Abstract
Based on the hypothesis that isoflavones are absorbed more efficiently from fermented than from non-fermented soy foods, we compared the urinary isoflavonoid excretion (UIE) after intake of miso soup or soy milk. We recruited 21 women with Japanese ancestry who consumed standardized soy portions containing 48 mg isoflavones. On day 1, half the women consumed soy milk, the other half started with miso soup. On day 3, the subjects ate the other soy food and on day 5, they repeated the first food. Each participant collected a spot urine sample before and an overnight urine sample after soy food intake. All urine samples were analyzed for the daidzein, genistein, and equol using liquid chromatography-mass spectrometry and were expressed as nmol per mg creatinine. We applied mixed models to evaluate the difference in UIE by food while including the baseline values and covariates. Relative to baseline, both groups experienced significantly higher UIE after consuming any of the soy foods. We observed no significant difference in UIE when soy milk was compared to miso soup (p = 0.87) among all women or in the seven equol producers (p = 0.88). Repeated intake of the same food on different days showed high reproducibility within subjects. These preliminary results indicate similar UIEs after consuming a fermented soy food (miso) as compared to a non-fermented soy food (soy milk). Therefore, recommendations favoring fermented soy foods are not justified as long as the intestinal microflora is capable of hydrolyzing the isoflavone glucosides from non-fermented soy foods.
Introduction
Soy foods may protect against chronic diseases1; 2. Isoflavones in soy beans, i.e., daidzein, genistein, and glycitein, are active substances that have chemical structures similar to mammalian estrogens3. In food, they are primarily found as beta-glucosides with and without additional malonates and acetate esters4. Once ingested, these conjugated isoflavones undergo hydrolysis by beta-glucosidases mainly from intestinal bacteria, releasing the principal bioactive aglucone (glucose free) form that is absorbed, whereas the highly water soluble glucosides are not absorbed5–8. Therefore, intestinal bacteria are crucial for the absorption and bioavailability of isoflavones5; 8; 9. It is the aglycones (sugar free forms) that show an affinity for estrogen receptors and have other non-hormonal effects on the cell machinery6. The isoflavone daidzein is metabolized to equol and O-desmethylangolensin (O-DMA) by gut bacteria and excreted predominantly through the urine10. The ability to produce equol is limited to 30–50% of the population but whether this metabolic feature results in more beneficial health effects from soy consumption remains uncertain9; 11. The extent of isoflavone metabolism varies among individuals and may be influenced by additional dietary factors12; 13.
Dietary isoflavonoids are specific to soy foods and urinary isoflavonoid excretion (UIE) serve as an excellent marker for the bioavailability of isoflavones14; 15. As a result of their rapid metabolism, urinary appearance of isoflavonoids reflects circulating levels when the timing of specimen collection is accurately considered8; 16; 17. Due to micro-organism induced hydrolysis in fermented soy products, e.g., tempeh and miso, the predominant form of isoflavones in these foods are aglucones. Consuming fermented soy foods was reported to lead to higher levels of absorption and urinary isoflavone recovery in some18–20 but not in other studies21–23. We hypothesized that women will excrete more isoflavonoids and produce more equol after consuming equivalent isoflavone amounts in one serving of miso soup than in one serving of soy milk because the aglucone form present in fermented soy products is more bioavailable than the conjugated isoflavones in the non-fermented soy milk.
Methods
Participants
We recruited a convenience sample through employees, families, and friends of the Cancer Research Center of Hawaii. Eligible participants were at least 18 years of age, at least 50% Japanese ancestry, current resident of Hawaii, and free of any known soy allergies. Women with Japanese ancestry were chosen because they were expected to have had previous soy food exposure and a more comparable gut flora than subjects with different ancestries24. During the intervention, none of the participants were taking antibiotics, food supplements, or probiotics that could alter the intestinal flora. The study protocol was approved by the Committee on Human Subjects at the University of Hawaii and all subjects gave written informed consent.
