Abstract
Frequency tuning of auditory cortical neurons is typically determined by integrating spikes over the entire duration of a tone stimulus. However, this approach may mask functionally significant variations in tuning over the time course of the response. To explore this possibility, frequency response functions (FRFs) based on population multiunit activity evoked by pure tones of 175 or 200 ms duration were examined within 4 time windows relative to stimulus onset corresponding to “on” (10–30 ms), “early sustained” (30–100 ms), “late sustained” (100–175 ms), and “off” (185–235 or 210–260 ms) portions of responses in primary auditory cortex (A1) of 5 awake macaques. FRFs of “on” and “early sustained” responses displayed a good concordance, with best frequencies (BFs) differing, on average, by less than 0.25 octaves. In contrast, FRFs of “on” and “late sustained” responses differed considerably, with a mean difference in BF of .68 octaves. At many sites, tuning of “off” responses was inversely related to that of “on” responses, with “off” FRFs displaying a trough at the BF of “on” responses. Inversely correlated “on” and “off” FRFs were more common at sites with a higher “on” BF, thus suggesting functional differences between sites with low and high “on” BF. These results indicate that frequency tuning of population responses in A1 may vary considerably over the course of the response to a tone, thus revealing a temporal dimension to the representation of sound spectrum in A1.
Introduction
A basic function of the auditory system is to analyze the frequency content of sounds. This analysis is reflected in a tonotopic representation of frequency maintained throughout the auditory pathway, including primary auditory cortex (A1) (e.g., Popper and Fay, 1992). Frequency tuning of auditory cortical neurons is typically determined by summing spikes occurring over the duration of a tone stimulus (e.g., Recanzone et al., 2000a) or within a restricted time window, which often includes only the initial “on” portion of the response to a tone (e.g., Fishman et al., 2000a). However, these approaches may mask or overlook functionally significant variations in tuning across different time segments of the response. Indeed, it is possible that spectral selectivity is temporally dynamic and varies depending upon whether spikes are integrated within a temporal window which includes the initial “on”, “sustained”, or “off” portion of responses. The importance of considering the temporal dynamics of neural responses is supported by the different functional roles ascribed to event-related potential components occurring at different latencies relative to stimulus onset (e.g., Näätänen, 1990). Furthermore, lesion and inactivation studies suggest that auditory cortex is critically involved in the processing of multiple sound attributes such as spectrum, pitch, and location (Heffner and Heffner, 1990; Talwar et al., 2001; Tramo et al., 2002; Malhotra et al., 2004). These attributes might be differentially encoded in temporally discrete portions of the neural response. Later neural activity may also reflect amplitude modulation (e.g., Bieser and Muller-Preuss, 1996), roughness and sensory dissonance (Fishman et al., 2000b; Fishman et al., 2001), or the behavioral salience of a stimulus, as demonstrated in visual and auditory cortices (e.g., Mehta et al., 2000; Supér et al., 2001; Selezneva et al., 2006).
Several studies have examined the temporal dynamics of frequency tuning of single neurons in auditory cortex (Loftus and Sutter, 2001; Qin et al., 2003, Moshitch et al., 2006; Qin et al., 2007) and have reported variations in frequency tuning between early and later portions of the response to a tone in cat auditory cortex (see Discussion for details). These findings have potentially important implications for the functional organization of auditory cortex and raise several issues that warrant further examination. First, it is unclear whether the use of anesthetics in two of the studies limits extrapolation of the findings to the awake state. Second, whether such variations in tuning are exhibited also by neural responses in A1 of primates is unknown. Finally, accumulating evidence from studies of many cortical areas strongly supports the relevance of neural population responses for information transmission in the brain (e.g., deCharms and Merzenich, 1996; Pasupathy and Connor, 2002; Petersen et al., 2002; Sanger, 2003; Samonds et al., 2004). For instance, synchronized responses of neural populations are more likely to drive neurons in downstream cortical areas to firing threshold than responses of individual single units (deCharms and Merzenich, 1996; Lisman, 1997; Eggermont, 2001). The potential significance of population activity for auditory perception is further reinforced by earlier work from our laboratory demonstrating concordance between population responses in monkey A1 and human psychophysics of consonance and dissonance perception (Fishman et al., 2001), auditory stream segregation (Fishman et al., 2004), the voice-onset time phonetic boundary (Steinschneider et al., 2003), critical bandwidth (Fishman and Steinschneider, 2006), and temporal pitch (Steinschneider et al., 1998). Given the potentially important role of population activity in auditory information processing, a key question is whether responses of local neural populations in A1 display temporally dynamic frequency tuning similar to that observed in previous single-unit studies.
Motivated by these considerations, the present study examines the temporal dynamics of frequency tuning of multiunit activity (MUA) evoked by contralaterally presented tones in A1 of awake macaques. Specifically, we investigate variations in frequency tuning of population responses occurring within four different time windows corresponding to initial “on”, “early sustained”, “late sustained”, and “off” portions of responses.
Materials and Methods
Five adult male macaque monkeys (Macaca fascicularis) were studied using previously described methods (Fishman et al., 2001; Steinschneider et al., 2003). Animals were housed in our AAALAC-accredited Animal Institute under daily supervision of laboratory and veterinary staff. All experimental procedures were reviewed and approved by the AAALAC-accredited Animal Institute of Albert Einstein College of Medicine and were conducted in accordance with institutional and federal guidelines governing the experimental use of primates. To minimize the number of monkeys used, other auditory experiments were conducted in the same animals during each recording session.
A. Surgical procedure
Animals were acclimated to the recording environment while sitting in custom-fitted primate chairs prior to surgery. Under pentobarbital anesthesia and using aseptic techniques, holes were drilled bilaterally into the dorsal skull to accommodate matrices composed of 18-gauge stainless steel tubes glued together in parallel. Tubes served to guide electrodes toward A1 for repeated intracortical recordings. Matrices were stereotaxically positioned to target A1. They were oriented at a 30 degree anterior-posterior angle and with a slight medial-lateral tilt in order to direct electrode penetrations perpendicular to the superior surface of the superior temporal gyrus, thereby satisfying one of the major technical requirements of one-dimensional CSD analysis (Vaughan and Arezzo, 1988). Matrices and Plexiglas bars, used for painless head fixation during the recordings, were embedded in a pedestal of dental acrylic secured to the skull with inverted bone screws. Peri- and post-operative antibiotic and anti-inflammatory medications were routinely administered. Recordings began after a 2-week post-operative recovery period.
B. Neurophysiological recordings
Recordings were conducted in an electrically shielded, sound-attenuated chamber. Sound attenuation was enhanced by the application of 3-inch foam wedges to most exposed surfaces of the chamber. Recordings in three of the five monkeys were conducted under passive, awake, listening conditions. Frequent visits by experimenters into the recording chamber in between stimulus blocks and delivery of preferred foods helped to maintain the animals in an alert state throughout the recordings. Recordings in two monkeys were conducted under active listening conditions wherein subjects performed a simple auditory discrimination task (detection of a randomly presented noise burst interspersed with test tones) to ensure attention to auditory stimuli. While the possibility of intermittent drowsiness cannot be excluded, particularly during recordings under passive conditions, no qualitative differences in results were observed between the two recording conditions.
Intracortical recordings were performed using linear-array multi-contact electrodes containing 14–16 recording contacts, evenly spaced at 150 μm (+/− 10%) intervals (Barna et al., 1981; Plexon, Inc.). Individual contacts were constructed from 25 μm-diameter stainless steel wires, and maintained at an impedance of about 200 kΩ. An epidural stainless steel guide tube positioned over the occipital cortex served as a reference electrode. In four monkeys, field potentials were recorded using unity-gain headstage pre-amplifiers, and subsequently amplified 5000 times by differential amplifiers (Grass, Inc.) with a frequency response down 6 dB at 3 Hz and at 3 kHz. Signals were digitized on-line at 3.4 kHz and averaged by computer (Neuroscan software and hardware, Neurosoft, Inc.) to yield auditory evoked potentials (AEPs). No significant aliasing in the AEPs is observed at this digitization rate, as the amplitude of signals at frequencies above 1500 Hz is negligible (< 0.1%) compared with that of signals that dominate the AEP and which fall below 200 Hz. In one monkey, neural signals were band-pass filtered from 3 Hz to 3 kHz (48 dB/octave) and digitized at 12 kHz using a RA16 PA Medusa 16-channel preamplifier connected via fiber-optic cables to a RX5 data acquisition system (Tucker-Davis Technologies, Inc.). One-dimensional current source density (CSD) analyses characterized the laminar pattern of net current sources and sinks within A1 generating the AEPs and were used to identify the laminar location of concurrently recorded MUA. CSD was calculated using a 3-point algorithm that approximates the second spatial derivative of voltage recorded at each recording contact (Freeman and Nicholson, 1975; Nicholson and Freeman, 1975). To derive MUA, signals were simultaneously high-pass filtered (48 dB/octave) at 500 Hz, amplified an additional 8 times, full-wave rectified, and then low-pass filtered (48 dB/octave) at 600 Hz prior to digitization and averaging to prevent signal aliasing (see Supér and Roelfsema, 2005 for a methodological review). MUA is a measure of the envelope of summed action potential activity of neuronal aggregates within a sphere of about 100 μm in diameter surrounding each recording contact (Legatt et al., 1980; Vaughan and Arezzo, 1988; Brosch et al., 1997; Supér and Roelfsema, 2005). Using an electrode impedance similar to that of the electrodes utilized in present study (100–300 kΩ), MUA and single-unit recordings have been shown to yield similar results in primary visual cortex, with MUA reflecting the average local activity and orientation tuning of closely spaced neurons in the vicinity of the electrode (Supér and Roelfsema, 2005). MUA data in the present study are derived from action potential activity primarily recorded within lower lamina 3, as identified by the presence of large amplitude initial current sinks that are balanced by concurrent superficial sources in upper lamina 3 (Steinschneider et al., 1992, 1994, 2008; Fishman et al., 2000a,b). Previous studies have localized the initial sinks to thalamorecipient zone layers of A1 (Müller-Preuss and Mitzdorf, 1984; Steinschneider et al., 1992; Sukov and Barth, 1998; Metherate and Cruikshank, 1999).
