Abstract
Previous research indicates that drug motivational systems are instantiated in structures that process information related to incentive, motivational drive, memorial, motor/habit, craving, and cognitive control processing. The present research tests the hypothesis that activity in such systems will be powerfully affected by the combination of drug anticipation and drug withdrawal. Event-related fMRI was used to examine activation in response to a pre-infusion warning cue in two experimental sessions that manipulated withdrawal status. Significant cue-induced effects were seen in the caudate, ventral anterior nucleus of the thalamus, the insula, subcallosal gyrus, nucleus accumbens, and anterior cingulate. These results suggest that withdrawal and nicotine anticipation produce (1) different motor preparatory and inhibitory response processing and (2) different craving related processing.
Drug withdrawal symptoms have long been considered to be a primary feature of drug addiction. For instance, classical conditioning theories of drug motivation such as those proposed by Wikler and Siegel emphasize avoidance of withdrawal as a primary explanation for the maintenance of addictive behavior (Wikler, 1973; Siegel, 1976). The central tenet of these theories is that the addicted organism takes drug primarily to avoid or reduce aversive withdrawal symptoms. Hence, there should be a strong relation between withdrawal severity and indices of drug motivation. Some recent research has supported the importance of withdrawal by showing that withdrawal indexes the likelihood that an addicted individual will return to drug use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004).
However, some data suggest that withdrawal is not a significant motivational force in addictive drug use. For instance, some studies have shown that relapse occurrence and drug self-administration are not consistently related to the severity or timing of withdrawal symptoms (McAuliffe, 1982; Shaham, Rajabi, & Stewart, 1996). Such data, and other evidence, have been used to support theories of addiction motivation that emphasize the role of drug reward and incentive processes that are mediated by mesotelencephalic dopaminergic structures and systems (Balfour, 1994, Robinson & Berridge, 1993). Such theories hold that psychomotor stimulants, and cues associated with their delivery, activate incentive-system structures such as the nucleus accumbens and other regions associated with the limbic cortical-ventral striatopallidal circuitry. This activation mediates rewarding effects and/or heightened approach motivation. These theories, and supportive evidence (Brody et al., 2002; Childress et al., 1999; Due, Huettel, Hall, & Rubin, 2002; Garavan et al., 2000; George et al., 2001; Naqvi, Rudrauf, Damasio & Bechara, 2007; Wilson, Sayette, Delgado & Fiez, 2005) have led some theorists to downplay the role of withdrawal in influencing drug motivation (Jaffe, 1989; Lyvers, 1998; Robinson & Berridge, 1993; Stewart, de Wit & Eikelboom, 1984).
There is much evidence to suggest that withdrawal is a clinically relevant feature of addiction motivation. Characteristic symptoms of nicotine withdrawal include drug craving, irritability, restlessness, sadness, worry, and decreased attention and can appear after only a few hours (Hughes, 1992; Hughes & Hatsukami, 1986; Hughes, Higgins, & Hatsukami, 1990; Parrott, Garnham, & Wesnes, 1996; Watkins, Koob, & Markou, 2000). Severity of these symptoms are typically mild to moderate, however, there is a substantial amount of evidence suggesting that withdrawal is a crucial factor accounting for relapse. For instance, smokers indicate that withdrawal symptoms motivate them to smoke and indeed, negative affect and other withdrawal symptoms prospectively predict relapse (Covey, Glassman, & Stetner, 1990; Kenford et al., 2002).
To investigate the role of withdrawal in addiction motivation we identified regions of interest on joint consideration of two factors: evidence that the region was linked to motivationally relevant events (e.g., drug cue or drug exposure, craving severity) and was linked theoretically in a drug motivational processes cascade. Some theorists have suggested functional or processing roles for the various brain regions in the context of drug motivation. For example, Breiter, Volkow, and others have forwarded models that implicate several linked processes and regions in drug seeking. Volkow et al. (2003) posits: (1) a system involving the ventral striatum that confers incentive value on drug cues; (2) a motivational drive system involving the OFC that energizes pursuit or drug seeking behavior; (3) a memory system involving the amygdala that processes memories about prior reinforcement and is especially involved in the processing of emotionally valenced memories; and (4) a cognitive control circuit that includes the anterior cingulate and prefrontal cortex, including the DLPFC, that applies top-down influence to process information related to response selection and monitoring. It is important to note that other regions are involved in the above processes and that these processes constitute only one model of drug seeking processing substrata. As Volkow et al., (2003a) notes, other brain regions and associated processes may be involved. For example, with increased rates of drug consumption, the organism will develop physical dependence, which will produce distress contingent upon reductions in drug use with interoceptive distress cues potentially cueing additional self-administration (Baker et al., 2004). In this regard, the insula has been empirically linked with craving intensity (Naqvi et al., 2007), which may be attributed to its role in integrating interoceptive cues that may provide signals of withdrawal status or distress. In addition, as drug self-administration becomes highly ingrained it acquires the properties of an automatic motor sequence that is triggered in an obligatory manner by drug associated stimuli (i.e., acquires the features of habit learning; Everitt & Robbins, 2005). This is why motor systems may be activated by drug cues via stimulus-response associations, with processing occurring in motor systems such as the dorsal striatum and the anterior ventral nucleus of the thalamus (Everitt & Robbins, 2005).
Prior theory regarding the systems involved in drug motivation and findings linking regions with drug motivation indicators (e.g., reaction to drug cues, direct drug exposure, craving) led us to target the following as ROI’s (followed by relevant citations to drug motivation effects): the amygdala (Franklin et al., 2007; Breiter et al., 1997; Breiter & Rosen, 1999; Childress et al., 1999; Stein et al., 1998), insula (Brody et al., 2002; Franklin et al., 2007; McBride et al., 2006), ventral striatum/nucleus accumbens (David et al., 2005; Franklin et al., 2007; Stein et al., 1998; the adjacent subcallosal gyrus was also included as per Breiter & Rosen, (1999), thalamus (Brody & George, 2007; Kufahl et al., 2008; McClernon et al., 2005; Stein et al., 1998; Volkow et al., 2003b), cingulate cortex/anterior cingulate (Brody et al., 2002; Franklin et al., 2007; McBride et al., 2006; McClernon et al., 2005; Stapleton et al., 2003; Stein et al., 1998), dorsal striatum (putamen & caudate: McBride et al., 2006; Stapleton et al., 2003; Volkow et al., 2003), superior frontal gyrus (Bonson et al., 2002; David et al., 2005; McClernon et al., 2005), orbital frontal cortex (Brody et al., 2002; Franklin et al., 2007; Martin-Solch et al., 2001; McBride et al., 2006), and dorsolateral prefrontal cortex (Brody et al., 2002; Franklin et al., 2007; McBride et al., 2006). These regions and circuits tend to receive dopaminergic innervations and are interconnected via glutaminergic projections (David et al., 2005; Volkow et al., 2003a), suggesting linked roles in drug motivational processing.