Data Collection
Demographic, familial, and medical information was collected by questionnaire. Regular soy intake was assessed with a 12-item questionnaire which elicited the frequency and average serving size of soy food consumed during the last 12 months25. To obtain a summary score, we multiplied the frequencies of intake for each of the 12 soy foods by the estimated isoflavone content from the food composition database maintained by the Nutrition Support Shared Resource at the Cancer Research Center of Hawaii. Isoflavone concentrations for soy foods in this database were primarily derived from an analysis of representative local foods4. An additional lifetime questionnaire estimated soy exposure since birth using the following stages: infancy (1 year), childhood (1–9 years), adolescence (10–19 years), early adulthood (20–29 years), and late adulthood (30 + years)26. Participants indicated the annual frequency of 4 categories of soy foods (tofu; soy beans and sprouts; soybean drink or milk; and other soy products) for each period. To obtain a summary score, we multiplied the frequency of intake for each of the four foods by the estimated isoflavone amount and added the total amounts of the four foods in each stage of life to determine an annual intake for each stage.
Intervention and Urine Collection
The 6-day study protocol included three spot urine collections after a soy-free day and three overnight urine collections after consumption of a standardized portion of miso soup or soy milk. According to our HPLC analyses, one serving of soy milk (250 mL of Edensoy® Original Organic Soy milk) contained 48.3 mg isoflavones as aglycone equivalents which was matched with 31 g of Haccho miso (again determined by HPLC) to be dissolved in one cup of water; 98% of the isoflavones in miso were aglycones but only 2% in the soy milk. During and one day before starting the intervention, participants were instructed to abstain from all soy foods other than those provided to them for the study. Half of the participants started with soy milk (group A) as follows: On day 1, at around 6 pm, the women collected a spot urine sample, consumed one container of soy milk, and collected all urine thereafter until they got up the next morning (overnight urine). On day 2, participants proceeded with their normal diets. On day 3, subjects followed the same protocol as day 1, but drank one serving of miso soup. On day 4, participants again returned to their normal diets. On day 5, subjects followed the same protocol as day 1 exactly, collected a spot urine sample at around 6 pm, consumed one container of soy milk, and collected all overnight urine. The other half of the participants (group B) followed the same protocol except starting with miso soup (day 1) followed by soy milk (day 3) and finally miso soup again (day 5). The urine collection containers contained ascorbic and boric acid to prevent bacterial contamination and degradation of analytes. The samples were stored in refrigerators and transported in chilled coolers. The urine samples were aliquoted into 2 ml vials and stored at −80°C until analyzed. Based on the collection times recorded by each subject, we calculated the total number of overnight collection hours.
Urine Analysis
