Abstract
Tibia fracture in rats evokes nociceptive, vascular, and bone changes resembling complex regional pain syndrome (CRPS). Substance P (SP) signaling contributes to the hindpaw warmth, increased vascular permeability, and edema observed in this model, suggesting that neurogenic inflammatory responses could be enhanced after fracture. Four weeks after tibia fracture we measured SP and calcitonin gene-related peptide (CGRP) protein levels in the sciatic nerve and serum. Hindpaw skin extravasation responses and SP receptor (NK1), CGRP receptor (calcitonin receptor-like receptor, CRLR) and neutral endopeptidase (NEP) protein levels were also determined. Gene expression levels of these peptides, receptors, and peptidase were examined in the DRG and skin. Spontaneous and intravenous SP-evoked extravasation responses were increased ipsilateral, but not contralateral to the fracture. Fracture increased SP and CGRP gene expression in the ipsilateral L4,L5 DRG and neuropeptide protein levels in the sciatic nerve and in serum, but had no effect on electrically-evoked SP and CGRP release. NK1 receptor expression was increased in the ipsilateral hindpaw skin keratinocytes and endothelial cells after injury, but CRLR and NEP expression were unchanged. Fracture also increased epidermal thickness, but had no effect on epidermal skin neurite counts. These results demonstrate that spontaneous and intravenous SP-evoked extravasation responses are enhanced in the ipsilateral hindlimb after fracture and that fracture chronically increases the expression of endothelial and keratinocyte NK1 receptors in the injured limb. We postulate that SP activation of these up-regulated NK1 receptors results in skin warmth, protein leakage, edema, and keratinocyte proliferation in the injured limb.
Keywords: fracture, substance P, microdialysis, calcitonin gene-related peptide, complex regional pain syndrome, neurogenic inflammation
1. Introduction
Previously proposed complex regional pain syndrome (CRPS) animal models have not reproduced the inciting trauma and the complex nociceptive, vascular and bone changes observed in the CRPS patient. CRPS type I is a frequent sequela of distal tibia [28] and radius fractures [1, 4]. The affected limb presents with an initial increase in skin temperature [5, 34], increased cutaneous protein extravasation [23], distal limb edema [32], pain, and allodynia [8, 33]. Periarticular osteoporosis also develops in the fracture limb [3, 28]. Recently we described a rat tibia fracture model that closely resembles the clinical scenario [12, 13, 26, 27]. After distal tibia fracture rats develop chronic unilateral hindlimb warmth, edema, facilitated spontaneous protein extravasation, allodynia, unweighting, increased spinal Fos, and periarticular osteoporosis, changes resembling those observed in CRPS I patients.
We postulate that the enhanced vascular permeability, edema, warmth, redness, and vasodilatation observed in CRPS could be attributable to an exaggerated neurogenic inflammatory response. Neurogenic inflammation is mediated by the activation of small diameter sensory afferents in the skin, triggering the peripheral release of substance P (SP) and calcitonin gene-related peptide (CGRP), which activate their cognate receptors in the dermal vasculature to induce protein extravasation and vasodilation. Centrally released SP facilitates sensitization of second-order spinal neurons via activation of the NK1 receptors on ascending spinal neurons, resulting in spontaneous pain and hyperalgesia [2, 21]. Recently we observed that the SP neurokinin 1 (NK1) receptor antagonist LY303870 partially reversed the warmth, edema, spontaneous extravasation, and allodynia observed after tibia fracture [12], evidence supporting our hypothesis that SP signaling contributes to the vascular and nociceptive sequelae of fracture. Furthermore, the intravascular injection of SP evoked enhanced extravasation and edema responses in the injured limb, suggesting that post-junctional facilitation of SP signaling contributes to the increased protein extravasation and hindpaw edema observed after fracture [13].
Several randomized controlled trials have reported that a 4–12 week course of high-dose glucocorticoid treatment can alleviate pain, hyperalgesia, and edema in CRPS patients [10, 11]. Chronic glucocorticoid treatment dose-dependently inhibits electrically-evoked extravasation responses and intravenous SP-evoked extravasation responses in the hindpaw skin of normal rats [14], indicating a glucocorticoid inhibitory effect on neurogenic inflammation. When high-dose glucocorticoids are chronically administered after tibia fracture in rats there is a reduction in hindpaw warmth, spontaneous protein extravasation, and edema [13]. Chronic glucocorticoid administration also blocks the facilitated SP-evoked extravasation and edema responses in the fracture hindpaw. Collectively, these data suggest that facilitated neurogenic inflammatory responses contribute to the development of vascular abnormalities in the rat tibia fracture model of CRPS. To further test this hypothesis the effects of fracture on SP and CGRP signaling in the cutaneous microvasculature were investigated.
2. Materials and methods
All experiments followed the guidelines of the IASP [36] and were approved by our institute’s Subcommittee on Animal Studies. Adult (10-month-old) male Sprague Dawley rats (Harlan, Indianapolis, IN) were used in all experiments. The animals were housed individually in isolator cages with solid floors covered with 3 cm of soft bedding. Fracture rats were fed and watered ad libitum, whereas control rats were pair-fed so as to match the diet of the fracture rats. During the experimental period the animals were fed Lab Diet 5012 (PMI Nutrition Institute, Richmond, IN), which contains 1.0% calcium, 0.5% phosphorus, and 3.3 IU/g of vitamin D3.
2.1 Surgery
Tibia fracture was performed under isoflurane anesthesia, as previously described by our group [12, 13]. The right hindlimb was wrapped in stockinet (2.5 cm wide) and the distal tibia was fractured using pliers with an adjustable stop (Visegrip, Petersen Manufacturing, Dewitt, NE) that had been modified with a 3-point jaw. The hindlimb wrapped in casting tape (Delta-Lite, Johnson & Johnson, Raynham, MA) so the hip, knee and ankle were flexed. The cast extended from the metatarsals of the hindpaw up to a spica formed around the abdomen. The cast over the paw was applied only to the plantar surface; a window was left open over the dorsum of the paw and ankle to prevent constriction when post-fracture edema developed. To prevent the animals from chewing at their casts, the cast material was wrapped in galvanized wire mesh, which was attached by wires inserted into holes drilled in the cast. After fracture and casting, the rats were given subcutaneous saline and 2 days of subcutaneous buprenorphine (0.3 mg/kg) for post-operative hydration and analgesia. At 4 weeks the rats were anesthetized with isoflurane and the cast removed with a vibrating cast saw. After 4 weeks cast immobilization all rats had functional union at the fracture site by manual examination. All extravasation and microdialysis experiments were performed the day after cast removal (29 days after fracture). All tissues used for ELISA, real-time PCR, Western blotting and immunohistochemistry studies were also collected the day after cast removal.
