified periodic breathing were similar to that seen with e-LFCNB (visual scoring OR 2.9, [CI: 1.8–4.5]; OR 2.7, [CI: 1.8–4]; OR 3.6, [CI 2.3–5.8]; respectively, all P < 0.001). A male dominance was noted in those with visually detected periodicity, as was noted with the spectrographic e-LFCNB phenotype: males 3.9% vs. females 1.2%, χ2 35.2, P < 0.001.
D. Relation of Medication Use to Narrow Band Elevated Low Frequency Cardiopulmonary Coupling
Subjects with e-LFCNB (considered as categorical variable) were more likely to take angiotensin converting enzyme inhibitors, loop diuretics, or tricyclic antidepressants. Using logistic regression, these associations were not significant after adjustments for age, sex, and body mass index. When considered as a continuous variable, there were statistically significant increases in those using converting enzyme inhibitors, diuretics, β- blockers, calcium blockers, and nitrates (Table 5). Following adjustment for age, sex, body mass index and the presence of self-reported heart failure, there was a significant increase in e-LFCNB in those using loop diuretics (F5, 4944 = 52.7.1, P = 0.002), calcium blockers (F5, 4944 = 52.3, P = 0.02) and β-blockers (F5,4944 = 51.5, P = 0.02), but not angiotensin converting enzyme inhibitors or nitrates.
Table 5.
Narrow Band Elevated-Low Frequency Coupling and Drugs
| Drug (total number using drug/ 5247) | e-LFCNB present (proportion using drug) | e-LFCNB absent (proportion using drug) | χ2, P | Drug present (e-LFCNB % TST) | Drug absent (e-LFCNB % TST) | P (t-test) |
|---|---|---|---|---|---|---|
| ACEI (699) | 0.16 | 0.13 | 11.3, 0.001 | 2.6 ± 5.9 | 1.9 ± 5.9 | 0.01 |
| Diuretics (784) | 0.17 | 0.15 | 3.1, 0.08 | 2.6 ± 7.5 | 1.9 ± 5.7 | 0.004 |
| Loop diuretic (218) | 0.05 | 0.04 | 3.8, 0.05 | 3.9 ± 10.4 | 1.9 ± 5.7 | < 0.001 |
| Digitalis (141) | 0.03 | 0.03 | 0.6, 0.43 | 2.9 ± 8 | 2 ± 5.9 | 0.08 |
| β-blockers (606) | 0.11 | 0.12 | 0.8, 0.36 | 3 ± 8.4 | 1.9 ± 5.5 | < 0.001 |
| Calcium blockers | 0.13 | 0.15 | 3.6, 0.06 | 3 ± 8.2 | 1.9 ± 5.5 | < 0.001 |
| Nitrates (134) | 0.03 | 0.03 | 0.9, 0.34 | 3.1 ± 8.6 | 2 ± 5.9 | 0.04 |
| Theophylline (93) | 0.02 | 0.02 | 0.002, 1.0 | 2.8 ± 9.6 | 2 ± 5.9 | 0.18 |
| Benzodiazepines (250) | 0.05 | 0.05 | 0.16, 0.69 | 1.6 ± 4.4 | 2 ± 6 | 0.2 |
| Antipsychotic (16) | 0.004 | 0.001 | 2.6, 0.1 | 1.9 ± 7.5 | 2 ± 6 | 0.92 |
| Antidepressant (154) | 0.02 | 0.03 | 3.1, 0.08 | 1 ± 2.3 | 1.1 ± 3.9 | 0.26 |
| Estrogen (747) | 0.09 | 0.16 | 32.4, < 0.001 | 1.1 ± 4.4 | 1.1 ± 3.7 | 0.71 |
e-LFCNB: elevated low frequency coupling, narrow band; TST: total sleep time. Estrogen effect not significant after adjusting for sex.
