Abstract
Mutant superoxide dismutase type 1 (MTSOD1), the most common known cause of familial amyotrophic lateral sclerosis (FALS), is believed to cause FALS as a result of a toxicity of the protein. MTSOD1s with full dismutase enzymatic activity (e.g., G37R) and without any enzymatic activity (e.g., G85R) cause FALS, demonstrating that the ability of MTSOD1 to cause FALS is not dependent on the dismutase activity; however, it remains unclear whether MTSOD1 dismutase activity can influence disease phenotype. In the present study, we selectively knocked down G85R expression in particular cell types of G85R mice. Results following knockdown of G85R in motor neurons (MNs)/interneurons of G85R mice were similar to results from a published study involving knockdown of G37R in G37R mice; however, G85R knockdown in microglia/macrophages induced a prolonged early and late disease phase while G37R knockdown in the same cells only affected late phase. These results show that: (i) MN as well as non-MN expression of G85R, like G37R, has a significant effect on disease in transgenic mice – indicating the role of non-cell autonomous degeneration in both dismutase active and inactive MTSOD1. (ii) The effect of MTSOD1 expression in microglia/macrophages varies with different mutants, and may be influenced by the MTSOD1’s dismutase activity.
Keywords: familial amyotrophic lateral sclerosis (FALS), ALS, superoxide dismutase type 1 (SOD1), motor neuron, neurodegeneration, microglia
Introduction
Amyotrophic lateral sclerosis (ALS) is a relentless motor system degeneration that results in death usually 3–5 years after the onset. Approximately 10% of cases are inherited, and 15% of these familial ALS (FALS) cases are caused by mutations of Cu/Zn superoxide dismutase type 1 (SOD1). Scientists have vigorously investigated mutant (MT) SOD1-induced FALS under the assumption that this disease involves pathways of motor neuron (MN) death that are similar to those operative in sporadic ALS.
MTSOD1 is believed to cause cell death by means of a toxic gain of function rather than a loss of function. The mechanism by which MTSOD1 exerts its toxicity, however, remains unclear. MTSOD1s with full dismutase enzymatic activity (e.g., G37R) and without any enzymatic activity (e.g., G85R) cause FALS (Borchelt et al., 1994), demonstrating that the ability of the mutant protein to cause FALS is not dependent on its dismutase activity. Despite evidence indicating that a gain of function of MTSOD1 leads to FALS, the presence or absence of dismutase activity of the MT enzyme may still affect the pathogenesis or phenotype of the disease.
One important issue related to ALS is whether MN death is non-cell autonomous, i.e., whether MN death is influenced by the production of pathogenic factors produced in other cell types besides MNs. Compelling data related to this topic and especially relevant to the present study came from the use of CreLoxP technology to decrease the expression of MTSOD1 in one neural cell type with a constant MTSOD1 expression in the remainder of cell types (Boillee et al., 2006a; Yamanaka et al., 2008). In these experiments, mice were generated that carried a Cre recombinase transgene (that can excise a “floxed” gene that is flanked by LoxP sites) under the control of a cell type-specific transcriptional control element along with a floxed G37R MTSOD1 (G37Rflox) transgene. These mice were crossed with a mouse carrying Cre expressed in MNs (under the Islet-1 or VAChT promoter) or microglia/macrophages (under the CD11b promoter) to determine the effect of disease following a knockdown in G37R expression in these cell types. A decrease in G37R expression in the G37Rflox mice in MNs and other neurons delayed the onset and early phase of disease, while a decrease in expression of G37R in microglia and activated macrophages had little effect on the onset or early phase of disease, but significantly increased disease duration because of slowing of the late phase of disease.
