Abstract
Despite growing interest in emotion regulation, the degree to which psychophysiological measures of emotion regulation are stable over time remains unknown. We examined four-week test-retest reliability of corrugator electromyographic and eyeblink startle measures of negative emotion and its regulation. Both measures demonstrated similar sensitivity to the emotion manipulation, but only individual differences in corrugator modulation and regulation showed adequate reliability. Startle demonstrated diminished sensitivity to the regulation instructions across assessments and poor reliability. This suggests that corrugator represents a trait-like measure of voluntary emotion regulation, whereas startle should be used with caution for assessing individual differences. The data also suggest that corrugator and startle might index partially dissociable constructs and underscore the need to collect multiple measures of emotion.
Recent years have witnessed an explosion of research aimed at understanding the psychological and neural mechanisms underlying emotion regulation. Emotion regulation involves complex and dynamic processes that are associated with activated emotions in pursuit of achieving one’s goals (Campos, Frankel, & Camras, 2004). This broad construct of emotion regulation, however, has been difficult to operationalize into a single process (Cole, Martin, & Dennis, 2004), and is better conceptualized as a continuum from unconscious, effortless, automatic regulation to conscious, effortful, voluntary regulation (Davidson, Jackson, & Kalin, 2000).
Based on Davidson’s (1998) definition of emotion regulation, our laboratory developed an experimental paradigm designed to assess the processes that are involved in the voluntary regulation of negative affect (Jackson, Malmstadt, Larson & Davidson, 2000). In this paradigm, subjects were instructed to either suppress, maintain, or enhance their emotional experience in response to standardized unpleasant pictures, while subjects’ affective state was objectively measured by two well-validated indices of emotion: corrugator electromyography (EMG) activity and eyeblink startle magnitude (Bradley, Codispoti, Cuthbert, & Lang, 2001). Results indicated that not only was the intended negative emotion generated but that subjects were able to voluntarily increase and decrease corrugator and startle measures of negative emotion in accordance with the instructions.
These peripheral physiological effects were replicated using the identical paradigm by our laboratory (Lee & Davidson, 2005) and others (Piper & Curtin, 2006). Studies employing variants of this paradigm have largely replicated these effects and extended them to positive pictures (Bernat, Cadwallder, Ward, & Patrick, 2004; Dillon & LaBar, 2005; Driscoll, Tranel, & Anderson, in press), threat-of-shock (Lissek et al., 2007), and emotional words (Deveney & Pizzagalli, 2008). Variants of our paradigm have also been successfully adapted for use with event-related potential (Krompinger, Moser, & Simons, 2008; Moser, Hajcak, Bukay, & Simons, 2006) and functional magnetic resonance imaging measures of the central nervous system (Ochsner & Gross, 2008).
Such group mean differences are, however, subject to marked variability across individuals. Individual differences in emotional responding are the rule rather than an exception (Hamann & Canli, 2004) as regulatory challenges vary significantly across individuals (Thompson, 1994). In particular, individual differences in emotion regulation are associated with various indices of healthy adaptation (John & Gross, 2004; Mauss, Cook, Cheng, & Gross, 2007), as well as vulnerability to, and resilience from, psychopathology (Davidson, 2003). However, unlike paper-and-pencil measures of emotion regulation (e.g., Gross and John, 2003, Nolen-Hoeksema, Parker, & Larson, 1994), the test-retest reliability of individual differences in psychophysiological measures of emotion regulation has never before been systematically examined. Thus, the degree to which such measures reflect stable, trait-like individual differences remains unknown. Consequently, it is difficult to implement studies (e.g., longitudinal, intervention, or extreme-groups) which are predicated on the assumption that psychophysiological measures of emotion regulation are trait-like, and to interpret studies reporting associations between such measures and other individual differences.