E. Association of Narrow Band Elevated Low Frequency Cardiopulmonary with Cardiovascular and Cerebrovascular Disease
There was increased e-LFCNB both as a categorical or continuous measure in hypertensive subjects and in those with self-reported stroke, ischemic heart disease, and congestive heart failure, but not in diabetics (Table 6). After adjustment for age, sex, body mass index, hypertension, and diabetes, only prevalent stroke remained associated with both categorical and continuous measures of e-LFCNB, while treated and total hypertension were associated only with the ECG biomarker as a continuous measure. There was a 2% higher hypertension prevalence for every 1% of e-LFCNB as a proportion of sampling windows across total sleep time. The odds ratio for prevalent stroke was 1.65 [CI: 1.19–2.29] in those with vs. without the presence of e-LFCNB. For every 1% increase in e-LFCNB as a proportion of sampling windows, there was a 3% increased odds of prevalent stroke.
Table 6.
Adjusted Associations of Narrow Band Elevated–Low Frequency Coupling with Cardiovascular Disease
| Disease | e-LFCNB as categorical variable - OR [C.I.], P | e-LFCNB as continuous variable - OR per 1% increase in e-LFCNB [C.I.], P |
|---|---|---|
| Treated hypertension | 1.05 [0.91–1.21], 0.51 | 1.02 [1.01–1.03], 0.006 |
| Total hypertension | 1.06 [0.92–1.22], 0.44 | 1.02 [1.01–1.04], 0.001 |
| Angina | 0.97 [0.75–1.26], 0.82 | 1 [0.98–1.01], 0.58 |
| Myocardial infarction | 1.23 [0.96–1.58], 0.11 | 1 [0.99–1.02], 0.8 |
| Coronary angioplasty | 0.83 [0.58–1.18], 0.29 | 1.01 [1–1.03], 0.17 |
| CABG | 1.13 [0.8–1.61], 0.49 | 0.96 [0.93–0.98], 0.001 |
| Stroke | 1.65 [1.19–2.29], 0.003 | 1.03 [1.01–1.06], 0.005 |
| All CVD | 1.19 [0.97–1.46], 0.1 | 1.00 [1.00–1.01], 0.74 |
| Heart failure | 1.3 [0.80–2.1], 0.29 | 1.02 [1–1.04], 0.08 |
Adjustments for categorical analysis: age, sex, race, body mass index, diabetes, apnea-hypopnea index ≥ 5. Adjustments for continuous analysis: age, sex, race, body mass index, diabetes, total e-LFC, apnea-hypopnea index ≥ 5. CABG: Coronary artery bypass graft; CVD: Cardiovascular disease (angina + myocardial infarction + CABG + percutaneous revascularization
DISCUSSION
The key findings of this analysis of the ECG-based sleep spectrogram in a community-dwelling cohort are: (1) Increasing age and male sex are associated with an increase in the prevalence of a biomarker of highly periodic low frequency cardiopulmonary oscillations, termed e-LFCNB. (2) The presence of e-LFCNB is a biomarker of severity of sleep disordered breathing, independent of age, sex, and body mass index. (3) The sleep spectrogram identifies a much larger proportion of individuals with periodic breathing-like respiratory oscillations during sleep than visual identification using current guidelines. (4) Use of diuretics, calcium blockers, and β-blockers was associated with increased e-LFCNB. (5) e-LFCNB was associated with prevalent stroke and hypertension.
We have previously shown that the e-LFCNB spectral biomarker is more likely seen in those with conventionally scored central apneas and predicts the acute induction of central apneas during application of continuous positive airway pressure therapy.4 Speculatively, e-LFCNB may reflect chemoreflex influences on sleep related breathing, regardless of the exact morphology of the scored event (central apnea, periodic breathing, or hypopnea), The finding that e-LFCNB is more prevalent in men is consistent with the known male predominance of periodic breathing in congestive heart failure,9 the greater severity of sleep disordered breathing in men in general,10 and the lower NREM sleep CO2 reserve in men.11 The latter may predispose to increased instability of respiratory control during sleep and increase the prevalence of e-LFCNB.
