Abstract
3,3′-Diindolylmethane (DIM) is a potential chemopreventive phytochemical derived from Brassica vegetables. In this study we characterized the effect of DIM on cell cycle regulation in both androgen dependent LNCaP and androgen receptor negative-p53 mutant DU145 human prostate cancer cells. DIM had an antiproliferative effect on both LNCaP and DU145 cells, as it significantly inhibited [3H]-thymidine incorporation. FACS analysis revealed a DIM mediated G1 cell cycle arrest. DIM strongly inhibited the expression of cdk2 and cdk4 protein and increased expression of the cell cycle inhibitor p27Kip1 protein in LNCaP and DU145 cells. Promoter deletion studies with p27Kip1 reporter gene constructs showed that this DIM-mediated increase in p27Kip1 was dependent on the Sp1 transcription factor. Moreover, using a dominant negative inhibitor of p38 MAPK, we showed that the induction of p27Kip1 and subsequent G1 arrest by DIM involves activation of the p38 MAPK pathway in the DU145 cells. Taken together, our results indicate that DIM is able to stop the cell cycle progression of human prostate cancer cells regardless of their androgen-dependence and p53 status, by differentially modulating cell cycle regulatory pathways. The Sp1 and p38 MAPK pathways mediate the DIM cell cycle regulatory effect in DU145 cells.
Keywords: 3, 3′-Diinolylmethane, Prostate cancer, Cell cycle arrest, p27Kip1, p38 MAPK, Cancer
1. Introduction
Prostate cancer is the second most frequently diagnosed cancer in men of the western world and is the leading cause of cancer mortality in older men [1]. Since early stage prostate cancers are androgen dependent for their growth and survival, surgical or chemical castration have been used as the main therapeutic approach against the disease [2]. Initial responsiveness of the tumor to androgen ablation therapy inevitably gives way to an androgen independent relapse that ultimately leads to patient mortality. Presently there is no generally effective treatment for androgen independent prostate cancer, underscoring the need for the development of novel alternative therapeutic strategies. Dietary and plant-based drugs have been suggested as possible alternative strategies in anti-cancer treatment. Among various groups of cancer chemotherapeutic agents, extensive experimental data have been generated suggesting a role for dietary indoles in the treatment of various cancers including prostate cancer.
3,3′-Diindolylmethane (DIM), the major in vivo product derived from the acid-catalyzed condensation of indole-3-carbinol (I3C), is a promising antitumor agent derived from Brassica vegetables. Several studies have indicated the pre-clinical efficacy of DIM against various epithelial cancers, including endometrial and mammary tumors [3, 4], and the pre-clinical efficacy of DIM against prostate cancer is currently under investigation. I3C and DIM are currently among the most popular adjunct therapies for recurrent respiratory papillomatosis (RRP) because of their effectiveness and low level of toxicity [5, 6]. The pronounced anticancer activity of DIM in rodents and humans has generated considerable interest in the modes of action of this indole. Since loss of cell cycle regulation has been implicated in tumor proliferation, it is possible that the inhibition of tumor growth by DIM could be partly due to modulation of the cell cycle.
Cellular proliferation is driven by the periodic association of cyclin dependent kinases (cdks) with their cyclin partners and controlled by kinase inhibitors. Progression from a quiescent G0/G1 phase to S phase is controlled by cyclin D/cdk4/6 and cyclin E/cdk2 mediated phosphorylation of pRb, subsequent release of E2F1 and transcription of early S phase genes [7]. Reduced levels of p27 Kip1 (p27), an inhibitor of cdk2, and increased levels of cdk2 and cyclin E are indicators of androgen independence and are associated with poor prognosis [8–10]. Several studies in our laboratories indicate the G1 phase as a target for dietary indole mediated anti-proliferative effects. Both DIM and I3C, have been shown to induce a G1 arrest in human breast cancer cells independent of estrogen receptor status [11, 12]. A G1 arrest was induced by I3C in androgen dependent LNCaP prostate tumor cells, while DIM exhibited androgen antagonist activity in these cells [13, 14].
We report here an investigation of DIM effects in androgen receptor (AR) positive, p53 wild type LNCaP cells and AR negative, p53 mutant DU145 cells. Both cell lines exhibited growth inhibition in response to DIM and the effects of DIM on cell cycle events were determined. Growth inhibition by DIM was accompanied by an arrest in the G1 phase of the cell cycle, a reduction in pRb phosphorylation, a decrease in cdk2 and cdk4 levels and an increase in p27 levels in both cell lines, regardless of their AR and p53 status. DIM treatment of LNCaP cells resulted in decreased cyclin E protein levels and an inhibition in cdk2 transcription, results not observed in DU145 cells. Treatment of DU145 cells with DIM resulted in an increase in p38 mitogen activated protein kinase (MAPK) activation, and the DIM mediated induction of p27 was reversed by inhibition of p38 MAPK, implicating this pathway in the DIM mediated G1 arrest. These investigations provide the first evidence that DIM treatment activates the p38 MAPK pathway leading to a G1 arrest in AR negative prostate cancer cells.
2. Materials and methods
2.1. Materials
All laboratory chemicals and SB202190 were purchased from Sigma-Aldrich (St. Louis, MO). DIM was obtained from LKT Laboratory Inc. (St. Paul, MN). [3H]-thymidine and [γ-32P]-ATP, and ECL reagents were purchased from Perkin-Elmer (Boston, MA). Antibodies to cdk2, cdk4, cdk6, cyclin D1, cyclin E, p21Cip1, secondary antibodies, and recombinant Rb protein were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to p27Kip1 were from Biocare Medical (Concord, CA). Anti-phospho-Rb-S807/S811, anti-phospho-p38-Thr180/Tyr182, and anti-p38 MAPK, were from Cell Signaling (Beverly, MA).
2.2. Cell culture
Human prostate carcinoma cell lines LNCaP and DU145 were obtained from American Type Culture Collection (Manassas, VA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco (Gaithersburg, MD). Fetal bovine serum (FBS) was supplied by Omega Scientific Inc. (Tarzana, CA). The LNCaP and DU145 cell lines were maintained as previously described [14]. Cultures used in subsequent experiments were at less than 40 passages.
2.3. Plasmid reporters and expression vectors
The p27 promoter-luciferase reporter constructs p27PF, p27No. 2, and p27No. 12 [15] were a kind gift from Dr. Toshiyuki Sakai. Cells were collected, transferred to a cuvette, and electroporated with a Bio-Rad Laboratories (Hercules, CA) gene-pulser using 3μg of plasmid. After electroporation, the cells were resuspended in media and plated at 1 ml/dish in 12-well multiplates. The cells were treated with DIM for 24 h. Cells were harvested, and luciferase activity was determined as previously described [16]. The dominant negative p38 MAPK was a generous gift from Dr. Roger J. Davis. Cells were transfected with the dominant negative p38 MAPK as described previously [17].