Thus, the aim of the present study was to determine the four-week test-retest reliability of individual differences in corrugator and startle measures of emotion regulation using a paradigm identical to that described in Jackson et al. (2000). To permit comparison with prior reports (Larson, Ruffalo, Nietert, & Davidson, 2000; 2005; Manber, Allen, Burton, & Kaszniak, 2000), the reliability of emotion modulation was also computed. On empirical and psychometric grounds, we hypothesized that individual differences in corrugator would show higher reliability than startle. First, in the only published report comparing the reliability of corrugator and startle measures of emotion modulation, Manber et al. (2000) found that corrugator exhibited much better two-week test-retest reliability (intraclass r = .61) than startle (intraclass r = .21). Second, psychometric theory (e.g., Nunnally, 1978) indicates that reliability is generally proportional to the number of constituent “items” or samples. By convention, the number of samples contributing to the estimate of corrugator (seconds) and startle (milliseconds) amplitude differs by two to three orders of magnitude.
Method
Participants
Fifty-nine undergraduates (29 female) were recruited (M = 20.2 years, SD = 1.8) from the University of Wisconsin-Madison. Participants were right-handed (Chapman & Chapman, 1987), free from psychiatric/neurological disorders, and had no prior exposure to the stimuli. Ten participants (4 female) with fewer than 50% eyeblink responses were excluded from analyses1, yielding a final sample of 49.
Stimuli
Pictures with the most similar ratings for men and women (Lang, Greenwald, & Bradley, 1999) were selected from the International Affective Picture System (Center for the Study of Emotion and Attention, 1999). Two sets of 76 negative pictures (assessment 1: valence M = 3.10, SD = 1.24, arousal M = 5.04, SD = 1.53; assessment 2: valence M = 3.08, SD = 1.53, arousal M = 5.01, SD = 1.53) and two sets of 26 neutral pictures (assessment 1: valence M = 4.98, SD = 1.19, arousal M = 2.29, SD = 1.92; assessment 2: valence M = 4.99, SD = 1.22, arousal M = 2.18, SD = 1.96) were matched on valence and arousal ratings, with no repetition of pictures across assessments.
Procedure
Subjects participated in two sessions separated by four weeks and conducted at the same time of day. Procedures were identical to those detailed previously (Jackson et al., 2000) and are only briefly described here. Prior to the experiment, 6 negative and 4 neutral pictures (paired with 4 startle probes) were presented to familiarize participants with the protocol and to permit them to practice regulation.
During the experiment, 102 pictures (8-s/picture; 12-s intertrial interval [ITI]) were presented in 17-picture blocks. Four seconds after picture onset, one of three auditory regulation instructions were presented: enhance (increase emotional response), suppress (decrease emotional response), or maintain (keep the initial intensity of the emotional response). Participants were instructed to continue regulating for 8 s following picture offset until they saw “Relax.” Negative pictures were paired with each of the 3 regulation cues, whereas neutral pictures were only paired with the maintain cue.2
Acoustic startle probes (95-dB, 50-ms) were presented at one of four times. Probe A was presented 3 s following picture onset to index emotion modulation. Probe B was presented 7 s following onset (i.e., 3 s following regulation cue) to index emotion regulation in the presence of the picture. Probes C (12 s) and D (15 s) were presented during the ITI to index emotion regulation in the absence of the picture.
EMG Data Collection and Reduction
EMG data recording and quantification was identical to our prior reports (Jackson et al., 2000). In brief, EMG from the corrugator supercilii and orbicularis oculi was continuously acquired. Signals were amplified (10 k) and filtered (1–400 Hz for corrugator; 1–800 Hz for startle). Corrugator EMG was scored for artifacts, segmented into 1-s Hamming-windowed chunks (50% overlap), and baseline-corrected (2-s) spectral power density (log10 µV2 for the 45–200-Hz EMG band) computed for three epochs: Pre-Instruction (0-4 s post-stimulus onset) to index emotion modulation, and Post-Instruction (4–8 s post-stimulus onset) and Post-Picture (0–8 s post-stimulus offset) to index emotion regulation. Eyeblink data were integrated, rectified (τ = 20 ms), and sampled (1000 Hz) from 50 ms before probe onset (baseline) until 250 ms following probe onset. Following baseline correction, peak magnitudes (µV) were computed as maximum minus blink onset (20–120 ms post-probe), and z-transformed within participants and assessment.
