Abstract
Objective
To assess whether monitoring sedation status using bispectral index (BIS) as an adjunct to clinical evaluation was associated with a reduction in the total amount of sedative drug used in a 12 h period.
Design
Prospective randomized controlled clinical trial.
Setting
Tertiary care neurocritical care unit.
Patients
Sixty-seven mechanically ventilated adult patients receiving continuous intravenous sedation with propofol.
Interventions
Sedation monitoring using clinical assessment with the Ramsay scale (Ramsay-alone group) or clinical assessment plus BIS monitoring (BIS-augmentation group). Subjects were randomized to Ramsay-alone (n = 35), or BIS-augmentation (n = 32). Nurses adjusted the dose of propofol to a Ramsay of 4, or a Ramsay of 4 and BIS between 60 and 70.
Measurements and Main Results
Patients in the BIS-augmentation group received significantly less propofol by volume (93.5 ml vs. 157.8 ml, respectively; P < .015), and had lower infusion rates (14.6 vs. 27.9 mcg/kg/min; P = .003). There is a lower risk of propofol infusion exceeding manufacturer’s recommended dosing guides in the BIS-augmentation group versus the Ramsay-alone group (0 vs. 23%, P = .0052). The BIS-augmentation group woke up much quicker than those in the Ramsay-alone group (1.2 vs. 7.5 min; P < .0001).
Conclusions
BIS-augmented sedation monitoring resulted in a marked reduction in the total dose of sedative used to achieve the same level of clinical sedation resulting in shortened time to wake up without any measurable adverse effects. Physiologic sedation assessment tools may provide a useful means of improving the care of sedated critically ill patients.
Keywords: Sedation assessment, Nursing care, Critical care, Neurofunction monitoring, Neurocritical care
Introduction
Critically ill patients often require continuous intravenous (IV) sedation to facilitate medical and nursing interventions such as mechanical ventilation, prevent recall of unpleasant events, and maintain a safe environment [1]. Oversedation can result in delayed weaning from mechanical ventilation and increased length of stay [2–4] while undersedation may result in patient recall of unpleasant events, increased oxygen consumption, ventilatory dysynchrony, and vital sign instability [5–11].
Currently, care givers most commonly use observational assessment, with tools such as sedation assessment scales, to monitor and inform sedation treatment decisions. These tools, however, were not designed for continuous assessment and lack adequate precision and interrater reliability [1, 12–17]. The Ramsay scale, a frequently used observational tool, is one such example. The Ramsay scale is a single-item, six-level scale in which the assessor provides a score between 1 and 6 that describe state and responsiveness to stimuli [18]. The original manuscript describes the scale:
Awake levels were: 1, patient anxious and agitated or restless or both; 2, patient co-operative, orientated, and tranquil; 3, patient responds to commands only. Asleep levels were dependent on the patient’s response to a light glabellar tap or loud auditory stimulus: level 4, a brisk response; 5, a sluggish response; and 6, no response [18].
Neurophysiological monitors such as the bispectral index (BIS) monitor (Aspect) and Sedline (Hospira) monitor have been proposed as a means of near-continuous assessment of patient level of sedation. The BIS value is a numerical value ranging from 0 to 100 and represents a signal processed electroencephalographic (EEG) value derived from proprietary software [19]. BIS-augmented sedation assessment has been extensively studied as a component of intra-operative care and found to be associated with a decrease in sedative use [20–22]; however, evidence supporting its value in ICU sedation monitoring is limited. BIS-augmented sedation assessment has therefore not been widely adopted into contemporary ICU practice [23, 24].
This study seeks to explore whether BIS monitoring provides additional value to traditional observational assessment in selecting an ideal level of patient sedation for ventilated patients in the ICU setting. The specific purpose of this study was to assess whether BIS monitoring can reduce sedative dose requirements while preventing undersedation events.
Methods
This randomized clinical trial divided subjects into two groups. Both groups received the standard of care for sedation assessment. One group received the standard of care plus BIS monitoring. The study was conducted over the course of a single clinical nursing shift (12 h).
Subjects and Setting
Subjects were considered eligible for study inclusion if they were adult, mechanically ventilated patients admitted to the neurocritical care unit (NCCU) of a tertiary care hospital with a primary neurological or neurosurgical diagnosis and currently receiving continuous intravenous (IV) sedation with propofol. Nurses and physicians in the NCCU had been using the Ramsay scale and BIS monitoring prior to the onset of this study and were familiar with both tools. Subjects were excluded if they had bifrontal brain injury as this may impair the reliability of electro-encephalographic-based monitoring (BIS and EEG), required deep (barbiturate coma) sedation, or were admitted for status epilepticus. The Institutional Review Board reviewed and approved the protocol.