Electrodes were moved with a microdrive and guided by on-line examination of click-evoked potentials. Test stimuli were delivered when the electrode channels bracketed the inversion of early AEP components and the largest MUA, typically occurring during the first 50 ms post-stimulus onset, was situated in the middle channels. Evoked responses to 50 presentations of each stimulus were averaged with an analysis window of 300 ms (including a 25-ms pre-stimulus baseline interval).
At the end of the recording period, monkeys were deeply anesthetized with sodium pentobarbital and transcardially perfused with 10% buffered formalin. Tissue was sectioned in the coronal plane (80 μm thickness) and stained for acetylcholinesterase and Nissl substance to reconstruct the electrode tracks and to identify A1 according to previously published physiological and histological criteria (Merzenich and Brugge, 1973; Wallace et al., 1991; Morel et al., 1993; Hackett et al., 1998). Based upon these criteria, all electrode penetrations considered in this report were localized to A1. However, the possibility that some sites situated near the lower-frequency anterolateral border of A1 were located in the rostral field R cannot be excluded.
C. Stimuli
Stimuli were generated and delivered at a sample rate of 100 kHz by a PC-based system using RP2 or RX8 modules (Tucker Davis Technologies, Inc.). Frequency response functions (FRFs) based on pure tone responses characterized the spectral tuning of the cortical sites. Pure tones used to generate the FRFs ranged from 0.15 to 18.0 kHz, as limited by the range of the headphones and speakers used. Stimuli were 175 ms and 200 ms in duration in three and two monkeys, respectively (including 10 ms linear rise/fall ramps), and were presented with a stimulus onset-to-onset interval of 658 ms. For three of the monkeys, resolution of FRFs was generally 0.1 kHz for frequencies below 2.0 kHz, 0.25 kHz for frequencies between 2.0 and 5.0 kHz, and 0.5 kHz for frequencies between 5.0 and 18.0 kHz. For two of the monkeys, resolution of FRFs was 0.25 octaves (or finer) across the 0.15 to 18.0 kHz frequency range tested. Effects of stimulus intensity on the temporal dynamics of frequency tuning were examined in one of the monkeys performing the behavioral task (stimulus levels tested: 40, 50, 60, and 70 dB SPL). At sites where stimulus intensity was varied, tone duration was 200 ms and frequency resolution of FRFs was finer than. 25 octaves. In three monkeys, pure tones were delivered monaurally at 60 dB SPL via a dynamic headphone (MDR-7502, Sony, Inc.) to the ear contralateral to the recorded hemisphere. Sounds were introduced to the ear through a 3-inch long, 60 cc plastic tube attached to the headphone for the monkeys recorded in the passive, awake state. In the two monkeys performing a behavioral task, stimuli were presented via a free-field speaker (Microsatellite; Gallo, Inc.) mounted at a contralateral azimuth of 60 degrees on a semi-circular speaker array located 1 meter away from the animal’s ear (Crist Instruments, Inc.). Sound intensity was measured with a sound level meter (type 2236; Bruel and Kjaer, Inc.) positioned at the ear of the animal. The frequency response of the headphone was flattened (+/− 3 dB SPL) from 0.2 to 17.0 kHz by a graphic equalizer (GE-60, Rane, Inc.). The frequency response of the speaker was essentially flat (+/− 5 dB SPL) over this range.
D. Data analysis
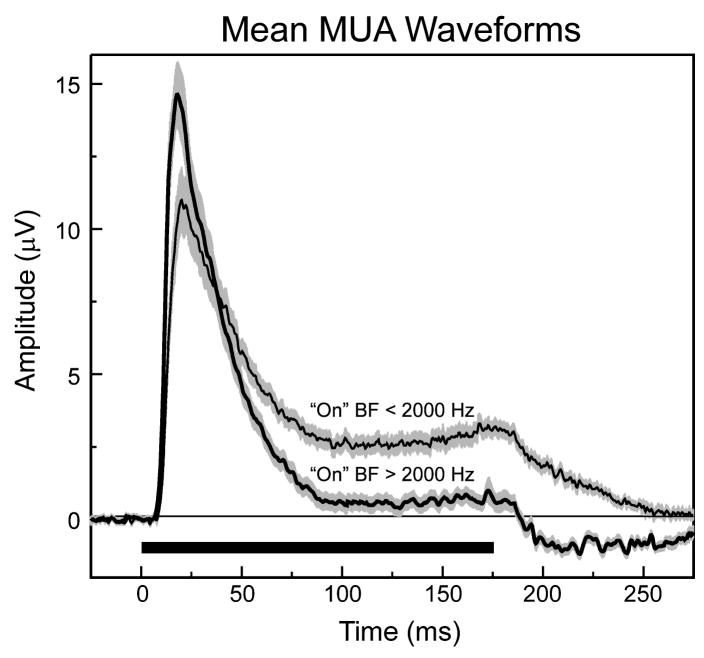
To examine differences in spectral tuning over the duration of the tone responses, FRFs were derived for 4 different time windows corresponding to the initial “on”, “early sustained”, “late sustained”, and “off” portion of responses. These time windows are illustrated in Figure 1, which depicts mean waveforms of MUA and CSD evoked by tones of frequency corresponding to the best frequency (BF; defined below) for the “on” portion of the response. Tone-evoked MUA centered in lower lamina 3 displays an abrupt onset, and an early peak occurring within the first 30 ms post-stimulus onset, followed by decay to a steady-state plateau of activity that persists until the end of the stimulus. All sites show sharp, short-latency (<15 ms) onset responses typical of A1 neurons (Recanzone et al., 2000a; Cheung et al., 2001; Lakatos et al., 2005). Time windows are delineated by transitions in the mean MUA waveform. As shown in Figure 1, these transitions, characterized by abrupt changes in waveform slope, often coincide with major CSD components recorded simultaneously with MUA across the laminar extent of A1. These prominent CSD components are characteristic of A1 laminar response profiles described in previous studies (e.g., Steinschneider et al., 2005; Fishman and Steinschneider, 2006; Steinschneider et al., 2008). Transitions in the mean MUA waveform, which potentially distinguish functionally distinct portions of the response, demarcate the four response time windows- “on”, “early sustained”, “late sustained”, and “off”-within which frequency tuning of MUA was evaluated (response portions labeled A, B, C, and D). For recordings in which stimulus intensity was held constant at 60 dB SPL, time windows relative to stimulus onset were defined as follows: “on”: 10–30 ms; “early sustained”: 30–100 ms; “late sustained”: 100–175 ms; and “off”: 185–235 ms (for 175 ms duration tones) and 210–260 ms (for 200 ms duration tones). These time windows are similar to those analyzed by other investigators (e.g., Recanzone, 2000b).
Figure 1.
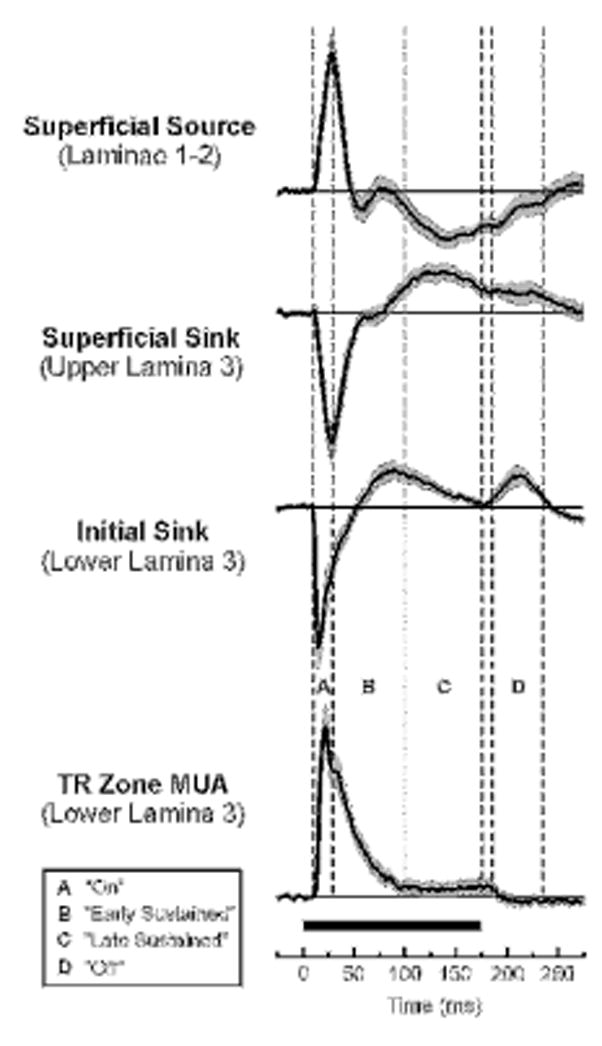
Mean waveforms of tone-evoked thalamorecipient (TR) zone MUA and simultaneously-recorded CSD components averaged across A1 sites. Averages include only responses to tones of 175 ms duration; N=45). Shading above and below the lines represents +/− SEM. CSD waveforms derived from AEPs recorded at 3 laminar depths display a source-sink current dipole configuration typical of responses in A1 and indicative of pyramidal cell activation. Vertical drop lines delineate four portions of responses, labeled A, B, C, and D, separately examined in the study, as defined in the legend. Boundaries of time segments correspond to transitions in the mean MUA waveform.
When examining effects of stimulus intensity on temporal dynamics of frequency tuning, time windows were referenced instead to the onset latency of the mean of responses evoked by all the tones presented at a given site used to define the FRF at a given sound level. Referencing time windows to response onset rather than to stimulus onset compensated for variations in response onset latency with stimulus intensity (with onset latencies generally decreasing with increasing sound level). Response onset latency was defined as the time at which MUA amplitude exceeded 4 standard deviations above the mean pre-stimulus baseline activity. Onset latencies defined by this criterion were in good agreement with latencies determined by visual inspection of the MUA waveforms. Time windows relative to response onset were defined as follows: “on”: 0–20 ms; “early sustained”: 20–90 ms; “late sustained”: 90–165 ms; and “off”: 200–250 ms (for 200 ms duration tones). These time windows delineated approximately the same portions of the MUA waveform as those defined by time windows referenced to stimulus onset for responses to tones presented at 60 dB SPL.