Results
Emotion Modulation
Corrugator
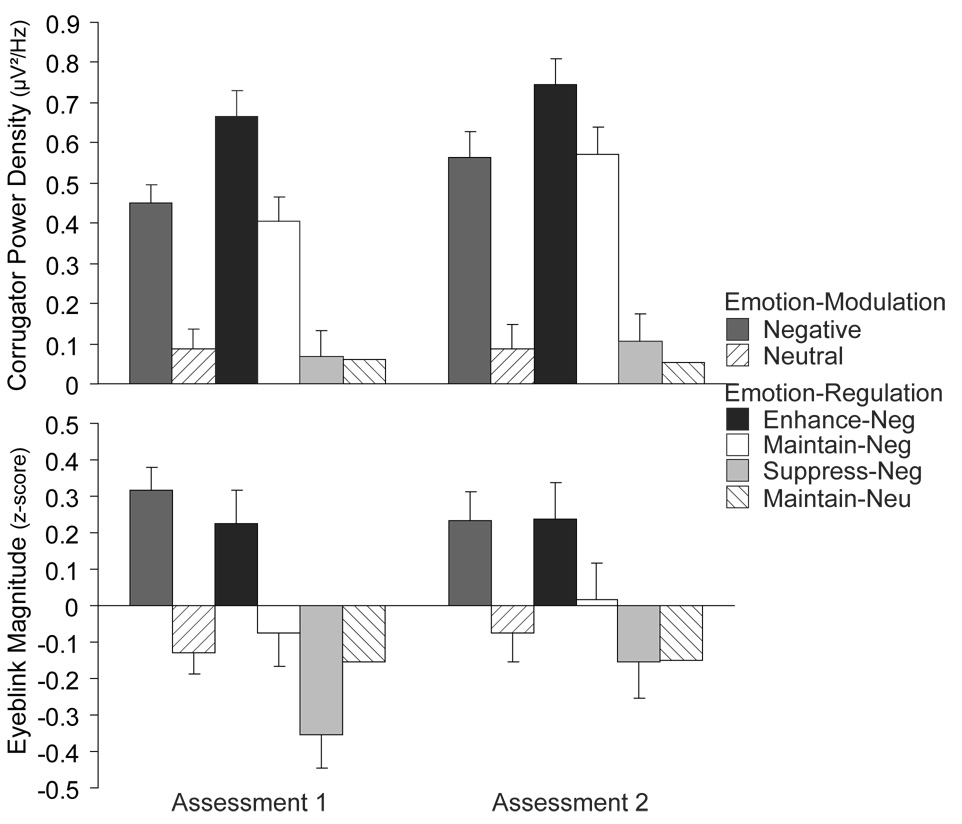
Corrugator was amplified by negative pictures (Figure 1)
Figure 1.
Mean emotion modulation and regulation at each assessment for corrugator (top) and startle (bottom; regulation reflects Probe B only). Error bars indicate the standard error of the mean difference from the relevant control condition.
Negative pictures increased corrugator activity, F(1,48) = 62.80, p < .001, ηp 2 = .57. This was moderated by a Valence × Assessment interaction (F(1,48) = 11.27, p < .01, ηp 2 = .19), driven by greater emotion modulation at the second assessment, t(48) = 3.36, p < .01. The main effect of Assessment was also significant, F(1,48) = 6.54, p = .01, ηp 2 = .12.
Corrugator modulation was stable
Individual differences in corrugator modulation (negative – neutral) exhibited high test-retest reliability3, r = .84, p < .001.
Startle
Startle was potentiated by negative pictures (Figure 1)
Negative pictures increased startle magnitudes, F(1,48) = 55.57, p < .001, ηp 2 = .54. No other effects were significant, ps > .17.
Startle modulation was not stable
Individual differences in startle modulation displayed low retest reliability, r = .16, p = .27.