2.4. Cell growth assay
Cell growth assay was performed as previously described [14]. LNCaP and DU145 cells were plated in 6-well plates and after 48 h for LNCaP cells and 24 h for DU145 cells, fed with fresh medium and treated with 0, 10, 30, or 50 μM DIM dissolved in DMSO for 24, 48, and 72 h. Cells were harvested using trypsin and resuspended in culture medium. Aliquots were diluted 50 fold in Isoton® II (Coulter Corp. Miami, FL) and 500 μL duplicates were counted in a Z1™ series Coulter Counter® and averaged.
2.5. Thymidine incorporation
Thymidine incorporation assay was performed as previously described [14]. Briefly, cells were plated in 24-well plates and treated with varying concentrations of DIM for 24 h. At the end of the treatment, 3 μCi of [3H]-thymidine was added to each well and allowed to incubate at 37°C for 3 h. Medium was removed and cells were washed three times with ice-cold 10% trichloroacetic acid, 500 μL of 0.3 N NaOH was then added to each well. The lysate was allowed to incubate for 1 h at room temperature. Aliquots (250 μL) were then transferred into scintillation vials and the amount of [3H]-thymidine incorporated into DNA was determined by scintillation counting.
2.6. Cell cycle analysis by flow cytometry
Prostate cancer cells were plated at 105 cells/well in 6-well plates and treated with 0, 10, 30, or 50 μM DIM in complete medium. Following treatment, cells were washed with phosphate-buffered saline and hypotonically lysed in 0.5 ml of DNA staining solution (0.5 mg/ml propidium, 0.1% sodium citrate, 0.05% Triton X-100). Nuclear-emitted fluorescence with wavelengths of >585 nm was measured with a Coulter® EPICS® XL™ flow cytometer. Ten thousand nuclei were analyzed from each sample at a rate of 300–500 nuclei/s. The percentages of cells within the G1, S, and G2/M phases of the cell cycle were determined by analysis with the Multicycle software MPLUS (Phoenix Flow Systems) in the Cancer Research Laboratory Microchemical Facility of the University of California, Berkeley.
2.7. Western blotting
After the indicated treatments, cells were harvested in lysis buffer (250 mM NaCl, 0.1% Triton X-100, 50 mM Tris/HCl, pH 7.3) containing protease and phosphatase inhibitors (50 μg/ml PMSF, 10 μg/ml aprotinin, 5 μg/ml leupeptin, 0.1 μg/ml NaF, 1 mM dithiothreitol (DTT), 0.1 mM sodium orthovanadate and 0.1 mM -glycerophosphate). Equal amounts of total cellular protein were mixed with loading buffer (25% glycerol, 0.075% SDS, 1.25 ml of 14.4 M 2-mercaptoethanol, 10% bromphenol blue, 3.13% stacking gel buffer) and fractionated by electrophoresis on 15% polyacrylamide, 0.1% SDS resolving gels. Rainbow marker purchased from Amersham (Piscataway, NJ) was used as the molecular weight standard. Proteins were electrically transferred to Immobilon-P membranes (Millipore, Billerica, MA) and blocked with 5% non-fat dry milk in 1X Western wash buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.05% Tween 20). Blots were subsequently incubated with antibodies against cdk2, cdk4, cdk6, cyclin D1, cyclin E, p21Cip1, and p27Kip1, followed by appropriate peroxidase –conjugated secondary antibody. Blots were visualized using ECL™ reagents from GE Healthcare (Piscataway, NJ), and fluorescence was detected using BioMax MR film from Kodak (Rochester, NY). Equal protein loading was determined by Ponceau S staining of blotted membranes, and reprobing of the membranes with anti-tubulin antibody.
2.8. Kinase assay
Prostate cancer cells were treated and lysed as described above and 500 μg of protein lysate was pre-cleared with protein A/G-plus agarose beads (Santa Cruz Biotechnology). cdk2 protein was immunoprecipitated using anti-cdk2 antibodies (5 μg) and protein A/G-plus agarose beads. Beads were washed three times with lysis buffer and once with kinase assay buffer (50 mM HEPES, 10 mM MgCl2, 5 mM MnCl2, 0.1 μg/mL NaF, 10 μg/mL betaglycerol phosphate, and 0.1 mM sodium orthovanadate). One-half of the immunoprecipitated sample was checked by Western blot analysis to confirm the IP and to compare the protein loading of each sample. The enzymatic activity of immunoprecipitated cdk2 was determined as previously described [11].
2.9. Real-Time Quantitative Reverse Transcription –PCR Analysis
Following treatment, total RNA was isolated according to the manufacturer’s protocol using the Aurum Total RNA Mini Kit from Bio-Rad. Reverse transcription was performed with 1 μg of total RNA using the iScript™ cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using SYBR Green Supermix with an iCycler® thermal cycler (Bio-Rad). Primers used to amplify p27 were: forward, 5-TTCTTTTCACTTCGGGCTGT3–3, and reverse, 5-CACAAAACATGCCACTTTGG-3. The data were collected and analyzed using the comparative Ct (threshold cycle) method using β-actin as the reference gene.