Daidzein, genistein, and equol were analyzed from urine by liquid chromatography-mass spectrometry using a triple quadruple TSQ Ultra system (ThermoFisher, San Jose, CA) with electrospray ionization in negative mode and multiple reaction monitoring27; 28. In brief, triply 13-C labeled internal standards of daidzein, genistein, and equol (University of St Andrews, UK) were added to 100 μL of urine and hydrolyzed for one hour at 37°C with glucuronidase and sulfatase (Roche Applied Sciences, Indianapolis, IN) followed by phase separation with diethyl ether29. The ether fractions were dried under nitrogen and re-dissolved in a 1:1 mixture of methanol/NaAc buffer (0.2 M, pH 5). 25 μL of this extract was analyzed by LC/MS-MS after separation on a BetaBasic C8-column (100 mm × 2.1 mm i.d., 3 μm) coupled to a BetaBasic C8-precolumn (10 mm × 2.1 mm i.d., 3 μm; both from ThermoFisher)27; 28; 30. The elution was performed with methanol/acetonitrile/water = 10/10/80 to 33/33/34 in 6 minutes, holding there for 1 minute before changing in 0.1 minute to the starting mixture for equilibration. Ammonium hydroxide (aqueous 2.5% at 10 μL/min) was infused to enhance the signal. Daidzein was monitored using the transitions (m/z) 253.020 to 222.988, 207.980, and 131.949; for genistein the transitions were 269.090 to 159.050, 133.035, and132.032, and for equol they were 241.130 to 134.950, 121.000, and 118.960. Limits of quantitation (LOQ) for all analytes were 1.5 nmol/L for daidzein and genistein and 3.0 nmol/L for equol for post-intervention samples and half of those values for pre-intervention samples due to differences in concentration steps prior to analysis. Mean intra- and inter-day coefficients of variations (CV) of LC/MS-MS quantitation for daidzein (422 nmol/L), genistein (35.9 nmol/L), and equol (9.9 nmol/L) were 8.3%, 13.6%, and 2.9%, and 7.3%, 17.9%, and 3.5%, respectively. Recoveries were 72–88%. Urinary creatinine concentrations were measured with a Roche-Cobas MiraPlus chemistry analyzer using a kit from Randox Laboratory (Crumlin, UK) that is based on a kinetic modification of the Jaffe reaction. LOQ was <15 μmol/L and the mean inter-assay CV was 0.8% at 187 μmol/L. The sum of dadzein, genistein, and equol was expressed in nmols of isoflavonoids per mg of creatinine. We also calculated the isoflavonoid excretion as nmols per hour based on urine weight, hours of urine collection, and isoflavonoid concentration in urine.
Statistical Analysis
All data management and statistical analyses were performed by using SAS, release 9.1 (SAS Institute, Cary, NC)31. To define equol producer status, we used 0.05 nmol per mg creatinine as a cut-off value. Two-sample t tests were used to compare study characteristics by group at baseline. Due to their non-normal distribution, we log transformed the UIE. We examined overall mean differences of UIE after soy milk and miso soup intake in one model that included all six UIE values for each woman. This examination was carried out using maximum likelihood estimation of a mixed general linear model that takes into account the covariance structure of the repeated measures within subjects32. The order (group A or B), day, age, weight, and equol producer status were included into the model as covariates. In addition, we computed adjusted least square means of UIE for the three food categories (no soy, soy milk, miso soup).
Results
We enrolled 21 participants with a mean age of 49.4 (range: 20–83) years (Table 1). The mean body weight was 58.0 with a standard deviation (SD) of 11.7 kg. All women reported at least 50% Japanese ancestry. Self-reported isoflavone intake during the previous 12 months varied among the participants with a mean of 20.1 (range: 0.1–85.6) mg/day. While self-reported early life soy intake was only 0.98 (range: 0–7.1) servings/day, adult soy intake was 1.7 (range: 0.2–10.2) servings/day. Although group B reported higher soy and isoflavone intake than group A during their previous life, none of the differences was statistically significant. The time during which overnight urine was collected covered a mean±SD of 10.8±1.5 hours and did not differ by group (11.0 vs. 10.5 hours; p = 0.67).
Table 1.