Emotion Regulation
Corrugator
Corrugator was sensitive to regulation across assessments (Figure 1)
The omnibus test4 of Instruction (enhance, maintain, suppress) was significant (F(2,96) = 50.25, p < .001, ε = .65, ηp 2 = .51) with pairwise contrasts in the predicted directions (enhance > maintain > suppress), ts(48) > 4.61, ps < .001.5 The Instruction × Assessment interaction was also significant (F(2,96) = 5.03, p < .01, ε = .81, ηp 2 = .10), driven by greater activity at the second assessment for maintain (t(48) = 3.69, p = .001) but not for suppress and enhance (ts(48) < 1.60, ps < .12). The main effect of Assessment was significant, F(1,48) = 8.32, p < .01, ηp 2 = .15.
Corrugator regulation was stable (Figure 2)
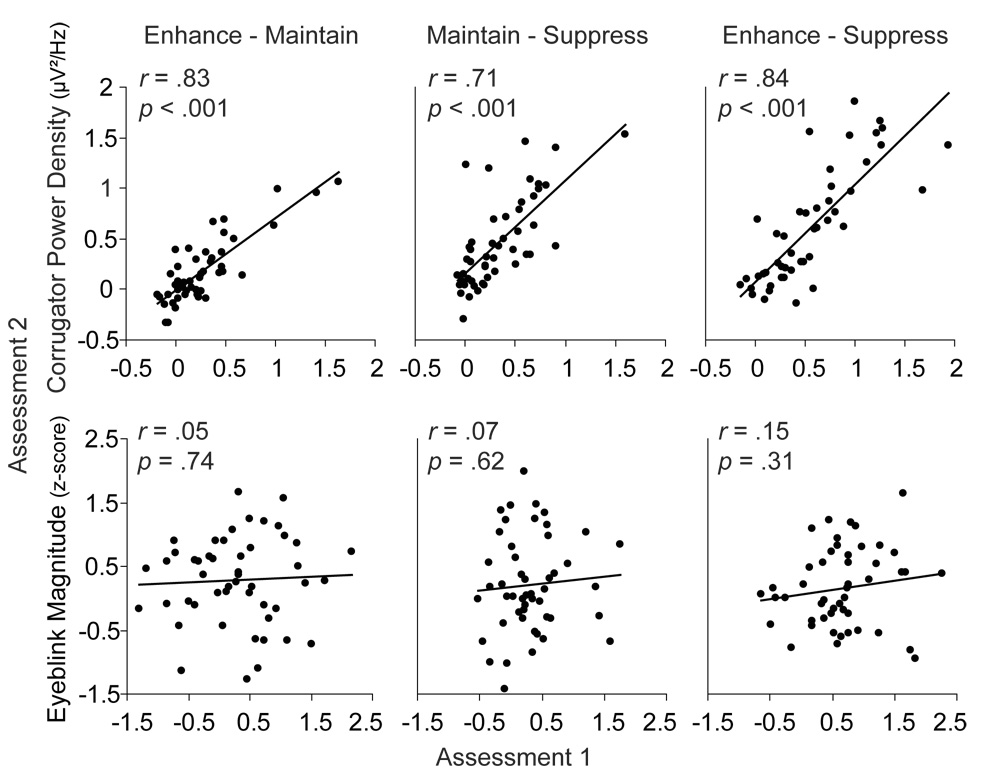
Figure 2.
Scatter plots demonstrating the test-retest correlations for corrugator (top) and startle (bottom; Probe B) for each regulation contrast.
Individual differences in corrugator regulation (enhance – maintain, maintain – suppress, enhance – suppress) demonstrated moderately high retest reliability, rs > .71, ps < .001.
Startle
Startle was sensitive to regulation at Probe B across assessments (Figure 1)
Collapsed across probes, the omnibus test of Instruction was significant (F(2,96) = 23.26, p < .001, ε = .96, ηp 2 = .33) with pairwise contrasts in the predicted directions, ts(48) > 2.62, ps < .01. However, these findings were qualified by an Instruction × Assessment interaction, (F(2, 96) = 14.71, p < .001, ηp 2 = .24), driven by significant regulation effects at the first (F(2,96) = 34.83, p < .001, ηp 2 = .57), but not the second assessment (F(2,96) = 2.14, p = .12, ηp 2 = .07).