The presence of e-LFCNB was associated with greater severity of sleep disordered breathing and sleep fragmentation, especially arousals and a reduction in stable sleep state (as indicated by high frequency coupling in the ECG-spectrogram). Mathematical modeling of periodic breathing12 predicts that increased chemoreflex sensitivity and loop gain,13 as well as arousals,13 increases the severity of sleep disordered breathing, and this prediction is supported in humans using proportional assist ventilation.14–16 The cause of an increased arousal index in individuals with e-LFCNB after adjusting for severity of sleep apnea remains unclear, but our findings raise the possibility that increased chemoreflex afferent input may have arousing influences, or that a low arousal threshold may contribute to ventilatory control instability. The percentage of subjects demonstrating e-LFCNB with mild, moderate, and severe sleep apnea in this dataset is remarkably similar to the prevalence of ventilatory control instability described by Younes et al.16
The ECG-derived spectrogram's detection of periodic breathing-type respiratory oscillations substantially exceeded that identified by visual detection of periodic breathing. Conventional scoring may be biased towards the scoring of obstructive hypopneas during periods of periodic breathing, and measurement of e-LFCNB could bring attention to parts of the polysomnogram where the probability of periodic breathing or central apneas is high; however, it is unlikely that visual and spectrographic scoring will match closely. Since the spectrogram is automated, objective, and maps the spectral dispersion of low frequency coupled cardiopulmonary oscillations, it could be a more accurate marker of periodic breathing.
The association of e-LFCNB with diuretic use is of interest as metabolic alkalosis is associated with a narrowing of the NREM sleep CO2 reserve.17,18 Although adjustment was made for self-reported heart failure, it remains possible that diuretic and β-blocker use is simply a marker for undiagnosed or unreported cardiac dysfunction. The e-LFCNB marker showed an increased prevalence in those with hypertension, ischemic heart disease, heart failure, and stroke. These associations were considerably diminished by adjustment for age, sex, body mass index, diabetes mellitus, and apnea-hypopnea index, although associations with hypertension and stroke persisted. There is an increased prevalence of periodic breathing following ischemic cerebrovascular disease, which is associated with increased sensitivity of the carotid chemoreflexes and hypocapnia, suggesting that stroke might cause elevated e-LFCNB.19 Hypertension might cause subclinical or undiagnosed cardiac dysfunction, a possible mechanism whereby hypertension might cause elevated e-LFCNB. An increase in e-LFCNB with increasing age was noted, after adjusting for sex, body mass index, and apnea-hypopnea index. However, while central apneas and periodic breathing are more common in the elderly,20 respiratory loop gain did not seem elevated in a recent investigation using proportional assist ventilation.21
Inconsistent associations were seen between e-LFCNB and surgical coronary revascularization, although no association was seen with coronary angioplasty or total coronary heart disease. While this is likely a chance association, autonomic effects of coronary or cardiac surgery22,23 or effects of cardiopulmonary bypass itself cannot be excluded.24
These findings should be interpreted with the caution that large sample sizes can result in statistically significant results that may not be clinically meaningful. Conversely, differences not evident with smaller groups can take advantage of a sample size such as that offered by the Sleep Heart Health Study. One additional limitation is that the stringency of our ECG entry criteria disproportionately excluded Native-American/Alaskans, diabetics, and those with heart failure. The impact of these exclusions on our results cannot be determined. Some of this loss can be avoided with meticulous attention to signal quality during recording (problematic if a specially trained technician is not present), multiple ECG leads, or alternate placements.
In summary, a distinct biomarker detected solely from ECG-based sleep spectrograms seems to provide insights into sleep physiology and pathology. It is not yet known whether the additional information provided by this measure will identify subgroups of individuals who differ with respect to risk of morbid sequelae of sleep apnea.