The BF of each cortical site and for each response window examined was defined as the pure tone frequency eliciting the maximal MUA centered in lower lamina 3 integrated over the duration of the response time window. MUA occurring in up to 3 adjacent electrode channels (a 450 μm laminar extent) located at or above the initial current sink was averaged together to ensure adequate sampling of maximally responsive neurons within lower portions of lamina 3 and to eliminate potential bias in the selection of MUA channels for analysis. MUA observed in 2 or more channels was averaged together if the BF during the “on” portion of the response was identical across channels and the amplitude of MUA recorded in a given channel exceeded 75% of the amplitude of MUA recorded in the channel displaying the maximal tone-evoked MUA during the “on” portion of the response. MUA was averaged over 3 adjacent channels in 22 sites and over 2 adjacent channels in 45 sites out of the 67 sites examined. As shown in Figure 1, MUA recorded in lower lamina 3 is temporally correlated with a prominent current dipole in the CSD characterized by co-located sinks that are balanced by concurrent, more superficial, current sources. While this current dipole configuration is consistent with activation of pyramidal neurons, MUA measures may also include action potentials of lamina 4 stellate cells and distal segments of thalamocortical axon terminals.
Frequency tuning of MUA was examined within each response time window and compared with the frequency tuning of MUA occurring during the initial “on” portion of the response. This comparison provides a measure of the deviation in frequency tuning within later time windows relative to the tuning of initial “on” responses typically used to define the spectral selectivity of A1 neurons and the tonotopic organization of auditory cortex.
Results
Results are based on responses evoked by pure tones at 67 sites in A1 of 5 macaque monkeys. A1 sites displaying multi-peaked FRFs during the “on” portion of the response were uncommon (7 sites in 5 animals) and are not considered in this report.
BFs of neural responses evoked by tones presented at 60 dB SPL occurring within the “on” time window ranged from 0.2 to 15.2 kHz and were anatomically distributed along the superior temporal gyrus in all animals according to the expected anterior-lateral to posterior-medial topographic gradients of low to high BF representation characteristic of A1 (Merzenich and Brugge, 1973; Morel et al., 1993; Recanzone et al., 2000a).
Frequency Tuning of MUA in A1 Varies Across Different Response Time Windows
Figure 2 shows FRFs for each of the four time windows examined at 6 representative sites in A1. FRFs for the “on” time window and for the other time windows are represented by white and black symbols, respectively. Negative values in the FRFs represent levels of MUA below those observed during the 25-ms pre-stimulus interval, and thus indicate response suppression during or immediately following tone stimulation. Waveforms of MUA evoked by tones of frequencies corresponding to the peaks of the FRFs for each of the time windows are shown superimposed in the insets for comparison (maximal MUA waveforms for the “on” time window and for later time windows are represented by gray and black lines, respectively). The “on” time window and later time windows are bounded by the dotted and solid vertical lines, respectively, in the insets. FRFs for “on” time windows are sharply tuned to a single frequency. “Early sustained” and “late sustained” time windows display BFs that are similar, although not necessarily identical to, the BF of “on” time windows (Figure 3, a, b, and c), or that are markedly different from the BF of “on” time windows (Figure 3, d, e, and f). Bandwidths of FRFs also vary across the different response time windows.
Figure 2.
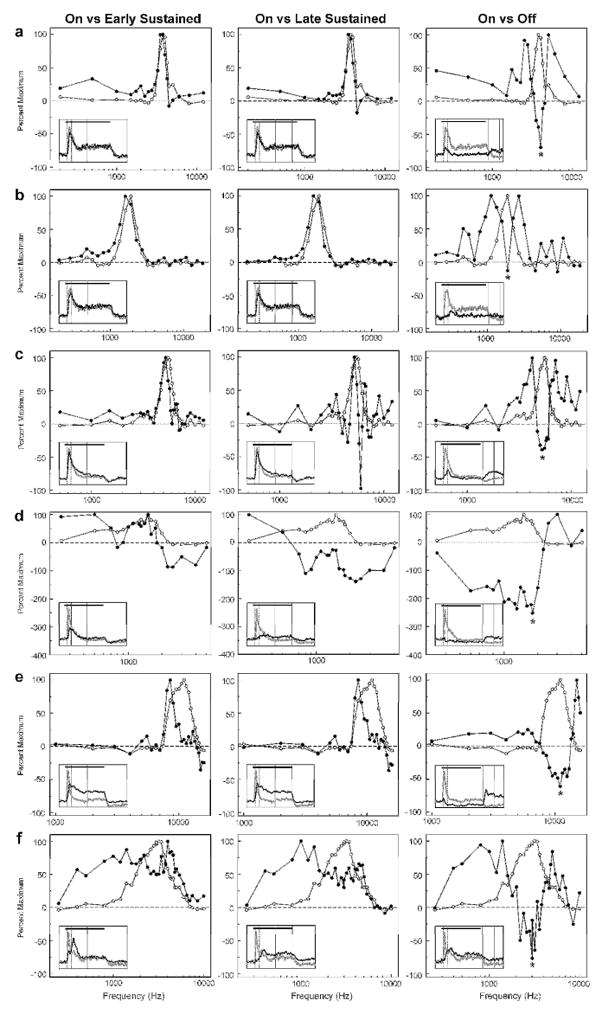
Variation in frequency tuning over the course of tone stimulation. FRFs and MUA from six representative A1 sites (a–f) are shown, illustrating changes in frequency tuning for “early sustained”, “late sustained”, and “off” portions of the response relative to the FRF for the “on” time window. At each site, FRFs for the time window indicated at the top of each column are compared with the FRF for the “on” time window, as represented by the black and white symbols, respectively. Insets within the FRF plots show superimposed waveforms of MUA evoked by the tone corresponding to the peak of the FRF for the “on” time window (gray curve) and for the time window indicated at the top of the column (black curve). Vertical dashed and solid lines in the insets delineate the boundaries of the “on” time window and the time window indicated at the top of the column, respectively. Tone duration is represented by the horizontal black line. At all sites shown, the FRF for the “off” time window is inversely related to the FRF for the “on” time window and displays a trough at the frequency corresponding to the peak of the FRF for the “on” time window (indicated by the asterisk). See text for details.
Figure 3.
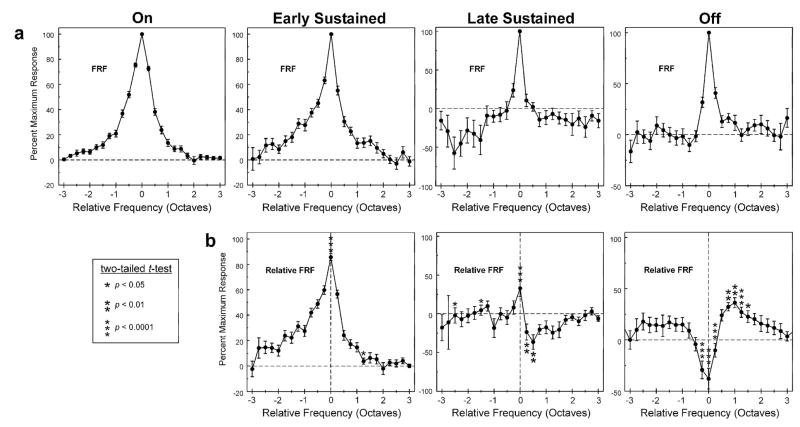
Mean FRF for each of the time windows examined in the study. a, Mean of FRFs expressed relative to the frequency of the peak of the FRF for each of the time windows examined in the study, as indicated above each column. Means represent the average of FRFs across A1 sites (N=67). Error bars represent +/−SEM. Bin width = 0.25 octave. b, Mean of FRFs expressed relative to the frequency of the peak of the FRF for the “on” time window for each of the time windows examined in the study (denoted as “Relative FRF”). Statistically significant differences (p <.05) between means of relative FRFs in b and corresponding means of FRFs in a are indicated by the asterisks. Significant differences represent a change in the FRF for the specified time window compared to the FRF for the “on” time window. Note the trough in the relative FRF for the “off” time window at a frequency corresponding to the peak of the FRF for the “on” time window. See text for details.
A striking and frequently observed feature of the FRF for the “off” time window is its inverse relationship to the FRF for the “on” time window. Specifically, FRFs for the “off” time window often display a trough (indicated by an asterisk in Figure 2) at a frequency corresponding to the peak of the FRF for the “on” time window (i.e., at the “on” BF). Furthermore, troughs in FRFs for the “off” time window are generally flanked by two side lobes extending over a limited spectral range above and below the trough. At some sites, the amplitude of responses evoked during the “off” time window is comparable to, or exceeds, that of responses evoked during the “on”, “early sustained”, and “late sustained” time windows.
To examine whether major trends in the temporal dynamics of frequency tuning observed at individual sites are evident across the entire sample of A1 sites examined, FRFs for each of the time windows were averaged across sites. Mean FRFs for each time window were derived as follows: The FRF at each site was normalized to the amplitude of the maximal response occurring within the specified time window and expressed as a function of frequency distance relative to the frequency of the tone eliciting the maximal response. The resultant FRF values were sorted into quarter-octave bins and then averaged across sites. If more than one data point of the FRF at a given site fell into the same quarter-octave frequency bin (which occurred in cases where the FRF was sampled at a resolution finer than a quarter-octave) the data point with the larger value was used. Due to occasional uneven sampling across the frequency range, the number of data points contributing to the mean FRF differs across quarter octave bins and ranges from 16 to 67.
Figure 3a shows mean FRFs for each of the four time windows analyzed. Mean FRFs spanned a 5-octave range above and below the BF of responses within each time window. For clarity only a 3-octave range above and below the BF is shown. Mean FRFs for all four response time windows display highly specific frequency tuning, with a single peak corresponding to the BF.
