Abstract
A mouse strain with a deleted acetylcholinesterase (AChE) gene (AChE knockout) shows a decreased inspiration time and increased tidal volume and ventilation. To investigate the respective roles of AChE in brain and muscle, we recorded respiration by means of whole-body plethysmography in knockout mice with tissue selective deletions in AChE expression. A mouse strain with the anchoring domains of AChE deleted (del E5+6 knockout mice) has very low activity in the brain and neuromuscular junction, but increased monomeric AChE in serum. A mouse strain with deletion of the muscle specific region of AChE (del i1RR knockout mice) exhibits no expression in muscle, but unaltered expression in the central nervous system. Neither strain exhibits the pronounced phenotypic traits observed in the complete AChE knockout strain. A third strain lacking the anchor molecule PRiMA, has no functional AChE and butyrylcholinesterase (BChE) in brain and an unaltered respiratory function. BChE inhibition by bambuterol decreases tidal volume and body temperature in del E5+6 and i1RR knockout strains, but not in PRiMA deletion or wild-type controls. We find that: (1) deletion of the full AChE gene is required for a pronounced alteration in respiratory phenotype, (2) BChE is involved in respiratory muscles contraction and temperature control in del E5+6 and i1RR knockout mice, and (3) AChE expression requiring a gene product splice to either exons 5 and 6 or regulated by intron1 influences temperature control.
Keywords: Respiration, Butyrylcholinesterase, Acetylcholine, Gene knockout, Temperature control, Mice
1. Introduction
Acetylcholine (ACh) influences respiratory control in the brainstem (Haji et al., 2000; Kubin and Fenik, 2004), through chemosensory mechanisms of central (Burton and Kazemi, 2000), and peripheral origins (Shirahata et al., 2007). Central cholinergic control may be critical in early life, since its dysfunction has been linked to the pathophysiology of the Sudden Infant Death Syndrome (Kinney et al., 1995). ACh is also the neurotransmitter at the neuromuscular junction whereby acetylcholinesterase (AChE) rapidly catalyzes its hydrolysis to prevent repetitive and uncoordinated binding to nicotinic receptors. Blockade of AChE by anticholinesterases such as chemical warfare agents produces rapid death by respiratory failure (Holmstedt, 1959; Sidell, 1994). Surprisingly, mice with complete deletion AChE (AChE knockout) survived despite the absence of AChE activity in all tissues (Xie et al., 2000). To ensure survival beyond the first week required liquid enriched food (Ensure®, Duysen et al., 2002) and ambient temperature maintenance near the thermo neutral zone (about 29 °C, Duysen et al., 2002; Sun et al., 2007). The defect of thermoregulation arises from down regulation of nicotinic receptor in the sympathetic ganglia (Sun et al., 2007). Interestingly the AChE knockout strain maintains central cholinergic pathways (Mesulam et al., 2002), and excess ACh resulting from diminished AChE (Hartmann et al., 2007) is compensated for by reduced cholinergic receptor levels (Bernard et al., 2003; Chatonnet et al., 2003; Li et al., 2003). Despite these compensations, AChE knockout mice have characteristic phenotypic traits of excessive cholinergic stimulation, including muscle fasciculations and body tremors, muscle weakness, pinpoint pupils, susceptibility to seizures and excessive salivation during stress (Duysen et al., 2002). AChE knockouts also show a pronounced respiratory phenotype consisting mainly in hyperventilation and increased chemoventilatory response to CO2 (Boudinot et al., 2004). The component of the system driving respiration (central command, muscle control) through which excess ACh produces these phenotypic traits is not known. To address this question, we compared mutants with selective deficits in AChE expression.
AChE is encoded by a single gene where alternative splicing yields a common catalytic domain (encoded by exons 2, 3 and 4) and 3 alternative 3′ splice variations (Li et al., 1993): (1) a rare non-splicing variant that encodes a monomeric AChER subunit, (2) a signal or H sequence, encoded by exon 5, that is cleaved and produces a dimer anchored to the plasma membrane AChEH through a glycophospholipid linkage, and (3) the WAT (tryptophan amphiphilic tetramerization) domain encodes the AChET. The WAT domain is essential for interaction of the catalytic subunits with ColQ (Krejci et al., 1997) and PRiMA (Perrier et al., 2002), two AChE associated proteins. ColQ anchors AChE in the basal lamina, whereas PRiMA anchors AChE in the plasma membrane. In PRiMA knockout mice, AChE is reduced to 2–3% of normal levels in brain and the residual enzyme is retained in the endoplasmic reticulum (Alexandre Dobbertin, Anna Hrabovska, Korami Dembele, Shelley Camp, Palmer Taylor, Eric Krejci and Véronique Bernard, unpublished). As in AChE knockout, m2 muscarinic receptors are not expressed in their normal membrane location (Dobbertin et al., unpublished). In the del E5+6 knockout strain, the deleted sequence prevents interaction of AChE with ColQ and PRiMA and transforms AChE in a soluble, shorter monomeric enzyme (Camp et al., 2008). The level of AChE expressed varies with the tissues, being very low in muscle and brain, but higher in serum. In i1RR knockout, the deletion of a short sequence in the intron 1 prevents expression in the skeletal muscle, but keeps expression intact in brain and spinal cord (Camp et al., 2008).
Herein, we compare phenotypes of three knock-out strains, corresponding to the differential accumulation of AChE in brain and muscle, with the reference strain of AChE knockout mice that express no AChE at all. Since a characteristic of AChE knockout mice is that the enzyme butyrylcholinesterase (BChE), plays a vital substituting role in these strains (Xie et al., 2000; Chatonnet et al., 2003; Hartmann et al., 2007), we investigated effects of BChE inhibition in the three mouse strains with impaired AChE function.
2. Methods
2.1. Animals
Animal studies were conducted in accord with European Union and French Government animal protection laws. Mice originating from UCSD (del E5+6 and i1RR knock-out strains) were bred in our facility in Gif-sur-Yvette, and PRiMA mice were transported from the University of Paris. Gene deletions in these mouse strains are indicated in Fig. 1 (Camp et al., 2008). Measurements were made on mice between ages 6 and 12 weeks.