2.10. Statistical Analysis
Unless indicated differently, the results are presented as means ± S.D. of at least three independent experiments. They were analyzed using the two-sided Student’s t-test (*P ≤ 0.05)
3. RESULTS
3.1. DIM inhibits proliferation of LNCaP and DU145 human prostate carcinoma cells
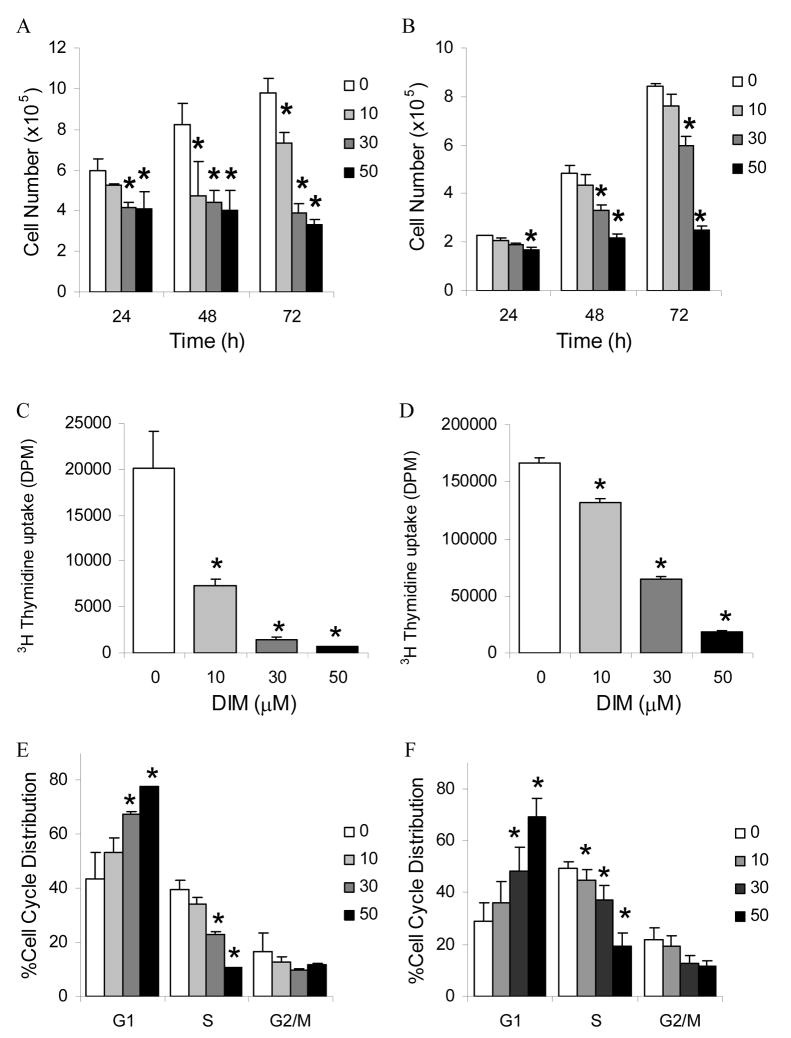
To assess the effects of DIM on cell growth, AR positive, p53 wildtype LNCaP cells were treated with 10, 30, and 50 μM concentrations of DIM for 24, 48, and 72 h. Treatment with DIM significantly inhibited the proliferation of the LNCaP human prostate carcinoma cell line in concentration- and time-dependent manners. Untreated LNCaP cells increased in cell number from 5.98×105 cells at 24 h to 8.26×105 cells at 48 h and 9.81×105 cells at 72 h (Fig. 1A). The number of LNCaP cells decreased following treatment with 10 μM DIM to 5.24×105 cells following 24 hours, and was 4.72×105 cells following 48 h, and 7.33×105 cells after 72 h. Treatment with 10 μM DIM resulted in a corresponding 12% inhibition of cell proliferation following 24 h, a 43% inhibition following 48 h, and a 25% inhibition following 72 h compared to untreated control. Treatment of LNCaP cells with 30 μM DIM resulted in 4.14×105 cells at 24 h, 4.41×105 cells at 48 h and 3.86×105 cells following 72 h. These numbers correspond to a 31% inhibition of cell growth following 24 h, a 50% inhibition of cell growth at 48 h, and a 61% inhibition of cell growth at 72 h. Finally, following 50 μM DIM treatment, the number of LNCaP cells was 4.08×105 at 24 h, 4.00×105 at 48 h, and 3.31×105 at 72 h. These numbers correspond to a 32% inhibition of cell proliferation following 24 h, a 52% inhibition at 48 h, and a 66% inhibition at 72 h compared to untreated cells.
Fig. 1. Inhibition of prostate cancer cell proliferation and cell cycle by DIM.
LNCaP (A) and DU145 (B) cells were treated with 0, 10, 30, or 50 μM DIM for 24, 48 and 72 h. Cell numbers were counted Values were expressed as mean ± SD. LNCaP (C) and DU145 (D) cells were treated as indicated for 24 h. DNA synthesis was determined by measuring thymidine uptake. Values were expressed as mean ± SEM. LNCaP (E) and DU145 (F) were treated with 0, 10, 30, or 50 μM DIM for 24 h. Cells were stained with propidium iodide. Flow cytometric analysis was performed for cell cycle distribution. Values were expressed as mean ± SD. Asterisks denote a significant difference compared with control at a level of P≤ 0.05.
Similarly, the effects of DIM on cell growth of AR negative, p53 mutant DU145 cells were assessed by treatment with 10, 30, and 50 μM concentrations of DIM for 24, 48, and 72 h. Untreated DU145 cells increased in cell number from 2.26×105 cells at 24 h to 4.82×105 cells at 48 h and 8.40×105 cells at 72 h (Fig. 1B). The number of DU145 cells decreased following treatment with 10 μM DIM to 2.07×105 cells following 24 hours, and was 4.33×105 cells following 48 h, and 7.59×105 cells after 72 h. Treatment with 10 μM DIM resulted in a corresponding 8%, 10% and 10% inhibition of cell proliferation following 24, 48, and 72 h of treatment, respectively. Similarly, treatment of DU145 cells with 30 μM DIM resulted in 1.89×105 cells at 24 h, 3.33×105 cells at 48 h and 5.95×105 cells following 72 h. These numbers correspond to a 16%, 31%, and 29% inhibition of cell growth following 24, 48, and 72 h respectivley. Following 50 μM DIM treatment, the number of DU145 cells was only 1.70×105 at 24 h, 2.17×105 at 48 h, and 2.49×105 at 72 h, corresponding to a 25%, 55%, and 70% inhibition at 24, 48, and 72 h respectively. Inhibition of DU145 cell proliferation by DIM was significantly different from control after only 24 h of treatment with 50 μM DIM. Treatment of DU145 cells with 30 μM DIM resulted in a significant decrease in proliferation after 48 h of exposure. This effect on proliferation remained significant after 72 h treatment with 30 μM DIM. The drug concentrations used in the present study were within the range used previously to document cellular effects of DIM [4, 11, 18, 19]; 10 μM DIM is within the range of levels achievable through dietary intake [14]. In both cell lines proliferation was significantly inhibited following 24 h treatment with 50 μM DIM. These results indicate a growth inhibitory effect of DIM in human prostate carcinoma cells irrespective of their AR or p53 status.