2.2 Skin extravasation assays
Spontaneous protein extravasation
The aim of this experiment was to determine whether tibia fracture increases spontaneous protein extravasation in the injured hindlimb after injury After 4 weeks immobilization the cast was removed and the next day Evans blue dye (50mg/kg, Sigma) was administered intravenously in a 50mg/ml solution in 0.9% saline. Exactly 24 h later, the rats were deeply anesthetized with isoflurane and transcardially perfused with 1000 ml of saline (0.9%), suspended 100 cm above the heart (50 mm Hg perfusion pressure). Then the plantar and dorsal skin on each hindpaw was excised from the base of the heel to the tip of the third digit and weighed. The excised tissue was placed in 4 ml of 99% formamide in a shaker bath at 55°C for 72 h. The extracted dye concentration was determined spectrophotometrically at a wavelength of 620 nm. Since Evans blue binds to serum albumin in vitro and in vivo, the dye content of the hindpaw skin provides an accurate measure of spontaneous protein extravasation into the interstitial space [29]. The spontaneous 24 h extravasation response in the hindpaw of the fracture limb was compared to the contralateral hindpaw in the intact limb and to the hindpaw extravasation response in naïve control rats.
SP-evoked extravasation
This experiment tested the hypothesis that tibia fracture enhances SP-evoked extravasation responses in the injured hindlimb at 4 weeks after injury, when compared with the contralateral intact hindlimb or normal controls. Five minutes after injection of Evans blue dye (50 mg/kg, Sigma) SP (10 ug/kg, Sigma) was injected intravenously into the internal jugular vein in a 10 mg/ml ringers solution. Five minutes after SP injection the rats were anesthetized with isoflurane, transcardially perfused as previously described, and the plantar and dorsal skin on each hindpaw was collected for dye content determination.
2.3 Dermal microdialysis
Electrically evoked extravasation and neuropeptide release
The aim of this experiment was to determine whether fracture increases electically-evoked neuropeptide release in the hindpaw skin at 4 weeks post-injury. Evans blue dye (25mg/kg) was injected into the jugular vein and 3 h later under isoflurane anesthesia two hollow plasmapheresis fibers (0.4 mm diameter, 3000 kDa cutoff; Dermal Dialysis, Mannheim, Germany), were inserted intradermally into the plantar skin of the right hind paw over a length of 1.5 cm, using a 25 G cannula. The two fibers were placed 5–6 mm apart parallel to the axis of the rat’s paw. The fibers were perfused with Ringer’s solution (Abbott Laboratories, Chicago, IL) at a constant flow rate of 1.5 ul/min using a microdialysis pump (Harvard Apparatus) and dialysate samples were collected every 30 min. After a baseline stabilization period of 90 minutes, the right sciatic nerve was electrically stimulated for 30 min (5 Hz, 0.5 ms pulse duration, 10 mA), followed by a washout period of 30 min. Throughout the microdialysis procedure the rectal temperature was monitored and maintained at 36–37°C. All elution aliquots were collected on ice and then immediately snap-frozen and stored at −80°C until analysis. After perfusion the dialysis fibers were dissected out of the hindpaw skin to confirm intradermal placement. Electrically-evoke neuropeptide release was measured in microdialysis samples that were pooled (dialysate from 8 fibers in 4 hindpaws per aliquot) to enhance detection of neuropeptides. SP and CGRP protein content was determined by ELISA as describe in Section 2.4.
2.4 ELISA procedure for sciatic nerve, dialysate, and serum SP and CGRP content
The aim of this experiment was to determine whether fracture induces increased SP and CGRP protein expression in the sciatic nerve or the serum at 4 weeks post-injury. The right sciatic nerve was collected under isoflurane anesthesia, immediately frozen, and weighed. Nerve samples were minced in 1 ml of 3:1 ethanol/0.7M HCl and homogenized for 20 s. The homogenates were shaken for 2 h at 4°C and centrifuged at 3000g for 20 min at 4°C. The supernatant was frozen and lyophilized, and the lyophilized product was stored at −80°C. All nerve and dialysis samples were assayed in duplicate using EIA kits for SP (Assay Designs) and for CGRP (Caymen Chemical). Rat serum was collected from the tail vein and the serum neuropeptide levels were determined using SP (Assay Designs) and CGRP (Phoenix Pharmaceuticals) EIA kits. Both assays followed the manufacturer’s protocols except that the SP standard was purchased from Sigma and reconstituted with the assay buffer in the SP EIA kit to the same standard concentrations recommended by the manufacturer.
2.5 RNA isolation, reverse transcription, and real-time PCR
These experiments tested the hypothesis that tibia fracture could chronically enhance neuropeptide signaling in the hindpaw skin, either by; 1) increasing SP and CGRP gene expression in the sensory afferent neurons innervating the hindpaw, or 2) by increasing gene expression for the SP or CGRP receptors in the hindpaw skin, or 3) decreasing the expression of neutral endopeptidase (NEP) in the hindpaw skin. NEP is a neuropeptide-specific peptidase that metabolizes SP and CGRP. At 4 weeks post-fracture the L4 and L5 DRGs ipsilateral to the fracture were excised under isoflurane anesthesia. RNA was extracted from the DRGs for SP (TAC1) and CGRP (CALCA) gene expression analysis. The right hindpaw dorsum skin was also collected for RNA extraction and PCR amplification of the NK1 (TACR1) and CGRP (CALCRL) receptors and the neuropeptide-specific peptidase neutral endopeptidase (NEP). The DRG and skin samples were first cut up in fine pieces in the lysis buffer, homogenized using a Polytron device (Brinkman Instruments Inc., Westbury, NY) and then centrifuged for 10 min at 12,000g. The supernatants were then processed and the total RNA was isolated using purification columns as directed (RNeasy Mini Kit; Qiagen, Valencia, CA). The purity and concentration of the purified RNA was determined spectrophotometrically. Subsequently, complementary DNA (cDNA) was synthesized using a reverse transcriptase iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA). One mg of RNA in a total volume of 20 ml was incubated at 25°C for 5 min followed by 42°C for 30 min and heat inactivation at 85°C for 5 min according to the manufacturer’s instruction. After incubation, the cDNA preparations were diluted 1:10 in RNase-free water prior to quantitative PCR (qPCR).
Real-time PCR amplification of TAC1 (forward primer: CGACCGCAAAATCCAACATG; reverse primer: AAAGAACTGCTGAGGCTTGG), CALCA (forward primer: CAGGCAGTTCCTTTGAGGTC; reverse primer: GCTCCCTGACTTTCATCTGC), and the internal control 18S (forward primer: AGGAATTGACGGAAGGGCAC, reverse primer: GTGCAGCCCCGGACATCTAAG) was performed on the ABI 7900HT sequencing detection system (ABI Prism) using SYBR® green reporter dye. Real-time PCR for the TACR1 and CALCRL receptors and NEP was performed using Taqman Universal PCR mix and Assay-on-Demand gene expression products (TACR1: Rn00562004, CALCRL: Rn00562334, NEP: Rn00561572, and 18S: Hs99999901s1, TaqMan MGB probes, FAM dye-labeled, Applied Biosystems, Foster City, CA). Mineral oil (8 ml) was loaded in each well to prevent loss of solution. Two ml of diluted cDNA, 0.5 ml of primers, and 2.5 ml of SYBR or Taqman Universal Master Mix were pipetted into each well. PCR parameters were 95°C, 5 min then followed by 95°C, 30 sec and 60°C, 60 sec for 40 cycles. Amplification kinetics for all PCR products were similar. The data from real-time PCR experiments were analyzed by the comparative Ct method as described in the manual for the ABI prism 7900HT real-time system. In each experiment samples were analyzed in triplicate or quadruplicate.