To determine whether these null effects were common to all of the regulation probes (B–D), or limited to those during (Probe B) or following (Probes C and D) picture presentation, we conducted analyses with Probe (B–D) as a factor. The omnibus test yielded an Assessment × Instruction × Probe interaction (F(4,192) = 3.70 p < .01, ε = .99, ηp 2 = .07), driven by Probe B exhibiting significant regulation effects for all regulation contrasts on both assessments (ps < .02), whereas other probes failed to show regulation effects or contrasts at the second assessment (ps > .12). No other effects were significant, ps > .33.6
Startle regulation at Probe B was not stable (Figure 2)
Individual differences in startle regulation at Probe B, which was sensitive to the regulation manipulation at each assessment, displayed poor reliability7, rs < .15, ps > .31.
Discussion
This study is the first to report the test-retest reliability of the corrugator EMG and eyeblink startle magnitude in the context of instructed regulation of negative emotion (Jackson et al., 2000) over a four-week interval. As expected, both corrugator and startle exhibited similarly strong emotion modulation at each assessment. Consistent with prior work (Manber et al., 2000), individual differences in corrugator modulation exhibited good test-retest reliability, whereas startle did not. Likewise, corrugator exhibited significant emotion regulation at both assessments and individual differences in regulation were reliable. In contrast, startle was sensitive to emotion regulation across assessments only during picture presentation, and its reliability during picture presentation was poor.
Consistent with our prediction, differential reliability of corrugator and startle is likely due to the vastly different amount of data on which each measure is based. Psychometric theory suggests that a response system that aggregates more data is inherently more stable (Nunnally, 1978; Tomarken, 1995). Notably, corrugator activity was averaged over several seconds, whereas startle magnitude was based on a modest number of single-sample peaks per condition. In addition, the reliability of emotion-modulated startle we observed was lower than those reported by Larson et al. (2000, 2005), which may be attributable to the different number of probes employed across studies. Despite the same number of trials per probe, reliability in the present study was based on only one probe, whereas Larson et al. averaged across three probes. For the comparable individual probe (i.e., negative – neutral at 4.5-s), Larson et al. (2000, 2005; personal communication) observed similarly low test-retest reliability, r = .20 (ns) and r = .30 (ns), consistent with the proposal that a greater number of probes should yield increased reliability.
Another plausible reason for the differential reliability, and perhaps differential sensitivity, of corrugator and startle measures of emotion regulation is that they might index partially dissociable aspects of emotion. Although both measures are sensitive to negative emotion and linked to amygdalar activation (e.g., Davis, Walker, & Lee, 1999; Lanteaume, Khalfa, Regis, Marguis, Chuavel, & Bartolomei, 2006), prior work indicates that startle is most strongly modulated by arousal, rather than valence, whereas corrugator modulation might reflect context-specific social communication (Bernat, Patrick, Benning, & Tellegen, 2006; Bradley, Codispoti, Cuthbert, et al.; 2001; Lang, Bradley, & Cuthbert, 1990). Consistent with this proposal, previous reports on the test-retest reliability of emotion-modulated startle demonstrated a change from valence-modulation at the first assessment to arousal-modulation at the second assessment (Manber et al., 2000) and showed that the arousal component of affective pictures most strongly contributed to the startle stability (Larson et al., 2000). Moreover, emotion regulation studies using both negative and positive pictures found that startle regulation was arousal-dependent (Bernat et al., 2004; Dillon & LaBar, 2005; Driscoll et al., in press), whereas corrugator regulation was valence-dependent (Bernat et al., 2004). Collectively, these observations suggest that corrugator and startle may be differentially modulated by valence and arousal during emotion regulation, resulting in our differential reliability and sensitivity of emotion regulation across measures over time.