3.2. DIM inhibits DNA synthesis in LNCaP and DU145 cells
To further characterize the growth inhibitory effects of DIM in human prostate carcinoma cells, the effect of DIM on DNA synthesis was determined by measuring thymidine uptake. DIM treatment showed a strong concentration-dependent inhibitory effect on thymidine uptake in both the LNCaP and DU145 cells. Treatment of LNCaP cells with 10 μM DIM for 24 h resulted in a 64% inhibition of DNA synthesis, 30 μM DIM led to a 93% inhibition, and 50 μM DIM led to a 96% inhibition of DNA synthesis (Fig. 1C). DNA synthesis in DU145 cells was inhibited by 21% after 24 h treatment with 10 μM DIM, treatment with 30 μM DIM led to a 61% inhibition of DNA synthesis and 50 μM DIM resulted in an 89% inhibition (Fig. 1D). All concentrations used significantly inhibited DNA synthesis in both LNCaP and DU145 cells.
3.3. DIM induces a strong G1 arrest in prostate cancer cells
Based on the growth and DNA synthesis inhibitory responses of DIM in LNCaP cells, we next examined its effect on cell cycle progression. As shown in Fig. 1E, DIM induced a G1 arrest in LNCaP cells. DIM treatment for 24 h resulted in accumulation of 54–78% of cells in G1 phase compared with control showing 44%. The observed increase in G1 cell population was accompanied by a decrease in the number of cells in both S phase as well as G2-M phase. Treatment of cells with DIM decreased proportion of S phase cells from 40% to 11%. The percentage of cells in G2-M phase decreased from 17% to 10% upon treatment with DIM.
Similar DIM treatments were administered to DU145 cells and cell cycle distribution analysis was performed to compare with the effects observed in LNCaP cells. A similar trend inG1 arrest was demonstrated, with a DIM concentration-dependent effect at 24 h treatment of DU145 cells as observed in LNCaP cells (Fig. 1F). DIM treatment resulted in an accumulation of 36–70% of cells in G1 phase compared to 29% in controls. The percentage of DU145 cells in S phase decreased from 49% in controls to 19% in 50 μM DIM treated cells. The proportion of cells in G2-M phase decreased from 22% in control to 12% in DIM treated cells. Thus, DIM-mediated growth inhibition of both LNCaP and DU145 cells correlated with G1 phase cell cycle arrest.
3.4. Effect of DIM on cell cycle regulatory molecules in LNCaP and DU145 cells
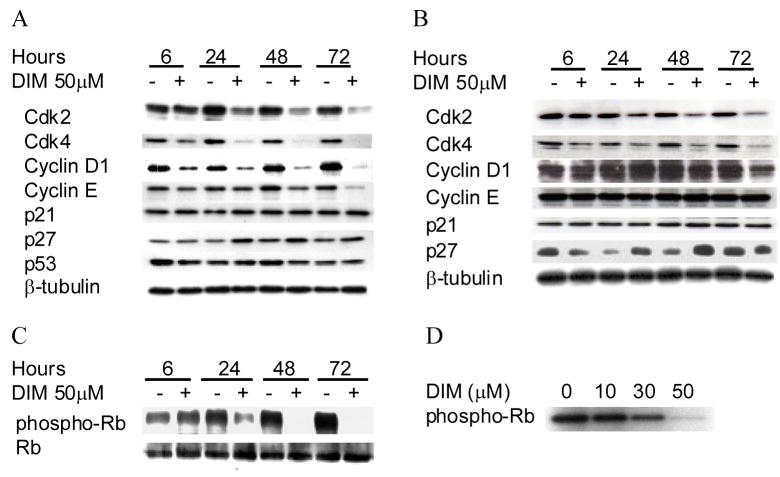
Based on the observation that DIM induces a G1 arrest in LNCaP and DU145 cells, we assessed by Western blot analysis the effect of DIM on cell cycle regulatory molecules that play important roles in G1-S cell cycle progression. As shown in Fig. 2A, DIM treatment induced a decrease in the protein level of cdk2 in AR positive-p53 wildtype LNCaP cells that was clearly visible after 24 h of treatment. The DIM effect was maintained after 72 h of treatment. The DIM-treated LNCaP cells also exhibited a decrease in protein levels of cdk4 and cyclin D1 that were visible after only 6 h of treatment. DIM also strongly inhibited cyclin E after 72 h in LNCaP cells. The DIM mediated downregulation of cdk2 and cdk4 was also observed in DU145 cells (Fig. 2B), but no cyclin inhibition was visible in DU145 cells. These results indicated that DIM-mediated cell cycle arrest in LNCaP and DU145 cells is associated with a decrease in protein levels of cell cycle regulatory molecules involved in cell cycle progression. However, cell cycle proteins are differentially regulated in AR negative-p53 mutant DU145 cells, as cyclin E down regulation is absent; while both cell types are arrested in G1.
Fig. 2. Effect of DIM on G1 cell cycle regulators in human prostate cancer cells.
Cells were treated with 50 μM DIM for indicated times. LNCaP (A) and DU145 (B) cells were collected, total cell ysates prepared and subjected to SDS-PAGE followed by western immunoblotting. Membranes were probed with indicated antibodies and visualized by ECL detection system. LNCaP (C) cells were treated for indicated times with 50 μM DIM, cells were collected and lysates prepared. Western blotting was performed using an antibody specific to pRb phosphorylated at Thr180/182 or an antibody specific to total pRb protein. LNCaP (D) cells were treated with indicated concentrations of DIM for 24 h and immunoprecipitated cdk2 phosphorylation of pRb was measured ex vivo by kinase assay. Results are representative of data collected from at least three experiments.
Because of the pronounced effect of DIM on cdk2 and cdk4 protein expression in LNCaP cells and DU145 cells, we raised the question of whether DIM treatment affected the phosphorylation of their in vivo substrate, pRb. Since DU145 cells do not express pRb, these investigations were only carried out in LNCaP cells using an antibody specific to pRb phosphorylated at Thr180/182. Consistent with cdk2 and cdk4 downregulation, DIM strongly inhibited pRb phosphorylation beginning at 24 h; pRb phosphorylation was absent at 48 and 72 h following treatment (Fig. 2C). Thus DIM treatment led to a time dependent decrease in pRb phosphorylation in LNCaP cells. To functionally test whether DIM had an effect on cdk2, we examined cdk2-mediated phosphorylation of exogenous pRB ex vivo. pRb phosphorylation by immunoprecipitated cyclin E/cdk2 complexes from LNCaP cells was strongly inhibited by DIM in a concentration dependent manner (Fig. 2D). This result is consistent with the large decrease of cdk2 protein observed in LNCaP cells after DIM treatment.
