Abstract
Theiler's murine encephalomyelitis virus (TMEV) infection directly induces many proinflammatory genes, including type I interferon (IFN) and a variety of cytokine genes. These virus-induced cytokines are a critical factor in developing TMEV-induced demyelinating disease. We have previously reported that the major activation signal for the cytokine genes is mediated via TLR3. In this study, we describe that TLR2 is upregulated via TLR3 signal and cooperatively participates in the expression of IL-6, IL-1β, CCL2, and CCL5 genes following TMEV infection. The expression of these genes was significantly impaired in both TLR2-deficient and TLR3-deficient primary astrocytes. However, the induction of type I IFNs was not affected by TLR2-deficiency in the primary cells. TMEV infection led to TLR2-mediated NF-κB activation, but not IRF3 or IRF7 activation critical for type I IFN production. More importantly, TLR3 was required for TMEV-induced early TLR2 upregulation in primary astrocytes leading to the production of TLR2-dependent cytokines such as IL-6. Interestingly, soluble factor(s) produced via TLR2/3-dependent signals appears to be partially associated with the downstream cytokine production. These results indicate that TMEV utilizes TLR3-induced TLR2 to induce inflammatory cytokines, which are critical to the development of immune-mediated demyelinating disease.
Keywords: Viral Immunity, CNS inflammation, TLRs, Cytokine gene activation
Introduction
To initiate an immune response against an invading pathogen, mammalian hosts use a variety of cellular receptors that recognize specific molecular structures on pathogens. These receptors, collectively termed pattern recognition receptors (PRRs), include Toll-like receptors (TLRs), nucleotide-binding oligomerization domain proteins, Card-domain containing RNA helicases, and dsRNA-activated protein kinase R (PKR) (Mogensen and Paludan 2005). Among the PRRs, TLRs have been the best described class. Ligation of distinct TLRs by different pathogen-associated molecules has the capacity to induce specific downstream intracellular signaling cascades and thus tailors the innate response to the invading pathogens, leading to inflammatory responses and subsequent adaptive immune responses (Akira 2006; Akira and Hemmi 2003).
TLR2 is known as the receptor for several microbial ligands, including bacterial lipopeptide and yeast zymosan (Means et al. 2000; Zhang and Ghosh 2001). TLR2 also mediates the production of proimflammatory cytokines in response to a virus, such as Herpes simplex virus 1 (HSV-1) (Kurt-Jones et al. 2004a) and Vaccinia virus (Zhu et al. 2007). TLR expression varies between different cell types and the pattern of expression is a critical factor for the innate immune response by the responding cells (Akira 2006). Low-levels of constitutive expression of messenger RNA encoding TLR2, TLR4, TLR5, and TLR9 were found in resting astrocyte cultures (Bowman et al. 2003). After treatment with a TLR2 ligand, lipoteichoic acid (LTA), microglia cells produced a variety of pro-inflammatory cytokines, such as tumor-necrosis factor-α (TNF-α, IL-1β, IL-6 and nitric oxide (Kinsner et al. 2006). Similarly, TLR2 signaling also mediates the production of CCL2 and CCL5 chemokines (Tsuboi et al. 2002). Moreover, these cytokines and chemokines have been implicated in the pathogenesis of various inflammatory neurological diseases such as Alzheimer's disease, Parkinson's disease and multiple sclerosis (Finch and Morgan 2007; Minagar et al. 2002; Mines et al. 2007; Rebenko-Moll et al. 2006; Wilms et al. 2007). Furthermore, TLR2 contributes to CNS injury in focal cerebral ischemia (Lehnardt et al. 2007) and the nerve injury-induced spinal cord glial cell activation (Kim et al. 2007). Therefore, TLR2 appears to play an important role in inflammatory responses of the CNS.