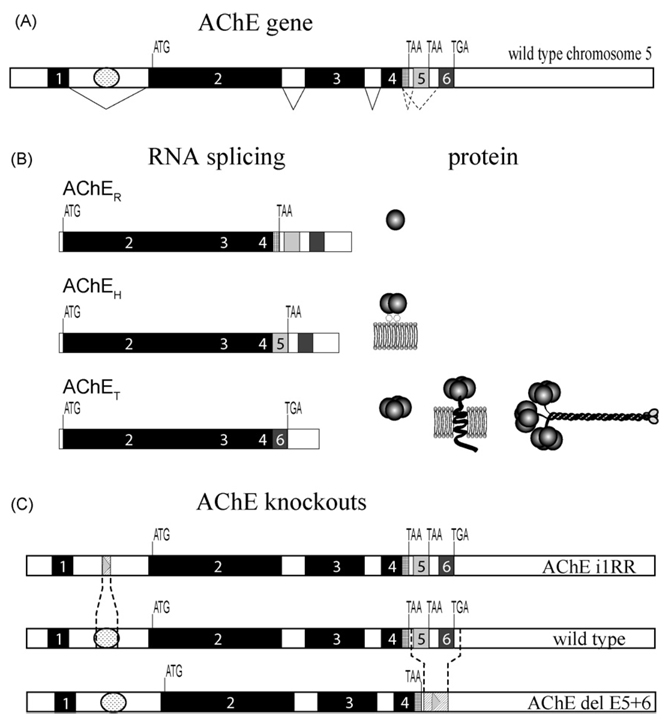
Fig. 1.
(A) AChE gene structure showing invariantly spliced exons 1–4 and alternatively spliced exons 5 and 6. ATG denotes translational start signal, TGA and TAA are translational stops. Invariant splicing is shown by solid lines while alternative splicing is indicated by dotted lines. The regulatory region in the first intron is depicted by a oval between exons 1 and 2. (B) Three AChE variants AChER, AChEH and AChET RNAs are produced by alternative splicing and generate a diversity of AChE molecular forms. (C) Map of the AChE gene with the wild-type and knockout mouse strains with the regions of deletion shown.
2.2. In vivo measurement of ventilation
Respiratory activity in adult mice of both sexes was measured using a barometric method (Bartlett and Tenney, 1970). The plethysmograph chamber (700mL), equipped with a temperature sensor, was connected through a small hypodermic needle to a reference chamber of the same volume. Pressure differences between the two chambers were measured with a differential pressure transducer (Validyne, DP-103-10) connected to a sine wave carrier demodulator (Validyne, CD15). The spirogram was stored on a PC computer (CED interface). Calibrations were made during each recording session by injecting 100 µL of air into the experimental chamber.
During recording sessions, mice were partially restrained by the tail, with a thin temperature probe permanently inserted in the rectum. Within the recording chamber, mice were placed in a small rectangular house opened at both ends that allowed body repositioning, but discouraged movements to chew on the probe. The animal’s nose was at the level of the open front end. The chamber was maintained at 27–28 °C and gas input and output closed during 2 min data collection sessions. Between sessions the chamber was flushed with fresh humidified air or gas mixtures.
2.3. Protocols
The mice were habituated to the chamber for 20 min the day before data collection. Ventilation at rest was initially measured in animals breathing room air (control). The chamber was then flushed with one of the following gas mixtures: 3%, 5% and 8% CO2 (mixed with 20% O2, balance N2) for hypercapnia protocols; 10% O2 balance N2 for the hypoxia protocol. In each session the animals were challenged with the four gas mixtures (4 min each), at 20 min intervals, with exposures in room air between gas challenges. Single mutant and control wild-type (WT) mice were recorded each afternoon and on distinct days in alternating order, and the results were averaged. In each record, we identified periods of breathing at rest, during quiet waking without limb, body or head movements. These periods of baseline breathing were analyzed as previously described (Boudinot et al., 2004). A computer program (ACQUIS1 software) measured the duration of inspiration (TI) and expiration (TE), breathing frequency (fR), tidal volume (VT) and ventilation (V̇E).
Separate experiments were conducted to determine the effects of butyrylcholinesterase (BChE) inhibition on ventilation and body temperature. After 15min of baseline data collection, BChE inhibitors bambuterol or tetraisopropyl pyrophosphoramide (iso-OMPA) were injected s.c. (10 µL/g), in physiologic saline or 3% DMSO in saline respectively.
2.4. Histology
The mice were deeply anesthetized with sodium chloral hydrate and were perfused transcardially with a mixture of 2% paraformaldehyde and 0.2% glutaraldehyde. Brainstem sections were histochemically stained for AChE and BChE activity by the Koelle–Friedenwald method (thiocholine method) with distinguishing inhibitors (Girard et al., 2007).
2.5. Drugs and data analysis
Bambuterol was a gift from Astra-Zeneca France. Iso-OMPA was purchased from Sigma–Aldrich. Results are expressed as means ± S.E.M. Respiratory responses to chemosensory challenges were analyzed using a two-way repeated-measures analysis of variance (ANOVA), and Bonferroni–Dunn post hoc tests. The factors of variation were genotype and treatment.
3. Results
A comparison of the three strains with partial AChE impairment to the respiratory phenotype ofAChE knockout mice that are devoid of AChE activity (Boudinot et al., 2004) is presented in Table 1 and Fig. 2.
Table 1.
Ventilation at rest in adult wild-type (+/+) and homozygous (−/−) mice for deletions of intron 1 (i1RR knockout), of exons 5 and 6 (del E5+6 knockout) of the AChE gene, or of the anchor molecule PRiMA.