3.5. DIM increases p27 protein expression
The cdk inhibitor p27 plays an important role in the regulation of G1-S transition by binding to and inhibiting kinase activity of cyclin E/cdk2, thus preventing entry of cells into S phase. To gain further insights into the mechanism of DIM-mediated G1 phase arrest, we determined its effect on p27 protein levels by immunoblotting. As shown in Fig. 2A, DIM treatment resulted in the increase of p27 protein level in LNCaP cells, which was clearly evident at the 24 h timepoint. p27 protein levels were also increased in DU145 cells, shown in Fig. 2B. This effect was evident at the 24 and 48 h time points in both cell lines, independently of their AR and p53 status. DIM had no effect on p21 protein levels in either cell line and there was no consistent change in p53 expression in LNCaP cells (Fig. 2A, B).
3.6. DIM increases p27mRNA
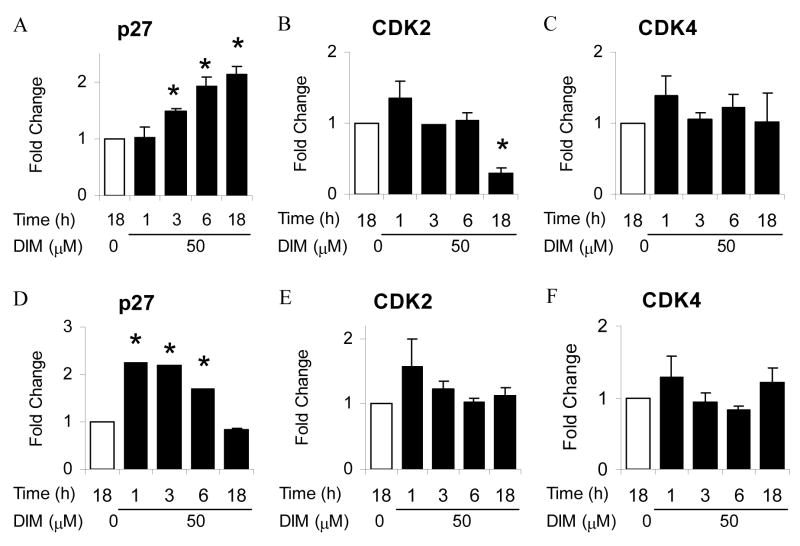
The observed induction of p27 protein and downregulation of cdk2 and cdk4 protein in LNCaP and DU145 cells led us to investigate early transcriptional regulation of these cell cycle regulators by DIM. Results presented in Fig. 3 show the effect of DIM treatment on p27, cdk2, and cdk4 mRNA levels in LNCaP (Fig. 3A, B, C), and DU145 (Fig. 3D, E F) cells. DIM treatment resulted in a significant 1.5-fold increase in p27 (Fig. 3A) mRNA in AR positive LNCaP cells following 3 h DIM treatment, and this induction increased to over 2-fold after 18 h. DIM treatment of AR negative DU145 cells resulted in a statistically significant 2-fold increase in p27 mRNA as rapidly as 1 h after treatment (Fig. 3D). This induction was maintained following 3 h exposure to DIM, followed by a progressive decrease to control levels by 18 h of exposure. These results indicate that the DIM-mediated induction of p27 protein is mediated by an increase in p27 gene transcription. Transcript levels of cdk2 were decreased significantly by 70% following 18 h of DIM treatment in LNCaP cells (Fig. 3B), whereas DIM did not affect transcript levels of cdk2 in AR negative, p53 mutant DU145 cells (Fig. 3E). Cdk4 transcription was not affected by DIM in either cell line after 18 h of treatment (Fig. 3C and 3F).
Fig. 3. Transcriptional regulation of cell cycle regulators by DIM.
LNCaP (A,B,C) and DU145 (D,E,F) cells were treated with 50 μM DIM for indicated times, harvested, RNA isolated and reverse transcription performed. Quantitative PCR was performed on RT samples using primers for indicated genes. Values were expressed as mean ± SD. Asterisk indicates significant difference compared with control at a level of P ≤ 0.05.
3.7. The DIM-induced increase in p27 expression is mediated by Sp1
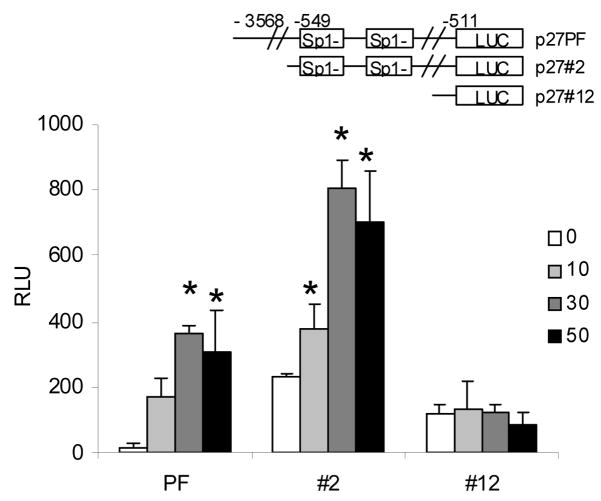
Since dietary indoles are known to influence transcription of G1 cell cycle mediators by modulating the activity of the Sp1 transcription factor in breast cancer cells [11, 20], we sought to determine whether Sp1 is involved in the DIM-mediated cell cycle regulation of prostate cancer cells. For these studies we used DU145 cells which exhibit a rapid and strong p27 induction following DIM treatment. The DU145 cells were transiently transfected with a series of progressive 5′ promoter deletion mutants of p27 as illustrated in Fig. 4. p27PF is a full length p27 promoter luciferase reporter, p27#2 is a deletion mutant of p27 promoter containing two Sp1 boxes. The p27#12 construct is a short deletion mutant of the p27 promoter depleted of the two Sp1 boxes. Luciferase activity was measured in control cells and cells treated with increasing concentrations of DIM for 24 h. DIM treatment of transfected DU145 cells resulted in a significant induction of p27 promoter luciferase activity in the full length and 549bp promoter fragment constructs. The deletion of two Sp1 promoter sites, however, completely ablated the DIM response. Although we can not exclude the possibility that other sequences in the p27 promoter are also required, these data suggest that the Sp1 sites may be essential for DIM-induced p27 induction in prostate cancer cells as it was reported for regulation of p21 in breast cancer cells [11].
Fig. 4. Activation of p27 promoter by DIM.
DU145 cells were transfected with the p27 promoter luciferase constructs illustrated. Cells were treated with 0, 10, 30, or 50 μM DIM for 24 hours, harvested, and luciferase assay performed. Values were expressed as mean ± SD. Asterisk indicates significant difference compared with control at a level of P ≤ 0.05.