Theiler's murine encephalomyelitis virus (TMEV) belongs to picornaviridae and contains a single-strand positive RNA genome. TMEV infection leads to the development of early acute disease in susceptible mice, resembling encephalomyelitis followed by late chronic demyelinating disease, and this virus-induced demyelinating disease is used as one of the relevant models for human multiple sclerosis (MS) (Dal Canto 1996; Dal Canto et al. 1995). Previous reports have shown that TMEV infection can directly induce the gene expression of proinflammatory cytokines and chemokines including TNF-α, IL-1, IL-6, CCL2, and CCL5 in primary astrocytes via the NF-κB pathway (Palma and Kim 2004; Palma et al. 2003). Also, we and others have shown that these proinflmmatory responses to TMEV infection are dependent on the presence of TLR3 (So et al. 2006), as well as PKR (Carpentier et al. 2007; Palma et al. 2003). However, these studies have not excluded the possibility that other PRRs and/or TLRs other than TLR3 are involved either cooperatively or independently in the induction of TMEV-induced innate cytokine responses.
Some viruses utilize more than one TLR to activate their proinflammatory responses (Thompson and Locarnini 2007). Therefore, it is conceivable that distinct TMEV components may interact with different TLRs or other intracellular detectors. In particular, highly TLR2-dpendent IL-6, CCL2 and CCL5 gene activation (Aravalli et al. 2005) is vigorously induced following TMEV infection (Palma et al. 2003; Rubio and Capa 1993). This TLR is associated with early cellular infiltration following physical injuries in the CNS (Babcock et al. 2006). In addition, TLR2 contributes to HSV-1-induced lethal encephalitis (Kurt-Jones et al. 2004b). Moreover, IL-6 is a key cytokine for promoting pathogenic T cells (Th17) and modulating antigen processing (Barrionuevo et al. 2008; McGeachy et al. 2007; Stumhofer et al. 2007). Therefore, we have assessed here the possibility that TLR2 may also be involved in the TMEV-induced signal transduction for cytokine gene activation.
In this study, we demonstrate that the induction of certain inflammatory cytokines in response to TMEV is mediated by TLR2-dependent signaling in addition to TLR3-mediated signals. Using TLR2-deficient primary astrocytes, we show that certain virus-induced cytokine production such as IL-6 is significantly impaired in comparison to wild-type cells. In contrast, type I IFN production is not affected by the absence of TLR2. These cytokine production patterns were similar to each other among different cell types, suggesting their cell-type independence. Further studies indicated that TLR3-mediated signal is a pre-requisite for TLR2 induction and subsequent TLR2-dependent cytokine production. These results strongly suggest that TLR2 is involved in the TMEV-induced signaling for activation of various cytokine genes associated with the initial inflammatory responses, in a TLR3-dependent manner.
Materials and Methods
Mice
C57BL/6 (B6, WT) and TLR2-deficient (TLR2-/-) and TLR3-deficient (TLR3-/-) mice on the C57BL/6 background were purchased from The Jackson laboratory (Bar Harbor, ME). TLR4-deficient C57BL/10ScNJ (TLR4-/-) and their normal control C57BL/10ScSnJ (B10) mice were also obtained from the same company. Mice were housed in the Center for Comparative Medicine of Northwestern University and used in accordance with the procedures approved by the Institutional Animal Care and Use Committee.
Cells and reagents
HEK 293 and C8D1A cell lines were purchased from American Type Culture Collection (Manassas, VA). All cells were cultured in DMEM supplemented with 10% fetal bovine serum. TLR2 ligand (LTA, Lipoteichoic acid from Staphylococcus aureus), TLR4 ligand (ultrapure LPS from E. coli 0111:B4), TLR7 ligand (R837) were obtained from InvivoGen (San Diego, CA). TLR3 ligand, poly (I-C) was purchased from Calbiochem (La Jolla, CA). All TLR ligands were directly added to cell culture media at indicated concentrations.
Preparation of primary glial cells
Primary glial cells were prepared from neonatal (0-3 day) mouse brains by differential shaking as described previously (Skias et al. 1987). The purity of astrocytes was greater than 95%, as determined by staining with anti-GFAP antibody (Dako, Carpenteria, CA).