Measures
Demographic data were abstracted directly from the electronic healthcare record. For both groups, observational assessments of sedation were scored using the Ramsay scale and documented as a routine component of nursing care. Within the BIS-augmented group, physiologic assessments of sedation were additionally scored using the BIS monitor and documented as routine component of nursing care; sedative use was measured as the rate of propofol (measured in milliliters) infused each hour as well as the total volume of drug infused in the 12 h study period; these data were obtained from chart abstraction. Recovery time was scored as the time in minutes and seconds beginning with the cessation of propofol infusion and ending with the time at which an independent assessor (advance practice nurse not affiliated with this study) deemed the patient to be awake enough to provide a reliable neurologic exam; the recovery time was obtained at 4 p.m. on the day of study. Undersedation events were scored as any self-initiated medical support device removal (intravenous or intra-arterial catheters, endotracheal tubes or cerebral pressure monitoring devices), or any period of ventilatory asynchrony; undersedation events were documented by the care nurse on an undersedation event form designed for this study. Injury severity and illness severity were hypothesized covariates of sedative use. Injury severity was scored using the Glasgow Coma Scale (GCS) the first GCS on admission to the NCCU was used for this measure. Illness severity was scored using the APACHE®IV score based on chart abstraction data in the first 24 h following admission.
Procedures
Prior to enrolling the first subject, and throughout the study, nurses were provided with a review of their education about sedation assessment using the Ramsay scale as well as with BIS monitoring. Nurses were given an education sheet and individual instruction with detailed information about interpreting BIS values that included a discussion on electromyographic (EMG) and signal quality index (SQI) as indicators of the extent to which the BIS is providing quality data. The legal representative of the patients was approached for the study within 24 h of being intubated, or with 24 h of admission to the NCCU if they arrived intubated. After obtaining consent, the subjects were randomized, via random number table, to either the Ramsay-only or BIS-augmentation group. The study period began at 8:00 a.m. on the morning following consent and lasted for 12 h (one nursing shift). Nurses were instructed to adjust the dose of propofol infusion to achieve a Ramsay score of 4 in the Ramsay-alone group and a Ramsay score of 4 plus a BIS value between 60 and 70 in the BIS-augmentation group. There were no specified assessment times nor frequency for either group, nor was a sedation algorithm provided; the nurses were free to self-determine their decision-making process. Propofol (10 mg/ml) concentrations were standard for all subjects and BIS monitoring was done with BIS VISTAtm monitors using the Quatro Sensors.
Both groups were treated according to the NCCU unit standards; every 2 h, Ramsay sedation assessments were performed and then the sedation was stopped so the nurse could obtain a neurological evaluation. In order to evaluate the best neurological function each patient was capable of displaying, the care nurse was instructed to reduce sedative infusion sufficiently to allowa patient to wake up and undergo a full clinical assessment. Recovery time, defined as the time to arouse sufficiently once the sedation was turned off such that an advance practice nurse (not associated with this study) could obtain a reliable comprehensive neurologic exam, was obtained and recorded at 4 p.m. on the day of study.
Analysis
The primary endpoint for this study was the total dose of sedative drug (propofol) used in 12 h; testing the hypothesis that BIS-augmented sedation reduces overall sedative use in neurocritically ill patients. The a priori power analysis resulted in a planned enrollment of 90 subjects (45 per group). To examine the primary endpoint, ANOVA was used to explore models of sedative use over the course of a 12 h nursing shift. Next, two covariate models were tested using the 4-step method of covariate analysis described by Cody and Smith [25]. The association of BIS augmentation and time to wake-up (recovery time) when sedation was stopped for neurologic examination was compared by using ANOVA. Finally we assessed whether there were differences in the number of events associated with undersedation for patients assigned to the BIS augmentation group compared with those assigned to Ramsay alone.