| i1RR +/+ (n = 6) | i1RR −/− (n=6) | P | % difference | |
|---|---|---|---|---|
| V̇E (mL/(g min−1)) | 2.01 ± 0.1 | 2.65 ± 0.14 | <0.01 | +32.5 |
| VT (µL/g) | 10.9 ± 0.3 | 13.3 ± 0.7 | <0.02 | +22 |
| fR (breaths/min) | 185 ± 11 | 204 ± 19 | NS | +10 |
| Ti (ms) | 105 ±7 | 89 ±5 | NS | −15 |
| TE (ms) | 232 ± 18 | 222 ± 30 | NS | −4 |
| TI/TTOT (%) | 31.5 ± 1.10 | 30.0 ± 2.68 | NS | −5 |
| VT/TI (µL/(g s−1)) | 106 ± 5.9 | 153 ± 13.2 | <0.01 | +44 |
| Weight (g) | 19.4 ± 0.67 | 14.5 ± 1.07 | <0.01 | −25 |
| Temperature (°C) | 37.4 ± 0.21 | 38.1 ± 0.26 | NS | +2 |
| Del E5+6 +/+ (n = 7) | Del E5+6 −/− (n=7) | |||
| V̇E (mL/(g min−1)) | 1.81 ± 0.11 | 2.57 ± 0.12 | <0.001 | +42 |
| VT (µL/g) | 10.9 ± 0.42 | 13.8 ± 0.60 | <0.01 | +27 |
| fR (breaths/min) | 167 ± 11 | 188 ±9 | NS | +12 |
| Ti (ms) | 109 ±8 | 80 ± 1 | <0.01 | −27 |
| TE (ms) | 266 ± 21 | 244 ± 15 | NS | −8 |
| TI/TTOT (%) | 29.7 ± 1.6 | 26.8 ±2 | NS | −10 |
| VT/TI (µL/(g s−1)) | 104 ± 9.5 | 166 ± 13 | <0.01 | +59 |
| Weight (g) | 23.9 ± 1.0 | 17.6 ± 1.7 | <0.01 | −26.5 |
| Temperature (°C) | 37.8 ± 0.2 | 37.8 ± 0.1 | NS | 0 |
| PRiMA +/+ (n = 6) | PRiMA −/− (n=8) | |||
| V̇E (mL/(g min−1)) | 1.62 ± 0.16 | 1.48 ± 0.10 | NS | −9 |
| VT (µL/g) | 10.4 ± 0.58 | 10.4 ± 1.02 | NS | 0 |
| fR (breaths/min) | 154 ±8 | 148 ±8 | NS | −4 |
| Ti (ms) | 113 ±4 | 118 ± 9.0 | NS | +4.5 |
| TE (ms) | 293 ± 20 | 307 ± 23.0 | NS | +5 |
| TI/TTOT (%) | 28.8 ± 1.5 | 28.2 ± 1.6 | NS | −2 |
| VT/TI (µL/(g s−1)) | 94 ± 7.4 | 93 ± 13.2 | NS | −1 |
| Weight (g) | 28.5 ± 2.3 | 30.1 ± 3.5 | NS | +5.5 |
| Temperature (°C) | 37.2 ± 0.2 | 37.1 ± 0.2 | NS | 0 |
| AChE +/+ (n = 9) | AChE −/− (n=9) | |||
| V̇E (mL/(g min−1)) | 1.34 ± 0.12 | 2.28 ± 0.19 | <0.001 | +70 |
| VT (µL/g) | 9.6 ± 1.1 | 18.8 ± 1.60 | <0.001 | +96 |
| fR (breaths/min) | 144 ±7 | 130 ± 12 | NS | −10 |
| Ti (ms) | 130 ±5 | 74 ± 4 | <0.001 | −43 |
| TE (ms) | 301 ± 20 | 448 ± 47 | <0.02 | +49 |
| TI/TTOT (%) | 30.9 ± 1.4 | 15.5 ± 1.6 | <0.001 | −50 |
| VT/TI (µL/(g s−1)) | 76 ± 10 | 264 ± 31 | <0.001 | +247 |
| Weight (g) | 24.9 ± 0.83 | 16.3 ± 1.10 | <0.001 | −34.5 |
| Temperature (°C) | 36.7 ± 0.4 | 36.6 ± 0.27 | NS | 0 |
Values for mice with deletion of the AChE gene (Boudinot et al., 2004) are presented for reference.
Fig. 2.
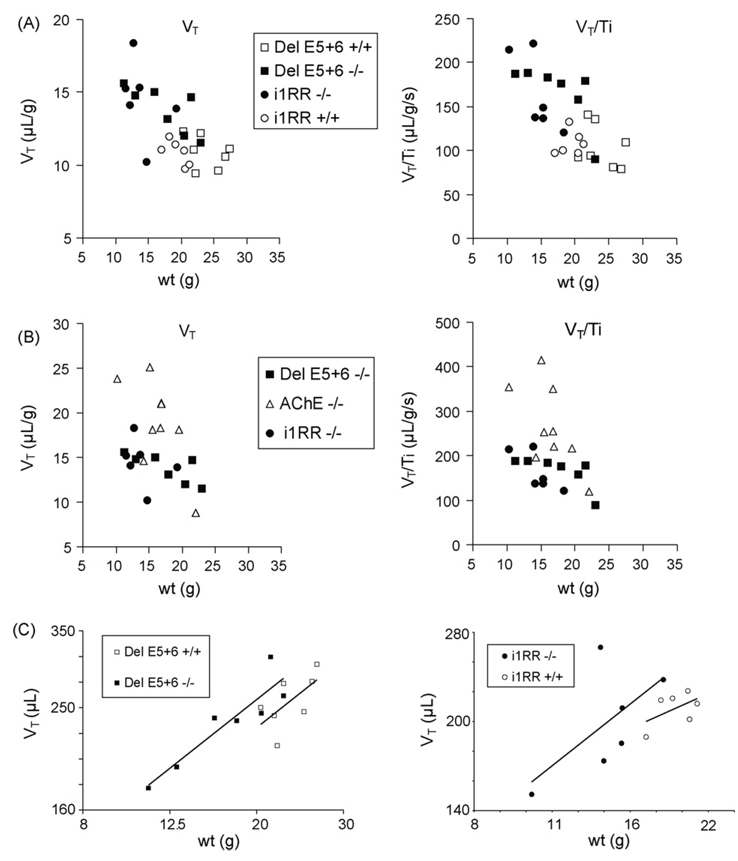
Tidal volume (VT) and mean inspiratory flow (VT/TI) plotted against body weight. (A) The phenotypes of individual del E5+6 knockout and i1RR knockout mice are largely overlapping and distinguished from their WT (+/+) controls. Note the wider dispersion of the mutants compared to WT animals. (B) The phenotypes of del E5+6 knockout and i1RR knockout mice are clearly distinguished from AChE knockouts with comparable weights. (C) The log–log linear regressions of tidal volume versus body weight for mutants and WT of the del E5+6 and i1RR knockout strains fit slighty distinct curves, indicating weak phenotypic differences.