Characteristics of the Study Population
| Group | Age (years) | Body weight (kg) | Early life soy intake (svgs/day) | Adult soy intake (svgs/day) | Mean Isoflavone intake* (mg/day) |
|---|---|---|---|---|---|
| A | 83 | 39.5 | 1.42 | 2.07 | 26.3 |
| A | 54 | 49.5 | 0.41 | 1.20 | 4.4 |
| A | 20 | 50.0 | 1.71 | 2.28 | 21.9 |
| A | 48 | 52.3 | 0.16 | 0.20 | 3.7 |
| A | 59 | 70.5 | 0.41 | 0.74 | 3.9 |
| A | 54 | 70.9 | 0.71 | 1.38 | 8.7 |
| A | 52 | 52.3 | 0.10 | 0.23 | 1.4 |
| A | 45 | 61.4 | 0.20 | 0.33 | 8.8 |
| A | 50 | 44.1 | 0.56 | 1.17 | 24.5 |
| A | 50 | 47.3 | 0.16 | 0.20 | 5.5 |
| B | 32 | 47.7 | 0.48 | 0.96 | 13.5 |
| B | 53 | 76.8 | 0.03 | 0.21 | 0.1 |
| B | 29 | 54.1 | 7.14 | 10.20 | 46.3 |
| B | 43 | 47.7 | 2.53 | 4.09 | 11.4 |
| B | 53 | 50.9 | 0.03 | 0.49 | 22.5 |
| B | 56 | 76.4 | 0.00 | 2.14 | 85.6 |
| B | 58 | 60.5 | 0.71 | 0.95 | 45.8 |
| B | 47 | 72.7 | 0.41 | 0.74 | 42.2 |
| B | 58 | 76.4 | 2.92 | 4.92 | 34.2 |
| B | 41 | 58.2 | 0.28 | 0.37 | 7.9 |
| B | 52 | 59.5 | 0.21 | 0.49 | 3.4 |
|
| |||||
| Mean ± std (A + B) | 49.4±21.9 | 58.0±11.6 | 0.98±1.6 | 1.7±2.3 | 20.1±21.1 |
|
| |||||
| Mean ± std (A) | 51.5±14.5 | 53.8±10.0 | 0.58±0.53 | 0.98±0.73 | 10.9±9.0 |
|
| |||||
| Mean ± std (B) | 47.5±9.6 | 61.9±11.1 | 1.3±2.1 | 2.32±1.9 | 28.4±24.3 |
|
| |||||
| p-value# | 0.68 | 0.11 | 0.82 | 0.27 | 0.38 |
Self-reported intake during the previous 12 months according to a food frequency questionnaire
Two-sample t tests using log transformed values for difference between group A and B
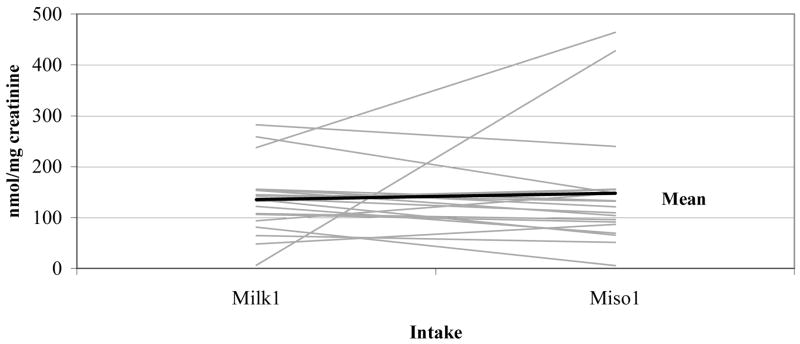
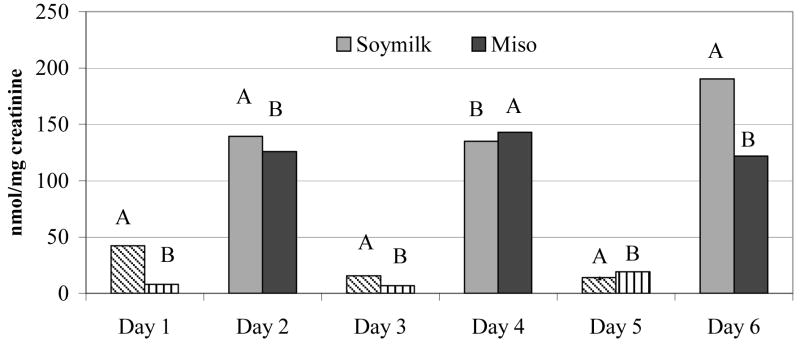
The unadjusted mean UIE was 135 nmol per mg of creatinine after first soy milk as compared to 148 nmol per mg of creatinine after first miso soup intake (Figure 2). When we expressed UIE as an hourly rate 33, the results remained unchanged; the respective medians for soy milk and miso soup were 6.39 and 6.57 μmol/hour. The correlation between the two UIE values expressed in different units was 0.95. Based on all six days, the mean UIEs on days after soy food intake were significantly higher than on the days without soy intake (p <0.001) (Figure 1). In a mixed model that included the baseline UIE values, the difference in UIE after soy milk versus miso soup consumption was not significant (p = 0.87). The respective adjusted mean UIEs for no soy, soy milk, and miso soup were 5.7, 116.9, and 111.7 nmol per mg of creatinine. Order (group A or B) was not significant (p = 0.98). Inclusion of body weight and age did not change the effect estimate for UIE (p = 0.19 and 0.38).
