Abstract
Siderophore production by marine-derived fungi has not been extensively explored. Three studies have investigated the ability of marine-derived fungi to produce siderophores in response to iron limitation [(Vala et al. in Indian J Mar Sci 29:339-340, 2000; Can J Microbiol 52:603-607, 2006); Baakza et al. in J Exp Mar Biol Ecol 311:1-9, 2004]. In all, 24 of 28 marine fungal strains were found to secrete hydroxamate or carboxylate siderophores; no evidence was found for production of catecholate siderophores. These studies did not determine the structures of the iron-binding compounds. More recently, a study of the natural products secreted by a marine Penicillium bilaii revealed that this strain produced the rare catecholate siderophore pistillarin when grown under relatively high iron concentrations (Capon et al. J Nat Prod 70:1746-1752, 2007). Additionally, the production of rhizoferrin by a marine isolate of Cunninghamella elegans (ATCC36112) is reported in this manuscript. The current state of knowledge about marine fungal siderophores is reviewed in light of these promising results.
Keywords: siderophores, fungi, marine
Introduction
Iron is required for growth of the vast majority of microorganisms (Templeton 2000; Sigel and Sigel 1998). Although iron is the fourth most abundant element on the earth's surface, it is only sparingly soluble in the aerobic, near neutral conditions under which most microbes grow (KSPFe(OH)3 = 10−39). In vast regions of the world's oceans, chlorophyll levels from photosynthetic microorganisms are unusually low leading to low levels of primary production, despite the fact that these waters are replete in major nutrients like nitrate, phosphate, and silicate. These high-nitrate-low-chlorophyll (HNLC) regions also coincide with very low iron levels, ranging from 20 pM to 1 nM in surface seawater (Morel et al. 2003; Morel and Price 2003; Moore et al. 2001; Johnson et al. 1997, 1994; Martin et al. 1994; O'Sullivan et al. 1991; Martin 1990; Martin and Fitzwater 1988). The recognition that the HNLC regions correlated with low iron concentrations led to the development of the Iron Hypothesis by John Martin (Martin 1990; Martin et al. 1991), which states that primary productivity in large areas of the world's oceans is limited by low iron concentrations. In addition to phytoplankton such as diatoms and cyanobacteria, fungi and heterotrophic bacteria constitute important microorganisms in the ocean that are also potentially limited by low iron levels in HNLC regions. Obligate and facultative marine fungi have been isolated from a wide variety of marine environments with greatest diversity found in coastal waters and sediments and lowest variance in open ocean waters.
To overcome this apparent lack of iron, microorganisms have evolved an elaborate mechanism to acquire Fe(III). Under aerobic growth conditions, fungi, bacteria, and other microorganisms produce siderophores, which are low molecular weight, virtually ferric-ion-specific compounds for the solubilization and sequestration of iron(III). Siderophores are generally produced under conditions of low iron availability and secreted into the surrounding environment where they can complex ferric-ion. Four mechanisms for return of siderophore-bound iron to the cells are known in fungi: (1) in the shuttle mechanism, the Fe(III)-siderophore complex is transported across the cytoplasmic membrane by siderophore specific transport proteins and the iron is reductively removed from the siderophore complex inside the cell (van der Helm and Winkelmann 1994; Winkelmann 1990; Emery 1971; Ardon et al. 1997, 1998); (2) in the taxicab mechanism, Fe(III) is transferred from extracellular siderophores to intracellular ligands across the cytoplasmic membrane (Müller et al. 1985a, b; Winkelmann and Huschka 1987); (3) in the hydrolytic mechanism, the Fe(III)-siderophore is transferred inside the cell by specific transport proteins and the metal is removed by simultaneous reduction and degradation of the siderophore by esterases (van der Helm and Winkelmann 1994; Adjimani and Emery 1987, 1988); and (4) in the reductive mechanism, the iron is reduced by membrane bound reductases and Fe(II) is transported into the cell, the siderophore remains outside the cell (van der Helm and Winkelmann 1994; Ecker et al. 1982; Lesuisse and Labbe 1994; Lesuisse et al. 1998; Dancis et al. 1992).
Fungal siderophores
Most fungi have been shown to produce siderophores under aerobic conditions during iron limitation with the notable exception of certain Saccharomyces species (Lesuisse and Labbe 1994; Neilands 1995). Most fungi secrete hydroxamate-type siderophores including fusarinines, coprogens, and ferrichromes. Many strains have been shown to simultaneously synthesize more than one type of hydroxamate siderophore (see, for e.g. Renshaw et al. 2002). Although most fungi produce hydroxamate-type siderophores, zygomycetes have been shown to produce the carboxylate siderophore rhizoferrin (Thieken and Winkelmann 1992; Drechsel et al. 1991). A few fungi also secrete phenolate type compounds under iron limitation, but the relevance of these in iron uptake is uncertain (Fekete et al. 1989; Jellison et al. 1991; Ismail et al. 1985).