DISCLOSURE STATEMENT
This was not an industry sponsored study. The ECG-derived sleep spectrogram software has been licensed by the Beth Israel Deaconess Medical Center to Embla, Inc. Dr. Thomas, Dr. Peng, Dr. Goldberger and Mr. Mietus are co-inventors of the technique, and hold the patent. In addition, Mr. Mietus is a research advisor and has financial interests in DynaDx, Inc., which develops computational software for physiologic signal analysis. Dr. Goldberger is a research advisor to DynaDx, Inc. Dr. Peng is a co-founder of DynaDx Inc.
ACKNOWLEDGMENTS
Performance site: Beth Israel Deaconess Medical Center, Boston, MA, USA
Financial support: This work was supported in part by the grants from the National Heart, Lung and Blood Institute R21 HL079248 (RJT), the Periodic Breathing Foundation, and following to ALG: G. Harold and Leila Y. Mathers Foundation, the James S. McDonnell Foundation, the NIH-sponsored Research Resource for Complex Physiologic Signals (UO1EB008577), and the Wyss Institute for Biologically Inspired Engineering.
APPENDIX
ECG-DERIVED CARDIOPULMONARY COUPLING ASSESSMENT
Cardiopulmonary Coupling Analysis and Generation of Sleep Spectrograms
The cardiopulmonary coupling technique is based on a continuous electrocardiogram (ECG) signal and employs Fourier-based techniques to analyze 2 features of the signal: (1) the variability of the cardiac interbeat (RR) interval series and (2) the fluctuations in QRS amplitude induced by respiration—the ECG-derived respiration (EDR) signal. These signals have 2 basic patterns—a high frequency component due to physiological sinus arrhythmia that reflects breath-to-breath fluctuations and a low frequency component that reflects cyclic variation across multiple breaths. Using the Fourier transform, the R-R interval time series and the associated EDR signals are first decomposed into a set of sinusoidal oscillations with specific amplitudes and phases at each frequency. Two factors are considered in evaluating the strength of the coupling between these 2 signals: (1) If, at a given frequency, both signals have relatively large oscillation amplitudes, then it is likely that these 2 signals are coupled with each other. This can be measured by computing the cross-spectral power, i.e., the product of the powers of the 2 individual signals at a given frequency. (2) If 2 oscillations at a given frequency are synchronized with each other (i.e., they maintain a constant phase relationship), this can be measured by computing the coherence of these signals. We use the product of the coherence and the cross-spectral power to weight these 2 effects to quantify the degree of the cardiopulmonary coupling.
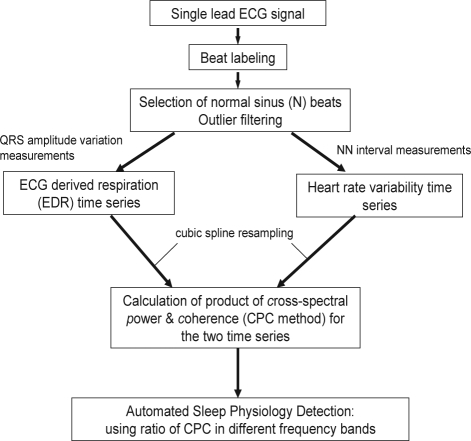
The sequential steps in the derivation of the cardiopulmonary coupling measures are diagrammed in Figures 1 and 2. Using a single-lead ECG, an automated beat detection algorithm1,2 is used to detect beats and classify them as either normal or ectopic based on their morphology and timing. In addition, amplitude variations in the QRS complex due to shifts in the cardiac electrical axis relative to the electrodes during respiration and changes in thoracic impedance are determined. These fluctuations in the mean cardiac electrical axis (typically between 1 degree and 12 degrees peak-to-peak) correlate with phasic changes in the respiratory cycle. From these amplitude variations a surrogate ECG derived respiratory signal (EDR) is obtained as previously described.3,4 A time series of normal-to-normal sinus (N-N) intervals and the time series of the EDR associated with these N-N intervals are then extracted from the original R-R interval time series. Outliers due to false or missed R-wave detections are removed using a sliding window average filter with a length of 41 data points where central points lying outside 20% of the window average are rejected. Since Fourier analysis requires evenly sampled data the resulting N-N interval series and its associated EDR signal are resampled at 2 Hz using cubic spline interpolation. At this sampling rate the Nyquist frequency allows detection of coupling frequencies up to 1 Hz. The cross-spectral power and coherence of these to signals are calculated over a 1024-sample (8.5-min) window using the Fast Fourier Transform applied to the 3 overlapping 512 sample sub-windows within the 1024 coherence window. In each sub-window the DC components and linear trends are removed and the data windowed using the Hanning (cosine) function before calculation of the Fourier Transform. The 1024 coherence window is then advanced by 256 samples (2.1 min) and the calculation repeated until the entire N-N interval/EDR series are analyzed.