3.8. DIM activates the p38 MAPK pathway in DU145 cells
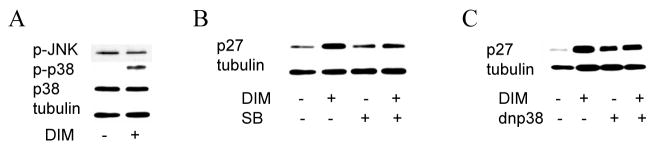
Because DIM treatment led to a robust p27 induction in DU145 cells and because p27 has been reported to be under the influence of p38 MAPK in androgen independent prostate cancer cells [21], we chose to pursue the mechanism of this induction in DU145 cells. Since DIM has been reported to activate the p38 MAPK pathway [22] and since the association of Sp1 with p38 MAPK has been reported to activate the p21 promoter [23], we hypothesized that the observed DIM-mediated induction of p27 may be mediated through p38 MAPK. As shown in Fig. 5A, we demonstrated that p38 MAPK phosphorylation increased following 24 h treatment of DU145 prostate cancer cells with 50 μM DIM, while the total expression levels of p38 MAPK were not affected, indicating an increase in p38 MAPK activation. To assess the specificity of p38 MAPK activation by DIM, JNK phosphorylation level was investigated as an example of another MAPK pathway. The level of hyperphosphorylated JNK was not changed by DIM treatment (Fig. 5A), emphasizing the specificity of DIM activity. The known selective pharmacological inhibitor of p38 MAPK, SB202190 [24], was able to reverse the induction of p27 by DIM (Fig. 5B), indicating that p38 MAPK may be a key target of DIM. The p27 up-regulation by DIM is lost when p38 MAPK is inhibited. To further characterize the involvement of p38 MAPK in the DIM regulation of the cell cycle, DU145 cells were transiently transfected with dominant negative p38 MAPK known to inhibit p38 MAPKactivity [17] (Fig. 5C). The result was a reversal of p27 induction by DIM as expected. Thus inhibition of p38 MAPK activity by two separate methods was sufficient to reverse the DIM mediated p27 induction in AR negative, p53 mutant DU145 cells.
Fig. 5. DIM activates p38 MAPK pathway in DU145 cells.
(A), DU145 cells were treated with or without 50 μM DIM for 24 hours, after which total lysates were prepared and Western blotting conducted. Membranes were probed with indicated antibodies and visualized by ECL detection system. (B), DU145 cells were treated with or without 50 μM DIM and the p38 MAPK inhibitor SB202190 (SB) and subjected to Western blot analysis as in A. (C), DU145 cells transfected with or without dominant negative p38 MAPK (dnp38), were treated with 50 μM DIM and subjected to Western blot analysis as in A.
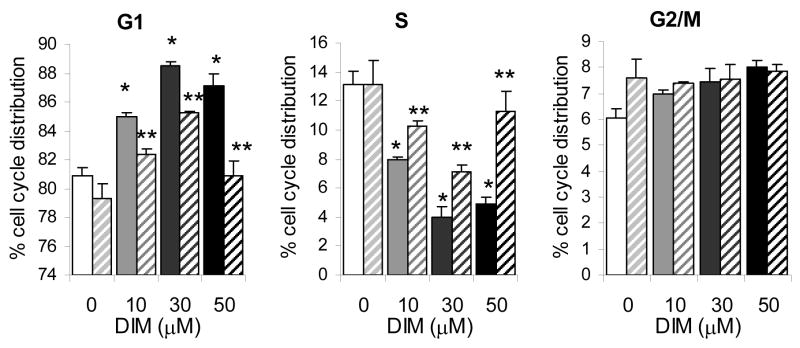
3.9. Inhibition of p38 MAPK activity reverses the DIM mediated G1 arrest in DU145 cells
These studies suggest that DIM treatment leads to a G1 arrest in both AR positive p53 wild-type LNCaP and AR negative p53 mutant DU145 human prostate cancer cells, and that DIM exerts this effect by a p38 MAPK-mediated induction of p27 in the DU145 cells. To confirm the latter, we further sought to determine if inhibition of p38 MAPK activity could lead to reversal of the DIM-mediated G1 arrest. As shown in Fig. 6, treatment of cells with DIM in combination with the p38 MAPK inhibitor SB202190 was able to reverse the effect of DIM on DU145 cell cycle distribution. The inhibitor alone had no significant effect on cell cycle distribution in control cells. Treatment of AR negative DU145 cells with 10 μM DIM resulted in an 85% accumulation of cells in G1; the proportion of cells in G1 decreased to 82% upon treatment with p38 MAPK inhibitor. 30 μM DIM treatment led to an accumulation of 88% of cells in G1, inhibitor reduced this proportion to 85%. Treatment of cells with 50 μM DIM resulted in 87% in G1 phase. Addition of SB202190 led to an accumulation of 80% of cells in G1. While addition of p38 MAPK inhibitor had no significant effect on proportion of cells in G1, in each case addition of inhibitor in combination with DIM resulted in a statistically significant reduction in proportion of cells in G1 compared to DIM treatment alone. 50 μM DIM treatment resulted in a reduction in S phase cell cycle distribution from 13% in controls to 5% in treated cells. The addition of p38 MAPK inhibitor restored S phase content cells to 11%. In each case the addition of SB202190 led to a statistically significant increase in S phase distribution compared to DIM treatment alone. These results indicate that the observed DIM-mediated G1 arrest in AR negative p53 mutant DU145 cells may be a direct result of p38 MAPK activation.
Fig. 6. The DIM-mediated cell cycle arrest of DU145 is p38 MAPK dependent.
DU145 cells were treated with DIM in the absence (solid bars) and presence (dashed bars) of the p38 MAPK inhibitor SB202190 for 24 hours and subjected to FACS analysis to determine percent cell cycle distribution. Results are expressed as mean ± SD (%). Asterisk indicates significant difference from untreated cells P ≤ 0.05. Double asterisk indicates significant difference from respective DIM-dose treated cells in the absence of p38 MAPK inhibitor with P ≤ 0.05.
4. Discussion
At present there is no effective therapy for androgen independent prostate cancer, and there is therefore a pressing need for new therapeutic approaches. Androgen independent prostate cancer is associated with the loss of the cdk2 inhibitor p27 [9], and the upregulation of cdk2 suggesting that therapies restoring p27 and inhibiting cdk2 may be useful approaches to restore cell cycle arrest and thus inhibit proliferation. Although DIM is an androgen receptor antagonist [14], its regulation of cell cycle in androgen dependent cells and efficacy against androgen independent prostate cancer has not been investigated. Here we show for the first time that DIM treatment inhibits prostate cancer cell proliferation regardless of androgen responsiveness and p53 status. We found that DIM profoundly inhibited progression of prostate cancer cells into S phase, and further identified that DIM treatment causes a G1 cell cycle arrest. The results of this study clearly demonstrate that DIM induces a G1 phase-specific cell cycle arrest with the induction of p27 and the degradation of the G1 phase-specific cell cycle regulatory proteins cdk2 and cdk4 and that DIM has a therapeutic potential in both androgen dependent and androgen independent prostate cancer. We further demonstrated that the induction of p27 in AR negative, p53 mutant DU145 cells was mediated through the p38 MAPK pathway and that inhibition of p38 MAPK activity was sufficient to prevent the DIM mediated G1 arrest in DU145 cells.