Fusarinines
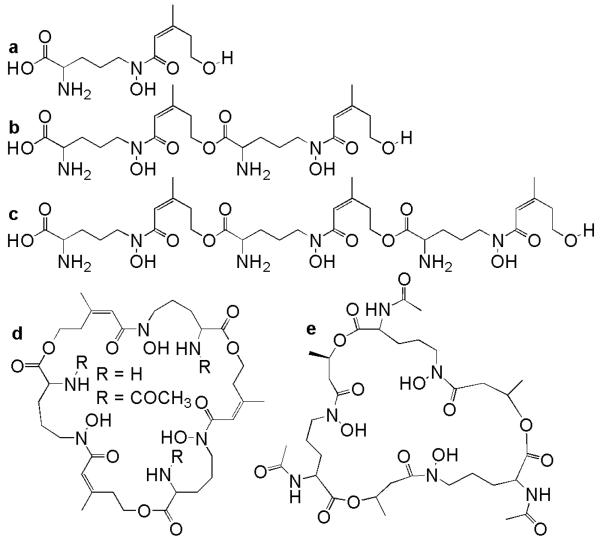
Cis and trans subunits of Nδ-anhydromevalonic acid-Nδ-hydroxy-L-ornithine are fundamental building blocks of many fungal siderophores; these subunits are called the cis- and trans-fusarinines. The fusarinines are hydroxamate siderophores composed of two (fusarinine A, Fig. 1b) or three cis-fusarinine subunits (fusarinine B, Fig. 1c) joined by labile α-amino ester bonds (Renshaw et al. 2002). Two cyclic trimers of cis-fusarinine have also been identified (fusarinine C and triacetylfusarinine C, Fig. 1d; Renshaw et al. 2002). Neurosporin (Fig. 1e) is a unique siderophore consisting of a cyclic of triester of Nα-acetyl-Nδ-hydroxy-Nδ-((R)-3-hydroxybutyryl)-D-ornithine polymerized via amino-acylester bonds between the carboxyl group of ornithine and the 3-hydroxy group of the butyric acid moiety (Renshaw et al. 2002). Neurosporin is particularly unusual since it is composed of D-ornithine while all other known fungal hydroxamate siderophores are derived from L-ornithine (Renshaw et al. 2002).
Figure 1.
Structures of the fusarinines (Renshaw et al. 2002). a cis-fusarinine; b fusarinine A; c fusarinine B; d fusarinine C (R=H, fusigen) and N,N′,N″-triacetylfusarinine C (R=COCH3, triacetylfusigen); e neurosporin.
Coprogens
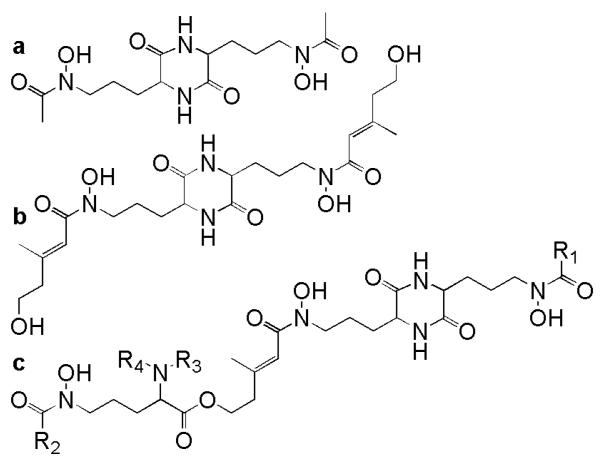
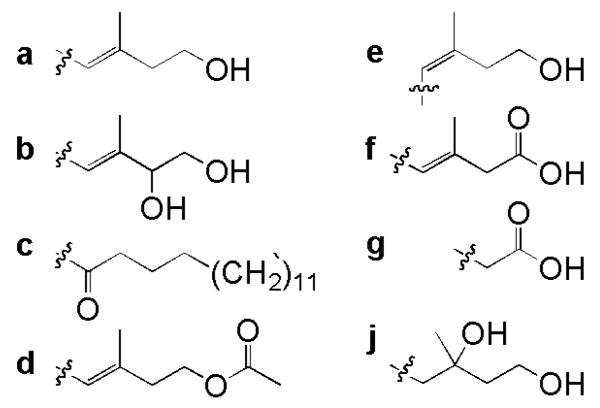
The coprogens are fungal siderophores composed of trans-fusarinine subunits. These linear siderophores take either dihydroxamate or trihydroxamate forms. Rhodotorulic acid (Fig. 3a; Renshaw et al. 2002) and dimerum acid (Fig. 3b; Renshaw et al. 2002) are the two known dihydroxamate coprogen siderophores. In each, two Nδ-acyl-Nδ-hydroxy-L-ornithines form a six membered diketopiperazine ring. In rhodotorulic acid, the acyl groups are acetic acid; in dimerum acid, the acyl groups are trans-anhydromevalonic acid. The trihydroxamate coprogens contain two Nδ-acyl-Nδhydroxy-L-ornithine subunits linked to form a diketopiperazine ring and a third Nδ-trans-anhydromevalonic acid-Nδ-hydroxy-L-ornithine subunit linked in a linear fashion through an ether bond (Figs. 2 and 3, Table 1). The four variable R groups of the coprogens include H, CH3, acetyl (Ac), anhydromevalonic acid (A), 4-hydroxy-anhydromevalonic acid (B), and palmitic acid (C). These diverse R groups alter the hydrophilic nature of the siderophores thus altering their solubility in water. The specific biological functions of these different substituents are not known.