To illustrate average changes in frequency tuning over time relative to tuning during the “on” portion of the response, the normalized FRF at each site and for each time window was first expressed as a function of frequency distance relative to the peak of the FRF for the “on” time window. Such FRFs are henceforth referred to as ‘relative FRFs’. The resultant relative FRF values were sorted into quarter-octave frequency bins and then averaged across sites. Thus, mean relative FRFs were used to compare the average tuning of responses during the “on” time window with that of responses occurring during later time windows. If there is no difference in tuning between “on” and later responses, then the value of the relative FRF at each quarter-octave frequency bin for a given later time window should be identical to the value of the non-relative FRF (i.e., the FRF shown in Figure 3a) for that time window at corresponding frequency bins.
Figure 3b shows the mean relative FRF for each of the time windows examined. The mean relative FRF for the “early sustained” time window shows a prominent peak at a relative frequency of zero, indicating that, on average, the tuning for this time window is comparable to that for the “on” time window. However, the mean value at the peak is about 85% rather than 100%, thus indicating that tuning within these time windows is not identical. A more marked discrepancy is observed between tuning for the “late sustained” and “off” time windows and that for the “on” time window. Unlike the mean FRF for the “late sustained” time window shown in Figure 3a, the mean relative FRF for the “late sustained” time window does not show a prominent peak at a relative frequency of zero (Figure 3b, middle graph), thus indicating a general lack of consistency in tuning between “late sustained” and “on” responses. Paralleling FRFs from the individual sites shown in Figure 2, the mean relative FRF for the “off” time window is inversely related to the mean FRF for the “on” time window, displaying a trough at a frequency corresponding to the BF of the “on” time window that is flanked by two side lobes beginning at about an octave above and below the BF of the “on” time window (Figure 3b, far right).
The difference between the mean frequency tuning for later time windows and that for the “on” time window is quantified by comparing the mean FRF with the corresponding mean relative FRF for each of the later time windows (i.e., mean values in plots of Figure 3a are compared with mean values in corresponding plots of Figure 3b). A statistically significant difference between the mean FRF and the mean relative FRF for a given time window indicates that average frequency tuning of the response within that time window differs from tuning of the response within the “on” time window. Statistically significant differences (two-tailed t-test; no correction for multiple comparisons) between the mean FRF and the mean relative FRF are observed for all time windows and are indicated by asterisks within the mean relative FRF plots. Corresponding p-values are shown in the box on the left in Figure 3. These results demonstrate that frequency tuning of responses within later time windows differs, on average, from that of responses within the “on” portion of responses.
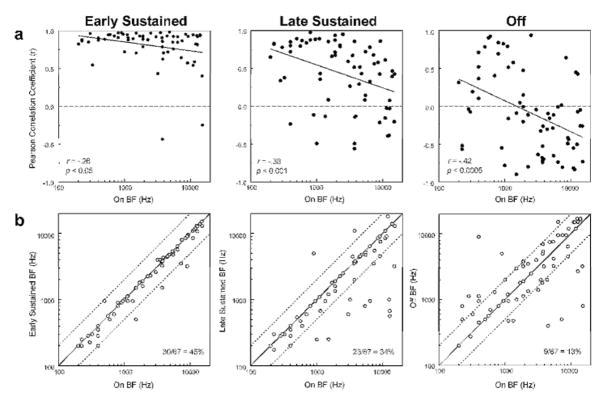
The degree of discrepancy between frequency tuning for the “on” time window and for later time windows is further evaluated by computing the correlation between the FRF for the “on” time window and the FRF for later time windows at each site. Results of this analysis are shown in Figure 4a, wherein the Pearson correlation coefficient computed between the FRF for the “on” time window and the FRF for later time windows is plotted as a function of the BF for the “on” time window. An intriguing and unexpected finding is that the correlation between the FRF for the “on” time window and that for later time windows depends upon the “on” BF, with lower correlation coefficients being more prevalent for sites with a higher “on” BF. Thus, for instance, negatively correlated (and hence inversely related) FRFs for “on” and “off” time windows are observed more frequently at sites with a higher “on” BF. This trend is quantified by statistically significant negative Pearson correlation coefficients (included in the plots) and represented by the negatively sloped regression lines shown superimposed on the plots. Negative correlations between “on” and “off” FRFs were statistically significant (p < 0.05) for 22 of the 67 sites examined.
Figure 4.
a, Pearson correlation coefficients quantifying the relationship between the FRF for the time window indicated at the top of each column and the corresponding FRF for the “on” time window plotted as a function of “on” BF. Correlation coefficients tend to decrease with increasing “on” BF (as represented by the significant negative Pearson correlation coefficients and negatively sloped regression lines shown in the plots). Correlation coefficients become increasingly negative for later time windows, indicating a greater prevalence of inversely tuned “on” and later responses. b, BF of “early sustained”, “late sustained”, and “off” responses are plotted against BF of “on” responses to illustrate changes in BF over the course of the response. Data points falling on the diagonal represent identical BFs for “on” and later responses. Values one octave above and below the “on” BF are represented by the dashed lines. Proportion of sites displaying identical BFs for the “on” time window and for the specified later time window is indicated in the plots. Deviations in BF tend to become more prevalent for later time windows.
Differences between the BF for later time windows and the BF for the “on” time window are represented in Figure 4b, wherein the BF for later time windows is plotted against the BF for the “on” time window. Identical BFs fall on the diagonal, whereas non-identical BFs deviate from the diagonal. Values corresponding to an octave above and below the “on” BF are indicated by the dashed lines. In agreement with the inverse relationship observed between the FRFs for the “on” and “off” time windows, many of the points do not fall on the diagonal for the “off’ time window and may deviate by more than 4 octaves from the “on” BF. The proportion of sites displaying identical BFs for “on” and “early sustained”, “late sustained”, and “off” time windows is 45%, 34%, and 13%, respectively, thus indicating a progressive decrease over time in the correspondence in frequency tuning between early and later portions of the neural response.
Temporal Dynamics of Frequency Tuning in A1 Depends on the BF of “On” Responses
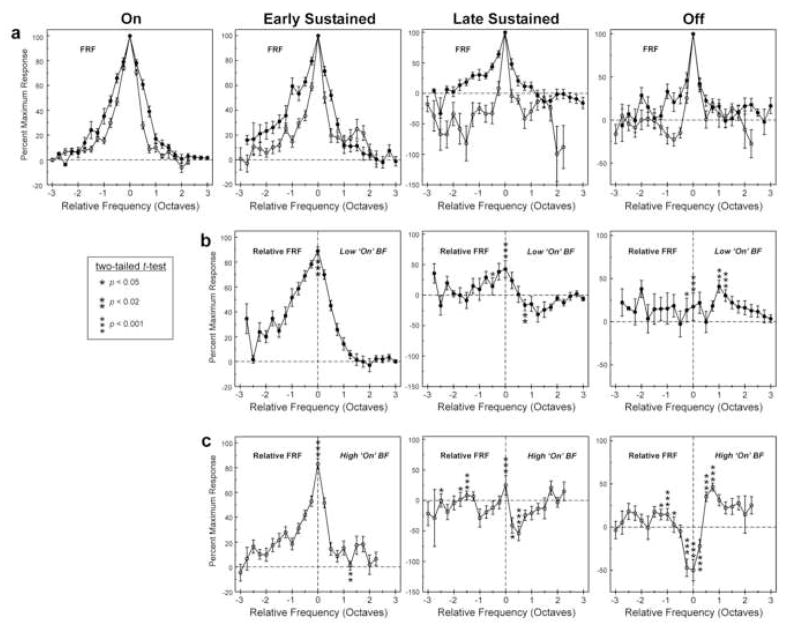
In light of the greater number of negative correlations observed between frequency tuning of “on” and later time windows at sites with a high “on” BF (Figure 4a), we examined whether the mean frequency tuning for each time window differed between sites with a low versus a high “on” BF. Sites with “on” BFs below 2 kHz (N=30) and above 2 kHz (N=37) were defined as having low and high “on” BFs, respectively. While this division is somewhat arbitrary, 2 kHz approximates the zero crossing of the regression line shown in Figure 4a for “off” responses and roughly corresponds to the logarithmic midpoint of the range of “on” BFs.
Figure 5a shows mean FRFs for sites with a low “on” BF (black symbols) and for sites with a high “on” BF (white symbols; same conventions as in Figure 3a). Frequency tuning bandwidth measured on a logarithmic scale is narrower for sites with a high “on” BF for all time windows examined (see Figure 6d for quantification of bandwidth differences). For both sites with a low and a high “on” BF, the mean relative FRF for the “early sustained” portion of the response displays a prominent single peak at a frequency corresponding to the BF of the “on” time window (Figures 5b and 5c). However, with the exception of the “off” time window for sites with a high “on” BF, mean relative FRFs for later time windows display diminished tuning specificity, thus reflecting a general lack of correspondence in tuning between “on” and later portions of the responses. As in Figure 3b, all time windows show statistically significant differences between the mean FRF and the mean relative FRF, thus indicating that frequency tuning of responses within later time windows differs, on average, from that of responses within the “on” portion of responses. In agreement with the greater prevalence of negative correlations between “on” and “off” FRFs observed for sites with a high “on” BF (Figure 4a), the mean relative FRF for the “off’ time window displays a trough at a frequency corresponding to the BF of the “on” time window only for sites with a high “on” BF.
Figure 5.
Mean FRFs (a) and Relative FRFs (b and c) for sites with a low (black symbols) and with a high (white symbols) “on” BF. Same conventions as in Figure 3. The range of data points along the abscissa differs for sites with low and high “on” BF due to the absence of data points at relative frequencies above +2.25 octaves for sites with high “on” BF and the absence of data points at relative frequencies below −2.75 for sites with low “on” BFs. See text for discussion.
Figure 6.