2.6 Homogenization and Western blotting procedure
These experiments tested the hypothesis that tibia fracture can induce chronic increases in hindpaw skin SP and CGRP receptor proteins and/or a decrease in NEP protein in the hindpaw skin. At 4 weeks after fracture the ipsilateral hindpaw dorsum skin was collected under isoflurane anesthesia and was homogenized in 56.8 mol/l Tris buffer, pH 6.8 with 1.8% (v/v) b-mercaptoethanol, 0.1% glycerol. The homogenate was centrifuged at 13,000 × g for 15 min at 4 °C. The supernatant was used for Western blot analysis to analyze the effect of fracture on SP (NK1) and CGRP (calcitonin receptor-like receptor, CRLR) receptor and NEP protein expression in the skin. Total protein concentration of the homogenate was measured using a Coomassie protein assay reagent (Pierce, Rockford, IL). Equal amounts of protein (100 mg) were subjected to SDS-PAGE (10% Tris-HCl acrylamide gel) and electrotransferred onto a polyvinylidene difluorided membrane. The blots were blocked overnight with 5 % non-fat dry milk in tris-buffered saline with 0.5% Tween-20 (TBST), incubated with primary antibody against specific proteins 1 hr on a rocking platform. Primary antibodies against NK1, CRLR, NEP, and actin, as well as HRP-conjugated secondary antibodies, were from Santa Cruz Biotechnology (Santa Cruz, CA). After washing in TBST three times, the blot was incubated with anti-goat or anti-rabbit HRP-conjugated secondary antibody for 1 h at room temperature, and washed again, then incubated in ECL plus chemoluminescence reagents (Amersham, Piscataway, NJ) and visualized by PhosphoImager (Typhoon, GE healthcare) and the band intensity was analyzed using ImageQuant 5.2 software (Molecular Dynamics, Piscataway, NJ). The specific protein/actin band intensity ratio represents the change of the specific protein after fracture.
2.7 Fluorescent immunohistochemistry
This experiment determined whether fracture increases epidermal thickness and SP NK1 receptor protein expression in keratinocytes and endothelial cells. At 4 weeks post-fracture rats were anesthetized with isoflurane and then transcardially perfused with 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4); the dorsal hind paw skin including sub-dermal layers was immediately removed and post-fixed in 4% paraformaldehyde (PFA) for 2 hours, then the tissues were treated with 30% sucrose in PBS at 4°C before embedding in OCT. Following embedding, 20-μm slices were made using a cryostat and mounted onto Superfrost microscope slides (Fisher scientific, Pittsburgh, PA). All sections were stored at −70°C until use for immunohistochemistry. Frozen sections were permeabilized and blocked with PBS containing 10% donkey serum and 0.3% Triton X-100 prior to primary antibody incubation. Sections were incubated with primary antibody diluted in PBS containing 2% serum at 4°C overnight. After washing in PBS, the sections were incubated with fluorophore-conjugated secondary antibody against the immunoglobulin of the species from which the primary antibody was generated. Upon detection of the first antigen, primary antibody from a different species against the second antigen was applied to the sections and visualized using an alternative fluorophore-conjugated secondary antibody. After three washes, the sections were mounted with anti-fade mounting medium (Invitrogen, Eugene, Oregon, CA). Images were visualized by a confocal microscope (Zeiss LSM510 Upright 2 photon; Carl Zeiss, Thornwood, NY). The following antibodies were used: rabbit anti-rat Substance P NK1 receptor (Sigma-Aldrich, Saint Louis, Missouri, diluted 1:8000), mouse anti-rat PECAM-1 (CD31) monoclonal antibody (Chemicon, Temecula, CA, diluted 1:50), monoclonal mouse anti-rat keratin, Pan Ab-1 (clone AE1/AE3) (Thermo Fisher Scientific, Fremont, CA, diluted 1:50), and FITC- (1:300) or cyanine dye 3 (1:500)-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
2.8 Intradermal neurite counting
This experiment determined whether tibia fracture causes distal nerve injury with loss of epidermal axons in the hindpaw plantar skin. Under isoflurane anesthesia a 2 mm diameter full thickness skin biopsy punch was removed from the mid plantar surface of the right hindpaw of 10 control and 9 experimental rats at 4 weeks post-fracture. Punches were immunolabeled against the pan-neuronal marker protein gene product (PGP) 9.5, a ubiquitin carboxyl-terminal hydrolase (Chemicon, Temecula, CA) using standard methods [17] that enable quantitation of individual epidermal axons. Slides were masked and randomized to conceal their identity until data acquisition was complete. All PGP9.5-immunolabeled neurites within the entire thickness of epidermis in four randomly chosen sections were counted.
2.9 Statistical analysis
An unpaired Student’s t-test was performed to evaluate between-group differences. The SP-evoked extravasation response in control rats was evaluated using a one-way ANOVA to compare the concentration-response at each time point and a Bonferroni post hoc test was used to determine differences between SP concentrations. All data are presented as the mean ± SE and differences are considered significant at p < 0.05 (Prism 4, GraphPad Software, San Diego, CA).
3. Results
3.1 Fracture chronically enhanced spontaneous and SP-evoked extravasation responses
Spontaneous protein extravasation responses were determined at 4 weeks after fracture by measuring the hindpaw skin Evans blue dye content 24 h after intravascular dye injection in 6 experimental and 7 control rats. Fig. 1A demonstrates that spontaneous extravasation increased 133% in the hindpaw skin ipsilateral (106 ± 14 ng/mg tissue wet wet), but not contralateral (49 ± 6 ng/mg) to the fracture, as compared to naive controls (45 ± 3 ng/mg). SP-evoked extravasation responses were also evaluated at 10 min after Evans blue dye injection and 5 min after intravenous SP injection in controls (n = 5) and fracture rats (n = 10). Fig. 1B illustrates that the SP-evoked dye extravasation was increased by 73% in the hindpaw skin ipsilateral (144 ± 15 ng/mg), but not contralateral (71 ± 6 ng/mg) to the fracture, as compared to controls (83 ± 5 ng/mg). These results demonstrate a regional post-junctional facilitation of SP induced extravasation responses in the microvasculature of the fracture hindlimb with a concurrent increase in spontaneous protein leakage in the hindpaw skin.
Figure 1.