Figure 3.
a Rhodotorulic acid, b dimerum acid, and c the coprogens (Renshaw et al. 2002). See Table 1 for R groups.
Figure 2.
Alkyl groups found in fungal hydroxamate siderophores.
Table 1.
Acyl groups of the coprogen siderophores.
| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| Coprogen | A | A | Ac | H |
| Coprogen B | A | A | H | H |
| Triornicin (isoneocoprogen I) | A | CH3 | Ac | H |
| Isotriornicin (neocoprogen I) | CH3 | A | Ac | H |
| Neocoprogen II | CH3 | CH3 | Ac | H |
| Nα-dimethyl coprogen | A | A | CH3 | CH3 |
| Nα-dimethyl neocoprogen I | CH3 | A | CH3 | CH3 |
| Nα-dimethyl isoneocoprogen I | A | CH3 | CH3 | CH3 |
| Hydroxycoprogen | A | B | Ac | H |
| Hydroxyneocoprogen I | CH3 | B | Ac | H |
| Hydroxyisoneocoprogen I | B | CH3 | Ac | H |
| Palmitoylcoprogen | A | A | H | C |
Ferrichromes
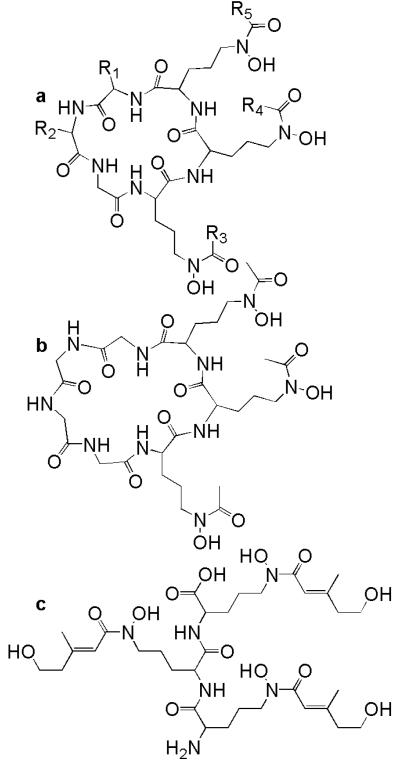
The ferrichromes are cyclic hexapeptide siderophores composed of three Nδ-acyl-Nδ-hydroxy-L-ornithine, two variable amino acids (alanine, serine, or glycine), and a glycine linked by way of peptide bonds (Fig. 4, Table 2; Jalal and van der Helm 1991). Five acyl groups have been identified so far: acetyl, malonyl, trans-β-methylglutaconyl, trans-anhydromevalonyl, and cis-anhydromevalonyl. The acyl groups are homogeneous in each ferrichrome, except for the asperchromes which are synthesized by Aspergillus ochraceous (Renshaw et al. 2002). Des(diserylglycyl)ferrirhodin and tetraglycylferrichrome do not follow the typical ferrichrome structure (Fig. 4b and c). Des(diserylglycyl)ferrirhodin is a linear trimer consisting of three Nδ-cis-anhydromevalonic acid-Nδ-hydroxy-L-ornithine subunits linked via peptide bonds (Jalal and van der Helm 1991). Tetraglycylferrichrome is a cyclic heptapeptide with three Nδ-acetyl-Nδ-hydroxy-L-ornithine residues and four glycine residues (Jalal and van der Helm 1991). Tetraglycylferrichrome is most similar to ferrichrome, which is a cyclic hexapeptide composed of three Nδ-acetyl-Nδ-hydroxy-L-ornithine residues and three glycine residues (Jalal and van der Helm 1991).
Figure 4.
Ferrichrome siderophores: a ferrichromes (see Table 2 for R groups), b tetraglycylferrichrome, and c des(diserylglycyl)ferrirhodin (Jalal and van der Helm 1991).
Table 2.