Results
Subject enrollment included 67 patients; enrollment was terminated following a planned interim analysis in which a significant clinical benefit was found in favor of the BIS-augmented group (P = .0146). Of the 67 subjects enrolled in the study, 35 were randomized to the Ramsay-alone group, while 32 received BIS-augmentation (Table 1). Subjects were representative of patients admitted to the NCCU. Age, gender, ethnicity, and weight were evenly distributed among the two groups. There were no differences between groups in terms of injury severity (GCS scores; P = .192) or illness severity (APACHE®IV scores; P = .113). Groups were also similar with respect to admission diagnosis (Table 1). Titrating sedative infusion using the BIS-augmentation strategy was associated with a nearly 50% reduction in the mean total dose of drug given over 12 h (93.5 ml, SD = 86.3) compared with the Ramsay-alone group (157.8 ml, SD = 119.2; P = .0146) (Table 2). A similar result was also seen when the mean weight-based hourly infusion rate (mcg/kg/h) was assessed (BIS-augmentation group 14.6 mcg/kg/min, SD = 12.2 versus Ramsay-alone group 27.9 mcg/kg/min, SD = 20.5; P = .0026). When the individual rates were examined for each hour of the study, there were 8 subjects in the Ramsay-alone group and no subjects in the BIS-augmentation group with documented infusion rates over 4 mg/kg/h (manufacturer’s recommended maximum dose) [26].
Table 1.
Admission emographics for subjects
| Variables | Measure | Ramsay-alone group N = 35 |
BIS-augmentation group N = 32 |
t-test of difference |
|---|---|---|---|---|
| Age | Mean (SD) | 54.8 (15.14) | 57.8 (19.82) | n.s. |
| Weight | Mean (SD) | 77.9 (18.17) | 83.1 (18.76) | n.s. |
| Gender | % female | 40.00% | 50.00% | n.s. |
| Caucasian | Percent | 57.14% | 56.25% | n.s. |
| African American | Percent | 31.43% | 37.50% | n.s. |
| Native American | Percent | 8.57% | 3.13% | n.s. |
| Pacific Asian | Percent | 2.86% | - | n.s. |
| Hispanic | Percent | - | 3.13% | n.s. |
| Admit GCS | Mean (SD) | 8.4 (2.64) | 7.6 (2.73) | n.s. |
| APACHE IV | Mean (SD) | 67.4 (20.28) | 75.64 (21.84) | n.s. |
| Admission diagnosis | ||||
| Hemorrhagic stroke a | 14 | 19 | ||
| Ischemic stroke | 5 | 1 | ||
| Traumatic brain injury | 7 | 3 | ||
| Encephalopathy | 3 | 5 | ||
| Spinal cord injury | 2 | 3 | ||
| Brain tumor | 3 | 1 | ||
| Myasthenic crisis | 1 | 0 |
n.s. = no significant difference
Includes subarachnoid, ubdural, and intraparenchymal emorrhage/hematoma
Table 2.
Summary statistics for sedative use (propofol) by group assignment
| Group | n | Propofol infusion rate (mg/kg/h) | Total propofol volume (ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 25th Quartile | Median | 75th Quartile | Mean (SD) | 25th Quartile | Median | 75th Quartile | ||
| Ramsay-alone | 35 | 1.69 (1.47) | 0.42 | 1.50 | 2.69 | 157.8 (119.2) | 61.1 | 149.1 | 235.2 |