In the present study, we carried out investigations of non-cell autonomous degeneration in G85Rflox mice similar to those carried out with G37Rflox mice (Boillee et al., 2006a; Yamanaka et al., 2008). Since G85R has no dismutase activity, while G37R has full dismutase activity, we reasoned that a comparison of our results with those obtained with G37Rflox transgenic mice might clarify the importance of the mutant’s enzymatic activity in different neural cell types in FALS in the following way. One could envision that overexpression of a dismutase-active MTSOD1, such as G37R, in a particular cell type could have neuroprotective activity (in addition to its toxicity) since oxidative stress has been implicated as a pathogenic factor in the disease; relevant to the issue of oxidative stress in particular cell types is a recent study that showed that overexpression of another antioxidant defense, Nrf2, in astrocytes ameliorated disease in the FALS transgenic mouse (Vargas et al., 2008). If the protective properties of a dismutase-active MTSOD1 in a particular cell type outweigh its toxicity, a knockdown of the MTSOD1 in this cell type would be expected to actually accelerate disease. In fact, results from a recent report showed that knockdown of G37R in Schwann cells ameliorated FALS, suggesting that synthesis of G37R in Schwann cells is neuroprotective (Lobsiger et al., 2009). On the other hand, if the toxicity in a particular cell type of a dismutase-active MTSOD1, such as G37R, outweighed its neuroprotective properties, a knockdown would be expected to extend the onset and/or duration of disease compared to that seen in a mouse with expression of that same active MTSOD1 in all cell types. One might expect that the amount of disease amelioration seen with a knockdown of a dismutase-active mutant in the same cell type would be less than that seen with knockdown of dismutase inactive MTSOD1 such as G85R – since G85R does not provide anti-oxidant activity.
Materials and methods
Mice and breeding
A G85R genomic clone generously provided by Dr. David Borchelt was used to generate a G85Rflox SOD1 transgenic mouse, as described (Wang et al., 2009). This mouse was crossed with Lhx3:Cre and CD11b:Cre mice (a gift from Drs. G. Kassiotis and G. Kollias), both of which have been previously described (Boillee et al., 2006a; Peng et al., 2007; Sharma et al., 1998). The G85Rflox mouse was prepared on a C57BL/6J background. The Lhx3:Cre (Peng et al., 2007; Sharma et al., 1998) and CD11b:Cre mice (Boillee et al., 2006a) were maintained for more than 6 generations on a C57BL/6J background before use. The primers used for genotyping were: SOD1 forward, 5′-CATAACTTCGTATAGCATACATTAT-3′, SOD1 reverse, 5′-CTTCTGCTCGAAATTGATGATGCCCT-3′. CD11b forward, 5′-ATTACCGGTCGATGCAACGAGT-3′, CD11b reverse, 5′-CAGGTATCTCTGACCAGAGTCA-3′. WTSOD1 transgenic mice (Wang et al., 2008) were used as controls.
Assessment of disease phenotype
Clinical assessment
Mice were weighed every two days and clinically assessed as previously described (Boillee et al., 2006a): the onset of disease was defined as peak weight before a decline; early phase of disease was the period from peak weight until loss of 10% of maximal weight; late phase of disease was the time from 10% loss in weight until death (when a mouse was unable to right itself within 20 seconds after being put on its back). In experiments monitoring disease parameters and survival, G85Rflox/Lhx3:Cre and G85Rflox/Cd11b:Cre double transgenic mice were compared with their littermate G85Rflox single transgenic mice. The results were compared using a one-way ANOVA variance analysis with the Newman–Keuls multiple comparison test.
Pathology
The pathology of the spinal cord was evaluated as previously described (Wang et al., 2008; Wang et al., 2002). Mice were anesthetized and perfused with ice-cold PBS, followed by 4% paraformaldehyde (PFA) using a protocol approved by the University of Chicago Animal Resources Center. The spinal cord was removed and fixed; special attention was given to the appearance and number of MNs in sections of the anterior horn of the lumbar cord.
Immunohistochemical evaluation
The expression pattern of SOD1 was determined by immunohistochemical staining using a rabbit antibody that reacts against mouse and human SOD1 (Stressgen, Ann Arbor, MI), a rabbit antibody that specifically recognizes the carboxyl end of mouse and human SOD1, as well as a human-specific anti-SOD1 antibody (Deng et al., 2006). The Nissl staining and glial fibrillary acidic protein (GFAP) antibody staining have been described (Wang et al., 2008). Rabbit anti-Iba1 antibody (Wako Pure Chemical Industries, Osaka, Japan) was used for microglial cell staining.
Studies of human SOD1 expression
In order to determine whether Cre was able to excise the G85Rflox, we co-electroporated this plasmid with and without CMV:Cre. For electroporation (Ghadge et al., 2006): a small window of the egg was made, the plasmid DNA (1 mg/ml) was mixed with 1% Fast injected into the central canal of the chick embryo with a glass microinjection pipette, and electroporation was performed by passing four pulses of current from electrodes on each side of the embryo. The open window was then covered with tape, and the eggs returned to the incubator. .