We predicted that we would see greater activity in these regions in the condition where individuals anticipated nicotine receipt while in withdrawal vs. while nicotine satiated. In essence, consistent with classic incentive motivational models (Robinson & Berridge, 1993, Everitt & Robbins, 2005; Bolles, 1967), we predicted that deprivation would increase the motivational value of drug cues associated with drug expectation. This prediction is also consistent with conditioning research that shows that internal states can enhance or devalue reinforcers (Rescorla & Wegner, 1972). Thus, we predicted that withdrawal, relative to ad libitum smoking, would increase activity in targeted brain regions when smokers anticipated nicotine infusion. Specifically, we believed that the combination of incipient drug receipt in the context of deprivation would increase activity in regions associated with incentive evaluation (because deprivation had inflated the value of drug), and that such incentive processing would elicit related processing regarding memories of past drug experience, habitual motor routines, and cognitive control resources because the well practiced drug self-administration behavior was unavailable (i.e., require inhibitory control). We used a saline-infusion control to determine if this withdrawal effect generalized to anticipation of a neutral stimulus.
In the present research, dependent smokers anticipated nicotine receipt in both withdrawn and non-withdrawn (i.e., ad libitum smoking) states. We focused on drug anticipation in this study since there is considerable evidence that anticipation of drug access is an effective instigator of drug motivational processing (Carter & Tiffany, 2001; Juliano & Brandon, 1998; Pomerleau, Pomerleau & Marks, 2000; Wertz & Sayette, 2001a; Wertz & Sayette, 2001b). We also decided to manipulate anticipation of drug delivery, rather than exposure to drug cues alone (without drug receipt), since we believed that anticipation of reinforcer access is the key motivational mechanism activated by conditioned stimulus exposure (Pavlov, 1997). In addition, we also believed that exposure to drug cues, without actual drug access, might elicit frustrative nonreward and distort motivational responding (Sayette & Hufford, 1994). For this reason, the present study examined the effect of withdrawal on regional brain activity while individuals anticipated imminent delivery of nicotine infusion.
Previous imaging studies have investigated the effects of nicotine deprivation on reaction to smoking cues. A recent neuroimaging study assessed the effect of short-term abstinence from smoking on regional brain activity in response to drug cues and revealed no effects of deprivation (McClernon, Hiott, Huettel & Rose, 2005). Similarly, an examination of neural response to a video depicting tobacco consumption behavior found that abstinence had a minimal effect on brain activation (McBride, Barrett, Kelly, Aw, & Dagher, 2005). Likewise, a third study found little effect of deprivation status on regional brain activity in response to smoking vs. nonsmoking videotape images (Franklin et al., 2006). In our view, these studies do not serve as sensitive tests of withdrawal effects because they did not involve anticipation of actual, incipient drug receipt in the magnet.
This study used functional magnetic resonance imaging (fMRI) to assess the anticipatory response of heavy smokers to warning cues that alerted them to forthcoming drug or saline delivery. Due to the physical limitations associated with fMRI data collection procedures, it was necessary to incorporate an intravenous infusion of nicotine in place of smoking a cigarette. Two pre-experimental sessions including nicotine infusion occurred prior to the fMRI experimental sessions to establish the reinforcing nature of the nicotine injection. Because actual nicotine infusions were used, and these might exert anterograde influence on subsequent responses, response to anticipation of a single nicotine infusion in both the withdrawn and non-withdrawn states was used as the primary outcome assessment time point.
Method
Participants
A total of 13 right-handed adults (6 females) with a mean age of 35.69 years (SD=11.08) from the greater Madison, Wisconsin area were recruited through newspaper and poster advertisements. People who smoked at least 10 cigarettes per day and had a minimum expired breath carbon monoxide (CO) level of 10 parts per million (ppm) were invited to participate. Mean number of years smoking and cigarettes smoked per day were 19.61 (SD=10.58) and 21.53 (SD=7.83), respectively. All participants reported a history of withdrawal symptoms upon past quit attempts and were free of heart disease, angina, and physical disabilities that would prevent exposure to MRI. Exclusion criteria comprised a history of metal in the body, psychiatric and substance use disorders other than tobacco use, current use of a smoking cessation treatment, pregnancy, or a significant desire or intention to quit smoking.
Procedure
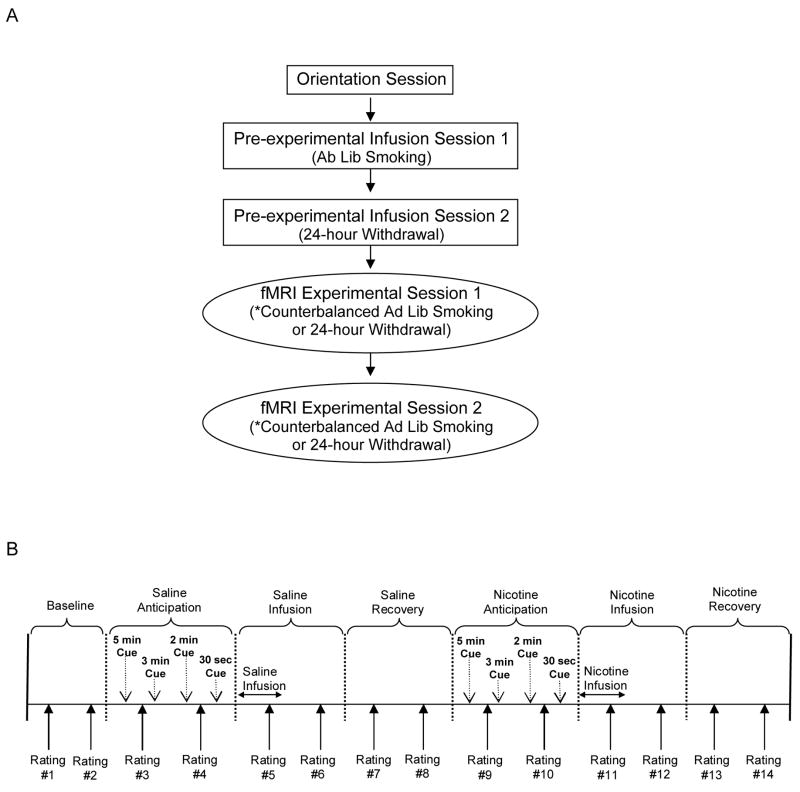
The general study procedure is illustrated in Figure 1A. Participants who passed an initial phone screen were invited to attend an Orientation Session that included collection of written informed consent, HIPAA authorization and a CO expired breath sample. Next, participants completed an electrocardiogram assessment of cardiac health and were assigned a counterbalanced smoking status order for the experimental sessions. The study consisted of two pre-experimental sessions designed to acquaint individuals with study procedures followed by two fMRI experimental sessions. All sessions were scheduled 1–2 weeks apart. Participants were reimbursed $100 for completion of each study session.
Figure 1.