| BIS-augmentation | 32 | 0.89 (0.99) | 0.29 | 0.6 | 1.65 | 93.5 (86.3) | 31.2 | 69.6 | 140.5 |
Sedation Assessment
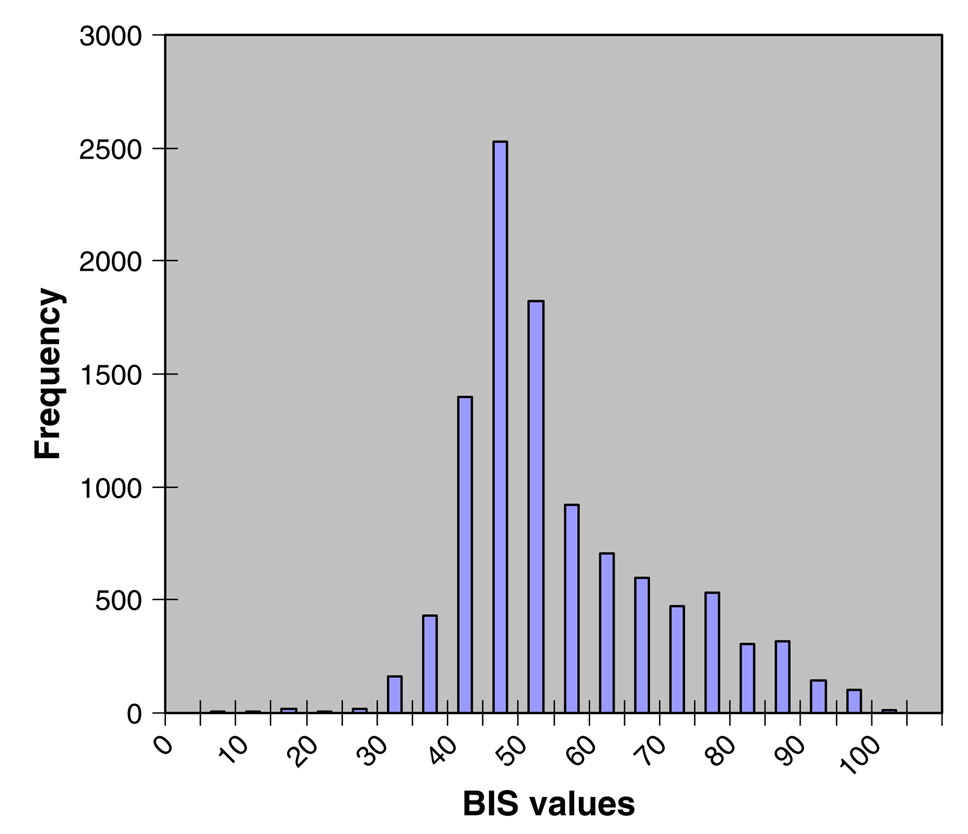
Ramsay scores for both groups are described in Table 3. There were no statistically significant differences noted in Ramsay scores nor in the distribution of scores for the two groups. In the Ramsay-alone group there were 163 observations (mean = 4); 7 observations were scored Ramsay = 1, and 29 as Ramsay = 6. In the BIS-augmentation group there were 152 observations (mean = 4); 6 observations were scored Ramsay = 1, and 36 as Ramsay = 6. For subjects assigned to the BIS-augmentation group, BIS values were downloaded directly from the BIS-Vista monitor to a USB drive and entered into a MySQL database. Values associated with high EMG values (>50) or with low SQI values (<50) were excluded from the analysis. There were 11,634 min of BIS monitoring with 941 min of EMG > 50 and 191 min of SQI < 50. The mean BIS value was 51.41 (median 47, standard deviation 14.35) across the remaining 10,502 min; scores ranged from 2 to 91 (see Fig. 1) and with an approximately normal distribution (positive skew = 0.87).
Table 3.
Descriptive statistics for Ramsay scores by group assignment
| Group assignment | N | Mean | SD | Distribution of Ramsay scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||||
| Ramsay-alone | 163 | 4.07 | 1.39 | 7 | 16 | 34 | 37 | 40 | 29 |
| BIS-Aug | 152 | 3.94 | 1.54 | 6 | 22 | 46 | 15 | 27 | 36 |
Fig. 1.
Distribution of BIS values for subjects randomized to the BIS-augmentation group
Oversedation and Undersedation
The difference in mean recovery time for the BIS-augmentation group (mean = 1.24 min, SD = 2.08) compared with the mean recovery time for the Ramsay-alone group (mean = 7.49 min, SD = 7.54) was significantly lower (P < .0001). There were no undersedation events during the 12 h course of study within either of the study groups.
Discussion
Our study found that BIS-augmentation resulted in patients receiving half as much sedative as those whose sedation was guided by observation only. Moreover, these patients also were significantly more likely to receive propofol at rates that exceed the manufacturer’s recommendation [26] compared with nurses who are provided with BIS data. BIS-augmented sedation assessment was also associated with a more rapid emergence from sedation. Decreasing sedative use in the BIS-augmented group appeared safe and was not complicated by any increase in undersedation events. In this study, propofol was the primary sedative for patients in the study and the only sedative that nurses adjusted during the study period. Fentanyl was prescribed as a prn bolus medication for 7 subjects in the Ramsay-alone group and 5 subjects in the BIS-augmentation group; there was no difference in the amount of fentanyl (Ramsay alone group = 350 mcg, BIS-augmentation group = 200 mcg) administered during the 12 h study period.