In order to determine the amount of knockdown of human SOD1 in MNs following Cre excision, we performed laser capture microdissection followed by real-time reverse transcriptase-polymerase chain reaction (qPCR). The lumbar spinal cord was dissected and then immediately frozen into OCT compound for storage at −80°C until further processing. To preserve the integrity of the RNA, section cutting and staining were carried out just before laser capture microdissection (Leica Microsystems, Wetzlar, Germany). In brief, the lumbar spinal cords were sectioned at 7 μm, mounted on glass-foil (Leica), and then immediatedly Nissl stained. MNs were identified according to previously published criteria (Wang et al., 2002). Approximately 1200 MNs from the anterior horn of each mouse spinal cord were collected into 75 μl of RLT buffer (Qiagen RNeasy Mini Kit, QIAGEN, Valencia, CA), and RNA was isolated using an RNeasy Mini Kit (QIAGEN). The RNA from the MNs was treated with DNase I (Invitrogen, Carlsbad, CA) to eliminate genomic DNA contamination followed by first-strand cDNA synthesis and qPCR using a SuperScript III two-step qPCR kit with Sybr green (Invitrogen). qPCR was performed on an iCycler Thermal Cycler machine (Biorad, Hercules, CA) using the following protocol: 1 cycle 50°C for 2 min, 1 cycle 95°C for 2 min; 40 cycles 95°C for 15s, 60°C for 30s. Details were previously published regarding the sequence of the primers and probes for SOD1 (Boillee et al., 2006a) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Lin et al., 2006). For analysis of qPCR data, expression levels of hSOD1 were calculated relative to expression of GAPDH.
Results
The preparation and breeding of G85Rflox transgenic mice
A G85Rflox transgenic mouse was generated, as previously described (Wang et al., 2009). The G85Rflox transgenic mice developed progressive paralysis culminating in death. These G85Rflox transgenic mice were crossed with either Lhx3:Cre or CD11b:Cre mice to generate G85Rflox/Lhx3:Cre and G85Rflox/CD11b:Cre mice respectively. The Lhx3:Cre mice express Cre in spinal and hindbrain MNs and a population of interneurons in the spinal cord (Peng et al., 2007; Sharma et al., 1998), while CD11b:Cre mice have expression of Cre confined to microglia and activated macrophages (Boillee et al., 2006a).
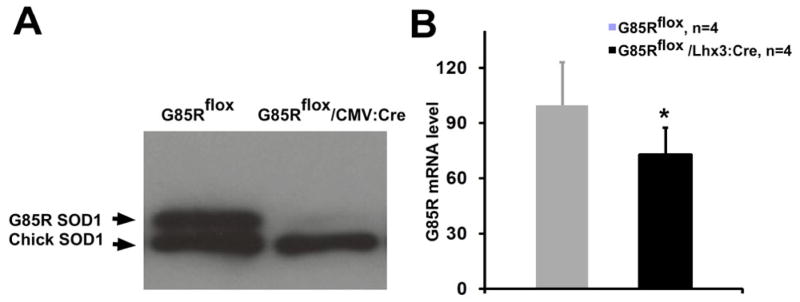
The ability of the G85Rflox transgene to be excised by Cre recombinase was initially demonstrated by comparing the expression of G85R in the chick embryo spinal cord following electroporation of G85Rflox genomic DNA with or without a CMV:Cre plasmid. A Western blot of the chick embryo spinal cord homogenate showed a prominent decrease in G85R expression when CMV:Cre was co-electroporated with G85Rflox DNA (Fig. 1A).
Figure 1.
Cre recombination of G85Rflox. A) Representative Western blot of chicken embryo spinal cord homogenates harvested one day after electroporation and immunostained with an anti-SOD1 antibody that reacts with both human and chick SOD1 (Stressgen). Co-electroporation of G85Rflox and CMV:Cre plasmids (left lane) led to a prominent decrease in G85R expression compared to electroporation of G85Rflox plasmid plus control vector alone (right lane). B) qPCR quantitation of human SOD1 mRNA. See Materials and Methods for details. G85R MTSOD1 mRNA levels in the spinal cord of G85Rflox/Lhx3:Cre mice (black) were decreased by ~25% compared to G85Rflox mice (gray shaded). The asterisk indicates P < 0.05.