DIM treatment induced a G1 arrest in both androgen dependent p53 wildtype LNCaP and androgen independent p53 mutant DU145 cells. However, the cell cycle modulator profile was differentially regulated in the two cell lines. The downregulation of cyclin E protein levels was present only in LNCaP cells. Since androgens have been shown to regulate cyclin E expression in coronary smooth muscle cells [25], it is possible that the lack of AR in DU145 is the reason cyclin E regulation is absent in these cells. The lack of regulation of cyclin E protein levels by DIM in DU145 cells suggests this cyclin is not essential towards induction of G1 arrest in these cells. The inhibition of cdk2 transcription by DIM is another difference between the AR-positive, p53 wildtype LNCaP and AR-negative, p53 mutant DU145 cells. Cdk2 transcription is known to be regulated by androgen in LNCaP cells [26], suggesting that DIM inhibition of cdk2 transcription in LNCaP cells is mediated through the AR. The observed differences in the mechanism of G1 arrest in LNCaP and DU145 cells may be attributable to the different genetic makeup of these two cell lines.
Members of the Rb family of proteins are known critical downstream targets of G1 specific cyclin/cdk complexes [7]. In the hypophosphorylated state, the Rb proteins associate with and inhibit the activity of E2F family transcription factors, which are involved in the transcription of key cell cycle regulatory proteins. Upon growth stimulus, the G1–specific cyclins/cdks phosphorylate Rb, causing the release of E2F factors and progression into S phase. Consistent with the DIM mediated inhibition of cdk2 and cdk4 protein expression, we showed that DIM also inhibited phosphorylation of pRb by cdk4 and cdk2 and inhibited the kinase activity of cellular cdk2 in LNCaP cells.
The p27 protein is a tumor suppressor protein that arrests cell cycle progression by binding to active cdk-cyclin complexes thereby inhibiting their activities [7]. In this study, we showed that DIM treatment induced the expression of p27 in AR positive, p53 wildtype LNCaP and AR negative, p53 mutant DU145 cells. This increased expression occurred at the transcriptional level and activated the p27 gene promoter. Because DU145 cells lack a functional p53 allele, this DIM-mediated induction of p27 is independent of p53. This is similar to the p53-independent induction of p27 expression in hepatoma cells described previously [27]. We also observed an inhibition of DIM mediated p27 expression when Sp1 sites were removed from the p27 promoter. This observation is consistent with many previous observations of transcriptional regulation by indoles mediated through Sp1, reviewed by Firestone et al. [28]. Since DIM has previously been described to influence the transcription of another cdk inhibitor, p21, via Sp1 activation in breast cancer cells [11] and since Sp1 has been found to be involved in the p38 MAPK mediated activation of p21 expression [23], it is likely that the DIM-mediated induction of p27 expression is mediated through the Sp1 transcription factor and p38 MAPK. Interestingly, the DIM-induced G1 arrest was accompanied by increased expression of p27, and not p21, in both LNCaP and DU145 cells, providing evidence for clear differences in p38 MAPK signaling in breast and prostate cancer cells.
The MAPK superfamily is known to play a vital role in eukaryotic cell proliferation, differentiation, and apoptotic responses to a wide range of extracellular stimuli [29]. Several studies have documented the involvement of the p38 MAPK pathway in the regulation of cell cycle progression, specifically the G1 phase [30, 31]. In addition, p27 expression has been reported to be regulated at the transcriptional level through activation of the p38 MAPK pathway in androgen independent PC-3 cells [21]. Our results demonstrate that the DIM-mediated activation of the p38 MAPK pathway leads to the transcriptional up-regulation of p27 in AR positive LNCaP and AR negative DU145, thus DIM-mediated activation of p38 MAPK acts as a sensor for the transcriptional up-regulation of p27 and suggests a possible molecular link between p38 MAPK signal transduction and the p27 transcriptional induction in prostate cancer. Interestingly, the effects of DIM on p27 expression specifically and on G1 arrest in general in AR negative DU145 were reversed by inhibition of p38 MAPK activity, demonstrating p38 MAPK as key target for regulation of the cell cycle by DIM in AR negative prostate cancer cells.
Several studies have suggested that the hormone refractory, advanced prostate cancer phenotype is associated with many cellular changes, such as the deregulation of cell cycle progression and cell survival signaling, and the inactivation of p53 [10], changes like those present in DU145 cells. Therefore, the findings presented here could be clinically significant considering that DIM treatment induces the p53-independent up-regulation of p27 leading to a G1. arrest. Moreover, conventional treatments, such as taxane-based chemotherapies, are currently found to be an ineffective treatment for patients with advanced disease. Therefore, the identification of novel targets and new treatment regimens are critical for the future control of advanced prostate cancer. The results of our studies suggest the activation of the p38 MAPK pathway as a potential target for the therapeutic treatment of androgen refractory prostate tumors.
In conclusion, the findings presented here demonstrate the anti-cancer potential of DIM in prostate. The ability of DIM to induce a cell cycle arrest in the AR positive, p53 wildtype LNCaP and AR negative, p53 mutant DU145 human prostate cancer cell lines strongly suggests that DIM should be considered for further efficacy studies. We have identified p38 MAPK activation leading to p27 induction in AR negative cells as a new cellular pathway for this interesting compound that possesses several useful biological activities, including significant antiproliferative activity. The results of the study presented here provide a strong basis for the further development of DIM as a novel agent that, alone or in combination with other compounds, may be useful for androgen refractory prostate cancer therapy and/or prevention.
Acknowledgments
Financial support for this work was provided by grants CA69056 and CA102360 from the NIH.