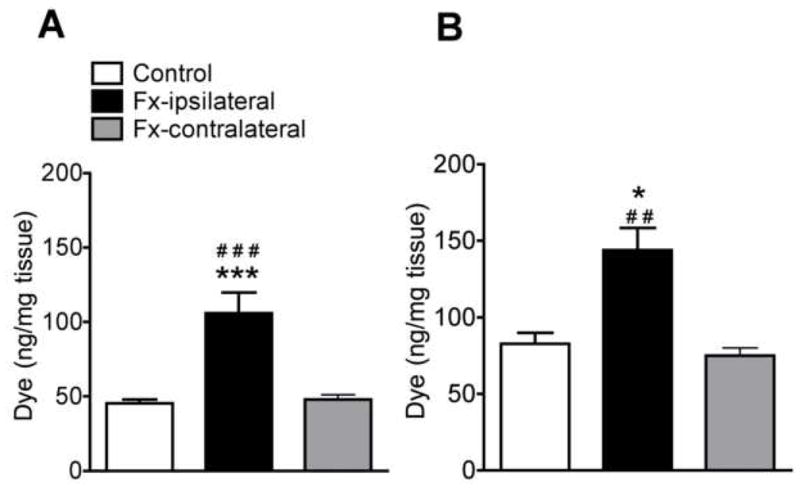
(A) Spontaneous dye extravasation 24 h after intravenous Evans blue dye injection. At 4 weeks after distal tibia fracture (Fx, n = 6) the dye content was increased by 133% in the ipsilateral, but not the contralateral hindpaw skin vs control rats (n = 7). (B) Intravenous substance P (SP) evoked dye extravasation in rats pretreated 5 min earlier with intravenous Evans blue dye. Five minutes after SP injection the hindpaw skin extravasation response was increased 73% in the ipsilateral, but not the contralateral hindpaw of fracture (n = 10) vs control rats (n = 5). All results are presented as the mean ± SEM in pg/mg wet tissue weight of the skin. *P < 0.05, and ***P < 0.001 for fracture vs control rat values, # P < 0.05, ## P < 0.01 for fracture vs contralateral hindpaw values.
3.2 Fracture had no effect on electrically-evoked SP and CGRP release
Figure 2 illustrates the effects of sciatic nerve electric stimulation on neuropeptide levels in hindpaw skin microdialysate. After intradermal insertion of dialysis fibers the SP and CGRP dialysate levels remained stable in both control and fracture hindpaws (n = 16 each cohort), except for SP levels in the fracture rats which declined over the 90 min stabilization interval. Sciatic stimulation increased SP and CGRP release in both the control and fracture hindpaws. Control and fracture hindpaw neuropeptide levels did not differ significantly either before or after sciatic stimulation.
Figure 2.
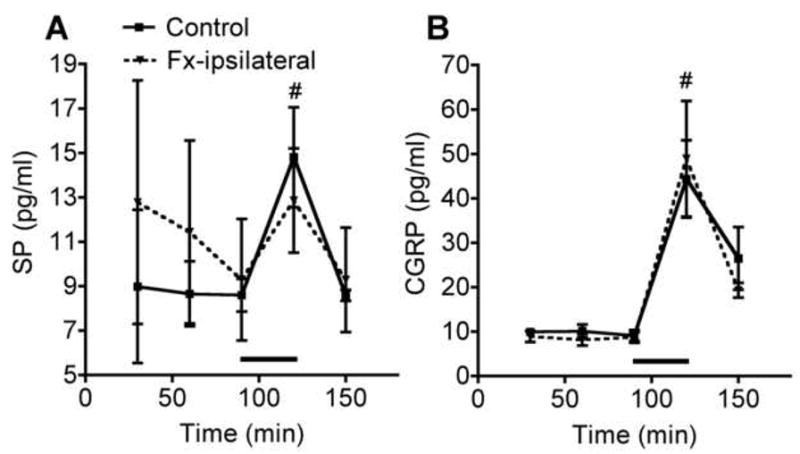
Time courses of SP (A) and CGRP (B) concentrations in the dialysate of intradermal microdialysis fibers after electrically stimulating the sciatic nerve. The black bars represent the interval of stimulation. SP and CGRP dialysate levels were increased after electrical stimulation in both control and fracture (Fx-ipsilateral) rats (n = 4 for each cohort), with no significant differences observed between treatment groups. #P < 0.05 for both fracture and control rats after 30 min stimulation vs their respective 90 min prestimulation baselines.
3.3 Up-regulation of SP and CGRP expression in the sciatic nerve and serum after fracture
SP and CGRP levels in the sciatic nerve were determined by EIA to assess the pre-junctional effects of fracture on neuropeptide signaling. Sciatic SP levels were 42% greater in fracture rats (Fig. 3A, 33 ± 12 pg/mg in fracture, n = 12 vs 23 ± 5 pg/mg in control groups, n = 12). CGRP levels were 53% higher after fracture (Fig. 3B, 168 ± 29 pg/mg in fracture n = 12 vs 110 ± 27 pg/mg in control groups, n = 12). Expression levels of TAC1 and CALCA genes, which encode the SP and CGRP peptides, were determined by RT-PCR of RNA isolated from the L4,L5 DRG. Fracture increased relative expression levels of the TAC1 and CALCA genes by 57% and 106% respectively (Fig. 3C, D, n = 16 per cohort), indicating an up-regulation of these two genes at the transcriptional level in the DRG.
Figure 3.
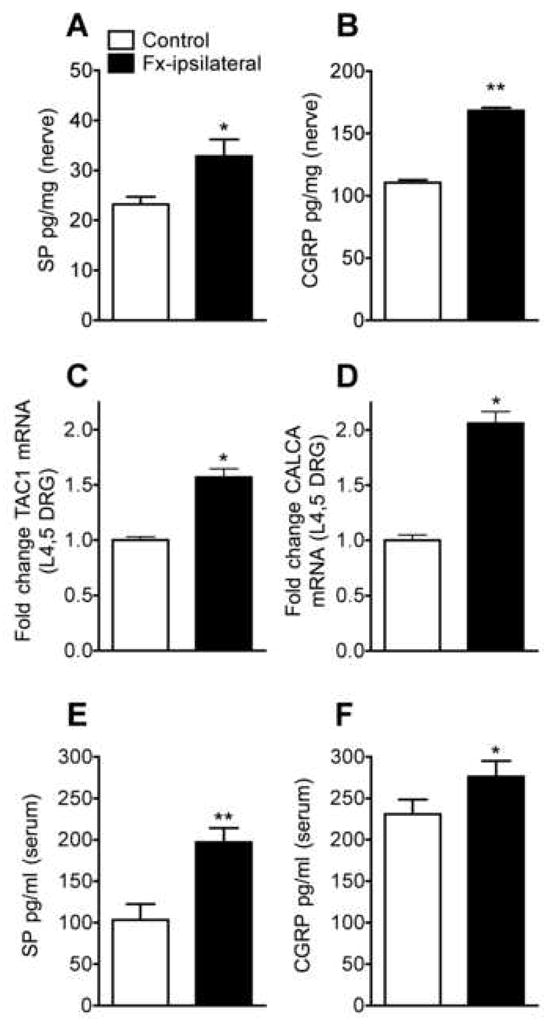
SP (A) and CGRP (B) content in the sciatic nerve as determined by EIA at 4 weeks after fracture. SP levels were increase 42% in fracture rats (Fx-ipsilateral) vs control rats (n = 12 per cohort). Expression levels of SP (TAC1) and CGRP (CALCA) genes were determined in the L4, L5 dorsal root ganglia by real-time PCR. Fracture increased the relative expression of the TAC1 gene (C) by 57% and the CALCA gene (D) by 106% (n = 16 per cohort). After fracture serum SP (E) and CGRP (F) protein levels increased by 91% and 20%, respectively. *P < 0.05, **P < 0.01 for fracture vs control rat values.