Published studies involving Lhx3:Cre have shown that β–galactosidase activity is restricted to MNs of the spinal cord and hindbrain as well as V2 interneurons of the spinal cord in F1 mice progeny from a cross between Lhx3:Cre mice with a Cre-reporter mouse that activates expression of β–galactosidase following Cre-mediated DNA recombination (Peng et al., 2007; Sharma et al., 1998). In order to quantitate the degree of MTSOD1 knockdown in MNs of the G85Rflox/Lhx3:Cre mice, we performed qPCR on laser-dissected spinal cord MNs from 4 G85Rflox/Lhx3:Cre and 4 G85Rflox mice (Fig. 1B). We found a mean decrease of ~25% (Fig. 1B) in MTSOD1 mRNA in G85Rflox/Lhx3:Cre vs. G85Rflox mice.
The CD11b:Cre mice that were used for this study were identical to the G37Rflox transgenic mice used by Boillée et al. (Boillee et al., 2006a). The latter studies showed that expression of β–galactosidase was restricted to activated macrophages and microglia in the F1 progeny from a cross of the CD11b:Cre mouse with a Cre-reporter mouse that activates expression of β–galactosidase following Cre-mediated DNA recombination (Boillee et al., 2006a). This study also showed that crosses between the CD11b:Cre and G37Rflox mice led to a human SOD1 knockdown of ~50% in cultured peritoneal macrophages and ~25% in cultured microglia.
A knockdown of expression of G85R in MNs and interneurons in G85Rflox transgenic mice delays the onset and early phase of disease as well as the pathology and immunohistochemical abnormalities
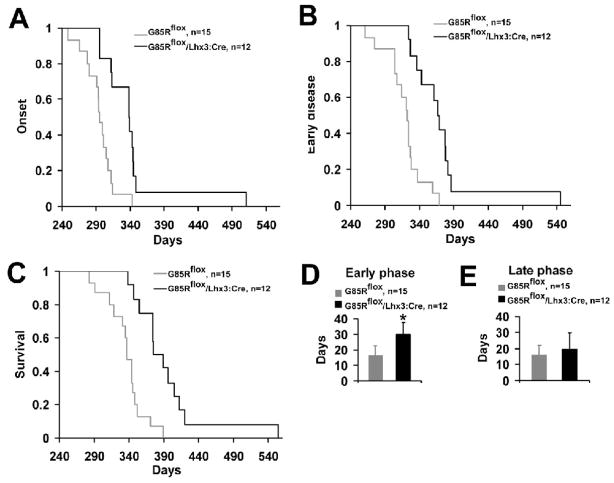
The clinical parameters of disease were compared between a group of 12 G85Rflox/Lhx3:Cre mice with a group of 15 G85Rflox mice. The mean onset of disease for G85Rflox/Lhx3:Cre mice was 342.7±56.4 days and significantly prolonged compared to the onset of 299.0±26.8 days for G85Rflox mice (P = 0.015) (Fig. 2A). In addition, there was a dramatic prolongation in the duration of early disease in G85Rflox/Lhx3:Cre vs. G85Rflox mice (P <0 .001) (Fig. 2B, 2D). As expected, survival of G85Rflox/Lhx3:Cre mice was significantly greater than G85Rflox mice (394.0±56.7 vs. 337.0±27.2 days, P = 0.002) (Fig. 2C). There was no significant difference in duration of the late disease, a time when clinical weakness was apparent, in the G85Rflox/Lhx3:Cre vs. the G85Rflox mice (20.0±9.7 vs. 16.0±5.9 days, P = 0.366) (Fig. 2E), indicating that the increased survival was a result of the delayed onset and prolonged early disease and not related to a change in the late disease.
Figure 2.
Plots of disease onset (A), end of early disease (B), and survival (C), and bar diagrams of the duration (with standard deviation) of the early phase (D) and late phase (E) of disease in G85Rflox/Lhx3:Cre vs. G85Rflox mice. In the Y axis of A–C, 1 refers to 100% of the number of animals, which is specified in the upper right. The asterisk indicates P < 0.001.