Viral infection
The BeAn strain of TMEV was propagated in BHK-21 cells grown in DMEM supplemented with 7.5 % donor calf serum. Viral titer was determined by plaque assay on BHK cell monolayers. UV-inactivation of TMEV was performed in a UV transluminator for 30 min on ice. UV inactivation was verified with plaque assays. For all infection experiments, cells were washed and subsequently incubated in infection media (DMEM supplemented with 0.1 % bovine serum albumin) with TMEV at various multiplicities of infection (MOIs).
RT-PCR and real-time PCR
Total RNA was purified using TRIzol Reagent (Invitrogen, Carlsbad, CA), according to the manufacture's instructions. First strand cDNA was synthesized by MMLV reverse transcriptase and oligo (dT)18, from 1-4 μg total RNA. The sense and antisense primer sequences used were as follows; IL-6 (5′-AGTTGCCTTCTTGGGACTGA-3′ and 5′-TCCACGATTTCCCAGAGAAC-3′); CCL5 (5′-GTGCCCACGTCAAGGAGTAT-3′ and 5′-GGGAAGCGTATACAGGGTCA-3′); CCL2 (MCP-1, 5′-AGCAGGTGTCCCAAAGAAGCTGTA-3′ and 5′-AGAAGTGCTTGAGGTGGTTGTGGA-3′); IL-1β (5′-TCATGGGATGATGATAACCTGCT-3′ and 5′-CCCATACTTTAGGAAGACACGGAT-3′); IFN-α4 (5′-AAGGCTCAAGCCATCCTTGTGCTA-3′ and 5′-TTGCCAGCAAGTTGGTTGAGGAAG-3′); IFN-β (5′-GGAAAGATTGACGTGGGAGA-3′ and 5′-CTGAGGCATCAACTGACAGG-3′); TLR2 (5′-CTCCCACTTCAGGCTCTTTG-3′ and 5′-AGGAACTGGGTGGAGAACCT-3′); TLR3 (5′-ATATGCGCTTCAATCCGTTC-3′ and 5′-CAGGAGCATACTGGTGCTGA-3′); GAPDH (5′-AACTTTGGCATTGTGGAAGG-3′ and 5′-ACACATTGGGGGTAGGAACA-3′); TMEV genome (5′-CCCAGTCCTCAGGAAATGA-AGG-3′ and 5′-TCCAAAAGGAGAGGTGCCATAG-3′). For quantitative analysis of the gene expression, real-time PCR was performed by using SYBR green I mastermix in an iCycler (Bio-Rad, Hercules, CA). GAPDH expression was assessed as an internal reference for normalization. Relative gene expression levels were expressed by the fold increase over the media-treated control samples. Every real-time PCR was performed in triplicates.
ELISA
The concentrations of IL-6 and CCL-2 (MCP-1) in culture supernatants were determined by using ELISA kits (BD Bioscience, San Diego, CA). The IFN-α level was determined with the ELISA kit purchased from PBL Biomedical Laboratories (Piscataway, NJ). Plates were read at 450 nm.
Transfection and reporter gene assay
HEK 293 cells were transfected with mouse TLR2 or TLR3 expression construct (InvivoGen) using Lipofectamine 2000 (Invitrogen). After 48 h, stable HEK 293 cells expressing TLR2 or TLR3 were selected with Blasticidin S (InvivoGen). These TLR-expressing HEK cells and parental HEK 293 cells were transfected with luciferase reporter genes for NF-κB and ISRE (provided by Dr. Maniatis, Harvard Univ. MA). In some experiments, cells were transfected with GAL4-IRF7 plus UAS(GAL)-luciferase reporter gene (kindly provided from Dr. Fitzgerald, University of Massachusetts, MA) to detect the activity of IRF7. In all cases, thymidine kinase driven Renilla luciferase reporter gene (pRL-TK, Promega) was co-transfected to normalize the transfection efficiencies. At 24 h after transfection, the gene activities were measured by dual-luciferase reporter assays (Promega, Madison, WI). The relative luciferase activity was expressed as a fold induction to the media-treated control sample. For gene transfection into primary astrocytes, the Amaxa nucleofector system for mouse astrocytes (VPG-1006, Amaxa Inc., Gaithersburg, MD) was used. After transfection, primary astrocytes were cultured for 48 h before infection.