Appendix Figure 1.
Sequential steps in the derivation of cardiopulmonary coupling measures.
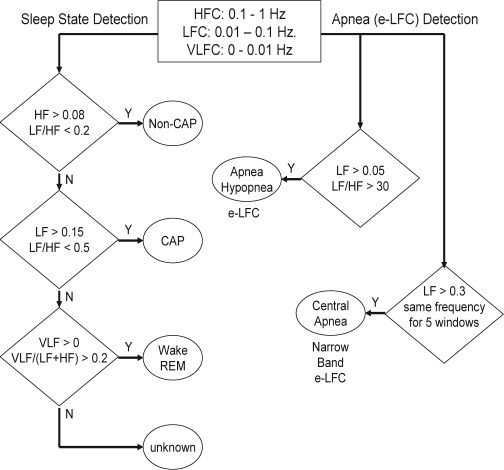
Appendix Figure 2.
Sequential steps in the detection of sleep physiology from cardiopulmonary coupling measures. HFC = High Frequency Coupling; LFC = Low Frequency Coupling; VLFC = Very Low Frequency Coupling; e-LFC = elevated-Low Frequency Coupling
For each 1024 window, the product of the coherence and cross-spectral power is used to generate a spectrogram of coupling powers at each frequency vs. time. This technique thus generates a moving average of the oscillatory frequencies of the coupling between heart rate and respiration. During sleep a predominance of power in the low-frequency band is associated with periodic sleep behaviors and periodic respiration during SDB, while a predominance of power in the high-frequency band is associated with physiologic respiratory sinus arrhythmia and deep sleep with stable respiration. To quantify the low and high frequency coupling power distributions, in each 1024 window the coherence and cross power product is used in calculating the ratio of the sum of the 2 maximal coherent cross power peaks in the low-frequency (0.01–0.1 Hz) band to the sum of the 2 maximal peaks in the high-frequency (0.1–0.4 Hz) band.
Prior analysis of polysomnographic data using cardiopulmonary coupling spectrograms indicated that the low and high-frequency coupling regimes have only weak correlation with standard sleep staging but did follow cyclic alternating pattern (CAP) scoring, whereas low-frequency coupling is associated with CAP and high-frequency coupling with non-CAP. It was also determined that the ratio of the sum of the 2 maximal peaks in the very low-frequency (0–0.01 Hz) to the combined power of the 2 maximal peaks in each of the low- and high-frequency bands could be used to estimate wake/REM periods where a predominance of power in the very low-frequency band is associated with wake/REM periods. For each of the 3 sleep states of non-CAP, CAP, and combined wake/REM, separate receiver-operator curves were calculated over a range of power thresholds, and the thresholds giving the maximum combined sensitivities and specificities for that state were selected as optimal for the detection of that state. Using these thresholds, sleep demonstrating predominantly non-CAP, CAP, and wake/REM states could be identified.