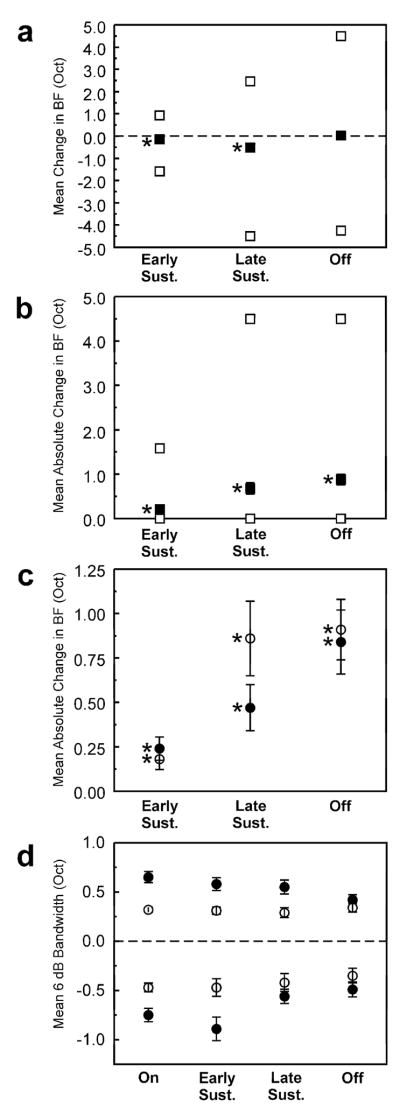
a, Mean change in BF (in octaves) relative to the BF for the “on” time window for each of the time windows examined (black symbols). Error bars represent +/− SEM (some of which are too small to be discernable in the figure). White squares above and below the black symbols represent the upper and lower range of variation in BF, respectively. Mean change in BF is statistically significant (one-sample t-test; p <.0001) for “early sustained” and “late sustained” time windows (as indicated by asterisks). b, Mean absolute change in BF (i.e., ignoring sign). Same conventions as in a. Mean absolute change in BF is statistically significant (one-sample t-test; p <.0001) for all time windows examined. c, Mean absolute change in BF for sites with a low (black symbols) and with a high (white symbols) “on” BF. Mean change in BF is statistically significant for all time windows and for both sites with a low and with a high “on” BF (one-sample t-test; p <.0005). d, Mean 6-dB bandwidth of the FRF for each of the time windows examined. Sites with “on” BFs above and below 2 kHz are represented by the white and black symbols, respectively. Mean upper and lower 6-dB bandwidths are represented by symbols above and below zero, respectively. Error bars represent +/− SEM. Variations in bandwidth across time windows are statistically significant only for lower 6-dB bandwidths at sites with a low “on” BF (one-way ANOVA, p <.01). Mean lower and upper bandwidths are significantly narrower at sites with an “on” BF above 2 kHz (two-way ANOVA, p <.0001).
Mean Change in Frequency Tuning Over Time
The mean deviation in BF between “on” and later time windows is quantified in Figure 6a. The upper and lower ranges of BF deviation are represented by the white square symbols. Mean deviation in BF is significantly different from zero for both “early sustained” and “late sustained” time windows (one-sample, two-tailed t-test, null-hypothesis of zero difference; p <.0001) and is negative, indicating that the BFs for these time windows tend to be lower than the BF for the corresponding “on” time window. Mean deviation in BF for “early sustained” and “late sustained” time windows is −.14 and −0.52 octaves, respectively. Mean deviation in BF for the “off” time window is not statistically significant (p >.05). This is because the deviation in BF for “off” time windows occurs in both positive and negative directions, thus yielding a mean deviation close to zero. However, mean absolute change in BF between “on” and “off” time windows is statistically significant, as is the mean absolute change in BF for “early sustained” and “late sustained” time windows (Figure 6b; one-sample, one-tailed t-test, null hypothesis of zero difference; p <.0001). Mean absolute deviation in BF for “early sustained”, “late sustained”, and “off” time windows is 0.21, 0.68, and 0.88 octaves, respectively. The upper limit of deviation in BF is 1.58, 4.5, and 4.5 octaves for “early sustained”, “late sustained”, and “off” time windows, respectively. Figure 6c shows mean absolute deviation in BF separately averaged for sites with a low “on” BF (black symbols) and with a high “on” BF (white symbols). Mean absolute change in BF is significantly different from zero for all time windows (one-sample, one-tailed t-test, null hypothesis of zero difference; p <.0005) both for sites with a low “on” BF and with a high “on” BF.
In addition to changes in BF, the bandwidth of frequency tuning may change over the course of the response to a tone. Moreover, given the greater prevalence of negative correlations between FRFs for the “on” and “off” time windows at sites with a high “on” BF, variations in tuning bandwidth may partly depend upon “on” BF. Figure 6d shows mean upper (data points above zero) and lower (data points below zero) 6-dB bandwidths (i.e., bandwidths at 50% of the FRF peak) of the FRF for “on”, “early sustained”, “late sustained”, and “off” time windows. Black and white symbols represent data for sites with a low “on” BF and with a high “on” BF, respectively. Mean lower 6-dB bandwidths of the FRF tend to be narrower (closer to zero) for later time windows than for the “on” time window (from two-way ANOVA; F = 4.07, p <.01). This overall effect of window is largely due to changes in bandwidth for sites with a low “on” BF (one-way ANOVA; F = 4.4, p <.01), with no statistically significant effect of window on mean lower 6-dB bandwidth obtained for sites with a high “on” BF (one-way ANOVA; F = 0.54, p >.05). Overall, there was no statistically significant effect of time window on mean upper 6-dB bandwidths (from two-way ANOVA; F = 1.5, p >.05). Mean 6-dB bandwidths are significantly narrower for sites with an “on” BF greater than 2 kHz (two-way ANOVA; upper and lower bandwidth F = 43.1 and 17.9, respectively; p <.0001), in general agreement with differences in the bandwidth of mean FRFs between sites with low and high “on” BF (see Figure 5a).
Effects of Stimulus Intensity
An important question is whether the findings obtained at a stimulus level of 60 dB SPL can be generalized to other stimulus levels. Effects of stimulus intensity on the temporal dynamics of frequency tuning were systematically examined in 22 sites in one monkey performing the previously described behavioral task. Tone intensity was varied in 10-dB increments from 40 to 70 dB SPL. Mean relative FRFs at each of the stimulus levels tested are shown superimposed for sites with a low “on” BF (N=13) and with a high “on” BF (N=9) in Figures 7a and b, respectively. While there are minor quantitative differences between relative FRFs in Figure 7 and those shown in Figures 3 and 5 (which may be due in part to the smaller sample of sites at which effects of stimulus level were systematically tested), results at the different tone intensities are qualitatively similar to those represented in Figures 3 and 5 based on responses to tones presented at 60 dB SPL. For all tone levels tested, peak of the mean relative FRF decreases with increasing time from the initial “on” portion of the response, thus indicating reduced consistency in tuning between “on” and later portions of the response. Note that the mean relative FRF shown for the “on” time window is identical to the mean FRF for the “on” time window, and is included to illustrate effects of stimulus intensity on tuning during the “on” portion of the response. Importantly, the inverse relationship between “on” and “off” FRFs observed for responses to tones presented at 60 dB SPL is observed at all of the stimulus levels tested at sites with a high “on” BF (indicated by the black arrow in the graph on the far right of Figure 7b). These findings suggest that time-dependent variations in frequency tuning are general features of population responses in A1 that persist at least over the 30-dB range of stimulus levels tested. Interestingly, the width of the relative FRF increases markedly with stimulus level at sites with a low “on” BF, whereas it is relatively insensitive to level at sites with a high “on” BF, further suggesting functional differences between sites with a low and a high “on” BF.
Figure 7.
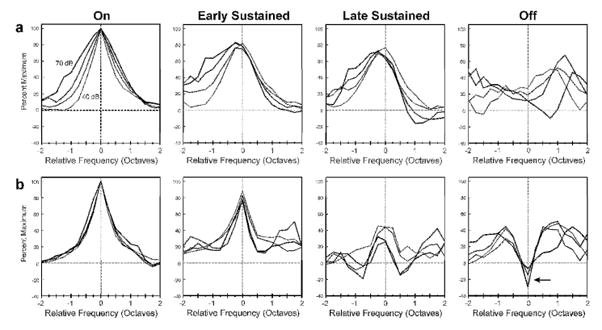
Effects of tone intensity on mean relative FRFs for each of the time windows examined. Same conventions as in Figure 3b. Tone intensity was varied in 10-dB increments from 40 to 70 dB SPL. Mean relative FRFs based on responses to tones presented at increasing stimulus levels are represented by lines of increasing thickness. Mean relative FRFs for sites with a low “on” BF (< 2.0 kHz; N=13) and with a high “on” BF (> 2.0 kHz; N=9) are plotted in a and b, respectively. Relative frequency range is limited to +/− 2.0 octaves to enhance display of differences among mean relative FRFs. Symbols and error bars are omitted for clarity. Note the trough (indicated by arrow) at a relative frequency of 0 octaves in mean relative FRFs for the “off” time window for sites with a high “on” BF.
Additional Evidence for Physiological Differences Between Sites with Lower and Higher “On” BFs
The significantly narrower frequency tuning at sites with a high “on” BF suggests a greater degree of lateral inhibition (suppression of responses by tones with frequencies adjacent to the BF) at sites with a high “on” BF than at sites with a low “on” BF. This conjecture is further supported in Figure 8, wherein mean waveforms of MUA evoked by tones (175 ms duration; 60 dB SPL; frequency = “on” BF) at sites with an “on” BF above (N=28) and below (N=17) 2.0 kHz are compared. Despite the larger amplitude of MUA occurring during the “on” portion of the response at sites with an “on” BF above 2.0 kHz (thick line), the mean amplitude of sustained activity is considerably lower than that at sites with an “on” BF below 2.0 kHz (thin line). Furthermore, during the “off” portion of the response, mean MUA is above baseline at sites with an “on” BF below 2.0 kHz and below baseline at sites with an “on” BF above 2.0 kHz. These findings suggest differences in inhibitory processes operating at sites with lower versus higher “on” BFs that may partly explain differences in the temporal dynamics of frequency tuning observed between sites with lower and higher “on” BFs.