EMSA
Nuclear and cytoplasmic extracts were prepared as described previously (Nakahira et al. 2002). The NF-κB consensus oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGG-3′) and the oligonucleotide corresponding to the ISRE region for ISG15 promoter (5′-GATCGGGAAAGGGAAACCGAAACTGAAGCC-3′) were used. Equivalent amounts of nuclear extracts (10 μg) were incubated with 32P-labled double-stranded oligonucleotide probes in binding buffer containing 10 mM HEPES-NaOH (pH 7.9), 1 mM EDTA, 30 mM NaCl, 0.1 % IGPAL, 1 mM DTT, 1 mg/ml bovine serum albumin, and 5 % glycerol, 40 μg/ml poly (dI-dC). After 30 min, the mixture was loaded on 5 % polyacrylamide gel in a 0.5 X Tris-borate-EDTA (TBE) buffer. In the supershift assay, nuclear extracts were incubated with antibodies for 30 min on ice before addition of the labeled probe. Mouse monoclonal antibody to p65 and rabbit polyclonal anti-IRF3 antibodies were purchased from Santa Cruz and Zymed (San Francisco, CA), respectively.
Silencing TLR2 and TLR3 gene expression
The mammalian expression vector, pSuper.retro.neo+gfp (OligoEngine, Seattle, WA) was used for expression of siRNA for TLR2 or TLR3, corresponding to the nucleotides of TLR2 (5′-TGACCTGAGTACTGTCTAT-3′) or TLR3 (5′-GAATCTTACTCACAACCAA-3′). These siRNA constructs were transfected into C8D1A mouse astrocyte cell line. Stably transfected cell lines were selected with 10 μg/ml G418 (Invitrogen).
Flow cytometric analysis of TLR2
Primary mouse astrocytes were cultured in the presence of TMEV for 3 h. The TLR2 expression level was analyzed after staining cells with PE-conjugated rat anti-mouse TLR2 IgG2b (eBioscience, San Diego, CA) using FACSCalibur (BD). The surface TLR2 level was expressed as the mean fluorescence intensity (MFI).
Statistical Analysis
Data are shown as the mean ± SD of one representative experiment from two to three independent experiments. The significance of differences in the mean values was determined by the two-tailed unpaired Student's t test using InStat Program (GraphPAD Software, San Diego, CA). p values of < 0.05 were considered to be statistically significant.
Results
The expression of select TMEV-induced cytokine genes, not type I IFN, requires TLR2
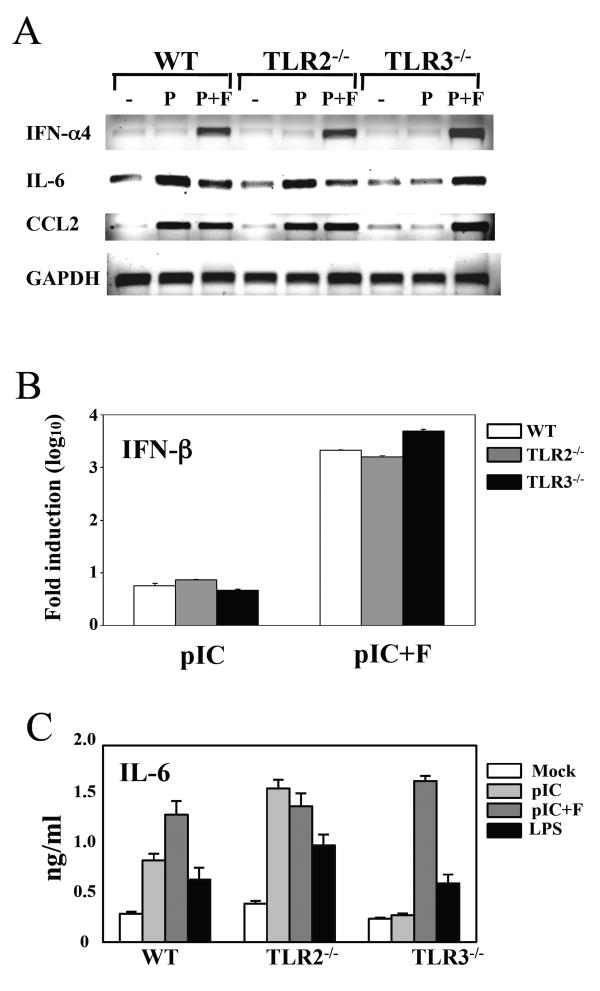
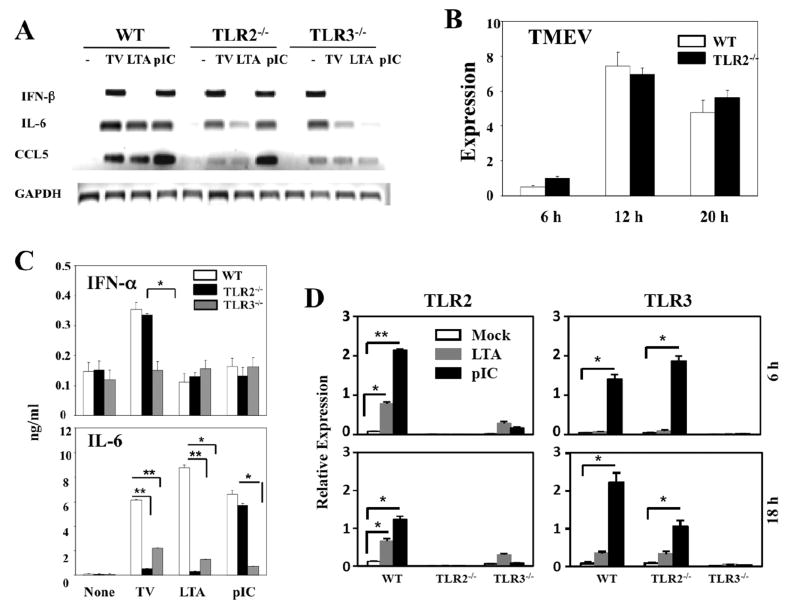