R groups of the ferrichrome siderophores.
| R1 | R2 | R3 | R4 | R5 | |
|---|---|---|---|---|---|
| Ferrichrome | H | H | CH3 | CH3 | CH3 |
| Ferrichrome A | CH2OH | CH2OH | F | F | F |
| Ferrichrome C | H | CH3 | CH3 | CH3 | CH3 |
| Ferrichrysin | CH2OH | CH2OH | CH3 | CH3 | CH3 |
| Ferricrocin | H | CH2OH | CH3 | CH3 | CH3 |
| Ferrirubin | CH2OH | CH2OH | A | A | A |
| Ferrirhodin | CH2OH | CH2OH | E | E | E |
| Malonichrome | H* | CH3* | G | G | G |
| Sake colorant A | CH2OH | CH3 | CH3 | CH3 | CH3 |
| Asperchrome A | CH2OH | CH3 | A | A | A |
| Asperchrome B1 | CH2OH | CH2OH | CH3 | A | A |
| Asperchrome B2 | CH2OH | CH2OH | A | CH3* | A* |
| Asperchrome B3 | CH2OH | CH2OH | A | A* | CH3* |
| Asperchrome C | CH2OH | CH2OH | D | A | A |
| Asperchrome D1 | CH2OH | CH2OH | A | CH3 | CH3 |
| Asperchrome D2 | CH2OH | CH2OH | CH3 | A | CH3 |
| Asperchrome D3 | CH2OH | CH2OH | CH3 | CH3 | A |
| Asperchrome E | CH2OH | CH2OH | E* | A* | A* |
| Asperchrome F1 | CH2OH | CH2OH | J* | A* | A* |
| Asperchrome F2 | CH2OH | CH2OH | A* | J* | A* |
| Asperchrome F3 | CH2OH | CH2OH | A* | A* | J* |
Rhizoferrin
Rhizoferrin (Fig. 5) is the only known carboxylate siderophore produced by fungi, specifically synthesized by members of the zygomycetes (Thieken and Winkelmann 1992). Rhizoferrin is composed of diaminopropane symmetrically acylated with citric acid via amine bonds to the terminal carboxylate of citric acid (Drechsel et al. 1991). Interestingly, both fungi and bacteria produce rhizoferrin; fungi produce only R,R-rhizoferrin (Drechsel et al. 1992) while a few bacteria produce enantio-rhizoferrin (S,S-rhizoferrin; Munzinger et al. 1999).
Figure 5.
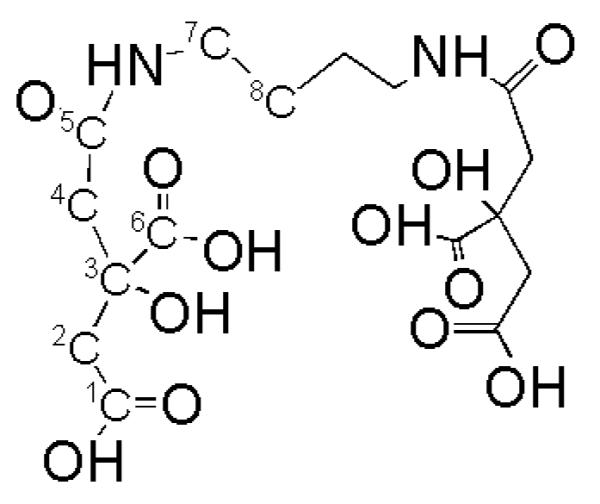
The structure of rhizoferrin, the polycarboxylate siderophore produced by zygomycetes (Drechsel et al. 1991).
Production of siderophores by marine-derived fungi
A series of three papers reported production of iron(III)-binding compounds by marine fungi, but the structures of these compounds were not determined (Vala et al. 2000, 2006). In the first report, Vala et al. (2000) disclosed that ten of thirteen marine fungal strains secreted iron(III)-binding compounds, based on positive ferric chrome-azurol sulfonate (Fe(III)-CAS) solution assays. The putative siderophore-producing strains included Cunninghamella elegans, Penicillium sp., P. chrysogenum, and P. funiculosum isolated from the water column; Monilia sp. and Paecilomyces variotii isolated from the surface of mangrove plants; and P. citrinum, Rhizopus sp., Syncephalastrum sp., and S. racemosum isolated from sediment (Vala et al. 2000). As might be anticipated from studies with terrestrial fungi, the four Zygomycetes (Cunninghamella elegans, Rhizopus sp., Syncephalastrum sp., and S. racemosum) were all found to synthesize carboxylate type siderophores as determined by spectrophotometric analysis (Vala et al. 2000). Interestingly, Paecilomyces variotii was found to produce both hydroxamate and carboxylate type siderophores, suggesting that carboxylate siderophores may also be produced by fungi outside the Zygomycota. Terrestrial Paecilomyces variotii have previously been shown to secrete the trihydroxamate siderophore ferrirubin (Renshaw et al. 2002). Carboxylate-type siderophores have not previously been detected in cultures of fungi outside the Zygomycota. All other siderophore-producing strains were found to generate hydroxamate type siderophores (Monilia sp., Penicillium sp., P. chrysogenum, P. citrinum, and P. funiculosum; Vala et al. 2000). No evidence was found for catecholate siderophores. Three strains were found not to produce siderophores: Alternaria sp. isolated from the water column, Dreschlera australiensis isolated from the surface of mangrove plants, and Ulocladium sp. isolated from sediment.