The steps in sleep state classification are as follows. First non-CAP is detected using the power thresholds giving the maximal sensitivity and specificity for non-CAP epoch-by-epoch detection. Specifically, a given minimum high-frequency power (> 0.08 normalized units [n.u.]) and a low-to-high ratio below a set value (< 2.0) is required. If an epoch is not detected as non-CAP CAP detection criteria are applied again using the thresholds giving maximal sensitivities and specificities for epoch-by-epoch detection. Here a given minimum low-frequency power (> 0.15 n.u.) and a low-to-high ratio above a set value (> 0.5) is required. Finally, if an epoch is not detected as either non-CAP or CAP, wake/REM is detected using the thresholds giving maximal sensitivity and specificity for its epoch-by-epoch detection. For this detection a minimum very low-frequency power (> 0 n.u.) and a minimum ratio of very low to combined low and high-frequency power (> 0.2) is required. The small percentage of epochs which are not detected as non-CAP, CAP, or wake/REM are classified as indeterminate. The amounts of detected non-CAP, CAP, and wake/REM are then expressed as the percentage of windows detected in relation to the total sleep period.
Cardiopulmonary Coupling Analysis and Estimation of Elevated Low Frequency Coupling (e-LFC) Subtypes
Analysis of the PhysioNet Sleep Apnea Database5 using the cardiopulmonary coupling technique indicated that elevated power in the low frequency coupling region coincided with periods of scored apnea/hypopnea. Sensitivities and specificities for minute-by-minute apnea/hypopnea detection were calculated for a range of low frequency coupling powers and low/high coupling ratios. Again receiver-operator curves were then calculated and the thresholds giving the maximum combined sensitivity and specificity for apnea/hypopnea detection was selected as optimal. These detection thresholds required that the minimum low frequency power be > 0.05 normalized units and that the low to high frequency ratio be > 30 to define periods of probable apnea/hypopnea, which we term elevated LFC (e-LFC). Thus, e-LFC is defined here as a subset of low frequency coupled cardiopulmonary oscillations, periods of which correlated significantly with periods of manually scored apneas and hypopneas in the PhysioNet Sleep Apnea Database.
Some spectrograms from the PhysioNet Sleep Apnea Database demonstrated periods of near-constant frequency spectral peaks in the e-LFC region that was reminiscent of the oscillations of heart rate variability seen in Cheyne-Stokes respiration in heart failure patients, which has a relatively constant cycle length. To explore this phenomenon further, we applied the algorithm to the PhysioNet Congestive Heart Failure Database,6 with the expectation that the database would provide more prolonged episodes with central periodic oscillations. Since the period of central apnea can be as slow as 120 seconds or longer we used the frequency band between 0.006 and 0.1 Hz to define narrow spectral band e-LFC (putative central sleep apnea, periodic breathing, or complex sleep apnea). We required (1) a minimum power in this band of 0.3 normalized units and (2) that the coupling frequency of each pair of consecutive measurements remains within 0.0059 Hz of each other over 5 consecutive sampling windows (totaling 16.9 continuous min). Periods of e-LFC not meeting these criteria were defined as broad spectral band e-LFC (putative obstructive sleep apnea). The amounts of broad and narrow spectral band coupling in e-LFC bands are again expressed as the percentage of windows detected in relation to the total sleep period. Thus, the narrow spectral band e-LFC identified periods with oscillations that have a single dominant coupling frequency, suggesting central sleep apnea or periodic breathing. The broad spectral band e-LFC identified periods with oscillations that have variable coupling frequencies, suggesting an alternate process, which we posited was dominance of anatomic upper airway obstructive processes. As it takes 16.9 min of continuous narrow-band cardiopulmonary coupling to reach the detection threshold, we estimated that this would be approximately equal to an averaged central apnea index of 5/h of sleep, assuming 6 h of sleep and a periodic breathing cycle length of approximately 35 sec. Thus the cardiopulmonary coupling technique can be used to detect apnea/hypopnea and differentiate these into obstructive vs. central.