Figure 8.
Mean waveforms of tone-evoked MUA averaged across sites with “on” BFs above 2 kHz (thick line; N=28) and with “on” BFs below 2 kHz (thin line, N=17). Tone frequency = “on” BF; intensity= 60 dB SPL; duration=175 ms, as represented by the horizontal black line. Shaded regions above and below the lines represent +/− SEM. Note the diminished sustained MUA and below-baseline MUA following tone offset at sites with an “on” BF above 2 kHz, suggesting greater overall inhibition.
Discussion
Summary of Findings
The present study demonstrates that the frequency tuning of population responses in lower lamina 3 of A1 is temporally dynamic throughout the response to a tone, and varies depending upon the particular time window analyzed. On average, frequency tuning of “on” and “early sustained” MUA is strongly positively correlated. However, tuning of “late sustained” responses is only weakly correlated with that of “on” responses. Strikingly, on average, tuning of “off” responses is inversely correlated with that of “on” responses. To our knowledge, this inverse relationship has not been previously reported. An additional and intriguing finding is that temporal variation in frequency tuning depends in part on the BF of “on” responses, thus suggesting functional differences between sites with lower and higher “on” BFs.
Relationship to Previous Studies
Several studies have systematically investigated the temporal dynamics of frequency tuning of individual neurons in A1 (Qin et al., 2003; Moshitch et al., 2006; Qin et al., 2007). Qin et al. (2003) examined changes in the frequency response area (FRA) and BF of single units in A1 of alert cats over the 500 ms duration of tone stimulation. Whereas changes in BF of up to 0.15 octave were observed for individual neurons over the course of tone stimulation, average variations in BF over time for the entire sample of cells studied were small (less than .05 octave) and were not statistically significant. This contrasts with the more marked and statistically significant changes in BF observed in the present study. On the other hand, a recent study by Qin et al. (2007) in awake cats reported differences between FRFs of “on” and “off” responses in over half of their sample of single A1 neurons, with some of these differences exceeding an octave. Whereas Qin et al. (2003) reported a statistically significant decrease in the bandwidth of tuning over the course of the response to a tone, in the present study a reduction in tuning bandwidth at later time windows was statistically significant only for lower 6-dB FRF bandwidths at sites with a low “on” BF. Explanations for these discrepancies include species differences, measurement of single- versus multi- unit responses, possible differences in the laminar location of the recorded neurons, that Qin et al. conducted separate analyses of changes in frequency tuning and bandwidth for each of three response types identified in their study (‘Tonic’, ‘Phasic-Tonic’, and ‘Phasic’), and that they examined changes in frequency tuning at a given neuron’s best SPL, in contrast with the present study in which intensity was generally held constant at 60 dB SPL. The present finding that FRF bandwidth is significantly narrower at sites with a high “on” BF (greater than 2 kHz) than at sites with a low “on” BF is consistent with previous reports of a negative correlation between characteristic frequency and tuning bandwidth in A1 of cats (Phillips and Irvine, 1981) and monkeys (Recanzone et al., 1999).
In the study by Moshitch et al. (2006), conducted in halothane anesthetized cats, two measures of changes in the frequency response area (FRA) of single A1 neurons over time were examined: “compactness”, which was defined as the area bounded by the tuning curve divided by the square of the length of the perimeter of the tuning curve (a measure of FRA shape), and mutual information, which quantifies the information about the stimulus contained in the response. Responses to tones of 115 ms duration were analyzed within nine contiguous time windows following tone onset, the first seven with a duration of 30 ms and the eighth and ninth windows with a duration of 100 ms. Moshitch and colleagues reported a decrease in compactness over the course of the response to tones following stimulus onset, suggesting an increase in the diffuseness of the FRA over time. Whereas mutual information tended to be highest within the first 70 ms of the response to a tone and to decrease over the duration of tone stimulation, there was still an appreciable amount of information about the stimulus frequency contained in later responses, including those occurring after tone offset, which sometimes lasted up to hundreds of milliseconds. These findings are broadly consistent with the results of the present study, where significant positive correlations between FRFs for the “on” and later portions of the response were obtained.
A number of studies have reported single units or multiunit clusters in A1 displaying multi-peaked FRFs (e.g., Sutter and Schreiner, 1991; Sutter, 2000; Kadia and Wang, 2003; Moshitch et al., 2006). A1 sites exhibiting multi-peaked tuning within the “on” portion of the response were rare and were not considered in the present study. However, an interesting possibility is that the multi-peaked FRFs reported in many of these previous studies were partly a result of integrating spikes over the entire duration of tone stimulation (e.g., see Kadia and Wang, 2003), an analysis which does not differentiate between initial “on” and later “sustained” neural activity. Indeed, Loftus and Sutter (2001) found that one of the peaks of a two-peaked tuning curve of single neurons in the posterior auditory field of the cat could be attributed to the tuning of earlier spikes and the other peak to the tuning of later spikes evoked by a tone. Importantly, these findings, along with the results of single unit studies of Qin and colleagues (Qin et al., 2003, 2007) indicate that temporally dynamic frequency tuning is displayed by single neurons and suggest that similar variations in tuning of population responses described here are not simply due to the integration of responses of neurons with disparate tuning properties (an issue discussed further below).
Present findings thus suggest that integrating spikes over the entire duration of a stimulus may not adequately characterize the tonotopic organization of auditory cortex and may mask functional differences between initial “on” and later portions of the response. Wang et al. (2005) showed that neurons in A1 of the awake marmoset respond in a sustained manner when they are stimulated with their “preferred” stimulus, defined as the stimulus (which could vary along one or more stimulus dimensions) evoking the maximal firing rate over the entire duration of the sound. However, characterizing the “preferred” stimulus of a population of A1 neurons may depend upon which portion of the response is analyzed. Indeed, the present study suggests that the “preferred” stimulus (at least when restricted to pure tones) for “on” responses may differ considerably from that for “late sustained” and “off” responses.
Possible Mechanisms
The mechanisms underlying the observed variations in frequency tuning over the course of the response to a tone in A1 are unclear. Frequency tuning during the “on” time window may be partly influenced by spectral splatter occurring at the onset of the tone. However, the comparable bandwidth of spectral tuning during the “on” time window and during later time windows suggests that effects of spectral splatter were minimal. Differences in tuning between earlier and later neural responses might be due to activation of the same population of neurons in A1 by thalamic and intracortical inputs with differing frequency tuning characteristics and latencies. In particular, frequency tuning of “on” responses may predominantly reflect tuning of thalamic afferents, whereas frequency tuning of later responses may be determined more by intracortical inputs (Matsubara and Phillips, 1988; Ojima and Takayanagi, 2004). Accordingly, intracellular studies of neurons in rat A1 suggest that thalamic inputs determine the sub-threshold responding range of auditory cortical neurons (Kaur et al., 2004; Liu et al., 2007), while subsequent, more narrowly tuned intracortical recurrent excitatory inputs (Liu et al., 2007) and more broadly tuned lateral inhibitory inputs (Wu et al., 2008) contribute to the enhanced frequency selectivity of supra-threshold responses of auditory cortical neurons.
The inverse relationship observed between frequency tuning during the “on” and “off” time windows may be explained by considering that neural activity evoked during each portion of the response in A1 reflects the sum of excitatory and inhibitory inputs (Volkov and Galazyuk, 1992; Kaur et al., 2004; Wu et al., 2008). If there is greater overall lateral inhibition at sites with a high “on” BF (as suggested in Figure 8), then removal of excitatory thalamic input following the termination of a tone would result in a net suppression of neural activity, as reflected by the corresponding trough observed in the mean relative FRF of “off” responses at a frequency corresponding to the “on” BF. Conversely, the side lobes flanking the trough in the mean relative FRF of responses occurring during the “off” time window may reflect a release from lateral inhibition following the termination of tone stimulation. That these side lobes occur at frequencies approximately 1 octave above and below the “on” BF may be related to the bandwidth of lateral inhibitory interactions proposed to underlie response suppression in A1, as observed in simultaneous masking studies (e.g., Shamma and Symmes, 1985; Kadia and Wang, 2003; Fishman and Steinschneider, 2006).
In evaluating the significance of the present results, an important consideration is whether the population responses represented by MUA reflect the activity of neurons with potentially different tuning properties and onset latencies (see Supér and Roelfsema, 2005). However, MUA displays many features that are similar to those of single-unit responses in A1, thus suggesting that the present findings are not simply an artifact of recording population activity. First, variations in frequency tuning over the course of a response to a tone have been observed for single A1 neurons in the studies of Qin et al. (2003, 2007). Second, multi-peaked tuning curves have been obtained for single neurons when summing activity over the entire duration of a tone stimulus, indicating that temporally distributed integration of spectral information occurs within individual neurons. Third, whereas significant differences in tuning bandwidth between single-unit and population responses would suggest that variations in frequency tuning of MUA observed in the present study reflect the integrated activity of many neurons, each with different tuning characteristics, mean 6-dB bandwidths of FRFs based on responses to 60 dB SPL tones in the present study are comparable to Q40 dB frequency tuning bandwidths (defined as the characteristic frequency divided by the bandwidth of the FRF at 40 dB above response threshold) reported in single-unit studies of A1 in anesthetized owl monkeys (Recanzone et al., 1999) and in awake macaques (Recanzone et al., 2000a).
On the other hand, whether or not the population activity recorded in this study reflects the aggregate activity of individual neurons with different response properties may be relatively inconsequential from the perspective of A1 function. In light of the functional relevance of population activity for cortical information processing and transmission (see Introduction), a primary rationale of the present study was to examine whether temporally dynamic frequency tuning reported in earlier single-unit studies is displayed also by population responses in A1. Indeed, that individual A1 neurons display temporally dynamic frequency tuning does not necessarily imply that these variations in tuning are reliably transmitted through subsequent stages of processing. Thus, the finding that population responses also display variations in tuning suggests that these differences over time are more likely to be reliably transmitted to other brain regions (deCharms and Merzenich, 1996; Lisman, 1997; Eggermont, 2001).