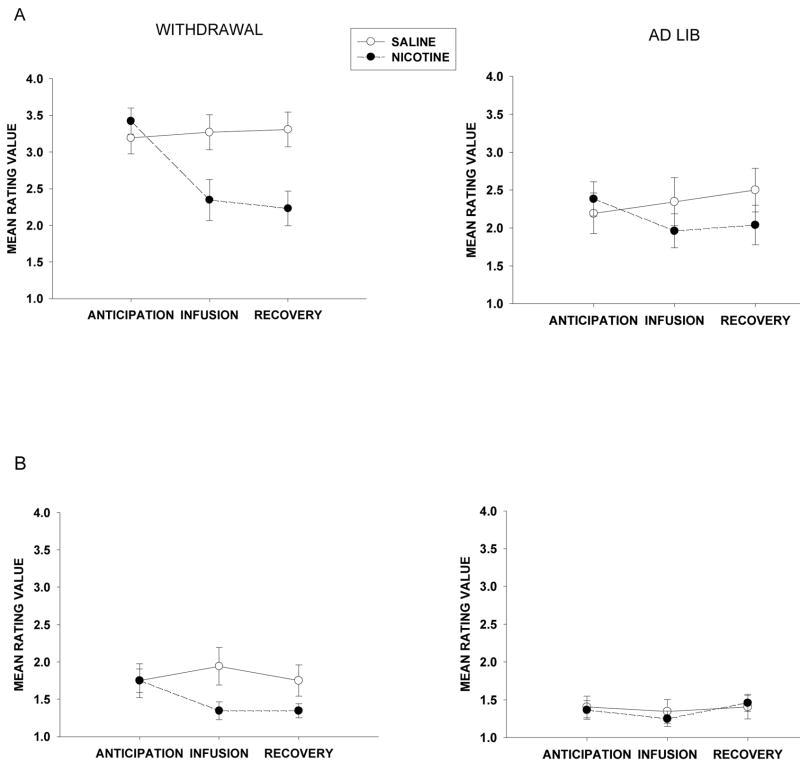
Experimental design and infusion procedure schedule. (A) After completing an orientation session assessing inclusion/exclusion criteria, participants completed two pre-experimental infusion sessions designed to establish the reinforcing effects of the nicotine infusion and two active fMRI experimental infusion sessions, with both sets of sessions counterbalanced for smoking status. (B) The infusion procedure used during the pre-experimental and experimental sessions included seven scan blocks, each lasting 5 min 30 sec and consisted of warning cues during the anticipation scans at 5 min, 3 min, 2 min, and 30 sec prior to the infusion. Saline or nicotine injections occurred at the beginning of the infusion scans and a recovery scan followed each infusion period. Participants completed two sets of mood and nicotine ratings during each scan block at 4 min and 1.5 min prior to infusion.
Experimental design
The first pre-experimental infusion session was held in a hospital exam room and CO level was collected to verify smoking status. During the session, non-withdrawn participants completed a set of paper-and-pencil questionnaires, underwent a brief medical exam, and were set-up with an intravenous (IV) needle. Next, participants completed the infusion protocol illustrated in Figure 1B while lying upright on an exam bed. This procedure comprised seven blocks that each lasted 5 min 30 sec and consisted of affect and drug effect ratings, verbal infusion warning cues, and IV injections of a 2 ml saline solution followed by a 2 ml nicotine solution containing 2 mg of nicotine. There were two sets of rating questions asked during each scan block and these occurred at 4 min and 1.5 min prior to the end of the block. Participants were asked to respond on a 4-point Likert rating scale to affect and drug effect questions. Four verbal infusion warning cues were presented during the anticipation scan blocks at 5 min, 3 min, 2 min, and 30 sec prior to infusion (e.g., “You will receive the saline/nicotine infusion in 5 min”). All injections occurred over a two-minute period immediately after the anticipation scan periods and saline infusion always preceded the nicotine infusion. At the end of the last trial, a post-infusion questionnaire was completed and the IV needle was removed.
The second pre-experimental infusion visit was a simulation session in which participants were assessed in an fMRI simulator room that was a replica of the fMRI environment including a hollow fMRI apparatus without the operational magnet. This session allowed participants to become familiar with experimental procedures while in withdrawal. All participants were instructed to abstain from smoking for 24 hours prior to this session. Abstinence was confirmed by a CO level below 10 ppm. The remainder of this session mimicked the procedures outlined above in Session 1, including the infusion of saline and nicotine. However, participants were positioned in a nonfunctional fMRI apparatus during the session.
Following successful completion of the pre-experimental conditions, two fMRI experimental infusion sessions counterbalanced for smoking status (24 hour withdrawal vs. ad lib smoking) were conducted in an active fMRI scanner following the same procedure as was used in the pre-experimental sessions. Affect and craving ratings were recorded using a button box and participants were given practice trials prior to the baseline scan to rehearse manual responses. The verbal infusion warning cues were presented through headphones while participants were in the scanner. Participants viewed a string of neutrally-valenced slides selected from the International Affective Picture System during fMRI scanning blocks to reduce fatigue and boredom.
Self-report assessments
At the beginning of the first pre-experimental session, participants were asked to complete a baseline battery of pencil-and-paper questionnaires that evaluated smoking behavior, withdrawal symptoms, and affect. The Fagerstrom Tolerance Questionnaire (FTQ; Fagerstrom, 1978) was used to assess level of nicotine dependence. This 8-item inventory includes questions designed to assess various components of smoking behavior and is correlated with biochemical measures of smoking heaviness. To evaluate baseline mood, the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) was employed. This schedule consists of 10 positive and 10 negative affect adjectives that are rated on a Likert scale ranging from 1 (very slightly or not at all) to 5 (Extremely) and pertains to feelings over the past 24 hours. Additionally, the 28-item Wisconsin Smoking Withdrawal Scale (WSWS; Welsch, 1999) was used to assess individual withdrawal symptom severity. The measure includes five subscales (e.g., anger, anxiety, concentration, craving, & hunger) and each item is rated on a 0 (strongly disagree) to 4 (strongly agree) Likert scale. Mean scores on the FTQ, PANAS Positive, PANAS Negative, and WSWS total score were 7.38 (SD=2.21), 25.76 (SD=7.15), 14.84 (SD=4.93) and 2.02 (SD=0.49), respectively. Finally, ratings of mood and subjective drug effects were collected during the fMRI scanning blocks and are shown in Table 1. Participants were asked to indicate how they felt on a 1 to 4 Likert scale with respect to two positive affect questions, two negative affect questions, and two nicotine questions.
Table 1.
Questions used for self-report ratings of positive affect, negative affect and nicotine effects.
| On a scale from 1 to 4, with 1 meaning very little and 4 meaning extremely, how… | |
| Positive affect | excited, interested, or enthusiastic do you feel? |
| alert, attentive, or determined do you feel? | |
| Negative affect | distressed or upset do you feel? |
| hostile or irritable do you feel? | |
| Nicotine effects | much of a buzz, rush, or high do you feel? |
| much of an urge or craving to smoke do you feel? |
fMRI acquisition and analysis
Images were collected using a General Electric (Fairfield, CT) Signa 3.0 Tesla high-speed magnetic imaging device, with a quadrature head coil. A total of 170 whole-brain functional image sets were collected per scan block, in volumes of 30 4mm sagittal echo-planar (EPI) slices (1-mm slice gap). A repetition time (TR) of 2 s was used, with an echo time (TE) of 30 ms, a 60° flip, and a field of view of 240 × 240 mm, with a 64 × 64 matrix, resulting in a 3.75 × 3.75 × 5 mm voxel size. Prior to collection of functional images, an inversion-recovery fast gradient echo anatomical scan consisting of 124 1 mm slices to assist with localization of function. Data from the two anticipation blocks were analyzed using the Analysis of Functional NeuroImage software (AFNI, version AFNI_2008_02_01_1144, May 22 2008). Data processing steps included offline reconstruction with a 1-voxel FWHM Fermi filter, field map correction to reduce distortion, 6-parameter rigid-body motion correction, removal of ghost and skull artifacts and application of a 6 mm FWHM gaussian blur to reduce noise. Timeseries were modeled with a least-squares fit of an ideal hemodynamic response to each individual warning cue, with motion parameters entered as covariates and a fourth order baseline to account for signal drift. The resultant beta-weights were converted to percent signal change, converted to standard Talairach space via the identification of anatomical landmarks on the IR scan, and smoothed with a 3-mm gaussian blur to account for anatomical differences across subjects.