A second report compared the production of siderophores by terrestrial and marine fungal strains (Baakza et al. 2004). The study included three fungi belonging to Zygomycota (Cunninghamella elegans, Rhizopus sp., and Syncephalastrum racemosum) and seven varieties of Ascomycota (Aspergillus flavus, A. niger, A. ochraceous, A. versicolor, Penicillium chrysogenum, P. citrinum, and P. funiculosum). For each fungus, siderophore production by a marine isolate was compared to siderophore production by a terrestrial isolate. With the exception of one marine strain of Aspergillus flavus, all studied fungi were shown to secrete siderophores in liquid culture (Baakza et al. 2004). All marine and terrestrial Zygomycetes were found to synthesize carboxylate-type siderophores by chemical and biological assays (Baakza et al. 2004). All marine and terrestrial strains belonging to Ascomycota except the marine Aspergillus flavus strain indicated above were shown to produce hydroxamate-type siderophores by chemical and biological assay. The relative concentrations of siderophores were determined by Fe(III)-CAS assay using standard curves prepared from desferrioxamine mesylate (DFOM, a commercially available bacterial siderophore). Interestingly, the marine isolates were found to produce greater quantities of siderophore than their terrestrial counterparts (Baakza et al. 2004). Marine and terrestrial C. elegans cultures contained the highest concentrations of siderophores with an estimated 1987.50 μg/mL and 1248.75 μg/mL DFOM equivalents, respectively.
The third report examined siderophore production by ten aspergilli, five from marine (Aspergillus sp., A. nidulans, A. niger, A. ochraceus, and A. versicolor) and five from terrestrial sources (A. duricaulis, A. fumigatus, A. niger, A. ochraceus, and A. versicolor; Vala et al. 2006). All ten were found to secrete hydroxamate-type siderophores; no evidence of catecholate or carboxylate-type siderophores was found (Vala et al. 2006). The concentrations of siderophores continued to increase for the fifteen days of analysis, with maximum concentrations occurring on day fifteen. In contrast to the results of the previous study (Baakza et al. 2004), terrestrial isolates generally produced the highest concentrations of siderophores, with the exception of A. versicolor. Overall, the highest concentration of siderophores was detected from a marine isolate of A. versicolor on day fifteen (182.5 μg/mL DFOM equivalents) (Vala et al. 2006). Marine Aspergillus sp. and A. niger produced the lowest concentrations of siderophores with only 14.4 μg/mL DFOM equivalents after fifteen days. Thus, both marine and terrestrial aspergilli were found to produce only hydroxamate-type siderophores based on chemical assays.
Structures of marine fungal siderophores: Pistillarin
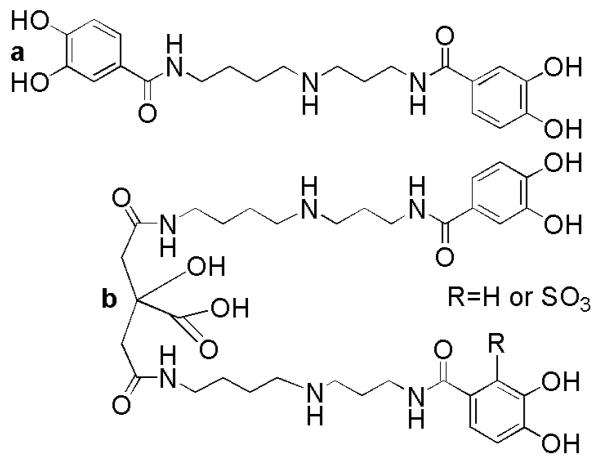
In the first report of structural characterization of a siderophore from a marine fungus, siderophore production by a marine isolate of Penicillium bilaii was investigated. P. bilaii was isolated from a boat ramp in Port Huon, Tasmania and was found to produce pistillarin (Fig. 6), a rare catechol siderophore, when grown under relatively high iron conditions on malt extract agar (Capon et al. 2007). A terrestrial isolate of Penicillium striatisporum collected nearby was found to produce similar aromatic polyketides and diketopiperazines but was not found to produce pistillarin (Capon et al. 2007). Pistillarin is a bitter compound produced by many macro fungi, which is known to coordinate iron(III) (Steglich et al. 1984). Interestingly, this compound contains an unusual 3,4-dihydroxycatechol moiety which was also identified in the marine bacterial siderophore petrobactin (Fig. 6; Barbeau et al. 2002; Bergeron et al. 2003; Hickford et al. 2004). Moreover, both are constructed from a spermidine scaffold. Not only are the structural features of pistillarin unusual for siderophores, but catechol-type siderophores have only rarely been isolated from fungi (Fekete et al. 1989; Jellison et al. 1991; Ismail et al. 1985).
Figure 6.
Structures of a pistillarin, a marine fungal siderophore (Capon et al. 2007) and b the petrobactins, marine bacterial siderophores (Barbeau et al. 2002; Bergeron et al. 2003; Hickford et al. 2004).