Rat serum SP and CGRP concentrations were determined to assess the systemic effect of fracture on serum neuropeptide levels. At 4 weeks post-fracture serum SP was elevated 91% (Fig. 3E, 197 ± 17 pg/ml, n = 9 vs. 103 ± 19 pg/ml, n = 9 in controls) and CGRP was also increased 19% (Fig. 3F, 276 ± 19, n = 10 vs. 231 ± 18, n = 10 in controls).
3.4 Fracture increased NK1 receptor expression in the skin, but had no effect on CRLR or NEP
The effects of fracture on SP (NK1) and CGRP (CRLR) receptor expression in the hindpaw skin were determined by Western blotting. Changes in expression of the neuropeptide degrading peptidase NEP were also evaluated. Representative specific bands of NK1, CRLR, NEP and actin are shown in Fig. 4D and the average digitized band intensities of NK1, CRLR, and NEP relative to actin are shown in Fig. 4A, B, and C respectively. Fracture increased NK1 receptor protein expression relative to actin by 74% (Fig. 5B, 0.49 ± 0.04, n=6 in fracture vs. 0.28 ± 0.04, n=6 in control groups). Fracture had no effect on CRLR or NEP protein expression levels (Fig. 4B, C). Similarly, fracture also significantly increased gene expression of the NK1 receptor (TACR1) by 93% (Fig. 4E, n = 10 each cohort), but had no effect on the CRLR receptor (CALCRL) or NEP genes (Fig. 4F, G), indicating that fracture up-regulated only the TACR1 gene in the skin at the transcriptional level.
Figure 4.
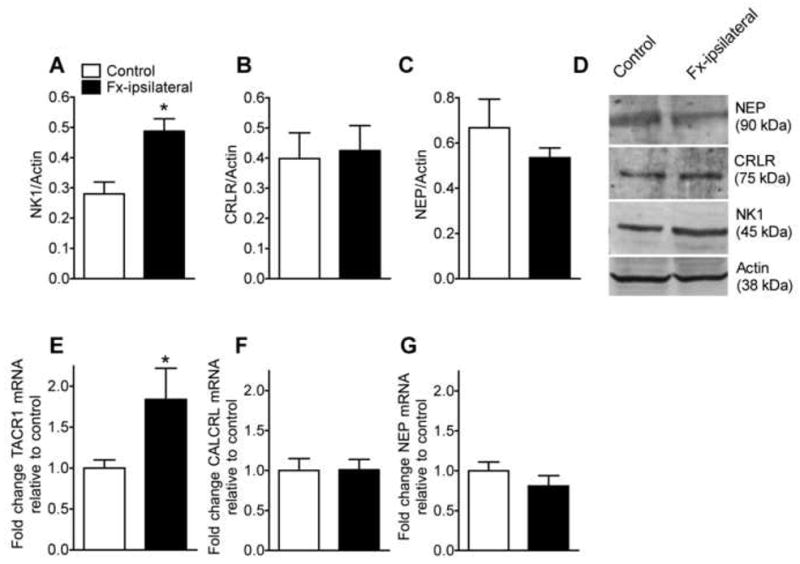
Effects of fracture on the expression of SP (NK1) and CGRP (CRLR) receptors and neutral endopeptidase (NEP) in the hindpaw skin. Protein levels were determined by Western blotting and gene expression was measured with real-time PCR. At 4 weeks after fracture NK1 (A) protein levels were increased by 74%, but no increase was observed in CRLR (B) or NEP (C) protein levels in skin (n = 6 per cohort). Panel D shows representative bands for the NK1 receptor, CRLR receptor, NEP, and actin from hindpaw skin collected from control and fracture (Fx-ipsilateral) rats. Similarly, fracture increased NK1 gene (TACR1) expression (E) by 93%, but had no effect on CRLR receptor gene (CALCRL) expression (F) or NEP gene expression (G). *P < 0.05 for fracture vs control rat values.
Figure 5.
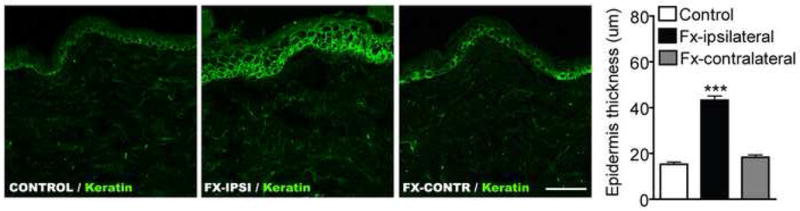
Immunostaining of keratin (a keratinocyte marker, green) in dorsal hindpaw skin sections from control rats and from the ipsilateral and contralateral hindpaws of fracture rats at 4 weeks post-injury (n = 5 per cohort). The thickness of the epidermal keratinocyte layer increased 280% in the injured hindlimb after fracture. ***P < 0.001 for fracture vs control rat values.
3.5 Fracture induced keratinocyte proliferation and increased keratinocyte and endothelial cell NK1
Figure 5 shows representative confocal microscopy of immunofluorescence staining for keratin (a keratinocyte marker, green) in dorsal hindpaw skin sections from control rats and from the ipsilateral and contralateral hindpaws of fracture rats at 4 weeks post-injury (n = 5 per cohort). There was a 283% increase in the thickness of the epithelial keratinocyte layer in the fracture limb (43.2 ± 1.8 um), but no change was observed in the contralateral paw (18.3 ± 1.0 um), as compared to controls (15.3 ± 1.0 um). Figure 6 presents representative immunostaining for NK1 receptors in keratinocyte and endothelial cells in dorsal hindpaw skin at 4 weeks post-fracture. Panel A demonstrates co-expression of NK1 (green) and keratin, (a keratinocyte marker, red) in the epidermis of controls, with an obvious increase in NK1 receptor expression in keratinocytes after fracture. Panel B presents representative dermal venules from control and fracture hindpaws, both immunostained for NK1 (green) and CD31 (an endothelial cell marker, red). These photomicrographs illustrate the dramatic up-regulation of NK1 receptors in dermal vascular endothelial cells after fracture.
Figure 6.