Results
Carbon monoxide and withdrawal
To test the effect of the withdrawal manipulation, a paired-samples t-test was conducted on the CO levels and WSWS rating scores for the two experimental fMRI infusion sessions. Examination of the CO recordings taken prior to the two experimental fMRI infusion sessions indicates that CO levels were significantly lower during the withdrawal session (M=4.30, SD=2.65) relative to the ad lib smoking session (M=23.07, SD=8.53), t12 =8.36, p=.00. Similarly, assessment of self-reported withdrawal severity indexed by WSWS total rating scores shows that participants experienced a significant increase in withdrawal symptoms during the experimental fMRI withdrawal infusion session (M=2.42, SD=0.45) versus the ad lib infusion session (M=1.98, SD=0.54), t12 =2.24, p=.04. These results confirm the success of the withdrawal manipulation.
Self-report data
Baseline ratings
To assess differences in baseline ratings between the withdrawal and ad lib sessions, paired samples t-tests comparing baseline ratings (i.e., the average of ratings 1 & 2) were conducted for the urge and negative affect ratings. The negative affect score was created by averaging across the two negative affect ratings (see Table 1). Results showed a significant difference between baseline urge ratings in the withdrawal (M=3.11, SD=0.86) relative to ad lib smoking (M=2.15, SD=0.82) conditions, t12 =3.36, p=.006. Similarly, ratings of negative affect were significantly different at baseline between the withdrawal (M=1.98, SD=0.99) versus ad lib smoking (M=1.28, SD=0.58) sessions, t12 =3.12, p=.009. Thus, withdrawal produced higher ratings of urge to smoke and negative affect compared to a non-withdrawn state at the beginning of the fMRI infusion session.
Analytic strategy
To assess the effect of warning cues and infusion on smoking urge, negative affect, positive affect and buzz ratings, a series of repeated measures ANOVAs were performed with Smoke Status (withdrawal vs. ad lib), Infusion (nicotine vs. saline) and Block (anticipation vs. infusion vs. recovery) as within-subject variables. The Block variable was created by averaging the two ratings during each recording block to produce a single rating for anticipation, infusion and recovery blocks. Planned Helmert orthogonal contrasts were performed on the ratings data to assess our a priori hypothesis that pre-infusion (i.e., anticipation block) ratings would be significantly greater than post-infusion ratings (i.e., infusion and recovery blocks) for urge and negative affect. Additionally, we predicted buzz ratings to peak immediately following infusion. Huynh-Feldt corrected p-values are reported for all within-subject effects to correct for possible violations of sphericity.
Urge, Affect and Buzz Ratings
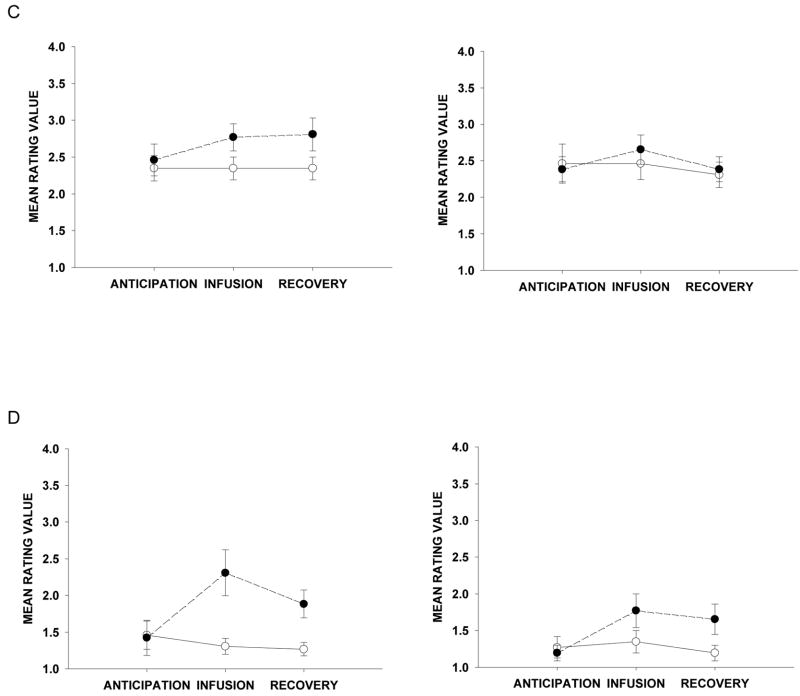
Self-report ratings of urge, affect and buzz collected during scanning blocks are depicted in Figure 2. A Helmert contrast analysis of urge data revealed a significant Infusion × Smoke Status × (Helmert) Block interaction, F(1,12) = 6.51, p =.025. In the following analyses, Block effects refer to effects obtained with the Helmert coding described above. This indicated that urge was greater in anticipation of infusion compared to post-infusion blocks (i.e., infusion and recovery blocks). Moreover, significant Infusion × Block, F(1,12) = 16.38, p =.002, and Smoke Status × Block, F(1,12) = 5.34, p =.039, interactions indicate that the impact of the warning cue on urge is significant in anticipation of nicotine when participants are in withdrawal.
Figure 2.
Self-report ratings during fMRI experimental infusion sessions. (A) Mean group ratings (± s.e.m.) for urge or craving to smoke. (B) Mean group ratings (± s.e.m.) for the average of two negative affect questions (i.e., distressed/irritable). (C) Mean group ratings (± s.e.m.) for the average of two positive affect questions (i.e., excited/alert). (D) Mean group ratings (± s.e.m.) for buzz/rush/high.
The next contrast analysis compared negative affect ratings pre-infusion (i.e., anticipation block) to post-infusion (i.e., infusion and recovery blocks). This analysis revealed a significant Infusion × Smoke Status × Block interaction, F(1,12) = 13.75, p =.003. Additionally, significant Infusion × Block, F(1,12) = 6.51, p =.025, and Smoke Status × Block, F(1,12) = 6.51, p =.025, interactions suggest that negative affect is greatest in anticipation of saline during the withdrawal condition. Finally, buzz rating contrast analyses comparing immediate post-infusion (i.e., infusion block) to the recovery block indicated a trend, F(1,12) = 4.03, p =.068, such that buzz ratings peaked following nicotine infusion when participants were in withdrawal and decreased post-infusion. No significant results were found comparing positive affect ratings during anticipation to post-infusion blocks (i.e., infusion and recover blocks), F(1,12) =.156, p =.700, or infusion to recovery, F(1,12) =.480, p =.502.
Neural response to warning cue
Analysis of blood oxygen level-dependent (BOLD) response focused on structures targeted a priori that have been associated with drug motivational processing systems and responses (e.g., the prefrontal cortex, nucleus accumbens, amygdala, anterior cingulate, & insula). Using anatomical masks from the AFNI Talairach dataset, average percent signal change was calculated for each structure. Signal change associated with the first warning (i.e., 5 min) was the strongest and most consistent across the targeted structures, so analysis was limited to the response to the first warning cue in each of the two anticipation blocks. Using percent signal change averaged over anatomical structure, repeated measures ANOVAs were performed with Smoke Status (withdrawal vs. ad lib) and Infusion (nicotine vs. saline) as within-subject variables (P < 0.05, uncorrected).