Because TLR2-mediated glial cell activation plays an important role in CNS inflammatory responses (Babcock et al. 2006; Kinsner et al. 2006; Zekki et al. 2002), we investigated whether TLR2 is also involved in the cytokine gene expression in mouse primary astrocytes following TMEV infection. Unlike the external treatment with poly I:C, intracellular transfection with poly I:C induced robust levels of IFN-α, CCL2 and IL-6 genes even in TLR3 KO astrocytes (Fig. 1). These results indicate that poly I:C transfection induces the stimulation of non-TLR3-mediated pathways, perhaps PKR and/or MDA-5-mediated signals. Hence, the external treatment method was used to stimulate the cells with poly (I-C) rather than transfection to minimize the non-TLR3-mediated cytokine stimulation. As shown in Fig. 2A, TLR2-deficiency did not affect on the type I IFN expression induced after TMEV infection, while the expression of IL-6 and CCL5 was drastically reduced, similar to that in TLR3-/- astrocytes. However, the poly (I-C)-induced expression of these cytokines was not significantly affected by the TLR2-deficiency. Low but detectable levels of cytokine gene activation were noted after stimulation of TLR2-/- astrocytes with LTA, a TLR2 ligand. The low level activation in TLR2-/- cells with a high concentration (10 μg/ml) of LTA may be attributable to LPS contamination (Morath et al. 2002). The poor cytokine gene induction in TLR2-deficient astrocytes was not due to the lack of viral replication, since WT and TLR2-/- astrocytes produced similar levels of viral RNA during the course of infection (Fig. 2B). Furthermore, the reduction in the cytokine gene expression of TLR2-/- astrocytes correlated with the reduced protein level as shown with IFN-α and IL-6 levels determined by ELISA (Fig. 2C). Thus, the mRNA levels correspond to the protein levels produced, indicating minimal post-translational modifications during the cytokine gene expression. Most interestingly, TLR3-/- astrocytes responded poorly to a TLR2 ligand, LTA (Fig. 2A and C). The TLR2 expression levels in TLR3-/- astrocytes were drastically lower than those in WT astrocytes, even after stimulation with TLR2 or TLR3 ligands (Fig. 2D). Therefore, it is likely that the low level of TLR2 expression on TLR3-/- astrocytes compromises the vigorous activation via TLR2. These results suggest that TLR3 enhances TLR2 expression on astrocytes.