3.1. PRiMA knockout mice
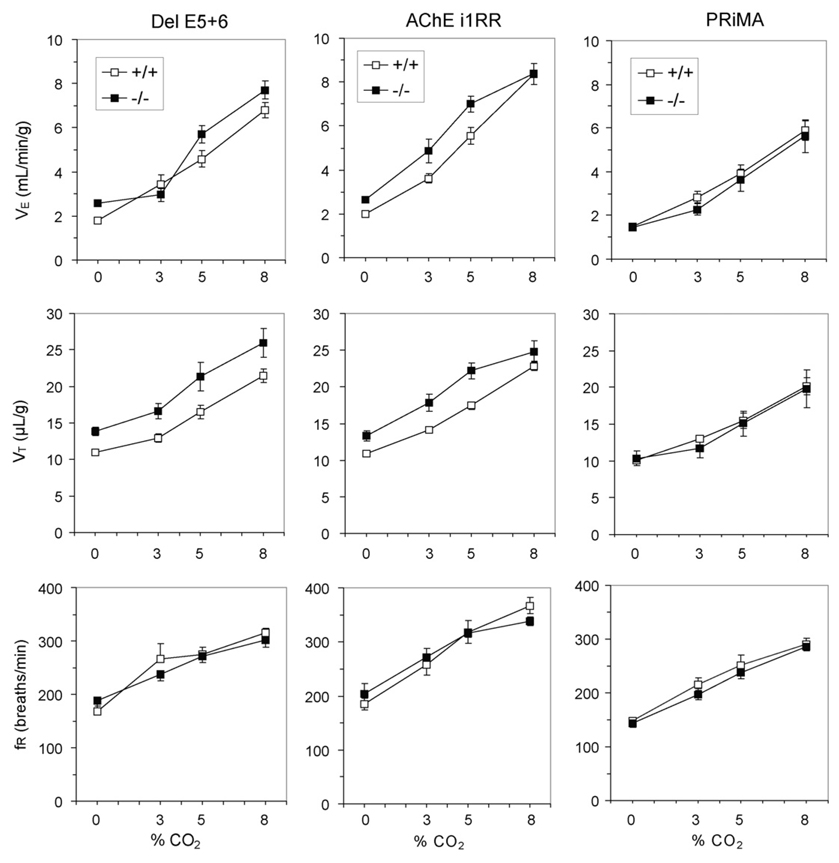
Despite the virtual lack of surface expression and complete retention of residual AChE within the neuron, PRiMAknockout mice are undistinguishable from their wild-type controls in terms of weight, body temperature and ventilation (Table 1). They respond to hypercapnic (Fig. 3) and hypoxic (Fig. 4) challenges similar to their WT controls, an indication of unaltered chemosensitivity.
Fig. 3.
Effects of CO2 on ventilation in del E5+6, i1RR and PRiMA wild-type (+/+) and nullizygous (−/−) mice.
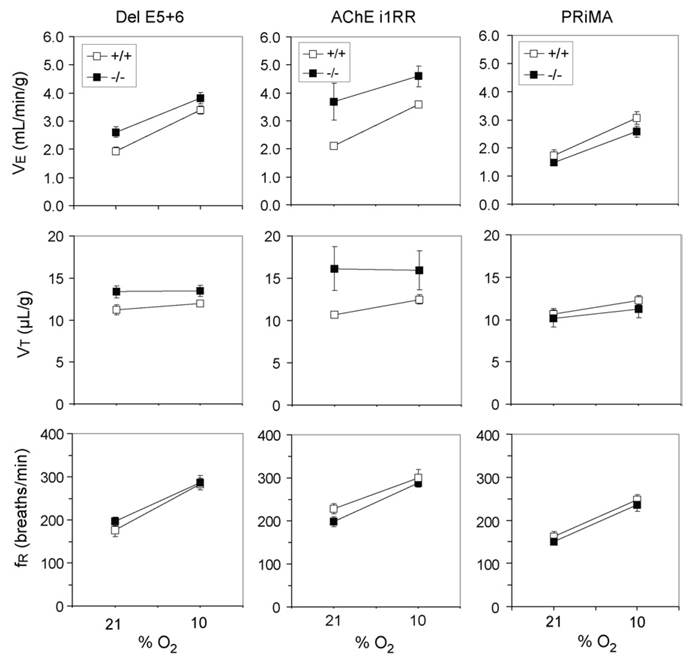
Fig. 4.
Effects of hypoxia on ventilation in del E5+6, i1RR and PRiMA wild-type (+/+) and nullizygous (−/−) mice.
3.2. i1RR knockout and del E5+6 knockout mice
3.2.1. Body weight and temperature
i1RR knockout and del E5+6 knockout mice were significantly smaller than their respective WT controls (Table 1). Body temperature in i1RR knockout and del E5+6 knockout mice was not significantly different from their respective WT controls. However, i1RR knockout mice tended to be slightly warmer than their controls (Table 1). To evaluate whether partial restraint during respiration recordings may affect body temperature, rectal temperature was measured in the same animals at different times. Measurements from each unrestrained animal on three different days gave similar values in WT (37.3 ± 0.09 °C) and i1RR knockout mice (37.4 ± 0.13 °C). Thus, i1RR knockout mice might be less adaptable to the stress of restraint than WT animals, resulting in higher temperature.
3.2.2. Ventilation and respiratory pattern
Ventilation and respiratory patterns reveal significant changes in i1RR knockout and in del E5+6 knockout mice (Table 1). These two strains showed higher tidal volume (V̇T), ventilation (VE) and mean inspiratory flow (VT/TI) than their respective WT controls. Respiratory timing parameters (TI, TE, fR and TI/TTOT) were largely unaffected in the two strains with the exception of a significantly shorter TI in del E5+6 knockout mice.
We examined if the elevated VT and VE (normalized by weight) observed in these two strains were an effect of allometry, since normalized VT was highest in the smallest animals. To take into account scaling factors, normalized VT and VT/TI were plotted against body weight (Fig. 2A). This shows that with comparable weights, i1RR knockout and del E5+6 knockout mice have comparable VT and VT/TI, with the smallest animals showing the largest differences with WT mice. The log–log linear regression curves of tidal volume (not normalized by weight) versus body weight for each knockout strain and their WT controls are very close (Fig. 2C), indicating that the two strains have at best a small phenotypic difference with WT animals.