Functional Implications
While it is possible that the population of neurons responding during the “sustained” and “off” portions of the response consists of different neurons with different tuning properties from those responding during the “on” portion of the response (as discussed above), the fact that neurons at the same anatomical location and cortical lamina in A1 may respond preferentially to markedly different frequencies depending upon the portion of the response analyzed nonetheless has potentially significant implications for the representation and processing of acoustic information within A1.
First, the good correlation between the tuning of “on” and “early sustained” responses and the comparatively poor correlation between the tuning of “on” responses and that of “late sustained” responses suggest that information reliably related to the frequency of a tone is represented largely within the first 100 ms of the response in A1. Moreover, the time-dependence of frequency tuning in A1 suggests that a topographic representation of sound frequency based upon the tuning of “late sustained” responses might differ markedly from the classic tonotopic organization based upon the tuning of “on” responses. This possibility is consistent with the observation that even a simple stimulus such as a pure tone can evoke a complex distributed and multi-focal pattern of activation within auditory cortex (Schreiner, 1998).
Second, the inverse relationship between “on” and “off” FRFs and the below-baseline trough commonly observed in “off” FRFs suggest a period of inhibition following the termination of a tone with a frequency equal to the “on” BF. Inhibition triggered by tone offset of subsequent tone responses may play an important functional role in the processing of sound sequences in A1. Such “forward inhibition” has been proposed as a mechanism of physiological forward masking (Calford and Semple, 1995; Brosch and Schreiner, 1997) and of auditory stream segregation in A1 (Fishman et al., 2004). In addition to forward inhibition, studies of responses to two-tone sequences in A1 of macaques have shown that the response to a second tone may be enhanced by the response to a preceding tone and that maximal response enhancement occurs when frequencies of the tones are approximately 1 octave apart from each other (Brosch et al., 1999). The two side lobes occurring approximately 1 octave above and below the “on” BF in the “off” FRFs observed in the present study may partly account for stimulus conditions inducing response enhancement in A1. Thus, the neural representation of the temporal structure of sounds may depend in part on the temporal dynamics of frequency tuning in A1.
Third, the observed differences in the temporal dynamics of frequency tuning between sites with lower and higher “on” BFs might be related to differential processing of acoustic cues used in sound localization for lower and higher frequency signals. Lower and higher frequency signals are localized via interaural time differences and interaural level differences, respectively (e.g., Macpherson and Middlebrooks, 2002). The reduced apparent inhibition in “late sustained” portions of responses to tones at sites with lower “on” BFs (Figure 8) is consistent with the importance of ongoing temporal cues for the localization of lower frequency signals, as represented by neural discharges phase-locked to cycles of binaural signals generating interaural phase differences in lower BF regions of A1 in cats and monkeys (e.g., Reale and Brugge, 1990; Scott et al., 2009).
Finally, the present findings are consistent with the possibility that later portions of neural responses in A1 represent stimulus or perceptual attributes other than frequency, such as pitch (Fishman and Steinschneider, 2008), frequency and amplitude modulation (e.g., Bieser and Muller-Preuss, 1996; Steinschneider et al., 1998; Fishman et al., 2000b, 2001; Atencio et al., 2007; Qin et al., 2008), or location (Ahissar et al., 1992). Later sustained neural activity in A1 may also reflect multisensory interactions (Schroeder and Foxe, 2005), behavioral salience (e.g., Mehta et al., 2000; Supér et al., 2001; Selezneva et al., 2006), or stimulus deviance (Ulanovsky et al., 2003). Temporally dynamic frequency tuning of neural populations in A1 may be relevant also for the cortical coding and categorization of biologically relevant sounds with dynamic spectra, such as species-specific vocalizations (Wang et al., 1995; Ghazanfar and Hauser, 2001). Thus, while speculative, the notion that various features of a sound might be represented in a ‘multiplexed’ manner within a single temporally distributed neural response in A1 remains an intriguing hypothesis to be explored in future work.
Acknowledgments
We are grateful to Dr. Steven Walkley, May Huang, Linda O’Donnell, Shirley Seto, and Jeannie Hutagalung for technical, secretarial, and histological assistance. We also thank two anonymous reviewers for helpful comments on a previous version of the manuscript.
Grants
Supported by National Institute of Deafness and Other Communications Disorders Grant DC-00657.
List of Abbreviations
- A1
Primary Auditory Cortex
- AEP
Auditory Evoked Potential
- BF
Best Frequency
- CSD
Current Source Density
- FM
Frequency Modulation
- FRA
Frequency Response Area
- FRF
Frequency Response Function
- MUA
Multiunit Activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahissar M, Ahissar E, Bergman H, Vaadia E. Encoding of sound-source location and movement: activity of single neurons and interactions between adjacent neurons in the monkey auditory cortex. J Neurophysiol. 1992;67:203–215. doi: 10.1152/jn.1992.67.1.203. [DOI] [PubMed] [Google Scholar]
- Atencio CA, Blake DT, Strata F, Cheung SW, Merzenich MM, Schreiner CE. Frequency-modulation encoding in the primary auditory cortex of the awake owl monkey. J Neurophysiol. 2007;98:2182–2195. doi: 10.1152/jn.00394.2007. [DOI] [PubMed] [Google Scholar]
- Barna J, Arezzo JC, Vaughan HG., Jr A new multicontact array for the simultaneous recording of field potentials and unit activity. Electroencephalogr Clin Neurophysiol. 1981;52:494–496. doi: 10.1016/0013-4694(81)90035-3. [DOI] [PubMed] [Google Scholar]
- Bieser A, Muller-Preuss P. Auditory responsive cortex in the squirrel monkey: neural responses to amplitude-modulated sounds. Exp Brain Res. 1996;108:273–284. doi: 10.1007/BF00228100. [DOI] [PubMed] [Google Scholar]
- Brosch M, Bauer R, Eckhorn R. Stimulus-dependent modulations of correlated high- frequency oscillations in cat visual cortex. Cereb Cortex. 1997;7:70–76. doi: 10.1093/cercor/7.1.70. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J Neurophysiol. 1997;77:923–943. doi: 10.1152/jn.1997.77.2.923. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schulz A, Scheich H. Processing of sound sequences in macaque auditory cortex: response enhancement. J Neurophysiol. 1999;82:1542–1559. doi: 10.1152/jn.1999.82.3.1542. [DOI] [PubMed] [Google Scholar]
- Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J Neurophysiol. 1995;73:1876–1891. doi: 10.1152/jn.1995.73.5.1876. [DOI] [PubMed] [Google Scholar]
- Cheung SW, Bedenbaugh PH, Nagarajan SS, Schreiner CE. Functional organization of squirrel monkey primary auditory cortex: responses to pure tones. J Neurophysiol. 2001;85:1732–1749. doi: 10.1152/jn.2001.85.4.1732. [DOI] [PubMed] [Google Scholar]
- deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hear Res. 2001;157:1–42. doi: 10.1016/s0378-5955(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Arezzo JC, Steinschneider M. Auditory stream segregation in monkey auditory cortex: effects of frequency separation, presentation rate, and tone duration. J Acoust Soc Am. 2004;116:1656–1670. doi: 10.1121/1.1778903. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Complex tone processing in primary auditory cortex of the awake monkey. II Pitch versus critical band representation. J Acoust Soc Am. 2000a;108:247–262. doi: 10.1121/1.429461. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Reser DH, Arezzo JC, Steinschneider M. Complex tone processing in primary auditory cortex of the awake monkey. I Neural ensemble correlates of roughness. J Acoust Soc Am. 2000b;108:235–246. doi: 10.1121/1.429460. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Steinschneider M. Spectral resolution of monkey primary auditory cortex A1 revealed with two-noise masking. J Neurophysiol. 2006;96:1105–1115. doi: 10.1152/jn.00124.2006. [DOI] [PubMed] [Google Scholar]
- Fishman YI, Steinschneider M. Pitch is represented by a rate-latency code in monkey primary auditory cortex. Assoc Res Otolaryngol Abs. 2008:679. [Google Scholar]
- Fishman YI, Volkov IO, Noh MD, Garell PC, Bakken H, Arezzo JC, Howard MA, Steinschneider M. Consonance and dissonance of musical chords: neural correlates in auditory cortex of monkeys and humans. J Neurophysiol. 2001;86:2761–2788. doi: 10.1152/jn.2001.86.6.2761. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Nicholson C. Experimental optimization of current source density technique for anuran cerebellum. J Neurophysiol. 1975;38:369–382. doi: 10.1152/jn.1975.38.2.369. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Hauser MD. The auditory behaviour of primates: a neuroethological perspective. Curr Opin Neurobiol. 2001;11:712–720. doi: 10.1016/s0959-4388(01)00274-4. [DOI] [PubMed] [Google Scholar]
- Hackett TA, Stepniewska I, Kaas JH. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol. 1998;394:475–495. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of bilateral auditory cortex lesions on sound localization in Japanese macaques. J Neurophysiol. 1990;64:915–931. doi: 10.1152/jn.1990.64.3.915. [DOI] [PubMed] [Google Scholar]
- Kadia SC, Wang X. Spectral integration in A1 of awake primates: neurons with single- and multipeaked tuning characteristics. J Neurophysiol. 2003;89:1603–1622. doi: 10.1152/jn.00271.2001. [DOI] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Pincze Z, Fu KM, Javitt DC, Karmos G, Schroeder CE. Timing of pure tone and noise-evoked responses in macaque auditory cortex. Neuroreport. 2005;16:933–937. doi: 10.1097/00001756-200506210-00011. [DOI] [PubMed] [Google Scholar]