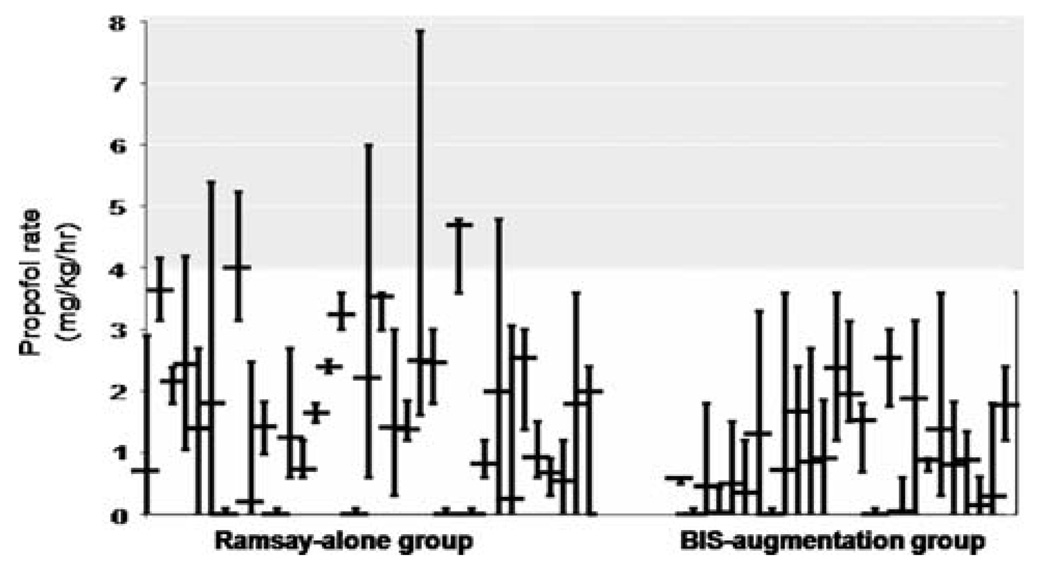
In the BIS augmentation group, the BIS value mean (51.4) and median (47) were below the BIS goal of 60–70. BIS values in healthy non-sedated volunteers will fall below 40 during sleep [27]. It is not possible in this sample to determine if patients had lower consciousness states due to sleep or due to sedation. A patient who is given light sedation is not prohibited from also falling asleep, but without a rigorous testing (e.g., polysomnography) it is not possible to fully test this hypothesis. There is general consensus that ICU patients are oversedated [28]. Less sedation, as seen in this study, may not equate with least sedation; patients may still be oversedated. Eight subjects in the Ramsay-alone group received propofol at a rate exceeding the manufacturer’s recommended maximum dose during at least one of the 12 h of study (Fig. 2). Data on complications from excessive propofol (e.g., cardio-vascular or hepatic changes) were not collected in this study but could be part of future studies.
Fig. 2.
Propofol infusion rates for the Ramsay-alone and BIS-augmentation group. Mean propofol infusion rates (mg/kg/h) are shown for each individual (with minimum and maximum hourly rates represented by vertical bars). Groups are separated by a space for visual clarity
Subject data were collected under the principle of intent-to-treat. Nine subjects (5 Ramsay-alone, 4 BIS-augmentation) received very little sedation (Fig. 2). Following subject recruitment and initiation of the study period, the propofol was weaned and the subject was extubated. Without knowing the decision-making process of each practitioner, it is not possible to determine if the method of assessment impacted the decision to extubate. There remains a significant difference between groups for both propofol volume (P = .019) and propofol rate (P = .012) when these subjects are excluded from the analysis.
The correlation between BIS values and Ramsay scores is low (Pearson correlation = −.28) when the mean BIS values for the hour preceding the Ramsay assessment are compared to the timed Ramsay assessment. However, given that sedation assessment with Ramsay is an intermittent assessment of the patient’s ability to respond to stimuli whereas neurofunction monitors provide a near-continuous assessment of the degree of cortical entropy based on a unilateral frontal EEG lead, this is not surprising. These two values represent two different domains of sedation assessment and are obtained from two different assessment methods; different methods of assessing different domains of a single construct are not expected to highly correlate [24].
Perfect sedation requires that the patient is neither over-nor undersedated. Given that both conditions exist in different patients, it is counter-intuitive to expect that BIS-augmented sedation would always result in less drug use. A study of BIS use for decreasing anesthesia awareness found that BIS use did not appear to reduce anesthesia awareness when compared to end-tidal anesthetic gas measurements [29]. Although BIS has been associated with reduced sedative use in earlier studies [20, 21], a more recent study found that BIS use did not decrease drug dosing [30].
Clinical Implications
This study provides support that the use of BIS monitoring, when combined with current methods of observational assessment, is associated with a decrease in the amount of sedative used to maintain an adequate level of sedation for neurocritically ill patients without an increased risk of undersedation. The results of the study are most clearly applicable to patients with neurological injuries but may be relevant to other populations.
Increased sedation is associated with higher risk of infection, prolonged length of mechanical ventilation, longer hospital stay, increased cost, and increased mortality [31–34]. Weinert found that although most patients are chronically oversedated, as few as 3% of the documented observational scores indicate oversedation [31]. Combining observational and physiologic assessment may enhance sedation management because nurses are being provided with more information than either tool provides independently. This more comprehensive and continuous assessment of the patient’s state was associated with a decrease in the incidence of oversedation.