There are three limitations of the current study that represent key challenges for future research. First, the present results are specific to the regulation of negative emotion. Future research should include both negative and positive stimuli in order to examine the differential contribution of valence and arousal to the stability of corrugator and startle measures of emotion regulation. Second, the acquisition of on-line subjective emotional experience would help to establish the degree to which self-reported success of emotion regulation parallel our results using psychophysiological measures. Third, the inference that individual differences in startle measure of emotion regulation are unreliable is based on a single probe during picture presentation. It will be important to replicate this effect using multiple probes of this period (Ditchter, Tomarken, & Baucom, 2002).
In conclusion, our data suggest that corrugator is the more adequate measure of stable, trait-like aspects of voluntary emotion regulation. Startle lacked the requisite temporal stability to reliably assess individual differences. Taken with the limited reliability of emotion-modulated startle, startle-based measures should be used with caution when making inferences about trait-like characteristics, at least as the paradigm is commonly used. The data also suggest that corrugator and startle may index partially dissociable constructs and underscore the importance of employing multiple measures of emotion.
Acknowledgments
We thank Linda Johnson, Rajat Singh, and Katherine Bolton for data collection and scoring, and Anand Lakshmanan, Gina Beguhn, and Larry Grieschar for technical support. This study was supported by NIMH grants R37-MH43454, R01-MH43454, and P50-MH069315 to RJD, and Korea Foundation for Advanced Studies scholarship to HL.
Footnotes
Although our rate of unusable datasets, 17%, is somewhat higher than the 9% reported by Jackson et al. (2000) for a single session, it is about half (29–31%) that reported by prior multi-session studies (Larson et al., 2000, 2005).
Regulation instructions are detailed in Jackson et al. (2000).
Pearson correlations were conducted, rather than intraclass correlations, because the main concern was whether individual differences were stable, regardless of mean differences across assessments (Larson et al., 2000, 2005).
Huynh-Feldt corrected p values, uncorrected degrees of freedom, and epsilon values less than one are reported. Post-hoc tests were corrected for multiple comparisons using a Bonferroni adjustment.
Exploratory analyses contraindicated the possibility that changes in peripheral physiology merely reflect a demand characteristic of our regulation instructions. Replicating Jackson et al. (2000), most (84%) subjects reported using “cognitive” (i.e., reappraisal) rather than “physiological” (e.g., inhibit outward expression) regulation strategies. The two post-hoc groups did not differ in corrugator or startle regulation at either assessment, ps > .29. Furthermore, corrugator and startle regulation was unrelated to conformity, indexed by the Marlowe-Crowne Social Desirability Scale (Crowne & Marlowe, 1960), ps > .2. Regulation was also unrelated to mood at the time of the laboratory session, as indexed by the Positive and Negative Affect Scales (Watson, Clark, & Tellegen, 1988), ps > .11. Positive and negative mood did not differ across assessments, ps > .67.
In contrast to prior work suggesting that women are more expressive or reactive to negative images (e.g., Bradley, Codispoti, Sabatinelli, et al., 2001), we found no significant interactions with Sex for corrugator or startle modulation, or for corrugator regulation. However, a Sex × Assessment × Instruction interaction was marginally significant for startle at Probe B, F(2,94) = 2.82, p = .08. This was driven by males who failed to show the maintain – suppress effect at the first assessment (p = .25) and females who failed to show the enhance – maintain effect at the second assessment (p = .96).
Additional analyses indicated that the differential stability of corrugator and startle regulation could not be attributed to 1) z-transformation, as similar results were found for raw startle; 2) gross differences in the range of individual differences across measures, as corrugator and startle displayed similar variances and comparable coefficients of variation; or 3) our use of difference scores, as the stability of individual differences in startle regulation (e.g., enhance minus suppress) was similar in magnitude to the stability of the constituent scores (e.g., enhance).
Contributor Information
Hyejeen Lee, University of Wisconsin–Madison.