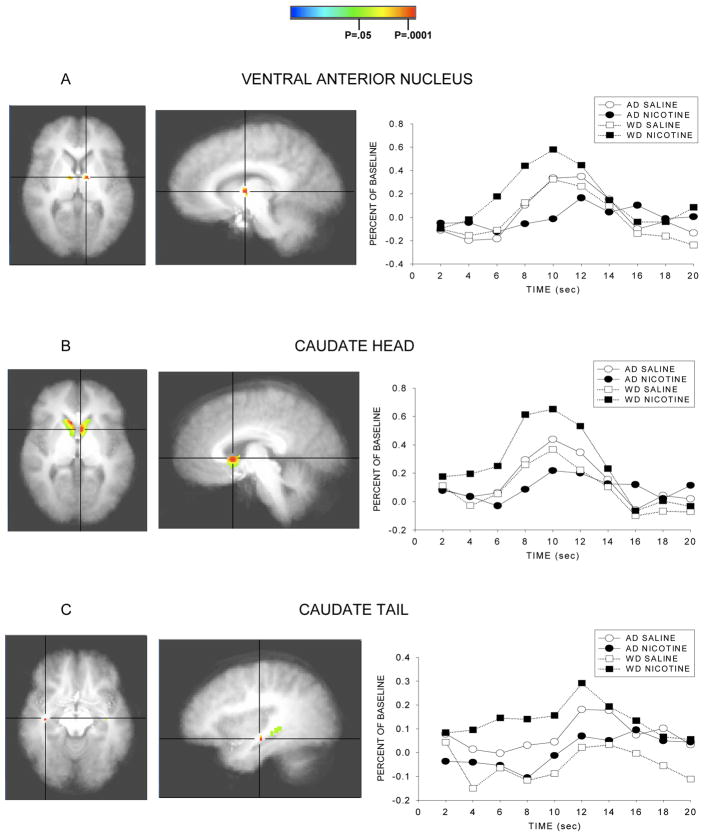
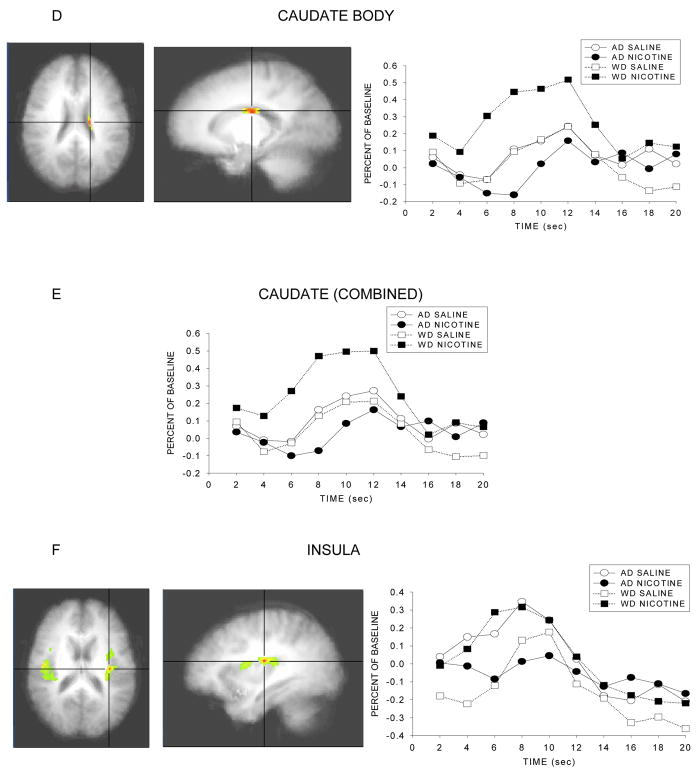
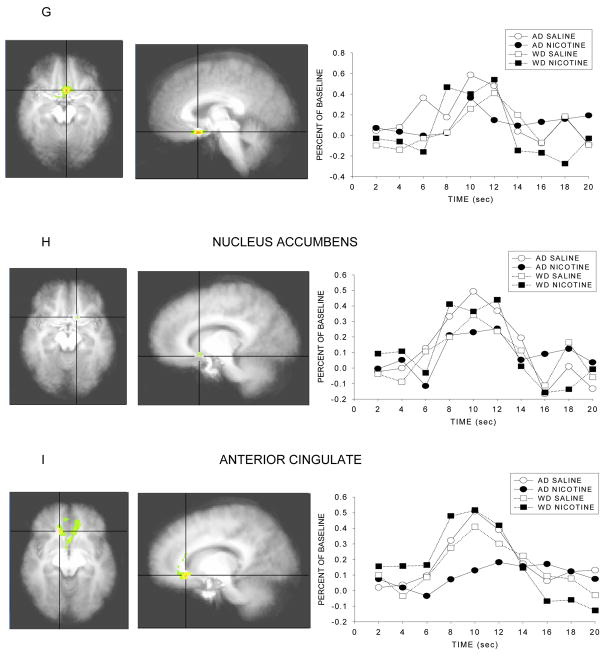
As predicted, significant Smoke Status × Infusion interactions were observed for several anatomical regions, such that the effect of infusion on response to the 5 min warning cue varies as a function of smoking/deprivation status and type of infusion. The nature of the interaction appeared to differ in different brain regions. For instance, in the case of the Ventral Anterior nucleus and the caudate (head, body), the source of the interaction was that especially high levels of activity were seen in individuals who anticipated nicotine receipt in the context of withdrawal, and especially low levels of activity were seen in individuals who anticipated nicotine following ad lib smoking. Thus, this effect appeared to reflect deprivation effects per se in the context of imminent drug receipt. Conversely, other structures such as the insula and the anterior cingulate tended to show high levels of activity except in the case where nicotine receipt was anticipated following ad libitum smoking. The F scores and p values for brain structures with significant interaction effects are presented in Table 2 and illustrations of the masked F scores and the time course of the response to the warning cue are depicted in Figure 3.
Figure 3.
Statistical activation maps and percent signal change during the withdrawn and ad lib smoking conditions. Activation maps illustrate statistically significant clusters in brain structures selected a priori (threshold at p<.05, uncorrected) and are centered on the voxel with the maximum interaction F score. Line graphs represent the percent signal change in brain activation to the 5 min nicotine warning cue as a function of time for both smoking conditions. Coordinates in Table 2.
Neural activity and baseline dependence ratings
To investigate whether differences in brain activation to the nicotine warning cue reflect individual differences in nicotine dependence and affect, the average activation in anatomical structures of interest (Table 2) was used to conduct correlation analyses with baseline FTQ, PANAS Postive, PANAS Negative, and WSWS total scores. We also examined the relationship between the difference in brain activation for the withdrawal and ad lib nicotine scans and mood and drug effect ratings recorded during the scanning blocks. The outcome variable of anticipatory neural activity was created for each anatomical structure by calculating the difference between the average percent signal change to the 5 min nicotine warning cue in the withdrawal versus ad lib smoking sessions (i.e., withdrawal percent signal change ad lib percent signal change). Thus, more positive values represent greater activation to the warning cue when in withdrawal versus non-withdrawal. WSWS score was a difference between WSWS total score in withdrawal versus ad lib smoking sessions. Mood and drug effect ratings were a difference between the average of ratings in the nicotine anticipation block in withdrawal and ad lib smoking sessions.
Table 2.