Figure 2. Urinary isoflavonoid excretion on days 2 & 4 comparing first soymilk and miso soup intake in 21 women*.
* Each line represents one woman.
Figure 1.
Median urinary isoflavonoid excretion before (day 1, 3, & 5) and after soy food intake (days 2, 4, & 6) in 21 Japanese-American women
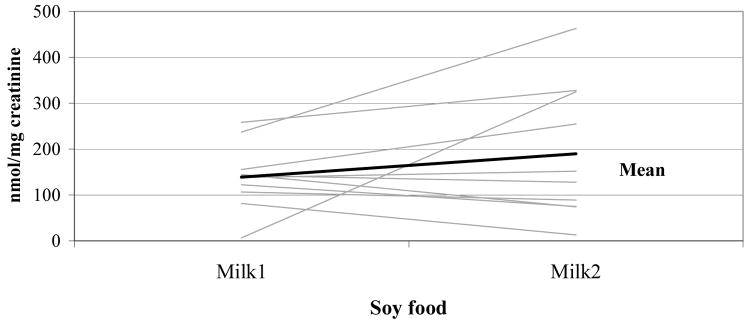
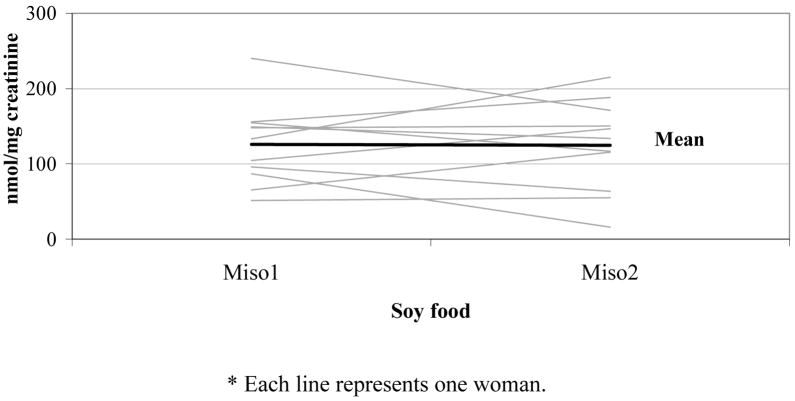
To assess the repeatability of UIE after consuming the same food, we compared the first and second soy milk intake for group A (Figure 3) and the first and second miso soup intake for group B (Figure 4). There was no significant difference in UIE between first and second intake for group A or for group B (p = 0.65 and 0.81).
Figure 3. Urinary isoflavonoid excretion after soymilk intake on days 2 & 6 in group A (n = 10)*.
* Each line represents one woman.
Figure 4. Urinary isoflavonoid excretion after miso soup intake on days 2 & 6 in group B (n = 11)*.
* Each line represents one woman.
Equol excretion was observed in seven of our 21 (33%) participants. Three of the seven women showed equol excretion on all feeding days, three only on the initial feeding day, and one on feeding days 2 and 4. We observed no significant difference in UIE between soy milk and miso soup for the seven equol producers (p = 0.88). Based on the mixed model, there was no difference in overall UIE between equol producers and non-producers (p = 0.87).