Alexander J. Shackman, University of Wisconsin–Madison
Daren C. Jackson, University of Wyoming
Richard J. Davidson, University of Wisconsin–Madison
References
- Bernat EM, Cadwallader M, Ward M, Patrick CJ. Emotion regulation during picture viewing: comparing responses to pleasant and unpleasant stimuli. Psychophysiology. 2004;41:S41. [Abstract] [Google Scholar]
- Bernat E, Patrick CJ, Benning SD, Tellegen A. Effects of picture content and intensity on affective physiological response. Psychophysiology. 2006;43:93–103. doi: 10.1111/j.1469-8986.2006.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Campos JJ, Frankel CB, Camras L. On the nature of emotion regulation. Child Development. 2004;75:377–394. doi: 10.1111/j.1467-8624.2004.00681.x. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention [CSEA-NIMH] The international affective picture system: Digitized photographs. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Chapman JP, Chapman LJ. The measurement of handedness. Brain and Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Cole PM, Martin SE, Dennis TA. Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development. 2004;75:317–333. doi: 10.1111/j.1467-8624.2004.00673.x. [DOI] [PubMed] [Google Scholar]
- Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychological Bulletin. 2000;126:890–906. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: Toward a synthesis. Psychophysiology. 2003;40:655–665. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Neurophysiologyl and neuropharmacology of startle and its affective modulation. In: Dawson ME, Schell AM, Bohmelt AH, editors. Startle modification. New York: Cambridge University Press; 1999. pp. 95–113. [Google Scholar]
- Deveney CM, Pizzagalli DA. The cognitive consequences of emotion regulation: An ERP investigation. Psychophysiology. 2008;45:435–444. doi: 10.1111/j.1469-8986.2007.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom BR. Startle modulation before, during and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43:191–196. doi: 10.1016/s0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dillon DG, LaBar KS. Startle modulation during conscious emotion regulation is arousal-dependent. Behavioral Neuroscience. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Driscoll D, Tranel D, Anderson SW. The effects of voluntary regulation of positive and negative emotion on psychophysiological responsiveness. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2008.03.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Current Opinion in Neurobiology. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality. 2004;72:1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8:132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Lanteaume L, Khalfa S, Regis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human agmydala. Cerebral Cortex. 2006;17:1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Stability of emotion-modulated startle during short and long picture presentation. Psychophysiology. 2005;42:604–610. doi: 10.1111/j.1469-8986.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- Larson CL, Ruffalo D, Nietert JY, Davidson RJ. Temporal stability of emotion-modulated startle response. Psychophysiology. 2000;37:92–101. [PubMed] [Google Scholar]
- Lee H, Davidson RJ. Individual differences in voluntary emotion regulation. Psychophysiology. 2005;42:S79. [Abstract] [Google Scholar]
- Lissek S, Orme K, Mcdowell DJ, Johnson LL, Luckenbaugh DA, Baas JM, Cornwell BR, Grillon C. Emotion regulation and potentiated startle across affective picture and threat-of-shock paradigms. Biological Psychology. 2007;76:124–133. doi: 10.1016/j.biopsycho.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Manber R, Allen JJB, Burton K, Kaszniak AW. Valence-dependent modulation of psychophysiolological measures: Is there consistency across repeated testing? Psychophysiology. 2000;37:683–692. [PubMed] [Google Scholar]
- Mauss IB, Cook CL, Cheng JYJ, Gross JJ. Individual differences in cognitive reappraisal: experiential and physiological responses to an anger provocation. International Journal of Psychophysiology. 2007;66:116–124. doi: 10.1016/j.ijpsycho.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Parker LE, Larson J. Ruminative coping with depressed mood following loss. Journal of Personality and Social Psychology. 1994;67:92–104. doi: 10.1037//0022-3514.67.1.92. [DOI] [PubMed] [Google Scholar]
- Nunnally JC. Psychometric theory. New York: McGraw-Hill; 1978. [Google Scholar]
- Piper ME, Curtin JJ. Tobacco withdrawal and negative affect: An analysis of initial emotional response intensity and voluntary emotion regulation. Journal of Abnormal Psychology. 2006;115:96–102. doi: 10.1037/0021-843X.115.1.96. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. The development of emotion regulation: Biological and behavioral considerations. Monographs of the Society for Research in Child Development. 1994;59:25–52. [PubMed] [Google Scholar]
- Tomarken AJ. A psychometric perspective on psychophysiological measures. Psychological Assessment. 1995;7:387–395. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]