3.2.3. Ventilation during hypercapnia and hypoxia
The ventilatory response to hypercapnia was unaltered in del E5+6 knockout mice. There were no significant interactions between genotype (WT or mutant) and ventilatory responses (V̇E, VT and fR) over the range of CO2 concentrations (Fig. 3). In i1RR knockout mice, however, the VE response curve to hypercapnia was significantly different than in WT controls (P < 0.02). This was due to a blunted response at the highest CO2 concentration, a possible consequence of muscle fatigue (Fig. 3).
Ventilatory responses to hypoxia were not different in del E5+6 knockout mice compared with their respective controls (Fig. 4), but in AChE i1RR knockout mice the VT response was significantly blunted (P < 0.02).
3.2.4. Comparison with AChE knockout mice
Tidal volume (VT) and mean inspiratory flow (VT/TI), the two parameters previously found to be the most affected in AChE knockout mice, are much lower in i1RR and del E5+6 knockout mice than in AChE knockout mice with comparable weights (Fig. 2B). The relative increases of VT and VT/TI in the two strains over their respective WT controls are only 18–28% of the increases observed in AChE knockout mice (Table 1). For example the increase in VT/TI in del E5+6 knockout mice (59% over WT),was a small fraction (24%) of the increase in AChE knockout mice (247% over WT, Table 1 and Fig. 2B). This was due to a smaller increase in VT and smaller decrease in TI. Thus, the two knockout mice strains do not present the pronounced phenotypic traits of AChE knockout mice.
3.3. Inhibition of butyrylcholinesterase
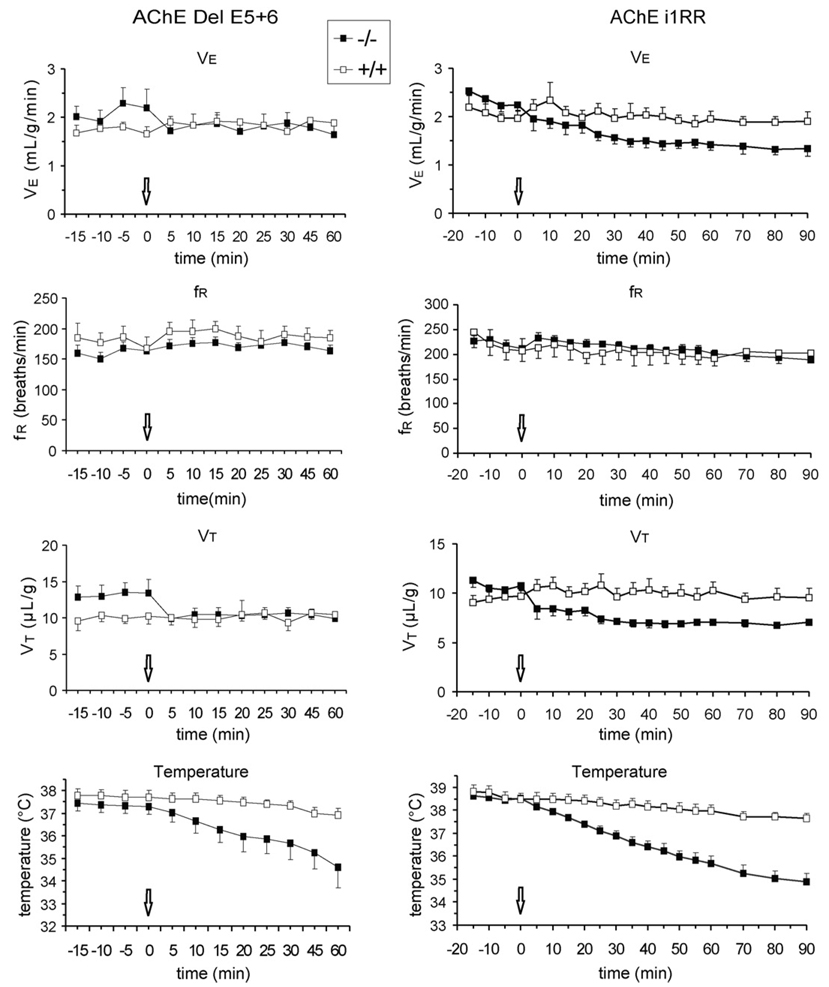
Inhibition of BChE by bambuterol (1 mg/kg) did not affect VE and fR in del E5+6 knockout mice compared with their WT controls (Fig. 5). However, within 5min of bambuterol administration, VT decreased by 25% in mutant mice and remained low 60 min later (P < 0.01), whereas the drug had no effect in WT mice. Body temperature progressively decreased in mutant mice and was significantly lower than in WT mice 20–60 min after administration (P < 0.05).
Fig. 5.
Effects of bambuterol (1 mg/kg, s.c.) administered to del E5+6 and i1RR wild-type (+/+) and nullizygous (−/−) mice. The arrows indicate the time of injection. Note in both mutant strains the rapid decrease of VT and the slow decrease of body temperature.del E5+6 knockout, n = 6; all other groups, n = 5.
In i1RR knockout mice, bambuterol did not affect breathing frequency, but VE and VT became significantly lower than in controls 30–90 min after administration. Body temperature also was lower at 20 min and the difference with WT mice widened with time (Fig. 5).