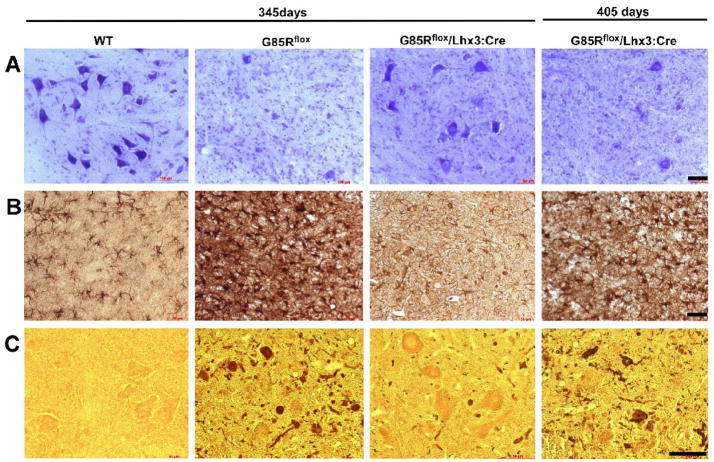
In order to compare the pathology in these mice, we examined the lumbar spinal cord anterior horn from non-transgenic, G85Rflox, and G85Rflox/Lhx3:Cre mice at 345 days and from G85Rflox/Lhx3:Cre mice at 405 days (Fig. 3). G85Rflox mice at 345 days demonstrated a significant loss of MNs by Nissl staining (Fig. 3, top row), vigorous astrocytosis by GFAP staining (Fig. 3, middle row), and abundant SOD1 aggregates (using rabbit antibody that recognizes the carboxyl end of SOD1) (Fig. 3, bottom row) in cells with MN morphology and in cell processes that was not apparent in control mice and not as prominent in G85Rflox/Lhx3:Cre mice (except at the 405 day time point). These results indicate that the pathological and immunohistochemical hallmarks of disease were apparent much earlier in G85Rflox mice than G85Rflox/Lhx3:Cre mice.
Figure 3.
Neuropathological and immunohistochemical studies of the anterior horn of the lumbar spinal cord. The first three columns show WTSOD1, G85Rflox, and G85Rflox/Lhx3:Cre mice at 345 days while the fourth column shows G85Rflox/Lhx3:Cre mice at 405 days with respect to Nissl staining (row A), GFAP immunoreactivity (row B), and SOD1 immunoreactivity (row C). The scale bar = 50μm
A knockdown of expression of G85R in microglia and activated macrophages of G85Rflox mice does not affect disease onset, but delays the early and late phase of disease
The mean onset of disease in the G85Rflox/CD11b:Cre mice (N = 9) was 322.0±54.6 days, while the mean onset in the G85Rflox mice (N = 15) was 295.0±22.2 days, which was not significant (P = 0.065) (Fig. 4A). There was, however, a significant prolongation of both the early (Fig. 4B, D) and late phase (Fig. 4E) of disease in the G85Rflox/CD11b:Cre (N = 9) vs. G85Rflox mice (N = 15) (early: 27.0±4.9 vs. 17.0±6.7, P = 0.005; late: 22.0±13.5 vs. 16.0±7.2, P = 0.03). As expected, when one adds the time of disease onset, early phase of disease, and late phase of disease, the survival of the G85Rflox/CD11b:Cre mice (N = 13) was significantly greater than G85Rflox mice (N = 15) (378.1±36.5 vs. 336.0±22.0, P = 0.002) (Fig. 4C).
Figure 4.
Plots of onset (A), end of early disease (B), and survival (C), and bar diagrams of the duration (with standard deviation) of the early phase (D) and late phase (E) of disease in G85Rflox/CD11b:Cre vs. G85Rflox mice. In the Y axis of A–C, 1 refers to 100% of the number of animals. Please note the number of mice used in the survival is greater than the numbers used to plot onset and end of early disease. The asterisk in (D) indicates P < 0.01 and in (E) indicates P < 0.05.
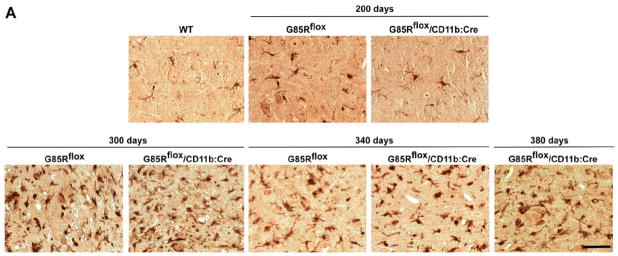
Pathological and immunohistochemical studies showed that microgliosis was not seen in G85Rflox or G85Rflox/CD11b:Cre mice at 200 days, but was prominent at 300 days in both mice, and continued throughout the disease (Fig. 5A). There was a similar amount of astrocytosis and SOD1-immunoreactive aggregation (using rabbit antibody that recognizes the carboxyl end of SOD1) in G85Rflox mice at 340 days and in G85Rflox/CD11b:Cre mice at 340 days and 380 days (Fig. 5B).