Abbreviations
- DIM
3,3′-Diinolylmethane
- I3C
Indole-3-carbinol
- DMSO
Dimethyl Sulfoxide
- RRP
recurrent respiratory papillomatosis
- AR
androgen receptor
- FACS
fluorescence-activated cell sorter
- MAPK
mitogen-activated protein kinase
- pRb
retinoblastoma protein
- cdk
cyclin-dependent kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer research. 1978;38:1410–3. [PubMed] [Google Scholar]
- 4.Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, et al. 3,3′-Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis. 2005;26:771–8. doi: 10.1093/carcin/bgi018. [DOI] [PubMed] [Google Scholar]
- 5.Wiatrak BJ. Overview of recurrent respiratory papillomatosis. Current opinion in otolaryngology & head and neck surgery. 2003;11:433–41. doi: 10.1097/00020840-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Auborn KJ. Therapy for recurrent respiratory papillomatosis. Antiviral therapy. 2002;7:1–9. [PubMed] [Google Scholar]
- 7.Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer research. 2000;60:3689–95. [PubMed] [Google Scholar]
- 8.Guo Y, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3:2269–74. [PubMed] [Google Scholar]
- 9.Macri E, Loda M. Role of p27 in prostate carcinogenesis. Cancer Metastasis Rev. 1998;17:337–44. doi: 10.1023/a:1006133620914. [DOI] [PubMed] [Google Scholar]
- 10.Karan D, Kelly DL, Rizzino A, Lin MF, Batra SK. Expression profile of differentially-regulated genes during progression of androgen-independent growth in human prostate cancer cells. Carcinogenesis. 2002;23:967–75. doi: 10.1093/carcin/23.6.967. [DOI] [PubMed] [Google Scholar]
- 11.Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23:1297–305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 12.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, et al. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. The Journal of biological chemistry. 1998;273:3838–47. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Hsu BAJ, Kinseth BAM, Bjeldanes LF, Firestone GL. Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer. 2003;98:2511–20. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]
- 14.Le HT, Schaldach CM, Firestone GL, Bjeldanes LF. Plant-derived 3,3′-Diindolylmethane is a strong androgen antagonist in human prostate cancer cells. The Journal of biological chemistry. 2003;278:21136–45. doi: 10.1074/jbc.M300588200. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Kamiyama J, Sakai T. Sp1 and NF-Y synergistically mediate the effect of vitamin D(3) in the p27(Kip1) gene promoter that lacks vitamin D response elements. The Journal of biological chemistry. 1999;274:32309–17. doi: 10.1074/jbc.274.45.32309. [DOI] [PubMed] [Google Scholar]
- 16.Cvoro A, Tzagarakis-Foster C, Tatomer D, Paruthiyil S, Fox MS, Leitman DC. Distinct roles of unliganded and liganded estrogen receptors in transcriptional repression. Molecular cell. 2006;21:555–64. doi: 10.1016/j.molcel.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Khurana A, Nakayama K, Williams S, Davis RJ, Mustelin T, Ronai Z. Regulation of the ring finger E3 ligase Siah2 by p38 MAPK. The Journal of biological chemistry. 2006;281:35316–26. doi: 10.1074/jbc.M606568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong C, Firestone GL, Bjeldanes LF. Bcl-2 family-mediated apoptotic effects of 3,3′-diindolylmethane (DIM) in human breast cancer cells. Biochemical pharmacology. 2002;63:1085–97. doi: 10.1016/s0006-2952(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 19.Riby JE, Firestone GL, Bjeldanes LF. 3,3′-diindolylmethane reduces levels of HIF-1alpha and HIF-1 activity in hypoxic cultured human cancer cells. Biochemical pharmacology. 2008;75:1858–67. doi: 10.1016/j.bcp.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. The Journal of biological chemistry. 2001;276:22332–40. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 21.Mukhopadhyay I, Sausville EA, Doroshow JH, Roy KK. Molecular mechanism of adaphostin-mediated G1 arrest in prostate cancer (PC-3) cells: signaling events mediated by hepatocyte growth factor receptor, c-Met, and p38 MAPK pathways. The Journal of biological chemistry. 2006;281:37330–44. doi: 10.1074/jbc.M605569200. [DOI] [PubMed] [Google Scholar]
- 22.Xue L, Firestone GL, Bjeldanes LF. DIM stimulates IFNgamma gene expression in human breast cancer cells via the specific activation of JNK and p38 pathways. Oncogene. 2005;24:2343–53. doi: 10.1038/sj.onc.1208434. [DOI] [PubMed] [Google Scholar]
- 23.Moon SK, Jung SY, Kim CH. Transcription factor Sp1 mediates p38MAPK-dependent activation of the p21WAF1 gene promoter in vascular smooth muscle cells by pyrrolidine dithiocarbamate. Biochemical and biophysical research communications. 2004;316:605–11. doi: 10.1016/j.bbrc.2004.02.096. [DOI] [PubMed] [Google Scholar]
- 24.Dadlani H, Ballinger ML, Osman N, Getachew R, Little PJ. Smad and p38 MAP kinase-mediated signaling of proteoglycan synthesis in vascular smooth muscle. The Journal of biological chemistry. 2008;283:7844–52. doi: 10.1074/jbc.M703125200. [DOI] [PubMed] [Google Scholar]
- 25.Bowles DK, Maddali KK, Dhulipala VC, Korzick DH. PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. American journal of physiology. 2007;293:C805–13. doi: 10.1152/ajpcell.00127.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lu S, Tsai SY, Tsai MJ. Regulation of androgen-dependent prostatic cancer cell growth: androgen regulation of CDK2, CDK4, and CKI p16 genes. Cancer research. 1997;57:4511–6. [PubMed] [Google Scholar]
- 27.Liu TZ, Chen CY, Yiin SJ, Chen CH, Cheng JT, Shih MK, et al. Molecular mechanism of cell cycle blockage of hepatoma SK-Hep-1 cells by Epimedin C through suppression of mitogen-activated protein kinase activation and increased expression of CDK inhibitors p21(Cip1) and p27(Kip1) Food Chem Toxicol. 2006;44:227–35. doi: 10.1016/j.fct.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3′-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. The Journal of nutrition. 2003;133:2448S–55S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 29.Roovers K, Assoian RK. Integrating the MAP kinase signal into the G1 phase cell cycle machinery. Bioessays. 2000;22:818–26. doi: 10.1002/1521-1878(200009)22:9<818::AID-BIES7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK, 3rd, et al. pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells. The Journal of biological chemistry. 1999;274:7341–50. doi: 10.1074/jbc.274.11.7341. [DOI] [PubMed] [Google Scholar]
- 31.Daly JM, Olayioye MA, Wong AM, Neve R, Lane HA, Maurer FG, et al. NDF/heregulin-induced cell cycle changes and apoptosis in breast tumour cells: role of PI3 kinase and p38 MAP kinase pathways. Oncogene. 1999;18:3440–51. doi: 10.1038/sj.onc.1202700. [DOI] [PubMed] [Google Scholar]