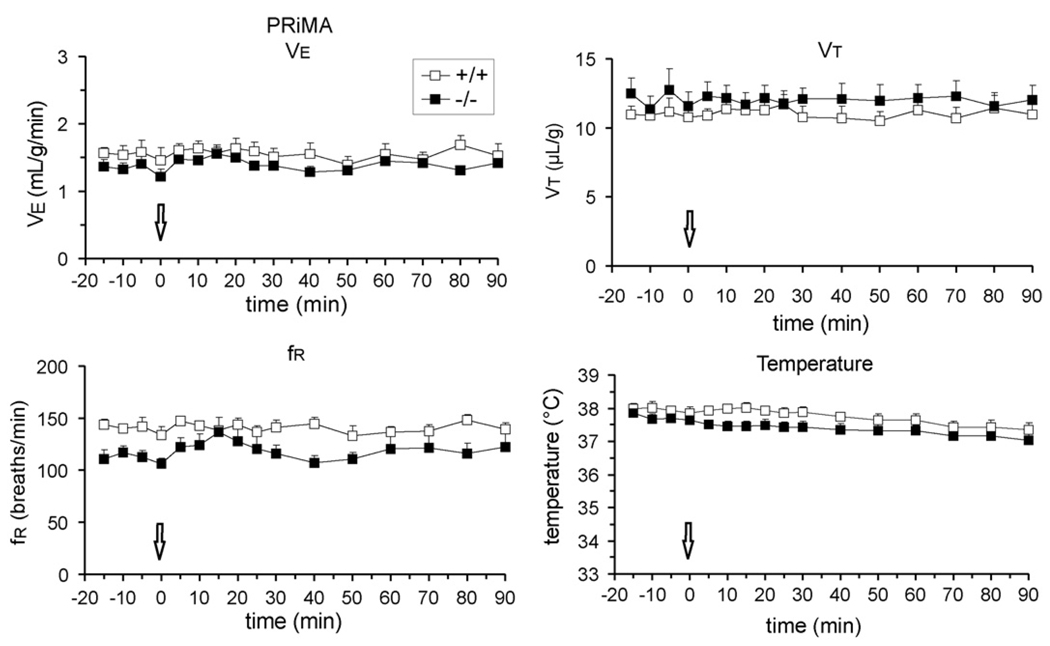
In PRiMA knockout mice bambuterol had no effect on breathing and body temperature (Fig. 6). Because BChE is anchored by PRiMA in the CNS, in PRiMA mutants, we also administered iso-OMPA (6 mg/kg s.c.), which, unlike bambuterol, penetrates the blood–brain barrier. V̇E, VT and fR did not significantly change from pre-injection control values 20 min (n = 3) and 60 min (n = 2) after administration (data not shown).
Fig. 6.
Effects of bambuterol (1 mg/kg, s.c.) administered to PRiMA wild-type (+/+) and nullizygous (−/−) mice. The arrows indicate the time of injection. Tidal volume and body temperature are unaffected in the mutants (n = 5) relative to their wild-type controls (n = 5).
3.4. Localization of AChE in respiratory brainstem areas
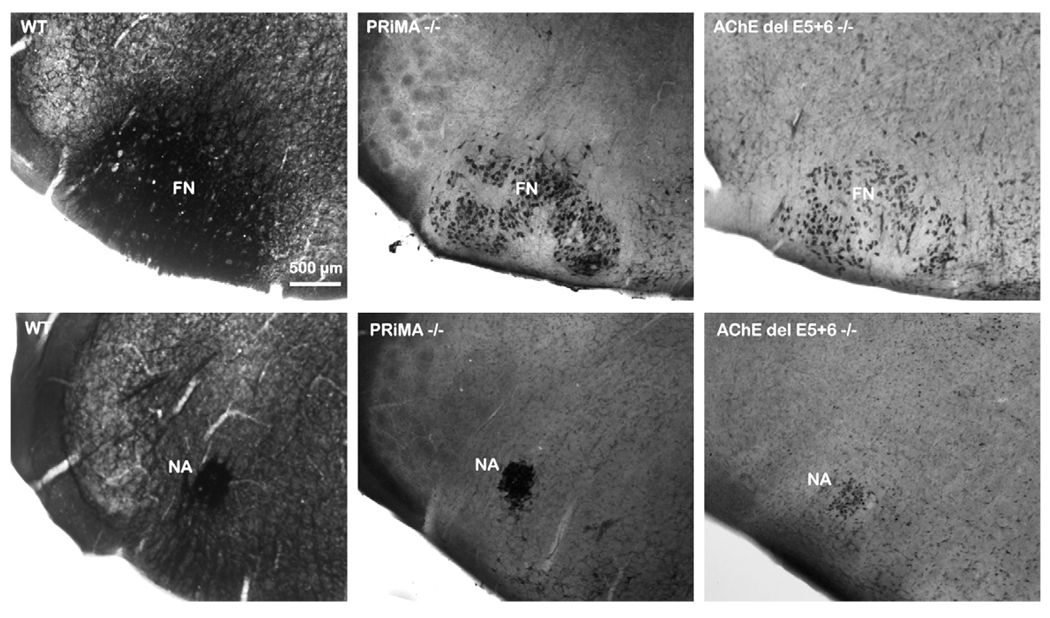
In the brainstem of both the PRiMA and del E5+6 knockout strains, AChE activity was restricted to the cell body and not found in the dendrites or axons (Fig. 7). In the mutants no labeling seems apparent in the area of the ventral respiratory group proximal to the nucleus ambiguus.
Fig. 7.
Histochemical detection of AChE activity by the Koelle–Friedenwald method in facial nucleus (FN) and nucleus ambiguus (NA) of WT, PRiMA and del E5+6 knockout mice. In WT mice, AChE activity is distributed homogeneously in the two nuclei and in neural processes. In both mutants, AChE labeling is restricted to cell bodies. No labeling is apparent in the ventral respiratory group ventral to the nucleus ambiguus.
4. Discussion
The striking outcome of this study is that AChE and BChE in the central nervous system (CNS) appear dispensable in the control of respiration. Compensatory changes that occur in the developmental processes result in an animal strain whose respiratory response to released ACh differs greatly from a mature animal where acute inhibition of AChE is produced chemically (Boudinot et al., 2005). Indeed, the complete absence of AChE and BChE in the CNS (PRiMA knockout) does not alter respiratory function, whereas a strain with a complete absence of AChE in muscle, but normal levels in brain (i1RR knockout), or another strain with dramatic reductions of AChE in brain and muscle (del E5+6 knockout), fail to reproduce the pronounced phenotypic traits of mice completely devoid of AChE expression (Boudinot et al., 2004). We verified that in the ventral brainstem respiratory-related areas of PRiMA and del E5+6 knockout mice, AChE is only detectable inside the neuron, in line with our findings that in the absence of PRiMA, AChE is retained in the endoplasmic reticulum thus preventing its secretion (Dobbertin et al., unpublished). Del E5+6 and i1RR knockout mice present a variety of behavioral phenotypes, such as impaired locomotion, of diminished intensity compared to AChE knockout mice (Camp et al., 2008), but no locomotor phenotype was detected in PRiMA knockout mice, in line with their unaltered weight and respiration.