Figure 5.
Immunohistochemical studies of the anterior horn of the lumbar spinal cord. (A) Iba1 immunostaining of G85Rflox and G85Rflox/CD11b:Cre mice at 200, 300 and 340 days, and also G85Rflox/CD11b:Cre mice at 380 days; a WTSOD1 transgenic mouse is shown as a control. (B) The three columns of the upper panel show WTSOD1, G85Rflox, and G85Rflox/CD11b:Cre mice at 200 days, while the three columns of the lower panel show G85Rflox and G85Rflox/CD11b:Cre mice at 340 days and G85Rflox/CD11b:Cre mice at 380 days with respect to GFAP (top row) and SOD1 immunoreactivity (bottom row). The scale bar =50 μm
Discussion
Although MTSOD1-induced FALS makes up only ~2% of the total number of ALS cases, it has provided a model system to explore the pathogenesis of this disease. There is convincing evidence that non-cell autonomous degeneration is important in the pathogenesis of MTSOD1-induced FALS, and that non-MN cell types make contribute to FALS pathogenesis. Although non-MN cell types play this important role, other reports show that transgenic mice with MTSOD1 expression restricted to neurons can develop MN degeneration and/or MND (Jaarsma et al., 2008; Wang et al., 2008). The recent demonstration of an abnormal accumulation of TAR DNA-binding protein (TDP-43) in non-MNs as well as MNs in sporadic ALS suggests that both sporadic ALS as well as FALS can be considered a disease involving many cell types (Geser et al., 2008). Non-cell autonomous degeneration is thought to underlie other neurodegenerative diseases.
A variety of studies have suggested that non-MN cell types make an important contribution to the pathogenesis of FALS (Clement et al., 2003; Beers et al., 2006; Di Giorgio et al., 2007; Nagai et al., 2007; Boillee et al., 2006a; Yamanaka et al., 2008). Data most relevant to the present study have come from studies of G37Rflox mice (Boillee et al., 2006a; Yamanaka et al., 2008). A variety of studies have suggested that non-MN cell types make an important contribution to the pathogenesis of FALS (Clement et al., 2003; Beers et al., 2006; Di Giorgio et al., 2007; Nagai et al., 2007; Boillee et al., 2006b; Yamanaka et al., 2008). . An important difference in the G85R flox mouse studies presented here from those concerning G37Rflox mice (Boillee et al., 2006a; Yamanaka et al., 2008) is that G85R has no dismutase activity while G37R has full activity (Borchelt et al., 1994). Although investigations have clearly shown that MTSOD1s with either no or full dismutase activity can lead to FALS, it still remained a possibility that activity of the mutant enzyme affected disease phenotype and the role of particular cell types in disease - for example, because oxidative stress or an aberrant enzymatic activity of the MTSOD1 contributed to the phenotype (Liochev and Fridovich, 2003). If FALS is influenced by a particular MTSOD1’s enzymatic activity, one would expect that knockdown in expression of the MTSOD1 from a cell type might affect disease variably depending on its dismutase activity. In fact, recent data suggest that the mutant’s dismutase activity in one particular cell type, Schwann cells, may actually be neuroprotective (Lobsiger et al., 2009); in this study, knockdown of G37R MTSOD1 in Schwann cells in G37Rflox/P0:Cre mice led to a significant acceleration of disease.
In the present study, G85R knockdown in MNs/interneurons of G85Rflox mice delayed the onset and prolonged the early phase of disease as well as survival of G85Rflox/Lhx3:Cre mice. These data are similar to those found by Boillée et al., in which knocking down G37R in MNs and other neurons primarily affected onset and early disease (Boillee et al., 2006a); a subsequent study in which G37R was crossed with VAChT:Cre mice, reported a delayed onset of disease with no change in disease duration (Yamanaka et al., 2008). The general similarity of these results occurred despite the use of different cell type-specific transcriptional control elements. The Lhx3:Cre mouse has Cre expression in MNs as well as interneurons (Peng et al., 2007; Sharma et al., 1998); of interest, a recent study of a FALS transgenic mouse reported prominent neuropathology in interneurons (in addition to MNs) (Martin et al., 2007). The Islet-1:Cre (Boillee et al., 2006a) has Cre expression in spinal cord MNs, other neuronal populations, and peripheral tissues, while the VAChT:Cre mice (Yamanaka et al., 2008) has Cre expression in post-natal MNs.