Brain regions of interest showing significant differences in neural activation between smoke status and infusion.
| Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Location | ROI mask (mm3) | Avg F-score | p-value | x | y | z |
| Ventral anterior nucleus | 543 | 18.5 | 0.001 | −10 | −5 | 6 |
| Caudate head | 3390 | 18.5 | 0.001 | −4 | 10 | 3 |
| Caudate tail | 1018 | 13.8 | 0.003 | 35 | −15 | −9 |
| Caudate body | 5613 | 7.5 | 0.018 | −17 | −15 | 23 |
| Caudate (combined) | 10021 | 13.8 | 0.003 | |||
| Insula | 29564 | 7.2 | 0.020 | −33 | −16 | 17 |
| Subcallosal gyrus | 3768 | 7.2 | 0.020 | −3 | 11 | −13 |
| Nucleus accumbens | 287 | 6.8 | 0.023 | −12 | 12 | −10 |
| Anterior cingulate | 22518 | 6.1 | 0.029 | 13 | 26 | −6 |
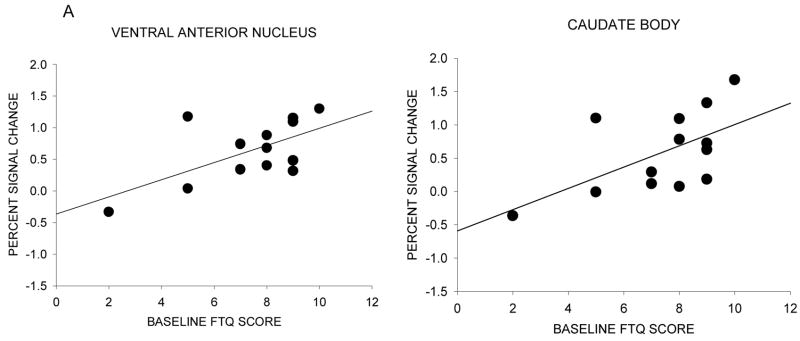
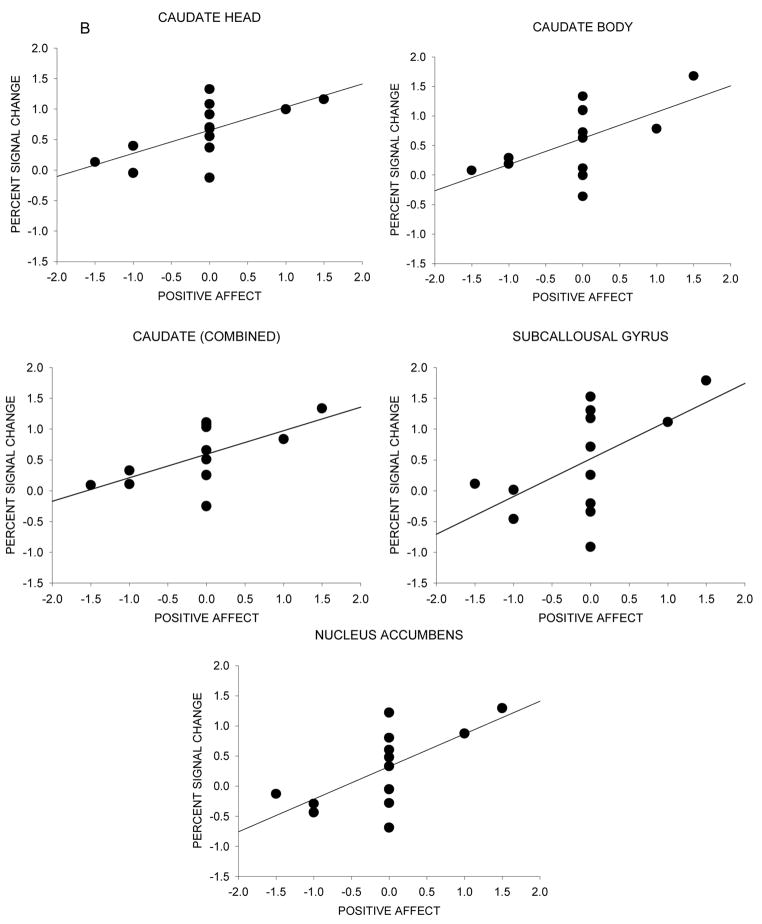
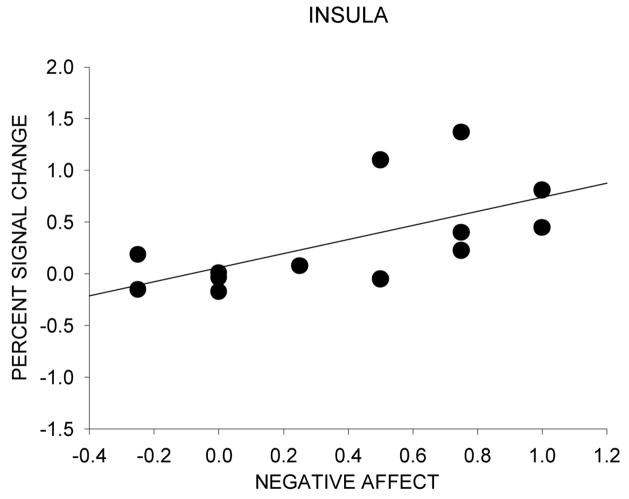
A significant positive correlation between baseline dependence level indexed by FTQ scores and neural response to the nicotine warning cue was found for the ventral anterior nucleus (r=0.62, p=0.02) and the caudate body (r=0.59, p=0.03) (Figure 4a). A significant positive correlation between positive affect and neural response to the nicotine warning cue was found for the caudate head (r=0.64, p=0.02), caudate body (r=0.58, p=0.04), caudate (combined) (r=0.62, p=0.02), subcallosal gyrus (r=0.56, p=05), and nucleus accumbens (r=0.66, p=0.01) (Figure 4b). A significant positive correlation between negative affect and neural response to the nicotine warning cue was found for the insula (r=0.63, p=0.02) (Figure 4c). No significant correlations were found between neural response to the nicotine warning cue and baseline PANAS and WSWS scores and drug effect ratings.
Figure 4.
Neural activation and individual differences in nicotine dependence level and affect during scanning sessions. The scatter diagram is of individual difference scores as a function of percent signal change between ad lib and withdrawal conditions for the nicotine warning cue. (A) Correlation between neural activity and baseline dependence (FTQ score). (B) Correlation between neural activity and the difference in positive affect ratings during the nicotine anticipation block for withdrawal - ad lib conditions. (C) Correlation between neural activity and the difference in negative affect ratings during the nicotine anticipation block for withdrawal - ad lib conditions.
Discussion
The role of withdrawal in the maintenance of drug addiction is controversial. Some theorists suggest that withdrawal is a critical factor that motivates the addicted individual to consume drug (Baker et al., 2004; Koob, Markou, Weiss & Schulties, 1993; Siegel, 1976; Solomon & Corbitt, 1974; Wikler, 1973) yet, other theories deemphasize the effect of withdrawal on addictive processes (Jaffe, 1989; Lyvers, 1998; Robinson & Berridge, 1993; Stewart, de Wit & Eikelboom, 1984). The present study investigated neural activity associated with anticipation of impending nicotine receipt. The goal of this study was to examine the effect of withdrawal on drug anticipation by manipulating withdrawal status in heavy smokers. We hypothesized that withdrawal would significantly increase brain activation in structures that were selected a priori based on previous research regarding brain circuitry known to reflect the motivational or incentive value of drug cues.