Fig. 1. Primary mouse astrocytes were prepared from WT, TLR2-/- or TLR3-/- mice.
(A) Astrocytes were treated with poly (I-C) (50 μg/ml, P) or poly (I-C) complexed with Fugene6 reagent (3 μl/μg poly I:C, P+F). Cells were also stimulated with LPS (5 μg/ml) or TLR4 ligand as controls. After 18 h of stimulation, gene expression levels were analyzed with RT-PCR. (B) IFN-β RNA levels were assessed using real-time PCR, and the results were presented as the fold-induction compared with uninfected cells. (C) IL-6 levels in culture supernatants were assessed with ELISA. Data shown are representative of two independent experiments.
Fig. 2. IL-6 and CCL5 gene expression induced by TMEV is impaired in TLR2-/- astrocytes.
(A) Primary mouse astrocytes prepared from WT, TLR2-/- and TLR3-/- mice were infected with TMEV (10 MOI) or treated with LTA (10 μg/ml) and poly(I-C) (pIC, 50 μg/ml) for 6 h. (B) The indicated gene expression was analyzed using RT-PCR. TMEV RNA levels at different times (6, 12, 20 h) in virus infected (20 MOI) cells were also assessed by real-time PCR. Quantitative expression of gene was calculated after normalization with GAPDH expression. (C) IFN-α, IL-6 levels in the supernatants from WT, TLR2-/- or TLR3-/- astrocytes after a 18 h-exposure to TMEV (10 MOI), LTA (10 μg/ml) or pIC (50 μg/ml) were assessed using ELISA. (D) Primary mouse astrocytes were prepared from WT, TLR2-/- and TLR3-/- mice. Cells were left untreated or treated with LTA (5 μg/ml) and poly(I-C) (50 μg/ml) for 6 or 18 h. TLR2 and TLR3 mRNA levels were determined using real-time PCR. The results are representative of 2-3 similar experiments. *, p<0.05 and **, p<0.01.
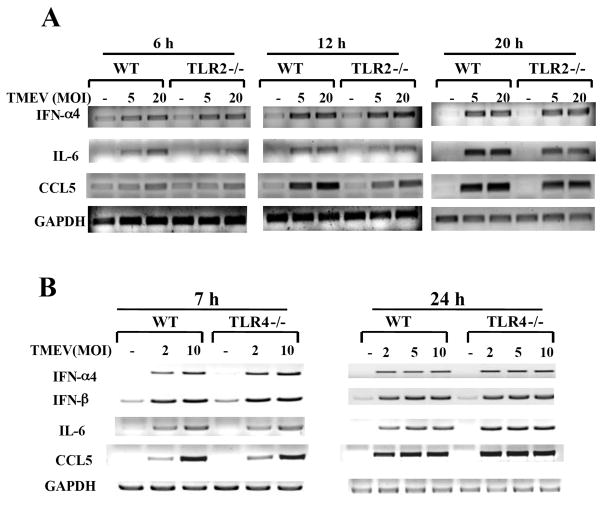
To examine the possibility that reduction of cytokine gene activation in TLR2-/- astrocytes reflects a delayed gene activation, cytokine mRNA levels were determined at 6, 12, 24 h post TMEV infection (Fig. 3A). Our results indicated that IL-6 and CCL5 gene expression levels, except IFN-α4, were consistently lower in TLR2-/- astrocytes than those in WT astrocytes at all these time points, although the levels were gradually increased in a time-dependent manner. These results suggest that those cytokine genes are also activated upon TMEV infection via signals mediated by other than TLR2, although the activation is inefficient and delayed. Since the astrocyte cultures contain greater than 95% of GFAP-expressing cells, a significant contribution to the reduction by other contaminated glial cell populations is unlikely. Interestingly, the activation of cytokine genes was significantly reduced in the absence of TLR3 (Fig. 2A), but not TLR4 (Fig. 3B). These results suggest that the activation of IL-6 and CCL5 genes induced following TMEV infection depends on the presence of both TLR2 and TLR3.