Knockdown of G85R in microglia/macrophages had no effect on onset (perhaps because microgliosis is minimal prior to disease onset), but increased disease duration (and survival), which is similar to what was found with G37Rflox/CD11b:Cre mice (Boillee et al., 2006a). The finding that MN as well as non-MN expression of G85R, like G37R, has a significant effect on disease in transgenic mice, demonstrates that non-cell autonomous degeneration occurs in disease caused by both dismutase active and inactive MTSOD1.
Although the effect of knockdown of the two different MTSOD1s in microglia/macrophages was similar in that they both only affected disease duration (perhaps because microgliosis is minimal prior to disease onset), G85R knockdown affected both the early and late phase of disease, while G37R knockdown only affected late disease - and to a much greater extent than seen with G85R knockdown: there was a statistically significant 59% increase in the duration of early disease in G85Rflox/CD11b:Cre vs. G85Rflox mice compared to no difference (~-3%) in early disease in G37Rflox/CD11b:Cre vs. G37Rflox mice; a 38% increase in late disease in G85Rflox/CD11b:Cre vs. G85Rflox mice that was much less than the ~2.5-fold increase in the duration of late disease in G37Rflox/CD11b:Cre vs. G37Rflox mice; a 43% increase in disease duration in G85Rflox/CD11b:Cre mice vs. G85Rflox mice compared to ~80% increase in disease duration of G37Rflox/CD11b:Cre vs. G37Rflox mice. The finding of a prolongation in early disease following G85R and not G37R knockdown is especially noteworthy considering that early disease was ~4 times longer in G37Rflox vs. G85Rflox mice.
One explanation for the differing results with respect to early and late disease following G37R vs. G85R knockdown in microglia may be related to the MTSOD1’s enzymatic activity. As noted above, recent data suggest that a dismutase active mutant SOD1 may in fact have a neuroprotective effect in at least one cell type, the Schwann cell (Lobsiger et al., 2009); in this case, knockdown of dismutase-active G37R in Schwann cells in G37Rflox/P0:Cre mice led to a significant acceleration in disease duration. In the present study, we found that both G37R and G85R expression in microglia were pathogenic since knockdown of either led to a prolongation in disease duration. Of special interest was the remarkable difference in the effect of the two MTSOD1s on early disease, i.e., there was a 59% increase in early disease in the G85Rflox/CD11b:Cre vs. G85Rflox mice, and no difference in early disease in G37Rflox/CD11b:Cre vs. G37Rflox mice. One interpretation of these results is that the dismutase-active G37R has both a neuroprotective as well as neurotoxic effect early in disease while the dismutase-inactive G85R only has a neurotoxic effect - perhaps because part of the toxicity during the early phase of disease is related to superoxide production by the microglia (that is checked by the dismutase activity of G37R). This presumed mixed effect (i.e., both a neurotoxic and neuroprotective effect) in microglia from dismutase-active G37R expression contrasts with a purely neurotoxic effect of dismutase-inactive G85R MTSOD1 – and may explain our finding that the knockdown of G37R in microglia/macrophages has a less significant ameliorative effect on early disease than G85R knockdown. With respect to late disease, it may be that there is less amelioration in disease caused by the knockdown in G85R expression in microglia compared to the G37R knockdown, perhaps because G85Rflox mice die from such an extremely short duration of late disease that there is insufficient time for a more prominent effect to be observed from the knockdown. In other words, the very progressive symptomatic late disease phase that rapidly leads to death caused by G85R is more difficult to slow down than one that is more slowly progressive and of longer duration, as in the case of G37R.