An analysis of self-report ratings of smoking urge, negative affect, positive affect and drug effects assessed during anticipation, infusion and recovery blocks, indicates that subjective response to the nicotine warning cue and infusion differs based on withdrawal status. Of note was the finding that participant urge ratings were greatest during withdrawal in anticipation of nicotine and decreased after the nicotine infusion, whereas there was no significant difference in urge ratings across scan blocks for the non-withdrawn condition. This suggests that the withdrawal manipulation was successful in affecting reactions to motivationally significant stimuli (i.e., nicotine warning cue).
The main purpose of this study was to assess neural activity in targeted brain regions in response to verbal warning cues that signaled nicotine infusion. We found differences in BOLD signal change as a function of deprivation and drug cue status in some of the ROI’s selected on a priori bases. Further analyses examined the correlation between neural activation and dependence to determine if response to the nicotine warning cue was sensitive to individual differences in dependence level.
Results showed significant differences in activation in the ventral anterior nucleus, the caudate (Head, Tail, Body), insula, subcallosal gyrus, ventral striatum (nucleus accumbens), and anterior cingulate. In some of these areas, notably the ventral anterior nucleus and the caudate, activity was high in the withdrawal + nicotine anticipation condition relative to all other conditions, but especially relative to the ad lib + nicotine anticipation condition. In the other areas (e.g., the insula, anterior cingulate, and to a lesser extent the nucleus accumbens) the biggest differences seemed to be due to the comparison of the ad libitum + nicotine anticipation condition with the other conditions. This pattern appeared to reflect a relative suppression of activity in response to nicotine infusion in the context of nicotine repletion and further anticipated receipt of nicotine. This characterization of regional differences in activity levels is somewhat simplistic as there was some variation in ordering of conditions across the latter group of regions. But the main pattern of findings in regions such as the insula and anterior cingulate is that activity is exceptionally low in the ad lib + nicotine anticipation condition (Figure 3h–i).
The two different patterns of findings raise questions about why different results were obtained in the various regions. An initial question is why the caudate and the ventral anterior nucleus show such differential activity in response to nicotine anticipation as a function of deprivation/satiation status. The fact that activity in both regions was correlated with the FTQ shows that activity in these regions was related to nicotine dependence (Baker et al., in press). This is somewhat remarkable because of restriction in range of the sample: i.e., fairly uniform, high levels of dependence (with the exception of some individuals with FTQ scores under 5; Fagerstrom, 1978).
Volkow et al., (2003) and others (Everitt & Robbins, 2005) argue that habit learning, where highly mapped behaviors are automatically elicited by conditioned stimuli, is mediated by the dorsal striatum (Ito et al., 2002). Thus, it may be that drug expectation or anticipation can serve as a conditioned stimulus that elicits self-administration motor programs such as drug self-administration behaviors, despite declarative knowledge that there will be no opportunity for actual drug self-administration. The fact that motor processing may be elicited, even though smoking behavior is impossible, is consistent with the automatic nature of this sort of processing (i.e., by-passing effortful and conscious cognitive control processes; Everitt & Robbins, 2005; Tiffany, 1990). Habit learning occurs in response to very large numbers of learning trials (Everitt & Robbins, 2005). Implicating the caudate in the processing of automatic drug self-administration motor responses is consistent with the correlation of caudate actiivty with FTQ score. FTQ scores largely reflect smoking rate (Baker et al., in press), and smoking rate, in turn, presumably determines the extent to which smoking is highly behaviorally mapped. The parallel activation of the ventral anterior nucleus along with the caudate could be explained by its strong role in caudate-thalamic functional connections in the development of synchronized behavioral sequences (e.g., Berezovskii & Oleshko, 1977). Thus, one account for the effects of withdrawal and anticipation on activation levels in the caudate and ventral anterior nucleus is that these structures reflect different levels of motor program processing when smokers anticipate nicotine. At first blush this is consistent with the notion that withdrawal, perhaps mediated via interoceptive cues, enhances processing in drug motivational systems. That is, when smokers anticipate nicotine, withdrawal produces much greater motor processing than does nicotine satiation.
The activity differences in the caudate and ventral anterior nucleus may reflect different levels of response inhibition. Research shows that the caudate and the thalamus are centrally involvedin response inhibition, including the inhibition of subliminally generated movements (Aron et al., 2003). According to these data, response inhibitionmay be manifested in reduced striatal activity due to input reductions or increased activation of inhibitory interneurons, which suppresses caudate activity. Therefore, the lower caudate activation in the condition where individuals expect nicotine following ad libitum smoking may reflect relatively high levels of response inhibition. According to this account response inhibition processing would be enhanced in conditions of low motivation (following ad libitum smoking) and attenuated by high levels of motivation (following deprivation). Thus, nicotine satiation may have produced levels of inhibition that exceeded levels produced when nicotine receipt was not anticipated (i.e., withdrawal + saline anticipation and ad lib + saline anticipation), while nicotine deprivation significantly reduced inhibition. In sum, the data from the caudate and ventral anterior nucleus suggest that withdrawal significantly modulates activity in motor processing systems when drug is anticipated or expected. This is reminiscent of the powerful drug expectancy effects in thalamic nuclei that were reported by Volkow et al., (2003b).
The other major pattern of findings is one in which effects appear to reflect primarily the reduced activity seen in response to drug anticipation in the context of drug repletion. To the extent that at least some of the implicated structures (e.g., nucleus accumbens) reflect incentive processing, we might assume that the results reflect lowered incentive value of nicotine administered in the context of drug repletion. This account does not accord well with the observation of consistently high responding in the condition where saline was anticipated in the state of repletion; i.e., why saline infusion would be so highly valued.
Perhaps a more likely account relies upon the role of structures such as the insula and the anterior cingulate in craving-relevant processing. There is strong evidence that the insula is critical to craving information processing, perhaps through its role in integrating interoceptive information such as affective information (Naqvi et al., 2007). If it is the case that interoceptive cues of drug deprivation and affective distress induce craving or urges to smoke (Baker et al., 2004), then it makes sense that there would be less craving and urge processing in the insula under conditions of drug repletion. Interestingly, insula activity was associated with self-ratings of negative affect (Figure 4c) such that increased negative affect was associated with increased insula activation as a function of withdrawal.
The anterior cingulate has been implicated in urge information processing through its role in error detection and the recruitment of cognitive control for conflict resolution (Curtin et al., 2007; McCarthy et al., in press; cf. Brody et al., 2004; Li & Sinha, 2008; R. McClernon et al., 2005; Volkow et al., 2003; Wilson et al., 2005). According to this perspective, the relatively low level of activity in the ad lib + nicotine condition is due to the fact that there is little conflict to detect: drug has been, and is, available. However, in all other conditions, conflict would be generated because drug has either been withheld chronically (deprivation) or it will not be delivered imminently (instead saline will be delivered). This account is based on the notion that present and anticipated deprivation can enhance urge processing. Therefore, these accounts suggest that activity patterns in the anterior cingulate and insula roughly reflect urge processing, which itself is a function of integrated feedback of internal state and error detection. One concern with regards to this account is that we did not observe significant associations between urge ratings and insula or anterior cingulate activity in this research. There was relatively little power to detect such effects, however.
There is some evidence that may reflect incentive enhancement caused by withdrawal. Anticipation of nicotine infusion elicited greater nucleus accumbens activity when it occurred in the context of deprivation than when it occurred in the context of repletion. This is consistent with our a priori hypothesis that withdrawal would inflate the incentive value of nicotine receipt. However, strong activation was also seen in conditions where individuals anticipated saline infusions. It is unclear why smokers would not respond more highly to nicotine anticipation during withdrawal than they responded to saline anticipation. It is possible that the high values in the saline anticipation condition reflect the fact that the saline trial was always first in each study session. Future research should remove the confound between cue administration sequence and cue type. Moreover, we might have observed stronger incentive effects regarding anticipation of nicotine delivery if we had presented subjects with the actual cues that normally precede smoking (e.g., gustatory cues). That is, incentive effects may be highly bound to the stimulus properties of the incentive cue.