Discussion
In this small intervention study among 21 women with Japanese ancestry, we observed no significant difference in overnight UIE after intake of one serving of soy milk or miso soup with equivalent isoflavone content. Expressing UIE as nmol per mg creatinine or as nmol per hour gave similar p-values33 and adjustment for potential confounders did not change the result. For the majority of subjects, the UIE did not vary by food (Figure 2). The results are contrary to our expectation of a higher UIE for miso soup that contains readily available aglucones, whereas the isoflavones in soy milk require hydrolysis prior to uptake. Repeated intake of the same food on different days showed high reproducibility within subjects. There was also no difference in UIE among the subgroup of seven equol producers or between them and the non-equol producers. The fact that one third of our participants excreted equol agreed with previous reports for Caucasians11 but is low for persons of Asian ancestry34.
These results agree with some previous reports21–23; 35; 36, but they are in conflict with others18–20; 37; 38. Urinary isoflavonoid recovery did not differ significantly after a meal of cooked soybeans, tofu, texturized vegetable protein, or tempeh35, between subjects who ingested soybean isoflavone glycosides or red clover isoflavone aglycones36, and in a cross-over trial that provided regular soy milk containing mostly glucosides and soy milk treated by probiotic bifidobacteria to produce aglucones21. Similarly, equivalent bioavailability was observed when isoflavones were given as glucosides as naturally present in soy drinks and after enzymatically hydrolyzing these drinks to produce aglucones23, and bioavailability of isoflavones was similar among American women after consumption of aglucone or glucoside tablets22. A comparison of isolated aglucones and conjugates isoflavones also showed identical uptakes for both8. On the other hand, a randomized cross-over trial described higher urinary isoflavonoid recovery after eating tempeh than soy bean pieces18, plasma concentrations over 24 hours were higher after the administration of aglucone tablets than an equivalent glucoside preparation19, isoflavone aglycones were absorbed in greater amounts than their glycosides in a comparison of regular soy milk, fermented soy milk, and β-glucosidase-treated soy milk20, and tempeh resulted in larger areas under the curve than textured vegetable protein38. Still another report demonstrated higher bioavailability of glucosides vs. aglucones when the area under the plasma curve after oral dosage of 50 mg of beta-glucosides (daidzin, genistin) was compared to that after intake of the equivalent amount of aglycones (daidzein, genistein)37.
Interventions that measured isoflavones in blood agree that the peak plasma level is achieved faster when aglucones as opposed to glucosides are consumed19; 20; 37; 38. In our study, it was not possible to assess the speed of isoflavone absorption during the first hours of consumption as described in studies that collected repeated blood samples8; 19; 20; 38. However, the parallel pattern of plasma isoflavone levels and UIE when plotted as a function of time has been shown8. Published reports are consistent in that isoflavones in liquids rather than solids are absorbed faster, but cumulative uptake may be higher for solids8; 38; 39. Therefore, fermentation of soy foods leads to faster absorption and higher peak levels, but it appears likely that similar amounts of isoflavonoids from non-fermented products will become available over time.
The results of this study have to be considered in light of several limitations, foremost the small sample size. Given the strong variation across subjects, the minimum difference by food that could have been detected with this study, given a power of 0.80, would have been 49 nmol per mg of creatinine. Because all subjects were free-living, we were not able to verify the food intake and the exact times of soy consumption. In fact, it appears that at least one subject may not have consumed the soy milk at all (Figure 2). Although our choice of two liquid soy foods eliminated bias due to the differential uptake according to the texture of the soy foods38; 39, the women may have consumed the study foods as part of a regular meal whose composition may have affected the uptake of the isoflavones38. On the other hand, previous studies do not indicate a major effect of regular diet on the excretion of isoflavones35; 36. Another issue was the lack of 24-hour urine collections. Overnight urine samples represent the urinary isoflavonoid excretion that occurred over 10–12 hours. Therefore, we have no information on isoflavonoid excretion during the next day. However, the time frame in our study covered approximately 70% of the total urinary isoflavone excretion8. Total excretion levels could have also been determined in 24-hour urine samples but extensive periods of urine collections tend to result in low compliance.