4.1. Inspiratory effort and chemosensitivity
Hyperventilation due to a doubling of VT characterizes AChE null mutants (Boudinot et al., 2004). Tidal volume and mean inspiratory flow were much lower in del E5+6 knockout and i1RR knockout mice of comparable weights, but unaltered in PRiMA knockout mice, showing that neither a complete absence of AChE function in the neuromuscular junction, nor the absence of AChE expression in brain in addition to the lack of expression in muscles can explain the phenotype of AChE null mutants.
The AChE null mutant strain presents an increased chemosensitivity to CO2 (Boudinot et al., 2004), presumably through up-regulation of some important central chemosensors located near the ventral surface of the medulla (Dev and Loeschcke, 1979; Mulkey et al., 2004), which are cholinoceptive and involve predominantly M3 receptors (Nattie and Li, 1990). The unaffected ventilatory response to CO2 in del E5+6 and PRiMA knockout mice was therefore unexpected. This might explain why tidal volume, regulated in part by CO2 chemosensitivity, is marginally or not affected at all in these mutants compared with AChE knockout mice. In i1RR knockout mice, the slightly blunted response to CO2 at a high concentration (8%), and the blunted VT response to hypoxia are likely to reflect an inability of these mice to sustain augmented muscle contractions.
4.2. Timing mechanisms
Respiratory frequency was unaffected in all three mutant strains as in AChE knockout mice, in which TI and TE are changed in opposite directions with TI markedly reduced. A smaller but significant reduction of TI was observed in del E5+6 knockout mice, but not in i1RR knockout mice, indicating that TI reduction correlates with the absence of functional AChE in the muscle but not in the brain. Hence TI reduction in AChE knockout mice does not result from the inability of respiratory muscles to maintain nerve-evoked tetanic contractions at high stimulus frequencies (Adler et al., 2004; Girard et al., 2005; Mouisel et al., 2006). Excess ACh is unlikely to decrease TI by stimulating ACh receptors present on neurons of the Pre-Bötzinger complex rhythym generator (Shao and Feldman, 2000) because the application of cholinergic agonists onto this generator isolated in brainstem slices produces the opposite: it increases the duration of inspiratory bursts. The target might be timing-controlling structures in the pons (Kubin and Fenik, 2004) and/or some hypothetical central cholinergic modulation of the Hering Breuer reflex.
4.3. Role of butyrylcholinesterase
Inhibition of BChE by bambuterol, a drug that does not penetrate the blood–brain barrier (Svensson, 1991), has no effect in WT animals but is lethal to AChE knockout mice (Xie et al., 2000; Chatonnet et al., 2003). Sub-lethal doses decrease VT (Boudinot et al., 2005), presumably by blocking BChE controlling presynaptically the release of ACh at the neuromuscular junction (Minic et al., 2003; Girard et al., 2007). A dose of 1 mg/kg (20 times the lethal dose in AChE knockout mice) decreased VT in del E5+6 knockout and i1RR knockout mice without any lethality, and was completely ineffective in PRiMA knockout mice. Thus BChE inhibition affects muscle contraction in mice which lack AChE at the neuromuscular junction, but is lethal only when AChE is completely absent in the animal. The survival of all mice of the three strains studied after BChE inhibition is thus surprising, and might be explained by the presence of AChE in the serum in these animals. Indeed the diffusion of ACh from the neuromuscular junction was documented in an historical experiment (Dale et al., 1936). This suggests that nerve released ACh can diffuse from the neuromuscular junction to the blood, where AChE and/or BChE may hydrolyze ACh. In this condition, the efficacy of the synaptic transmission should be maintained inside the neuromuscular junction by a gradient of diffusion.
Mature AChE null mutants are normothermic, but these animals show a marked hypothermia by post-natal day 15 (Duysen et al., 2002; Boudinot et al., 2004) due to a reduced nicotinic function in sympathetic ganglia (Sun et al., 2007). Since bambuterol decreased body temperature in del E5+6 and i1RR knockout mice, these animals also present a dysfunction of thermoregulatory mechanisms, conferring a critical role to BChE. A role for BChE in neurotransmission and thermoregulation has indeed been recently demonstrated in BChE knockout strains (Duysen et al., 2007).
5. Conclusions
Taken together, the present results show that the pronounced respiratory phenotype in AChE knockout mice cannot be explained by AChE deficit in the muscle and/or in the CNS. This phenotype might be caused by altered chemosensitivity to CO2, which is not up-regulated in the three mutants studied here. Both AChE del E5+6 knockout and AChE i1RR knockout mice show altered thermoregulatory mechanisms requiring compensation by BChE.
Acknowledgements
We thank Gérard Sadok who wrote the Elphy program and Sandra Autran for genotyping. This study was supported by the Centre National de la Recherche Scientifique (CNRS), the European Community (QLG2-CT2001-01467 “Brainstem Genetics”), Ministère de la Recherche (ACI BDP#57), Fondation pour la RechercheMédicale, NIH grant PO1ES10337 to PT and by grants from Inserm, Association Française contre les Myopathies (AFM), Agence Nationale de la Recherche (ANR Neuroscience), Bonus Quality Research Université Paris Descartes to EK. EK is supported by the CNRS.