Structures of marine fungal siderophores: Rhizoferrin
A second marine fungal siderophore has been characterized from marine-derived Cunninghamella elegans ATCC36112, reported here. The siderophore was identified by electrosprayionization mass spectrometry (ESI-MS), tandem MS, and NMR. C. elegans ATCC36112 is a hydrocarbon degrading marine fungus isolated from estuarine mud (Cerniglia and Perry 1973). C. elegans strains have been isolated from a wide variety of environments ranging from marine sources to terrestrial soil samples and were repeatedly isolated from coastal seawater (Vala et al. 2000; Baakza et al. 2004). C. elegans ATCC36112 was grown at room temperature in a modified artificial seawater medium, without the added vitamins, for 5 to 10 days with shaking at 170 rpm (Martin et al. 2006). Siderophores were isolated from the acidified supernatant (pH 2 to 3) using XAD-2 and a water/methanol step gradient. Siderophores were further purified by reversed phase HPLC using an ODS-AQ column (10 mm ID × 250 mm length) with a linear water/methanol gradient in 0.05% trifluoroacetic acid. The molecular mass of the purified compound was determined by ESI-MS to be m/z 437.1 on a Micromass QTOF2 Quadrupole Time-of-Flight spectrometer in positive ion mode dissolved in water/methanol with formic acid. Daughter ions were generated by collision with argon gas and the fragmentation pattern analyzed (data not shown). Repeated loss of 46 amu was evident in the fragmentation pattern (from m/z 437 to 391 to 345) and is indicative of the presence of two citrate moieties. Loss of 46 amu corresponds to decarboxylation of the citrate moiety and is a signature for the presence of citrate (Küpper et al. 2006). A major peak at m/z 263 corresponded to loss of a citryl moiety from the native siderophore while a peak at m/z 173 corresponds to the citryl fragment. The siderophore was tentatively identified as rhizoferrin based on the mass and fragmentation pattern.
The identification of this siderophore as rhizoferrin was confirmed by one and two dimensional NMR. Approximately 7 mg of purified sample in 95% water/5% D2O (500 μL) was analyzed by 1H, 13C, APT, 1H-1H COSY, HSQC, and HMBC on a 600 MHz UNITY INOVA 600 NB NMR System. These data were evaluated and compared to published values (Table 3; Drechsel et al. 1992). These data confirm that the siderophore produced by C. elegans ATCC36112 is rhizoferrin. The identification of rhizoferrin from C. elegans ATCC36112 agrees with previous studies which indicated that marine-derived C. elegans strains produced carboxylate-type siderophores (Vala et al. 2000; Baakza et al. 2004). Rhizoferrin has also been isolated from terrestrial strains of C. elegans (Thieken and Winkelmann 1992).
Table 3.
1H and 13C NMR data for rhizoferrin comparing published values (Drechsel et al. 1991, 1992) with observed values.
| C no. | 1H Published | 1H Observed | 13C Published | 13C Observed |
|---|---|---|---|---|
| 1,1' | 176.2 | 176.7 | ||
| 2,2' | 2.60, 2.83 | 2.80, 3.08 | 45.8 | 46.3 |
| 2.57, 2.80 | 2.77, 3.05 | |||
| 3,3' | 76.3 | 76.7 | ||
| 4,4' | 2.47, 2.58 | 2.66, 2.79 | 47.3 | 47.8 |
| 2.44, 2.55 | 2.64, 2.76 | |||
| 5,5' | 173.6 | 174.0 | ||
| N, 7.74 | N, 8.12 | |||
| 6,6' | 179.3 | 179. 8 | ||
| 7,7' | 2.96 | 3.16 | 41.5 | 42.0 |
| 8,8' | 1.28 | 1.48 | 28.3 | 28.6 |
Conclusions
Two marine fungal siderophores have been structurally characterized: the unusual catecholate siderophore pistillarin (Capon et al. 2007) and the polycarboxylate siderophore rhizoferrin. Both siderophores were isolated from near-shore fungal isolates collected from marine sediment (C. elegans ATCC36112) or from the surface of a marine boat ramp (P. bilaii). Tantalizing results from early studies indicated that most marine fungi secrete siderophores; 24 of 28 marine fungal strains were found to produce siderophores based on chemical and biological assays. It will be fascinating to determine the structures of siderophores produced by marine fungi and to investigate the interactions between these molecules, marine fungi and bacteria, and their environment.
Acknowledgments
This publication was made possible by Grant Number P2PRR016478 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. NMR data were collected at the Oklahoma State University Statewide shared NMR facility. Special thanks to Prof. Alison Butler for conducting the mass spectral analyses.