The homogenous ethnic background of the women with regular soy intake in the past was a strength because it reduced possible variation in UIE due to the development of specialized intestinal flora adapted to the breakdown of soy foods24. Individual differences in this cross-over study were minimized since the subjects served as their own controls and baseline UIE values were included in the statistical models. Results could be different in other ethnic groups who were not exposed to soy foods since childhood24. The wide age range in our study, as well as unmeasured genetic and lifestyle factors, e.g., alcohol intake and exercise, may have also affected isoflavone uptake. Although UIE does not directly assess isoflavone absorption, the use of urine seemed adequate to estimate isoflavone bioavailability due to the high correlation of isoflavone appearance patterns in plasma and urine8, the general high correlation between plasma and urine values when samples are collected correctly33; 40, and the strong correlation between plasma and urine values as determined in different experimental settings16; 17.
The interest in isoflavone uptake from fermented soy foods is based on the idea that the aglucones may be more readily bioavailable and more beneficial to human health20. Due to the continued ambiguity, our study examined this question using an ethnically homogeneous population, a cross-over design, two liquid soy foods, and a timed urine collection. The results do not support the idea that the consumption of fermented soy foods results in higher isoflavonoid exposure than the intake of unfermented soy foods. Future studies with repeated blood and/or urine collections over more than 24 hours among a larger study population are necessary to assess the speed of isoflavonoid uptake. At this time, the overall evidence does not justify nutritional recommendations that favor fermented soy foods as long as the intestinal microflora is capable of hydrolyzing the isoflavone glucosides from unfermented soy foods.
Acknowledgments
We are grateful for the Meiji-Yasuda Foundation for supporting this intervention, the study participants, and Laurie Custer for isoflavonoid analysis of the urine specimens. Thanks to Beth Hopping for her help with the manuscript. Support by NCI (CA71789) is appreciated. None of the authors had a conflict of interest related to this project.
References
- 1.Barnes S, Boersma B, Patel R, Kirk M, Darley-Usmar VM, Kim H, Xu J. Isoflavonoids and chronic disease: mechanisms of action. Biofactors. 2000;12:209–215. doi: 10.1002/biof.5520120133. [DOI] [PubMed] [Google Scholar]
- 2.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 3.Barnes S, Peterson G, Grubbs C, Setchell K. Potential role of dietary isoflavones in the prevention of cancer. Adv Exp Med Biol. 1994;354:135–147. doi: 10.1007/978-1-4899-0939-8_10. [DOI] [PubMed] [Google Scholar]
- 4.Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J Agric Food Chem. 1999;47:977–986. doi: 10.1021/jf9808832. [DOI] [PubMed] [Google Scholar]
- 5.Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J Nutr. 1995;125:2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. J Nutr. 2000;130:654S–655S. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KD, Brown NM, Zimmer-Nechemias L, Brashear WT, Wolfe BE, Kirschner AS, Heubi JE. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr. 2002;76:447–453. doi: 10.1093/ajcn/76.2.447. [DOI] [PubMed] [Google Scholar]
- 8.Franke AA, Custer L, Hundahl S. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50:141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- 10.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Soya--a dietary source of the non-steroidal oestrogen equol in man and animals. J Endocrinol. 1984;102:49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]
- 11.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 12.Lampe JW, Gustafson DR, Hutchins AM, Martini MC, Li S, Wahala K, Grandits GA, Potter JD, Slavin JL. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol Biomarkers Prev. 1999;8:699–707. [PubMed] [Google Scholar]
- 13.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 14.Seow A, Shi CH, Franke AA, Hankin H, Lee HP, Yu MC. Isoflavonoid levels in spot urine predict frequency of dietary soy intake in a population-based sample of middle-aged Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–140. [PubMed] [Google Scholar]