The patient who is maintained in a state of conscious sedation receives minimal sedative infusion and will quickly awaken when the sedation is removed [35]. Subjects in the BIS-augmentation group had a shorter recovery time than did their counterparts in the Ramsay-alone group. The positive implications of this result are clinically relevant. It is routine practice and important to awaken patients from sedation for the purpose of obtaining a neurologic exam. A decrease in the amount of time it takes to begin that exam will reduce the negative patient outcomes associated with halting sedation [16]. The additional implication is that if the patient requires less time to arouse from sedation, then it follows that the nurse will also experience a shortened time for which he/she is required to monitor for emergence from sedation, thus saving nursing time and effort, and freeing the nurse to engage in other tasks.
There is no standardized definition of undersedation; in an effort to be as objective as possible, for this study, undersedation was defined as any self-device removal event, or ventilatory asynchrony (document by the respiratory therapist) [7]. During the 12 h study period, there were zero undersedation events in either group. A Ramsay score of 1 is defined as being awake and anxious and agitated, or restless, or both [18]. As shown in Table 3, documentation of Ramsay scores equal to 1 were not significantly different for the Ramsay-alone group (7 instances) compared to the BIS-augmentation group (6 instances). It must be noted that this study was not powered to detect a difference in undersedation events, and undersedation events are notably rare. This study explored 12 h of care for a discrete patient population. Should these results be replicated in a larger study that extends over a longer period of time, they would support continued use of BIS as an adjunct to current sedation assessment tools.
Limitations
While our study did meet its primary goals, there is insufficient clinical data to precisely define the ideal goal and range for BIS scores and therefore the target range chosen for this study (60–70) may have been too narrow. It is not known how nurses interpreted the relationship between target and observed scores. For example, a subject may have had a BIS value of 59, and the nurse responsible for adjusting sedative infusion rate may have considered that to be ‘‘close enough’’ therefore electing not to adjust the infusion rate while a different nurse may have increased the infusion rate for the same BIS score.
The Hawthorne effect is a phenomenon wherein the participants in a study alter their behavior because they know they are being observed, which will in turn bias the results, often toward increasing a type II error. To preserve clinical equipoise and diminish the threat of introducing a Hawthorne effect, nurses were informed that the principal aim of the study was to correlate BIS and Ramsay with GCS scores while at the same time being instructed to achieve the specified sedation goals. While every subject in the study was cared for by an ICU nurse, not all nurses provide identical care; nursing personal and professional attributes could influence sedation [5]. There are a large number of medications that may directly or indirectly affect sedative use (e.g., narcotics). Data on concurrent medication use were not part of this study and is therefore a limitation.
Data were collected during a single 12 h day shift in a neurocritical care unit to increase internal validity, but this also limits external validity. As a preliminary study of augmenting sedation assessment it is logical to first explore the intervention over a discrete time frame during which only 1 nurse would care for 1 patient. We cannot state from this study, whether nurses managing patients with the Ramsay-alone approach would continue to oversedate their patients, given the opportunity to care for them for several days at a time. Longer-term studies that include individual nursing characteristics are needed to assess this. The choice to include only neurocritical care patients is supported by published work that supports BIS for brain-injured patients; future studies will include other patient populations [36].
The choice of sedation assessment tool is also very important. The Ramsay scale was used because it was standard-of-care for this hospital and the nurses were most familiar with Ramsay, thus we could compare standard-ofcare to standard-of-care-plus-BIS. Although at the time that this study was started the Ramsay scale was presumed to be a validated tool [37, 38], a recent study finds the Ramsay scale is not reliable [12] and thus it could be argued that the results of this study might have been different with a more validated sedation assessment tool such as the Richmond Agitation-Sedation Scale (RASS) [39]. While BIS provided continuous information, it is not known how often the RN assessed sedation using BIS. Tools such as Ramsay and RASS are impractical for continuous assessment and the standard-of-care was applied. It is unknown whether more frequent assessments using Ramsay would have yielded similar results.