REFERENCES
- 1.Moody GB, Mark RG. Development and evaluation of a 2-lead ECG analysis program. Comput Cardiol. 1982:39–44. [Google Scholar]
- 2.Mark RG, Moody GB. Arrhythmia analysis, automated. In: Webster JG, editor. Encyclopedia of medical devices and instrumentation. Wiley; 1988. pp. 120–130. [Google Scholar]
- 3.Moody GB, Mark RG, Zoccola A, Mantero S. Derivation of respiratory signals from multi-lead ECGs. Comput Cardiol. 1985;12:113–6. [Google Scholar]
- 4.Moody GB, Mark RG, Bump MA, et al. Clinical validation of the ECG-derived respiration (EDR) technique. Comput Cardiol. 1986;13:507–10. [Google Scholar]
- 5. http://www.physionet.org/physiobank/database/apnea-ecg.
- 6. http://www.physionet.org/physiobank/database/chfdb.
REFERENCES
- 1.Terzano MG, Parrino L, Smerieri A, et al. Atlas, rules, and recording techniques for the scoring of cyclic alternating pattern (CAP) in human sleep. Sleep Med. 2002;3:187–99. doi: 10.1016/s1389-9457(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 2.Thomas RJ. Cyclic alternating pattern in the electroencephalogram: what is its clinical utility? Sleep. 2007;30:553–5. doi: 10.1093/sleep/30.5.553. [DOI] [PubMed] [Google Scholar]
- 3.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–61. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RJ, Mietus JE, Peng CK, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30:1756–69. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 6.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 7.Moody GB, Mark RG, Zoccola A, Mantero S. Derivation of respiratory signals from multi-lead ECG's. Comput Cardiol. 1985;12:113–6. [Google Scholar]
- 8.Moody GB, Mark RG, Bump MA, et al. Clinical validation of the ECG-derived respiration 9EDR) technique. Comput Cardiol. 1986;13:507–10. [Google Scholar]
- 9.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 10.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 11.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep. 2006;29:95–103. doi: 10.1093/sleep/29.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2008;93:271–87. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 13.Younes M, Ostrowski M, Atkar R, Laprairie J, Siemens A, Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. J Appl Physiol. 2007;103:1929–41. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 14.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 16.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 17.Dempsey JA, Smith CA, Przybylowski T, et al. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560:1–11. doi: 10.1113/jphysiol.2004.072371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama H, Smith CA, Rodman JR, Skatrud JB, Dempsey JA. Effect of ventilatory drive on carbon dioxide sensitivity below eupnea during sleep. Am J Respir Crit Care Med. 2002;165:1251–60. doi: 10.1164/rccm.2110041. [DOI] [PubMed] [Google Scholar]
- 19.Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocapnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171:1048–52. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- 20.Launois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11:87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol. 2007;581:291–8. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalisnik JM, Avbelj V, Trobec R, et al. Effects of beating- versus arrested-heart revascularization on cardiac autonomic regulation and arrhythmias. Heart Surg Forum. 2007;10:E279–87. doi: 10.1532/HSF98.20071055. [DOI] [PubMed] [Google Scholar]
- 23.Brown CA, Wolfe LA, Hains S, Ropchan G, Parlow J. Heart rate variability following coronary artery bypass graft surgery as a function of recovery time, posture, and exercise. Can J Physiol Pharmacol. 2004;82:457–64. doi: 10.1139/y04-076. [DOI] [PubMed] [Google Scholar]
- 24.Zeitlhofer J, Asenbaum S, Spiss C, et al. Central nervous system function after cardiopulmonary bypass. Eur Heart J. 1993;14:885–90. doi: 10.1093/eurheartj/14.7.885. [DOI] [PubMed] [Google Scholar]