References
- Adjimani JP, Emery T. Iron uptake in Mycelia sterilia EP-76. J Bacteriol. 1987;169:3664–3668. doi: 10.1128/jb.169.8.3664-3668.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjimani JP, Emery T. Stereochemical aspects of iron transport in Mycelia sterilia EP-76. J Bacteriol. 1988;170:1377–1379. doi: 10.1128/jb.170.3.1377-1379.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon O, Weizman H, Libman J, et al. Iron uptake in Ustilago maydis: studies with fluorescent ferrichrome analogues. Microbiology UK. 1997;143:3625–3631. doi: 10.1099/00221287-143-11-3625. [DOI] [PubMed] [Google Scholar]
- Ardon O, Nudelman R, Caris C, et al. Iron uptake in Ustilago maydis: tracking the iron path. J Bacteriol. 1998;180:2021–2026. doi: 10.1128/jb.180.8.2021-2026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baakza A, Vala AK, Dave BP, et al. A comparative study of siderophore production by fungi from marine and terrestrial habitats. J. Exp. Mar. Biol. Ecol. 2004;311:1–9. [Google Scholar]
- Barbeau K, Zhang G, Live DH, Butler A. Petrobactin, a photoreactive siderophore produced by the oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus. J Am Chem Soc. 2002;124:378–379. doi: 10.1021/ja0119088. [DOI] [PubMed] [Google Scholar]
- Bergeron RJ, Huang G, Smith RE, et al. Total Synthesis and Structure Revision of Petrobactin. Tetrahedron. 2003;59:2007–2014. [Google Scholar]
- Capon RJ, Stewart M, Ratnayake R, et al. Citromycetins and bilains A-C: New aromatic polyketides and diketopiperazines from Australian marine-derived and terrestrial Penicillium spp. J. Nat. Prod. 2007;70:1746–1752. doi: 10.1021/np0702483. [DOI] [PubMed] [Google Scholar]
- Cerniglia CE, Perry JJ. Crude oil degradation by microorganisms isolated from the marine environment. Z. Allg. Mikrobiol. 1973;13:299–306. doi: 10.1002/jobm.3630130403. [DOI] [PubMed] [Google Scholar]
- Dancis A, Roman DG, Anderson GJ, et al. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake and transcriptional control by iron. Proc Natl Acad Sci USA. 1992;89:3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel H, Metzger J, Freund S, et al. Rhizoferrin - a novel siderophore from the fungus Rhizopus microsporus var. rhizopodiformis. BioMetals. 1991;4:238–243. [Google Scholar]
- Drechsel H, Jung G, Winkelmann G. Stereochemical characterization of rhizoferrin and identification of its dehydration products. BioMetals. 1992;5:141–148. [Google Scholar]
- Ecker DJ, Passavant CW, Emery T. Role of two siderophores in Ustilago sphaerogena regulation and biosynthesis and uptake mechanisms. Biochim Biophys Acta. 1982;720:242–249. doi: 10.1016/0167-4889(82)90047-7. [DOI] [PubMed] [Google Scholar]
- Emery T. Role of ferrichrome as a ferric ionophore in Ustilago maydis. Biochemistry. 1971;10:1483–1488. doi: 10.1021/bi00784a033. [DOI] [PubMed] [Google Scholar]
- Fekete FA, Chandhoke V, Jellison J. Iron-binding compounds produced by wood-decaying basidiomycetes. Appl Environ Microbiol. 1989;55:2720–2722. doi: 10.1128/aem.55.10.2720-2722.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickford SJ, Küpper FC, Zhang G, et al. Petrobactin sulfonate, a new siderophore produced by the marine bacterium Marinobacter hydrocarbonoclasticus. J Nat Prod. 2004;67:1897–1899. doi: 10.1021/np049823i. [DOI] [PubMed] [Google Scholar]
- Ismail A, Bedell GW, Lupan DM. Siderophore production by the pathogenic yeast Candida albicans. Biochem Biophys Res Comm. 1985;130:885–891. doi: 10.1016/0006-291x(85)90499-1. [DOI] [PubMed] [Google Scholar]
- Jalal MAF, van der Helm D. Isolation and structural identification of fungal siderophores. In: Winkelmann G, editor. CRC Handbook of Microbial Iron Chelates. 1st edn. CRC Press Boca Raton; 1991. pp. 235–270. [Google Scholar]
- Jellison J, Chandhoke V, Goodell B, Fekete FA. The isolation and immunolocalization of iron-binding compounds. Appl Microbiol Biotechnol. 1991;35:805–809. [Google Scholar]
- Johnson KS, Coale KH, Elrod VA, et al. Iron photochemistry in seawater from the equatorial Pacific. Marine Chem. 1994;46:319–334. [Google Scholar]
- Johnson KS, Gordon RM, Coale KH. What controls dissolved iron concentrations in the world ocean? Marine Chem. 1997;57:137–161. [Google Scholar]
- Küpper FC, Carrano CJ, Kuhn J-U, Butler A. Photoreactivity of iron(III)-aerobactin: photoproduct structure and iron(III) coordination. Inorg Chem. 2006;45:6028–6033. doi: 10.1021/ic0604967. [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Labbe P. Reductive iron assimilation in Saccharomyces cerevisiae. In: Winkelmann G, Winge DR, editors. Metal Ions in Fungi. Marcel Dekker; New York: 1994. pp. 149–178. [Google Scholar]