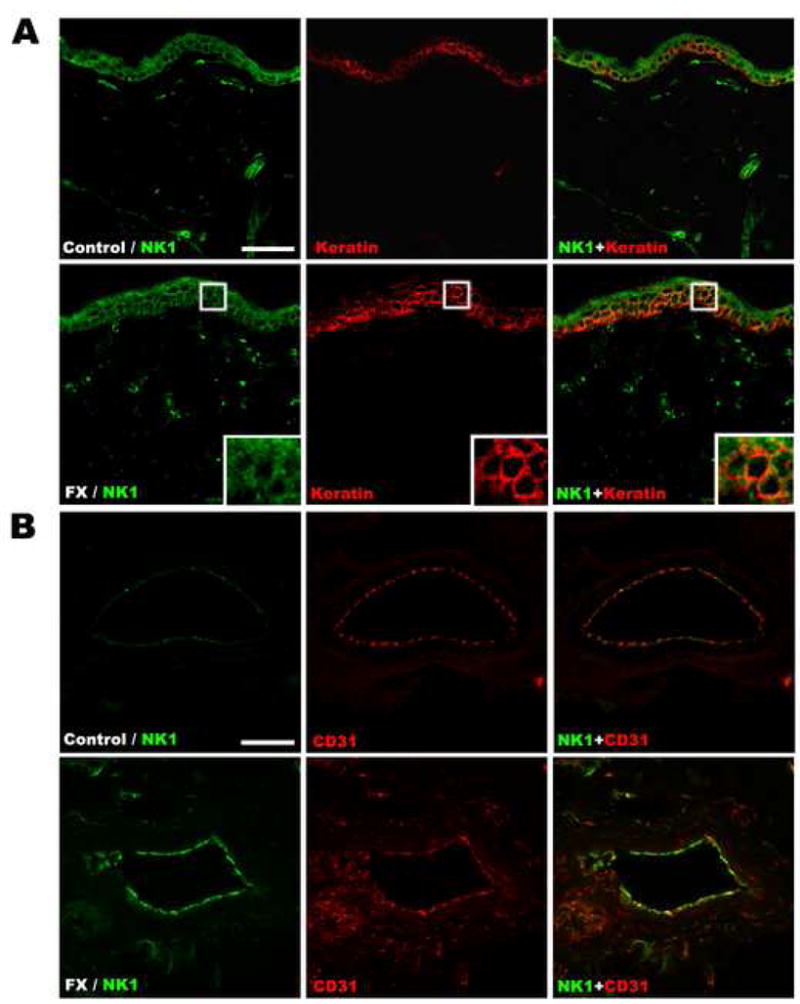
Representative fluorescence photomicrographs of NK1 receptor expression in keratinocyte and endothelial cells in the dorsal hindpaw skin at 4 weeks post-fracture. (A) Co-immunostaining of NK1 (green) and keratin, (a keratinocyte marker, red) in the epidermis demonstrates increased NK1 receptor expression in keratinocytes after fracture. (B) Co-immunostaining of NK1 (green) and CD31 (an endothelial cell marker, red) in the endothelium of a dermal venule demonstrates the dramatic up-regulation of NK1 receptors in cutaneous vascular endothelial cells after fracture. Scale bar in panel A = 50 μm, panel B = 20 μm.
3.7 Fracture had no effect on sensory epidermal nociceptive innervation in the hindpaw skin
The effect of fracture on cutaneous sensory innervation was examined by counting epidermal PGP9.5 immunolabeled neurites in hindpaw plantar skin punch biopsies obtained at 4 weeks post-fracture. Fig. 7 illustrates that epidermal neurite counts in the fracture rats (n = 9, 338 ± 71) did not significantly differ from counts in control rats (n = 10, 414 ± 56).
Figure 7.
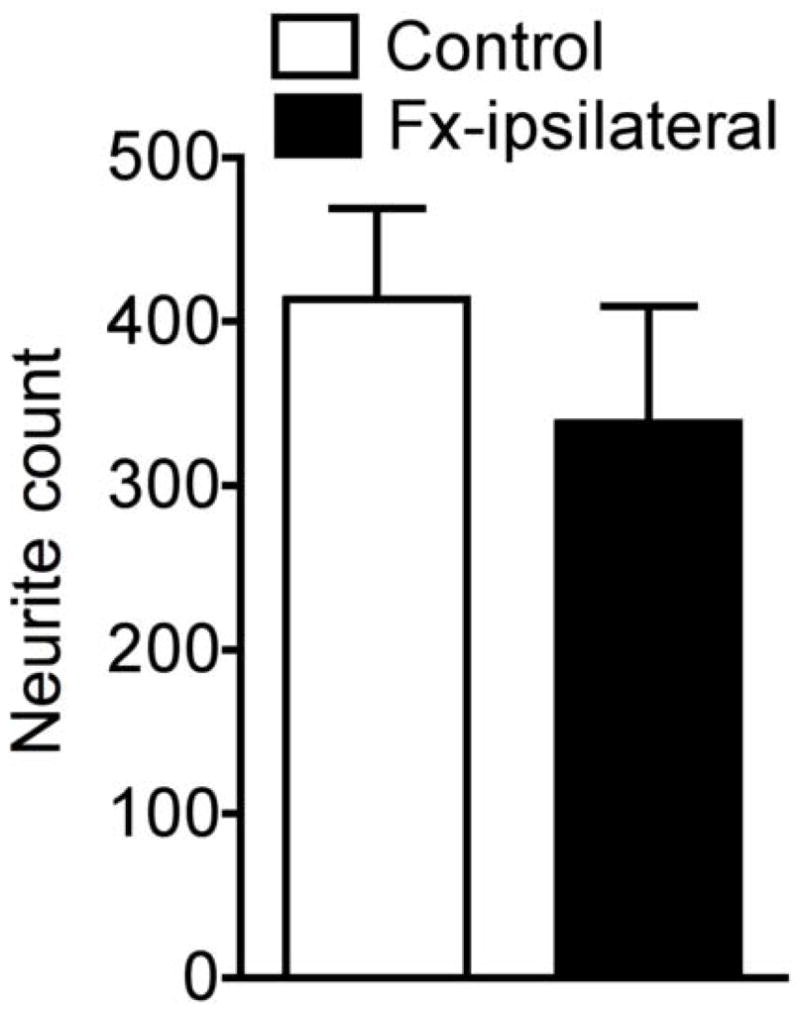
The effect of fracture on epidermal neurite counts in the hindpaw skin as determined using PGP9.5 immunolabeling. Compared to samples obtained from control rats, there was no significant change in neurite density in the ipsilateral hindpaw at 4 weeks after fracture.
Discussion
CRPS patients have chronically increased plasma protein leakage into the skin of the injured limb [23]. Facilitated protein extravasation increases interstitial fluid flow and when this exceeds the rate of lymphatic drainage it induces distal limb edema, an important component of CRPS [32]. Similarly, tibia fracture and casting in rats induces chronic facilitation of spontaneous protein extravasation and edema in the injured limb that can be partially reversed with an NK1 receptor antagonist [12]. In the current study we investigated the mechanisms mediating the enhanced neurogenic signaling observed in the hindpaw skin at 4 weeks post-fracture. Spontaneous protein extravasation was dramatically increased in the hindpaw skin in the ipsilateral, but not the contralateral hindlimb at 4 weeks post-fracture (Fig. 1A). Intravenous SP injection evoked an extravasation response within 5 min in the hindpaw skin of normal rats and at 4 weeks post-fracture this SP-evoked extravasation response was up-regulated in the hindpaw skin ipsilateral, but not contralateral to the injury (Fig. 1B). Collectively, these results support the hypothesis that enhanced post-junctional SP signaling in the injured hindlimb contributes to the increase in hindpaw spontaneous protein extravasation and edema observed after fracture.