Conclusion
Sedation assessment augmented with neurophysiological monitoring should be considered for the routine use of monitoring and caring for neurocritically ill patients who require sedation. Sedation assessment augmented by BIS monitoring was associated with a decrease in the amount of propofol used to maintain a safe level of sedation. Compared to subjects who were sedated and monitored using only an observational measure of sedation, subjects in the BIS-augmentation group experienced significantly shorter recovery times when sedation was interrupted for a neurological examination. There was no difference in the number of undersedation events associated, and therefore BIS monitoring provides a safe adjunct to current sedation assessment. Physiologic sedation assessment tools with EEG-derived parameters should not be seen as a possible replacement for nursing judgment but rather they should be incorporated, and studied, as an adjunct and a compliment to observational methods of sedation assessment. This small study supports the concept of BIS-augmented sedation assessment as a tool to safely reduce sedative use in mechanically ventilated neurologically ill patients. Additional studies with more diverse populations and larger samples sizes will be beneficial in determining the ultimate role of EEG-derived monitors in sedation assessment.
Acknowledgment
This research is supported by NIH T32 NR07091 Interventions to Prevent and Manage Chronic Illness and Aspect Medical Systems, Inc.
Contributor Information
DaiWai M. Olson, Department of Medicine-Neurology, Duke University Medical Center, Durham, NC 27710, USA, e-mail: dmOlson@email.unc.edu
Suzanne M. Thoyre, School of Nursing, University of North Carolina at Chapel Hill, Chapel Hill, NC 27516, USA
Eric D. Peterson, Division of Cardiology, Duke Clinical Research Institute, Duke University Medical Center, Durham, NC 27710, USA
Carmelo Graffagnino, Division of Neurology, Neuroscience Intensive Care Unit, Duke University Medical Center, Durham, NC 27710, USA.
References
- 1.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guide-lines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 2.Arbour RB. Using the Bispectral Index to assess arousal response in a patient with neuromuscular blockade. Am J Crit Care. 2000;9(6):383–387. [PubMed] [Google Scholar]
- 3.Carrasco G. Instruments for monitoring intensive care unit sedation. Crit Care. 2000;4(4):217–225. doi: 10.1186/cc697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollef MH, Levy NT, Ahrens TS, Schaiff R, Prentice D, Sherman G. The use of continuous I.V. sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114(2):541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 5.Weinert CR, Chlan L, Gross C. Sedating critically ill patients: Factors affecting nurses’ delivery of sedative therapy. Am J Crit Care. 2001;10(3):156–165. quiz 166–157. [PubMed] [Google Scholar]
- 6.Flaishon R, Windsor A, Sigl J, Sebel PS. Recovery of consciousness after thiopental or propofol. Bispectral index and isolated forearm technique. Anesthesiology. 1997;86(3):613–619. doi: 10.1097/00000542-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Mion LC, Minnick AF, Leipzig RM, Catrambone CD, Johnson ME. Patient-initiated device removal in intensive care units: a national prevalence study. Crit Care Med. 2007;35(12):2714–2720. doi: 10.1097/01.ccm.0000291651.12767.52. [DOI] [PubMed] [Google Scholar]
- 8.Tung A, Lynch JP, Roizen MF. Use of the bis monitor to detect onset of naturally occurring sleep. J Clin Monit Comput. 2002;17(1):37–42. doi: 10.1023/a:1015404803637. [DOI] [PubMed] [Google Scholar]
- 9.Boulain T. Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Association des Reanimateurs du Centre-Ouest. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1131–1137. doi: 10.1164/ajrccm.157.4.9702083. [DOI] [PubMed] [Google Scholar]
- 10.Grap MJ, Glass C, Lindamood MO. Factors related to unplanned extubation of endotracheal tubes. Crit Care Nurse. 1995;15(2):57–65. [PubMed] [Google Scholar]
- 11.Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM. Costs of adverse events in intensive care units. Crit Care Med. 2007;35(11):2479–2483. doi: 10.1097/01.CCM.0000284510.04248.66. [DOI] [PubMed] [Google Scholar]
- 12.Olson D, Lynn M, Thoyre SM, Graffagnino C. The limited reliability of the Ramsay scale. Neurocrit Care. 2007;7(3):227–231. doi: 10.1007/s12028-007-0069-x. [DOI] [PubMed] [Google Scholar]
- 13.Tallgren M, Pettila V, Hynninen M. Quality assessment of sedation in intensive care. Acta Anaesthesiol Scand. 2006;50(8):942–946. doi: 10.1111/j.1399-6576.2006.01094.x. [DOI] [PubMed] [Google Scholar]