- Lesuisse E, Simon-Casteras M, Labbe P. Siderophore-mediated iron uptake in Saccharomyces cerevisiae: the sit1 gene encodes a ferrioxamine B permease that belongs to the major facilitator superfamily. Microbiology. 1998;144:3455–3462. doi: 10.1099/00221287-144-12-3455. [DOI] [PubMed] [Google Scholar]
- Martin JD, Ito Y, Homann VV, et al. Structure and membrane affinity of new amphiphilic siderophores produced by Ochrobactrum sp. SP18. J Biol Inorg Chem. 2006;11:633–641. doi: 10.1007/s00775-006-0112-y. [DOI] [PubMed] [Google Scholar]
- Martin JH. Glacial-interglacial CO2 change: the iron hypothesis. Paleoceanography. 1990;5:1–13. [Google Scholar]
- Martin JH, Coale KH, Johnson KS, et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature. 1994;371:123–129. [Google Scholar]
- Martin JH, Fitzwater SE. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature. 1988;331:341–343. [Google Scholar]
- Martin JH, Gordon RM, Fitzwater SE. The case for iron. Limnol Oceanogr. 1991;36:1793–1802. [Google Scholar]
- Moore JK, Doney SC, Glover DM, et al. Iron cycling and nutrient-limitation patterns in surface waters of the World Ocean. Deep Sea Res Pt II. 2001;49:463–507. [Google Scholar]
- Morel FMM, Milligan AJ, Saito MA. Marine Bioinorganic chemistry: the role of trace metals in the oceanic cycles of major nutrients. In: Turekian KK, Holland HD, editors. Treatise on Geochemistry. Elsevier Science Ltd.; Cambridge, UK: 2003. [Google Scholar]
- Morel FMM, Price NM. The biogeochemical cycles of trace metals in the oceans. Science. 2003;300:944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- Müller G, Barclay SJ, Raymond KN. The mechanism and specificity of iron transport in Rhodotorula pilmanae probed by synthetic analogs of rhodotorulic acid. J Biol Chem. 1985a;260:13916–13920. [PubMed] [Google Scholar]
- Müller G, Isowa Y, Raymond KN. Stereospecificity of siderophore-mediated iron uptake by Rhodotorula pilmanae as probed by enantiorhodotorulic acid and isomers of chromic rhodotorulate. J Biol Chem. 1985b;260:13921–13926. [PubMed] [Google Scholar]
- Munzinger M, Taraz K, Budzikiewicz H, et al. S,S-rhizoferrin (enantio-rhizoferrin) - a siderophore of Ralstonia (Pseudomonas) pickettii DSM 6297 - the optical antipode of R,R-rhizoferrin isolated from fungi. BioMetals. 1999;12:189–193. [Google Scholar]
- Neilands JB. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- O'Sullivan DW, Hanson AK, Miller WL, et al. Measurement of Fe(II) in surface water of the equatorial Pacific. Limnol Oceanogr. 1991;36:1727–1741. [Google Scholar]
- Renshaw JC, Robson GD, Trinci APJ, et al. Fungal siderophores: structures, functions and applications. Mycol. Res. 2002;106:1123–1142. [Google Scholar]
- Sigel A, Sigel H, editors. Volume 35 of Metal Ions in Biological Systems. Marcel Dekker; New York: 1998. Iron Transport and Storage in Microorganisms, Plants and Animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich W, Steffan B, Stroech K, Wolf M. Pistillarin, a characteristic metabolite of Clavariadelphus pistillaris and several Ramaria species (Basidiomycetes) Z Naturforschung, C. 1984;39C:10–12. [Google Scholar]
- Templeton DM. Molecular and Cellular Iron Transport. Marcel Dekker; New York: 2000. [Google Scholar]
- Thieken A, Winkelmann G. Rhizoferrin: a complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes) FEMS Microbiol Lett. 1992;73:37–41. doi: 10.1016/0378-1097(92)90579-d. [DOI] [PubMed] [Google Scholar]
- Vala AK, Vaidya SY, Dube HC. Siderophore production by facultative marine fungi. Indian J. Mar. Sci. 2000;29:339–340. [Google Scholar]
- Vala AK, Dave BP, Dube HC. Chemical characterization and quantification of siderophores produced by marine and terrestrial aspergilli. Can. J. Microbiol. 2006;52:603–607. doi: 10.1139/w06-012. [DOI] [PubMed] [Google Scholar]
- van der Helm D, Winkelmann G. Hydroxamates and polycarboxylates as iron transport agents (siderophores) in fungi. In: Winkelmann G, Winge D, editors. Metal Ions in Fungi. Marcel Dekker; New York: 1994. pp. 39–98. [Google Scholar]
- Winkelmann G. Structural and stereochemical aspects of iron transport in fungi. Biotechnol Adv. 1990;8:207–231. doi: 10.1016/0734-9750(90)90013-2. [DOI] [PubMed] [Google Scholar]
- Winkelmann G, Huschka H. Molecular recognition and transport of siderophores in fungi. In: Winkelmann G, van der Helm D, Neilands JB, editors. Iron Transport in Microbes, Plants and Animals. VCH; Weinheim: 1987. pp. 317–336. [Google Scholar]