At 4 weeks after fracture there was an increase in SP and CGRP protein levels in the ipsilateral sciatic nerve (Fig. 3A,B) and increased SP and CGRP gene expression in the corresponding DRGs (Fig. 3C,D). Surprisingly, there was no increase in electrically-evoked sciatic nerve release of SP or CGRP into the plantar hindpaw interstitial fluid (Fig. 2), nor was there any change in peripheral nerve terminal counts in the plantar hindpaw epidermis (Fig. 7). Serum SP and CGRP levels actually increased after fracture (Fig. 3E), but the effects of increased circulating neuropeptides would be systemic and not restricted to the injured hindlimb. Another possible mechanism for a regionally restricted increase in spontaneous extravasation could be down-regulation of the neuropeptide metabolizing peptidase, neutral endopeptidase (NEP), in the injured limb. Fracture had no effect on NEP mRNA levels or NEP protein expression (Fig. 4 C,G). Fracture did up-regulate SP mRNA levels and NK1 receptor protein in the ipsilateral hindpaw skin (Fig 4 A,E), but there was no effect on CGRP receptor gene or protein expression in the skin (Fig. 4 B,F). Further evidence for fracture-induced post-junctional facilitation of SP signaling comes from the intravenous SP experiments that demonstrate enhanced extravasation responses in the ipsilateral, but not contralateral hindpaw after fracture (Fig. 1B). In addition, immunostaining in dorsal hindpaw skin sections demonstrated up-regulation of NK1 receptors on venule endothelial cells co-labeled with the endothelial marker CD31 (Figure 6B). NK1 receptor expression was also increased in the epithelial keratinocytes (Fig. 6A), associated with a 280% increase in epithelial thickness (Fig. 5). Substance P is a potent mitogen and in vitro studies indicate that keratinocyte NK1 receptor activation can stimulate cellular proliferation [18, 24, 31]. Collectively, these results support the hypothesis that SP acts on endothelial NK1 receptors to induce spontaneous protein extravasation in normal rats and that after fracture there is a regional increase in endothelial and keratinocyte NK1 receptors, resulting in enhanced microvascular permeability, edema, cutaneous vasodilatation, warmth, and epithelial hypertrophy in the injured hindlimb.
Several similarities are observed between the rat fracture model and CRPS. Scintigraphic studies with radio-labeled immunoglobulin G demonstrate increased spontaneous protein leakage in the affected limb of most CRPS patients early in the disease process [23], similar to the increase observed in spontaneous protein extravasation in the fracture hindlimb (Fig. 1A). Serum SP levels are elevated in CRPS patients [30] and in the fracture rats (Fig. 3E). Electrically-evoked protein extravasation and vasodilatation responses are enhanced in the edematous skin of CRPS patients, direct evidence of facilitated neurogenic inflammation[35]. When SP is infused into the brachial artery of normal subjects it evokes NK1 receptor mediated vasodilatation, flushing, and edema in the hand, similar to the clinical picture of vasodilatation, warmth, cutaneous redness, and edema in the CRPS limb [19, 20]. Intravascular SP injection evoked facilitated protein extravasation (Fig 1B) and edema [13] in the injured hindlimb of fracture rats, evidence of enhanced post-junctional SP signaling after fracture. Similarly, CRPS patients demonstrate enhanced protein extravasation responses to microdialyzed SP [15]. Collectively, these data indicate a facilitation of post-junctional SP endothelial signaling in both the tibia fracture model and in CRPS patients.
It is important to note several differences between the rat fracture model and the experimental data derived in CRPS patients. First, while there was a 17% reduction in the density of epidermal neurites in the fracture hindpaw compared to naïve controls, this effect did not reach statistical significance (Fig. 7), but in CRPS patients there is a statistically significant 29% reduction in the density of the epidermal neuritis in the affected limb [22].
Another difference between the rat fracture model and the clinical microdialysis studies is that dialyzed SP-evoked extravasation is enhanced in the limb contralateral to the CRPS affected extremity in 45% of patients [15]. This group also observed contralateral facilitation of electrically-evoked vasodilatation in CRPS patients, but no contralateral facilitation of electrically-evoked extravasation responses [16]. In the rat fracture model spontaneous protein extravasation and intravenous SP-evoked extravasation was unchanged in the contralateral hindlimb (Fig. 1A, B), evidence that there is no facilitation of SP signaling in the contralateral limb after fracture (Fig. 1B). The contralateral facilitation of SP-evoked extravasation observed in 45% of CRPS patients may indicate that some trauma patients have a genetic predisposition in towards the development of CRPS, but contralateral facilitated spontaneous extravasation and limb edema is rarely seen in CRPS patients [23, 32]. Again, the differences between the contralateral effects of trauma in the rat fracture model and CRPS patients may be attributable to species differences or perhaps to the interval between injury and testing, which in the rat fracture study was only 4 weeks, but in the clinical studies averaged 2 years.
Neurogenic inflammation is also mediated by the release of the CGRP from sensory neurons [9, 25]. CGRP does not affect vascular permeability but does induce neurogenic vasodilatation via a direct (i.e., endothelium-independent) relaxation of vascular smooth muscle. CGRP containing sensory nerves directly innervate the smaller arteries, coming into close apposition of smooth muscle cells expressing the CGRP (CRLR) receptor. After fracture there was an increase in CGRP (CALCA) mRNA levels and protein expression in the ipsilateral L4, L5 dorsal root ganglia and sciatic nerve (Fig. 3B, D). Fracture had no effect on electrically-evoked CGRP release into the interstitial fluid (Fig. 2B), but serum CGRP levels were elevated after fracture (Fig. 3F), similar to the increase in CGRP serum levels observed in CRPS patients [6, 7]. Fracture had no effects on CGRP receptor (CALCRL) mRNA or CRLR protein levels in the hindpaw skin (Fig 4). We did not perform laser Doppler studies to determine whether fracture facilitated vasodilatation responses to intravascular CGRP in the injured hindlimb, but consider this an unlikely scenario since there was no increase in sciatic stimulation-evoked CGRP interstitial release after fracture and no increase in CALCRL mRNA or CRLR protein levels in the hindpaw skin. Furthermore, we previously observed that the NK1 antagonist LY303870 dramatically inhibited hindpaw warmth after fracture, suggesting that facilitated SP signaling mediates the increase in hindpaw skin temperature after fracture [12].