References
- Adler M, Manley HA, Purcell AL, Deshpande SS, Hamilton TA, Kan RK, Oyler G, Lockridge O, Duysen EG, Sheridan RE. Reduced acetylcholine receptor density, morphological remodeling, and butyrylcholinesterase activity can sustain muscle function in acetylcholinesterase knockout mice. Muscle Nerve. 2004;30:317–327. doi: 10.1002/mus.20099. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir. Physiol. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Bernard V, Brana C, Liste I, Lockridge O, Bloch B. Dramatic depletion of cell surface m2 muscarinic receptor due to limited delivery from intracytoplasmic stores in neurons of acetylcholinesterase-deficient mice. Mol. Cell Neurosci. 2003;23:121–133. doi: 10.1016/s1044-7431(03)00034-4. [DOI] [PubMed] [Google Scholar]
- Boudinot E, Taysse L, Daulon S, Chatonnet A, Champagnat J, Foutz AS. Effects of acetylcholinesterase and butyrylcholinesterase inhibition on breathing in mice adapted or not to reduced acetylcholinesterase. Pharmacol. Biochem. Behav. 2005;80:53–61. doi: 10.1016/j.pbb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Boudinot E, Emery MJ, Mouisel E, Chatonnet A, Champagnat J, Escourrou P, Foutz AS. Increased ventilation and CO2 chemosensitivity in acetyl-cholinesterase knockout mice. Respir. Physiol. Neurobiol. 2004;140:231–241. doi: 10.1016/j.resp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Burton MD, Kazemi H. Neurotransmitters in central respiratory control. Respir. Physiol. 2000;122:111–121. doi: 10.1016/s0034-5687(00)00153-5. [DOI] [PubMed] [Google Scholar]
- Camp S, De Jaco A, Zhang L, Marquez M, De la Torre B, Taylor P. Acetylcholinesterase expression in muscle is specifically controlled by a promoter-selective enhancesome in the first intron. J. Neurosci. 2008;28:2459–2470. doi: 10.1523/JNEUROSCI.4600-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet F, Boudinot E, Chatonnet A, Taysse L, Daulon S, Champagnat J, Foutz AS. Respiratory survival mechanisms in acetylcholinesterase knockout mouse. Eur. J. Neurosci. 2003;18:1419–1427. doi: 10.1046/j.1460-9568.2003.02867.x. [DOI] [PubMed] [Google Scholar]
- Dale H, Feldberg W, Vogt M. Release of acetylcholine at voluntary motor nerve endings. J. Physiol. 1936;86:353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev NB, Loeschcke HH. Topography of the respiratory and circulatory responses to acetylcholine and nicotine on the ventral surface of the medulla oblongata. Pflugers Arch. 1979;379:19–27. doi: 10.1007/BF00622900. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Li B, Darvesh S, Lockridge O. Sensitivity of butyrylcholinesterase knockout mice to (−)-huperzine A and donepezil suggests humans with butyrylcholinesterase deficiency may not tolerate these Alzheimer’s disease drugs and indicates butyrylcholinesterase function in neurotransmission. Toxicology. 2007;233:60–69. doi: 10.1016/j.tox.2006.11.069. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Stribley JA, Fry DL, Hinrichs SH, Lockridge O. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Brain Res. Dev. Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- Girard E, Bernard V, Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and the control of synaptic responses in acetylcholinesterase knockout mice. Life Sci. 2007;80:2380–2385. doi: 10.1016/j.lfs.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Girard E, Barbier J, Chatonnet A, Krejci E, Molgo J. Synaptic remodeling at the skeletal neuromuscular junction of acetylcholinesterase knockout mice and its physiological relevance. Chem. Biol. Interact. 2005;157–158:87–96. doi: 10.1016/j.cbi.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Haji A, Takeda R, Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol. Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- Hartmann J, Kiewert C, Duysen EG, Lockridge O, Greig NH, Klein J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007;100:1421–1429. doi: 10.1111/j.1471-4159.2006.04347.x. [DOI] [PubMed] [Google Scholar]
- Holmstedt B. Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol. Rev. 1959;11:567–688. [PubMed] [Google Scholar]
- Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF. Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science. 1995;269:1446–1450. doi: 10.1126/science.7660131. [DOI] [PubMed] [Google Scholar]
- Krejci E, Thomine S, Boschetti N, Legay C, Sketelj J, Massoulie J. The mammalian gene of acetylcholinesterase-associated collagen. J. Biol. Chem. 1997;272:22840–22847. doi: 10.1074/jbc.272.36.22840. [DOI] [PubMed] [Google Scholar]
- Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir. Physiol. Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Li B, Duysen EG, Volpicelli-Daley LA, Levey AI, Lockridge O. Regulation of muscarinic acetylcholine receptor function in acetylcholinesterase knockout mice. Pharmacol. Biochem. Behav. 2003;74:977–986. doi: 10.1016/s0091-3057(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Camp S, Taylor P. Tissue-specific expression and alternative mRNA processing of the mammalian acetylcholinesterase gene. J. Biol. Chem. 1993;268:5790–5797. [PubMed] [Google Scholar]
- Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O. Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyze acetylcholine. Neuroscience. 2002;110:627–639. doi: 10.1016/s0306-4522(01)00613-3. [DOI] [PubMed] [Google Scholar]
- Minic J, Chatonnet A, Krejci E, Molgo J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockoutmouseneuromuscular junctions. Br. J. Pharmacol. 2003;138:177–187. doi: 10.1038/sj.bjp.0705010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouisel E, Blondet B, Escourrou P, Chatonnet A, Molgo J, Ferry A. Outcome of acetylcholinesterase deficiency for neuromuscular functioning. Neurosci. Res. 2006;55:389–396. doi: 10.1016/j.neures.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li AH. Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J. Appl. Physiol. 1990;69:33–41. doi: 10.1152/jappl.1990.69.1.33. [DOI] [PubMed] [Google Scholar]
- Perrier AL, Massoulie J, Krejci E. PRiMA: the membrane anchor of acetylcholinesterase in the brain. Neuron. 2002;33:275–285. doi: 10.1016/s0896-6273(01)00584-0. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBotzinger complex inspiratory neurons. J. Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M, Balbir A, Otsubo T, Fitzgerald RS. Role of acetylcholine in neurotransmission of the carotid body. Respir. Physiol. Neurobiol. 2007;157:93–105. doi: 10.1016/j.resp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Sidell FR. Clinical effects of organophosphorus cholinesterase inhibitors. J. Appl. Toxicol. 1994;14:111–113. doi: 10.1002/jat.2550140212. [DOI] [PubMed] [Google Scholar]
- Sun M, Lee CJ, Shin HS. Reduced nicotinic receptor function in sympathetic ganglia is responsible for the hypothermia in the acetylcholinesterase knockout mouse. J. Physiol. 2007;578:751–764. doi: 10.1113/jphysiol.2006.120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson LA. Mechanism of action of bambuterol: a beta-agonist prodrug with sustained lung affinity. Agents Actions Suppl. 1991;34:71–78. [PubMed] [Google Scholar]
- Xie W, Stribley JA, Chatonnet A, Wilder PJ, Rizzino A, McComb RD, Taylor P, Hinrichs SH, Lockridge O. Postnatal developmental delay and supersensitivity to organophosphate in gene-targetedmice lacking acetylcholinesterase. J. Pharmacol. Exp. Ther. 2000;293:896–902. [PubMed] [Google Scholar]