The current results present intriguing evidence that incipient receipt of nicotine may reveal differences in preparation or inhibition of motor responses related to self-administration and differences in urge processing: Differences that are accounted for by the joint effects of actual drug anticipation and drug deprivation status. Anticipation of nicotine receipt may activate self-administration motor sequences, and these may be modulated by state of nicotine/smoking deprivation. To the extent that activity in the insula and anterior cingulate reflect craving or urge processing, the least craving processing is seen when the smoker has been smoking ad libitum and anticipates receiving more nicotine. In sum, this research agrees with prior research and theory that emphasizes the important role of drug withdrawal and drug availability in influencing drug motivational processing (e.g., Juliano & Brandon, 1998; McBride et al., 2006; Wertz & Sayette, 2001b).
Some recent research suggests that withdrawal does not significantly affect regional brain activity in response to smoking cues (McBride et al., 2006; McClernon, Hiott, Huettel & Rose, 2005). The McClernon and the McBride studies used drug cues in the form of pictures and video clips depicting smoking-related objects and behavior, but these stimuli may not be as effective in eliciting drug motivational processing as is anticipation of actual drug delivery. Further, these visual cues were not followed immediately by actual drug consumption and this may have evoked feelings of frustration rather than anticipation. In contrast, the present research shows that withdrawal can reliably affect regional brain activity as manifested in single stimulus-presentation trials (e.g., single anticipation episodes). This suggests that real events or experiences, even if limited in number, may be sufficiently powerful so that they can be used effectively in future imaging studies (vs. repeated exposures to pictorial images).
Although the current data suggest that withdrawal modulates activity in brain regions implicated in drug motivation, the study sample size is relatively small and further research is clearly warranted. For instance, withdrawal-induced increases in the targeted structures should be related to behavioral measures of drug motivation. Future research should also examine response to drug cues in individuals who differ significantly in level of drug dependence. If such work suggests that withdrawal induced modulation of motivational processing is sensitive to dependence level, this measure could be used as an intermediate phenotype of dependence. Finally, we have attempted to draw inferences about the meaning of activation patterns observed in this research. As in all such attempts, inferences are challenged by the fact that brain regions may be involved in numerous processing tasks, and multiple regions may participate and interact in processing functions.
Acknowledgments
The authors thank the staff at the University of Wisconsin, Center for Tobacco Research and Intervention for their support. This research was supported by a Transdisciplinary Tobacco Use Research Center Award (TBB) P50DA19706 from the National Institutes on Drug Abuse.
References
- Aron AR, Schlaghecken F, Fletcher PC, Bullmore ET, Eimer M, Barker R, et al. Inhibition of subliminally primed response is mediated by the caudate and thalamus: evidence from functional MRI and Huntington’s disease. Brain. 2003;126:713–723. doi: 10.1093/brain/awg067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Moffitt T, Caspi A, Conti D. NIH Monograph #22: The Nicotine Dependence Phenotype. U.S. Government Printing Office; The nicotine dependence phenotype: Translating theoretical perspectives and extant data into recommendations for genetic mapping. (in press) [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Balfour DJ. Neural mechanisms underlying nicotine dependence. Addiction. 1994;89:1419– 23. doi: 10.1111/j.1360-0443.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Berezovskii VK, Oleshko NN. Electrophysiological characteristics of caudate-thalamic connections. Neurophysiology. 1977;9:431–436. [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–86. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Breiter HC, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Brieter HC, Rosen BR. Functional magnetic resonance of brain reward circuitry in the human. Annals of the New York Academy of Sciences. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, et al. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL. Neuroimaging of Nicotine Dependence: A Bioassay for Medications Development. In: George TP, editor. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007. pp. 275–289. [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–90. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Weirs RW, Stacy AW, editors. Handbook of implicit cognition and addiction. Thousand Oaks, CA: SAGE; 2006. pp. 233–250. [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–41. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Coury A, Phillips AG. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. Journal of Neuroscience. 1997;17:4849–55. doi: 10.1523/JNEUROSCI.17-12-04849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue- induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58:345–52. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, et al. Marijuana withdrawal in humans: effects of oral THC or divalproex. Neuropsychopharmacology. 2004;29:158–70. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under control of a drug-associated cue. Journal of Neuroscience. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kufahl P, Li Z, Risinger R, Rainey C, Piacentine L, Wu G, et al. Expectation modulates human brain responses to acute cocaine: A functional magnetic resonance imaging study. Biological Psychiatry. 2008;63(2):222–230. doi: 10.1016/j.biopsych.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Li CSR, Sinha R. Neuroscience & Biobehavioral Reviews. 3. Vol. 32. 2008. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction; pp. 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers M. Drug addiction as a physical disease: the role of physical dependence and other chronic drug-induced neurophysiological changes in compulsive drug self-administration. Experimental and Clinical Psychopharmacology. 1998;6:107–25. doi: 10.1037//1064-1297.6.1.107. [DOI] [PubMed] [Google Scholar]
- Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. Experimental Brain Research. 2001;139:278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- McAuliffe WE. A test of Wikler’s theory of relapse: the frequency of relapse due to conditioned withdrawal sickness. International Journal of Addiction. 1982;17:19–33. doi: 10.3109/10826088209054607. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McCarthy DE, Curtin JJ, Piper ME, Baker TB. Negative reinforcement: Possible clinical implications of an integrative model. In: Kassel JD, editor. Substance abuse and emotion. Washington DC: American Psychological Association; (in press) [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Marks JL. Abstinence effects and reactivity to nicotine during 11 days of smoking deprivation. Nicotine and Tobacco Research. 2000;2:149–57. doi: 10.1080/713688130. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive- sensitization theory of addiction. Brain Research Reviews. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–8. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Current Opinion in Neurobiology. 1997;7:191–7. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. Journal of Neuroscience. 1996;16:1957–63. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. Morphine analgesic tolerance: its situation specificity supports a Pavlovian conditioning model. Science. 1976;193:323–5. doi: 10.1126/science.935870. [DOI] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, Villemagne VL, Dannals RF, Grayson RG, et al. Intravenous nicotine reduces cerebral glucose metabolism: a preliminary study. Neuropsychopharmacology. 2003;28:765–772. doi: 10.1038/sj.npp.1300106. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewica J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI Study. American Journal of Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychological Review. 1984;91:251–68. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. American Journal of Psychiatry. 1991;148:621–6. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. The Journal of Clinical Investigation. 2003a;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-T, Ma Y, Fowler JS, Shu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulant in cocaine abusers. The Journal of Neuroscience. 2003b;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7:354–61. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychology of Addictive Behaviors. 2001a;15:268–71. [PMC free article] [PubMed] [Google Scholar]
- Wertz JM, Sayette MA. A review of the effects of perceived drug use opportunity of self-reported urge. Experimental and Clinical Psychopharmacology. 2001b;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence. Implications of a conditioning theory for research and treatment. Archives of General Psychiatry. 1973;28:611–6. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neuroscience. 2004;7:211–4. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine and Tobacco Research. 2005;7:637–45. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–54. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]