- Legatt AD, Arezzo J, Vaughan HG., Jr Averaged multiple unit activity as an estimate of phasic changes in local neuronal activity: effects of volume-conducted potentials. J Neurosci Methods. 1980;2:203–217. doi: 10.1016/0165-0270(80)90061-8. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nat Neurosci. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus WC, Sutter ML. Spectrotemporal organization of excitatory and inhibitory receptive fields of cat posterior auditory field neurons. J Neurophysiol. 2001;86:475–491. doi: 10.1152/jn.2001.86.1.475. [DOI] [PubMed] [Google Scholar]
- Macpherson EA, Middlebrooks JC. Listener weighting of cues for lateral angle: the duplex theory of sound localization revisited. J Acoust Soc Am. 2002;111:2219–2236. doi: 10.1121/1.1471898. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Hall AJ, Lomber SG. Cortical control of sound localization in the cat: unilateral cooling deactivation of 19 cerebral areas. J Neurophysiol. 2004;92:1625–1643. doi: 10.1152/jn.01205.2003. [DOI] [PubMed] [Google Scholar]
- Matsubara JA, Phillips DP. Intracortical connections and their physiological correlates in the primary auditory cortex AI of the cat. J Comp Neurol. 1988;268:38–48. doi: 10.1002/cne.902680105. [DOI] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Brugge JF. Representation of the cochlear partition of the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cruikshank SJ. Thalamocortical inputs trigger a propagating envelope of gamma-band activity in auditory cortex in vitro. Exp Brain Res. 1999;126:160–174. doi: 10.1007/s002210050726. [DOI] [PubMed] [Google Scholar]
- Morel A, Garraghty PE, Kaas JH. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol. 1993;335:437–459. doi: 10.1002/cne.903350312. [DOI] [PubMed] [Google Scholar]
- Moshitch D, Las L, Ulanovsky N, Bar-Yosef O, Nelken I. Responses of neurons in primary auditory cortex A1 to pure tones in the halothane-anesthetized cat. J Neurophysiol. 2006;95:3756–3769. doi: 10.1152/jn.00822.2005. [DOI] [PubMed] [Google Scholar]
- Muller-Preuss P, Mitzdorf U. Functional anatomy of the inferior colliculus and the auditory cortex: current source density analyses of click-evoked potentials. Hear Res. 1984;16:133–142. doi: 10.1016/0378-5955(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci. 1990;13:201–288. [Google Scholar]
- Nicholson C, Freeman JA. Theory of current source density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol. 1975;38:356–368. doi: 10.1152/jn.1975.38.2.356. [DOI] [PubMed] [Google Scholar]
- Ojima H, Takayanagi M. Cortical convergence from different frequency domains in the cat primary auditory cortex. Neurosci. 2004;126:203–212. doi: 10.1016/j.neuroscience.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Population coding of shape in area V4. Nat Neurosci. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- Petersen RS, Panzeri S, Diamond ME. Population coding in somatosensory cortex. Curr Opin Neurobiol. 2002;12:441–447. doi: 10.1016/s0959-4388(02)00338-0. [DOI] [PubMed] [Google Scholar]
- Phillips DP, Irvine DR. Responses of single neurons in physiologically defined primary auditory cortex (AI) of the cat: frequency tuning and responses to intensity. J Neurophysiol. 1981;45:48–58. doi: 10.1152/jn.1981.45.1.48. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR, editors. Neurophysiology. Springer-Verlag; New York: 1992. The Mammalian Auditory Pathway. [Google Scholar]
- Qin L, Chimoto S, Sakai M, Wang J, Sato Y. Comparison between offset and onset responses of primary auditory cortex ON-OFF neurons in awake cats. J Neurophysiol. 2007;97:3421–3431. doi: 10.1152/jn.00184.2007. [DOI] [PubMed] [Google Scholar]
- Qin L, Kitama T, Chimoto S, Sakayori S, Sato Y. Time course of tonal frequency- response-area of primary auditory cortex neurons in alert cats. Neurosci Res. 2003;46:145–152. doi: 10.1016/s0168-0102(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Qin L, Wang J, Sato Y. Heterogeneous neuronal responses to frequency-modulated tones in the primary auditory cortex of awake cats. J Neurophysiol. 2008;100:1622–1634. doi: 10.1152/jn.90364.2008. [DOI] [PubMed] [Google Scholar]
- Reale RA, Brugge JF. Auditory cortical neurons are sensitive to static and continuously changing interaural phase cues. J Neurophysiol. 1990;64:1247–1260. doi: 10.1152/jn.1990.64.4.1247. [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Response profiles of auditory cortical neurons to tones and noise in behaving macaque monkeys. Hear Res. 2000b;150:104–118. doi: 10.1016/s0378-5955(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Guard DC, Phan ML. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J Neurophysiol. 2000a;83:2315–2331. doi: 10.1152/jn.2000.83.4.2315. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol. 1999;415:460–481. doi: 10.1002/(sici)1096-9861(19991227)415:4<460::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperative synchronized assemblies enhance orientation discrimination. Proc Natl Acad Sci U S A. 2004;101:6722–6727. doi: 10.1073/pnas.0401661101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger TD. Neural population codes. Curr Opin Neurobiol. 2003;13:238–249. doi: 10.1016/s0959-4388(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Schreiner CE. Spatial distribution of responses to simple and complex sounds in the primary auditory cortex. Audiol Neurootol. 1998;3:104–122. doi: 10.1159/000013785. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Foxe J. Multisensory contributions to low-level, ‘unisensory’ processing. Curr Opin Neurobiol. 2005;15:454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Scott BH, Malone BJ, Semple MN. Representation of dynamic interaural phase difference in auditory cortex of awake rhesus macaques. J Neurophysiol. 2009 doi: 10.1152/jn.00678.2007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selezneva E, Scheich H, Brosch M. Dual time scales for categorical decision making in auditory cortex. Curr Biol. 2006;16:2428–2433. doi: 10.1016/j.cub.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Shamma SA, Symmes D. Patterns of inhibition in auditory cortical cells in awake squirrel monkeys. Hear Res. 1985;19:1–13. doi: 10.1016/0378-5955(85)90094-2. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Fishman YI, Arezzo JC. Spectrotemporal analysis of evoked and induced electroencephalographic responses in primary auditory cortex A1 of the awake monkey. Cereb Cortex. 2008;18:610–625. doi: 10.1093/cercor/bhm094. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Fishman YI, Arezzo JC. Representation of the voice onset time VOT speech parameter in population responses within primary auditory cortex of the awake monkey. J Acoust Soc Am. 2003;114:307–321. doi: 10.1121/1.1582449. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Reser DH, Fishman YI, Schroeder CE, Arezzo JC. Click train encoding in primary auditory cortex of the awake monkey: evidence for two mechanisms subserving pitch perception. J Acoust Soc Am. 1998;104:2935–2955. doi: 10.1121/1.423877. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Schroeder CE, Arezzo JC, Vaughan HG., Jr Speech-evoked activity in primary auditory cortex: effects of voice onset time. Electroencephalogr Clin Neurophysiol. 1994;92:30–43. doi: 10.1016/0168-5597(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Tenke CE, Schroeder CE, Javitt DC, Simpson GV, Arezzo JC, Vaughan HG., Jr Cellular generators of the cortical auditory evoked potential initial component. Electroencephalogr Clin Neurophysiol. 1992;84:196–200. doi: 10.1016/0168-5597(92)90026-8. [DOI] [PubMed] [Google Scholar]
- Steinschneider M, Volkov IO, Fishman YI, Oya H, Arezzo JC, Howard MA., 3rd Intracortical responses in human and monkey primary auditory cortex support a temporal processing mechanism for encoding of the voice onset time phonetic parameter. Cereb Cortex. 2005;15:170–186. doi: 10.1093/cercor/bhh120. [DOI] [PubMed] [Google Scholar]
- Sukov W, Barth DS. Three-dimensional analysis of spontaneous and thalamically evoked gamma oscillations in auditory cortex. J Neurophysiol. 1998;79:2875–2884. doi: 10.1152/jn.1998.79.6.2875. [DOI] [PubMed] [Google Scholar]
- Super H, Roelfsema PR. Chronic multiunit recordings in behaving animals: advantages and limitations. Prog Brain Res. 2005;147:263–282. doi: 10.1016/S0079-6123(04)47020-4. [DOI] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex V1. Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- Sutter ML. Shapes and level tolerances of frequency tuning curves in primary auditory cortex: quantitative measures and population codes. J Neurophysiol. 2000;84:1012–1025. doi: 10.1152/jn.2000.84.2.1012. [DOI] [PubMed] [Google Scholar]
- Sutter ML, Schreiner CE. Physiology and topography of neurons with multipeaked tuning curves in cat primary auditory cortex. J Neurophysiol. 1991;65:1207–1226. doi: 10.1152/jn.1991.65.5.1207. [DOI] [PubMed] [Google Scholar]
- Talwar SK, Musial PG, Gerstein GL. Role of mammalian auditory cortex in the perception of elementary sound properties. J Neurophysiol. 2001;85:2350–2358. doi: 10.1152/jn.2001.85.6.2350. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Shah GD, Braida LD. Functional role of auditory cortex in frequency processing and pitch perception. J Neurophysiol. 2002;87:122–139. doi: 10.1152/jn.00104.1999. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- Vaughan HG, Jr, Arezzo JC. The neural basis of event-related potentials. In: Picton TW, editor. Human-Event Related Potentials, EEG Handbook, Revised Series. 3. Elsevier; Ireland: 1988. pp. 45–96. [Google Scholar]
- Volkov IO, Galazyuk AV. Peculiarities of inhibition in cat auditory cortex neurons evoked by tonal stimuli of various durations. Exp Brain Res. 1992;91:115–120. doi: 10.1007/BF00230019. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Kitzes LM, Jones EG. Chemoarchitectonic organization of the cat primary auditory cortex. Exp Brain Res. 1991;86:518–526. doi: 10.1007/BF00230525. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu T, Snider RK, Liang L. Sustained firing in auditory cortex evoked by preferred stimuli. Nature. 2005;435:341–346. doi: 10.1038/nature03565. [DOI] [PubMed] [Google Scholar]
- Wang X, Merzenich MM, Beitel R, Schreiner CE. Representation of a species-specific vocalization in the primary auditory cortex of the common marmoset: temporal and spectral characteristics. J Neurophysiol. 1995;74:2685–2706. doi: 10.1152/jn.1995.74.6.2685. [DOI] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 2008;58:132–143. doi: 10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]