- 14.Chisholm CJ, Zurica J, Mironov D, Sciacca RR, Ornstein E, Heyer EJ. Comparison of electrophysiologic monitors with clinical assessment of level of sedation. Mayo Clin Proc. 2006;81(1):46–52. doi: 10.4065/81.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson BD, Kane-Gill SL. Sedation assessment in critically ill adults: 2001–2004 update. Ann Pharmacother. 2004;38(11):1898–1906. doi: 10.1345/aph.1E167. [DOI] [PubMed] [Google Scholar]
- 16.Olson DM, Graffagnino C, King K, Lynch JR. Toward solving the sedation-assessment conundrum: bispectral index monitoring and sedation interruption. Crit Care Nurs Clin North Am. 2005;17(3):257–267. doi: 10.1016/j.ccell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Abbott/American Association of Critical-Care Nurses; Saint Thomas Health System Sedation Expert Panel Members. Consensus conference on sedation assessment. A collaborative venture by Abbott Laboratories, American Association of Critical-Care Nurses, and Saint Thomas Health System; Crit Care Nurse; 2004. pp. 33–41. [PubMed] [Google Scholar]
- 18.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2(920):656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86(4):836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gan TJ, Glass PS, Windsor A, et al. BIS Utility Study Group. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 1997;87(4):808–815. doi: 10.1097/00000542-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Song D, Joshi GP, White PF. Titration of volatile anesthetics using bispectral index facilitates recovery after ambulatory anesthesia. Anesthesiology. 1997;87(4):842–848. doi: 10.1097/00000542-199710000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006;20(1):81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Leblanc JM, Dasta JF, Kane-Gill SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006;40(3):490–500. doi: 10.1345/aph.1E491. [DOI] [PubMed] [Google Scholar]
- 24.Olson DM, Thoyre SM, Auyong DB. Perspectives on sedation assessment in critical care. AACN Adv Crit Care. 2007;18(4):380–395. doi: 10.1097/01.AACN.0000298630.53276.be. [DOI] [PubMed] [Google Scholar]
- 25.Cody RP, Smith JK. Applied statistics and the SAS programming language. 5th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2006. [Google Scholar]
- 26.Ahlen K, Buckley C, Pulsford AH. AstraZeneca’s response to the review by Wysowski and Pollock regarding deaths reported in association with propofol use. Anesthesiology. 2007;107(1):175. doi: 10.1097/01.anes.0000268507.33332.a3. author reply 176. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson T, Patel J, Sleigh JW. Sleep patterns in intensive care unit patients: a study using the bispectral index. Crit Care Resusc. 2001;3(2):86–91. [PubMed] [Google Scholar]
- 28.Devlin JW, Nasraway SA., Jr Reversing oversedation in the intensive care unit: the role of pharmacists in energizing guide-line efforts and overcoming protocol fatigue. Crit Care Med. 2008;36(2):626–628. doi: 10.1097/01.CCM.0000299844.38883.5E. [DOI] [PubMed] [Google Scholar]
- 29.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358(11):1097–1108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 30.Lindholm ML, Brudin L, Sandin RH. Bispectral index monitoring: appreciated but does not affect drug dosing and hypnotic levels. Acta Anaesthesiol Scand. 2008;52(1):88–94. doi: 10.1111/j.1399-6576.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 31.Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Crit Care Med. 2007;35(2):393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues GR, Jr, do Amaral JL. Influence of sedation on morbidity and mortality in the intensive care unit. Sao Paulo Med J. 2004;122(1):8–11. doi: 10.1590/S1516-31802004000100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anis AH, Wang XH, Leon H, Hall R. Economic evaluation of propofol for sedation of patients admitted to intensive care units. Anesthesiology. 2002;96(1):196–201. doi: 10.1097/00000542-200201000-00034. [DOI] [PubMed] [Google Scholar]
- 34.Ostermann ME, Keenan SP, Seiferling RA, Sibbald WJ. Sedation in the intensive care unit: a systematic review. JAMA. 2000;283(11):1451–1459. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 35.Kost M. Manual of conscious sedation. Philadelphia: W.B. Saunders; 1998. [Google Scholar]
- 36.Deogaonkar A, Gupta R, Degeorgia M, et al. Bispectral Index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32(12):2403–2406. doi: 10.1097/01.ccm.0000147442.14921.a5. [DOI] [PubMed] [Google Scholar]
- 37.Haberthur C, Lehmann F, Ritz R. Assessment of depth of midazolam sedation using objective parameters. Intensive Care Med. 1996;22(12):1385–1390. doi: 10.1007/BF01709555. [DOI] [PubMed] [Google Scholar]
- 38.Schulte-Tamburen AM, Scheier J, Briegel J, Schwender D, Peter K. Comparison of five sedation scoring systems by means of auditory evoked potentials. Intensive Care Med. 1999;25(4):377–382. doi: 10.1007/s001340050861. [DOI] [PubMed] [Google Scholar]
- 39.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]