In summary, the present study suggests that spontaneous and intravenous SP-evoked extravasation responses are enhanced in the ipsilateral hindlimb after fracture, an effect mediated by a regional increase in dermal endothelial NK1 expression. We postulate that the up-regulated expression of NK1 receptors in the cutaneous keratinocytes and endothelial cells of the injured limb mediate the increased vascular permeability, edema, warmth, and keratinocyte proliferation that we observe in the tibia fracture model of CRPS. These findings closely resemble the clinical scenario and suggest that enhanced neuropeptide signaling mediates the vascular and cutaneous pathophysiology of CRPS.
Acknowledgments
This study was supported by National Institutes of Health Grants DK67197 (WSK) and NS42866 (ALO), and Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service Grant F4516I (WSK). The authors declare they have no conflicts of interest with the research presented in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after colles’ fracture. J Bone Joint Surg. 1990;72 B:105–110. doi: 10.1302/0301-620X.72B1.2298766. [DOI] [PubMed] [Google Scholar]
- 2.Benoliel R, Eliav E, Mannes AJ, Caudle RM, Leeman S, Iadarola MJ. Actions of intrathecal diphtheria toxin-substance P fusion protein on models of persistent pain. Pain. 1999;79:243–253. doi: 10.1016/s0304-3959(98)00170-5. [DOI] [PubMed] [Google Scholar]
- 3.Bickerstaff DR, Charlesworth D, Kanis JA. Changes in cortical and trabecular bone in algodystrophy. Br J Rheumatol. 1993 Jan;32(1):46–51. doi: 10.1093/rheumatology/32.1.46. [DOI] [PubMed] [Google Scholar]
- 4.Bickerstaff DR, Kanis JA. Algodystrophy: an under-recongnized complication of minor trauma. Br J Rheumatol. 1994;33:240–248. doi: 10.1093/rheumatology/33.3.240. [DOI] [PubMed] [Google Scholar]
- 5.Birklein F, Riedl B, Claus D, Neundorfer B. Pattern of autonomic dysfunction in time course of complex regional pain syndrome. Clin Auton Res. 1998;8:79–85. doi: 10.1007/BF02267817. [DOI] [PubMed] [Google Scholar]
- 6.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001 Dec 26;57(12):2179–84. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 7.Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta orthopaedica Belgica. 1998 Dec;64(4):448–51. [PubMed] [Google Scholar]
- 8.Blumberg H, Janig W. Clinical manifestations of reflex sympathetic dystrophy and sympathetically maintained pain. In: Wall PD, Melzack R, editors. Textbook of pain. 3. Edinburgh: Churchill Livingstone; 1994. pp. 685–697. [Google Scholar]
- 9.Brain SD. Sensory neuropeptides in the skin. In: Geppeti P, Holzer P, editors. Neurogenic Inflammation. Boca Raton: CRC Press; 1996. pp. 229–244. [Google Scholar]
- 10.Braus DF, Krauss JK, Strobel J. The shoulder-hand syndrome after stroke: a prospective clinical trial. Ann Neurol. 1994;36:728–733. doi: 10.1002/ana.410360507. [DOI] [PubMed] [Google Scholar]
- 11.Christensen K, Jensen EM, Noer I. The reflex sympathetic dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653–655. [PubMed] [Google Scholar]
- 12.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004 Mar;108(1–2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006 Mar;121(1–2):158–67. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Kingery WS, Guo T, Agashe GS, Davies MF, Clark JD, Maze M. Glucocorticoid inhibition of neuropathic limb edema and cutaneous neurogenic extravasation. Brain Res. 2001 Sep 21;913(2):140–8. doi: 10.1016/s0006-8993(01)02763-9. [DOI] [PubMed] [Google Scholar]
- 15.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003 Sep;183(1):197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 16.Leis S, Weber M, Schmelz M, Birklein F. Facilitated neurogenic inflammation in unaffected limbs of patients with complex regional pain syndrome. Neuroscience letters. 2004 Apr 15;359(3):163–6. doi: 10.1016/j.neulet.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995 Oct;45(10):1848–55. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 18.McGovern UB, Jones KT, Sharpe GR. Intracellular calcium as a second messenger following growth stimulation of human keratinocytes. The British journal of dermatology. 1995 Jun;132(6):892–6. doi: 10.1111/j.1365-2133.1995.tb16944.x. [DOI] [PubMed] [Google Scholar]
- 19.Newby DE, Sciberras DG, Ferro CJ, Gertz BJ, Sommerville D, Majumdar A, Lowry RC, Webb DJ. Substance P-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. British journal of clinical pharmacology. 1999 Sep;48(3):336–44. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NA, Webb DJ. Intra-arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. British journal of clinical pharmacology. 1997;43:493–499. doi: 10.1046/j.1365-2125.1997.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 22.Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006 Feb;120(3):235–43. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Oyen WJ, Arntz IE, Claessens RM, Van der Meer JW, Corstens FH, Goris RJ. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response? Pain. 1993 Nov;55(2):151–7. doi: 10.1016/0304-3959(93)90144-E. [DOI] [PubMed] [Google Scholar]
- 24.Paus R, Heinzelmann T, Robicsek S, Czarnetzki BM, Maurer M. Substance P stimulates murine epidermal keratinocyte proliferation and dermal mast cell degranulation in situ. Archives of dermatological research. 1995;287(5):500–2. doi: 10.1007/BF00373436. [DOI] [PubMed] [Google Scholar]
- 25.Peroutka SJ. Neurogenic inflammation and migraine: implications for the therapeutics. Molecular interventions. 2005 Oct;5(5):304–11. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- 26.Sabsovich I, Guo TZ, Wei T, Zhao R, Li X, Clark DJ, Geis C, Sommer C, Kingery WS. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008 Jul 31;137(3):507–19. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008 Aug 15;138(1):47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993 May;75(3):450–2. doi: 10.1302/0301-620X.75B3.8496220. [DOI] [PubMed] [Google Scholar]
- 29.Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Meth. 1983;8:41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- 30.Schinkel C, Gaertner A, Zaspel J, Zedler S, Faist E, Schuermann M. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. The Clinical journal of pain. 2006 Mar–Apr;22(3):235–9. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka T, Danno K, Ikai K, Imamura S. Effects of substance P and substance K on the growth of cultured keratinocytes. The Journal of investigative dermatology. 1988 Mar;90(3):399–401. doi: 10.1111/1523-1747.ep12456487. [DOI] [PubMed] [Google Scholar]
- 32.Veldman PHJM, Reynen HM, Arntz IE, Goris RJA. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–1016. doi: 10.1016/0140-6736(93)92877-v. [DOI] [PubMed] [Google Scholar]
- 33.Verdugo R, Campero M, Ochoa JL. Phentolamine sympathetic block in painful polyneuropathies. II. Further questioning of the concept of “sympathetically maintained pain”. Neurol. 1994;44:1010–1014. doi: 10.1212/wnl.44.6.1010. [DOI] [PubMed] [Google Scholar]
- 34.Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain. 2001;124:587–599. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- 35.Weber M, Birklein F, Neundorfer B, Schmelz M. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001 Apr;91(3):251–7. doi: 10.1016/S0304-3959(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 36.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