- 15.Maskarinec G, Singh S, Meng L, Franke AA. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiol Biomarkers Prev. 1998;7:613–619. [PubMed] [Google Scholar]
- 16.Grace PB, Taylor JI, Low YL, et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev. 2004;13:698–708. [PubMed] [Google Scholar]
- 17.Ritchie MR, Morton MS, Deighton N, Blake A, Cummings JH. Plasma and urinary phyto-oestrogens as biomarkers of intake: validation by duplicate diet analysis. Br J Nutr. 2004;91:447–457. doi: 10.1079/BJN20031062. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins AM, Slavin JL, Lampe JW. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J Am Diet Assoc. 1995;95:545–551. doi: 10.1016/S0002-8223(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 19.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 20.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–2296. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 21.Tsangalis D, Wilcox G, Shah NP, Stojanovska L. Bioavailability of isoflavone phytoestrogens in postmenopausal women consuming soya milk fermented with probiotic bifidobacteria. Br J Nutr. 2005;93:867–877. doi: 10.1079/bjn20041299. [DOI] [PubMed] [Google Scholar]
- 22.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003;77:1459–1465. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 23.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002;132:2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 24.Song KB, Atkinson C, Frankenfeld CL, Jokela T, Wahala K, Thomas WK, Lampe JW. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J Nutr. 2006;136:1347–1351. doi: 10.1093/jn/136.5.1347. [DOI] [PubMed] [Google Scholar]
- 25.Williams AE, Maskarinec G, Hebshi S, Oshiro C, Murphy S, Franke AA. Validation of a soy questionnaire with repeated dietary recalls and urinary isoflavone assessments over one year. Nutr Cancer. 2003;47:118–125. doi: 10.1207/s15327914nc4702_2. [DOI] [PubMed] [Google Scholar]
- 26.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–3094. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 27.Franke AA, Custer L, Wilkens L, LeMarchand L, Goodman MT, Kolonel LN. LC/PDA/MS analysis of dietary phytoestrogens from human urine and blood. J Chromatography B. 2002;777:43–57. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 28.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis) J Nutr. 2003;133:2262–2267. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 29.Franke AA, Custer LJ, Wang W, Shi SJ. HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med. 1998;217:263–273. doi: 10.3181/00379727-217-44231. [DOI] [PubMed] [Google Scholar]
- 30.Dai Q, Franke AA, Yu H, Shu XO, Jin F, Hebert JR, Custer LJ, Gao YT, Zheng W. Urinary phytoestrogen excretion and breast cancer risk: evaluating potential effect modifiers endogenous estrogens and anthropometrics. Cancer Epidemiol Biomarkers Prev. 2003;12:497–502. [PubMed] [Google Scholar]
- 31.SAS Institute Inc. SAS OnlineDoc 9.1.2. Cary, NC: SAS Institute Inc; 2004. [Google Scholar]
- 32.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- 33.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am J Clin Nutr. 2006;84:406–413. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 34.Akaza H, Miyanaga N, Takashima N, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, Wang HJ, Murphy PA, Hendrich S. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J Nutr. 2000;130:798–801. doi: 10.1093/jn/130.4.798. [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda N, Pomeroy S, Nestel P. Absorption in humans of isoflavones from soy and red clover is similar. J Nutr. 2002;132:2199–2201. doi: 10.1093/jn/132.8.2199. [DOI] [PubMed] [Google Scholar]
- 37.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 38.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, Zimmer-Nechemias L, Wolfe B, Setchell KD. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 39.de Pascual-Teresa S, Hallund J, Talbot D, Schroot J, Williams CM, Bugel S, Cassidy A. Absorption of isoflavones in humans: effects of food matrix and processing. J Nutr Biochem. 2006;17:257–264. doi: 10.1016/j.jnutbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Fanti P, Asmis R, Stephenson TJ, Sawaya BP, Franke AA. Positive effect of dietary soy in ESRD patients with systemic inflammation--correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol Dial Transplant. 2006;21:2239–2246. doi: 10.1093/ndt/gfl169. [DOI] [PubMed] [Google Scholar]