There are other interpretations as to why the present results using G85R mice may have showed differences in the duration of early disease following knockdown in microglia/macrophages compared to results from the published investigation involving the G37R knockdown (Boillee et al., 2006a). A shorter disease duration of human FALS has been reported to be associated with increased instability of a MTSOD1 (Sato et al., 2005). Therefore, the more ameliorative effect on early disease duration that occurred following knockdown of G85R vs. G37R in microglia/macrophages could be related to the fact that G85R is more unstable than G37R (Fukada et al., 2001); however, it may be that part of the reason that enhanced MTSOD1 stability is more likely to lead to a longer disease duration than that seen with an unstable MTSOD1 is related to the dismutase activity of the stable MTSOD1s. Another possible explanation for the difference in results seen with the knockdown of G85R vs. G37R could relate to the aggregation potential of the mutants.
If the difference in effect of G85R vs. G37R knockdown in microglia is in fact related to the dismutase activity of the MTSOD1 in microglia, then a gain of SOD1 dismutase function (as well as a toxicity and loss of function of MTSOD1) can affect disease. Both a gain as well as a loss of function have been implicated in the toxicity of mutant proteins associated with a number of other neurodegenerative diseases including another MND, bulbospinal muscular atrophy (Thomas et al., 2006). Our results suggest that a treatment of FALS may vary in effectiveness depending on the particular MTSOD1 that induces disease and the time when treatment is administered.
Acknowledgments
This work was supported by National Institutes of Health [NS049333-01 to RPR], ALS Association Foundation [#1211 to RPR], and Les Turner ALS Foundation [to RPR]. We thank Drs. George Kollias and George Kassiotis of the Alexander Fleming Biomedical Sciences Research Centre for the use of the CD11b:Cre mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006a;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006b;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994;91:8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O’Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada K, Nagano S, Satoh M, Tohyama C, Nakanishi T, Shimizu A, Yanagihara T, Sakoda S. Stabilization of mutant Cu/Zn superoxide dismutase (SOD1) protein by coexpressed wild SOD1 protein accelerates the disease progression in familial amyotrophic lateral sclerosis mice. Eur J Neurosci. 2001;14:2032–2036. doi: 10.1046/j.0953-816x.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- Ghadge GD, Wang L, Sharma K, Monti AL, Bindokas V, Stevens FJ, Roos RP. Truncated wild-type SOD1 and FALS-linked mutant SOD1 cause neural cell death in the chick embryo spinal cord. Neurobiol Dis. 2006;21:194–205. doi: 10.1016/j.nbd.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28:2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Mutant Cu,Zn superoxide dismutases and familial amyotrophic lateral sclerosis: evaluation of oxidative hypotheses. Free Radic Biol Med. 2003;34:1383–1389. doi: 10.1016/s0891-5849(03)00153-9. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Boillee S, McAlonis-Downes M, Khan AM, Feltri ML, Yamanaka K, Cleveland DW. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci U S A. 2009;106:4465–4470. doi: 10.1073/pnas.0813339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CY, Yajima H, Burns CE, Zon LI, Sisodia SS, Pfaff SL, Sharma K. Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron. 2007;53:813–827. doi: 10.1016/j.neuron.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Nakanishi T, Yamamoto Y, Andersen PM, Ogawa Y, Fukada K, Zhou Z, Aoike F, Sugai F, Nagano S, Hirata S, Ogawa M, Nakano R, Ohi T, Kato T, Nakagawa M, Hamasaki T, Shimizu A, Sakoda S. Rapid disease progression correlates with instability of mutant SOD1 in familial ALS. Neurology. 2005;65:1954–1957. doi: 10.1212/01.wnl.0000188760.53922.05. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Thomas PS, Jr, Fraley GS, Damian V, Woodke LB, Zapata F, Sopher BL, Plymate SR, La Spada AR. Loss of endogenous androgen receptor protein accelerates motor neuron degeneration and accentuates androgen insensitivity in a mouse model of X-linked spinal and bulbar muscular atrophy. Hum Mol Genet. 2006;15:2225–2238. doi: 10.1093/hmg/ddl148. [DOI] [PubMed] [Google Scholar]
- Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sharma K, Deng HX, Siddique T, Grisotti G, Liu E, Roos RP. Restricted expression of mutant SOD1 in spinal motor neurons and interneurons induces motor neuron pathology. Neurobiol Dis. 2008;29:400–408. doi: 10.1016/j.nbd.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Wild-type SOD1 overexpression accelerates disease onset of a G85R SOD1 mouse. Hum Mol Genet. 2009;18:1642–1651. doi: 10.1093/hmg/ddp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]