Fig. 3. TLR2 is important for maximal production of IL-6 in primary mouse astrocytes.
Primary mouse astrocytes were isolated from WT and TLR2-/- (A), or TLR4-/- (C) mice. Total RNAs from cells infected with TMEV were subjected to RT-PCR to determine IFN-α4, IFN-β, IL-6, and CCL5 mRNA levels.
TMEV-infection induces TLR2-dependent NF-κB activation
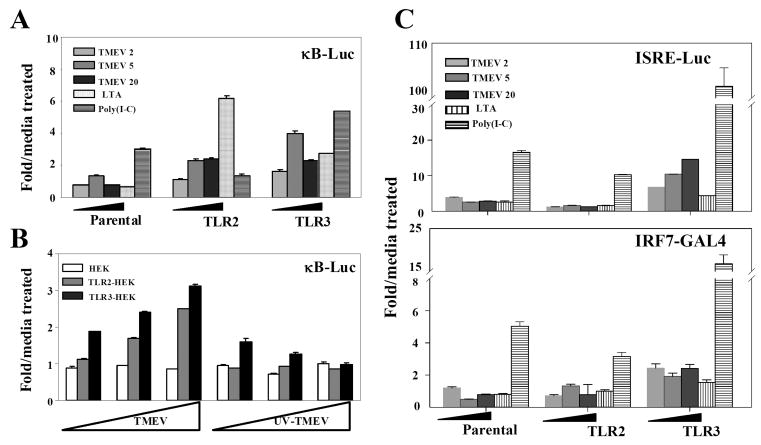
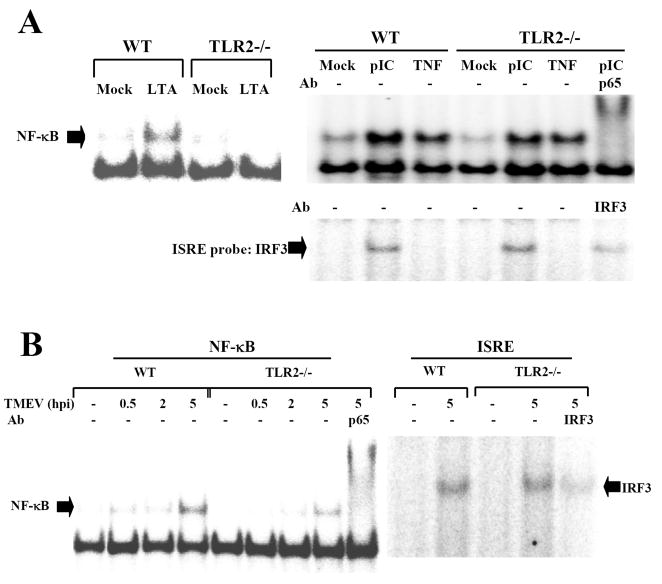
TLR2 mediates NF-κB activation in many different types of cells. In addition, TMEV infection induces the activation of NF-κB and IRF3 via TLR3 (So et al. 2006). However, it is not yet known whether TMEV infection also induces TLR2-mediated signals. Thus, we examined whether TMEV-induced NF-κB and IRF3/7 activation involves TLR2. For these studies, human embryonic fibroblast HEK293 cells, which do not express TLRs, were used to determine the function of TLRs. HEK293 cell lines stably expressing TLR2 or TLR3 were generated after transfection to conduct reporter gene assays for NF-κB activation induced by these TLRs. These cell lines evidently expressed the corresponding TLRs, hence their specific ligands were able to stimulate NF-κB activation (Fig. 4A). Viral infection resulted in activation of NF-κB in TLR2-expressing HEK293 cells, similar to that of TLR3-expressing cells. However, this activation was not detectable after infection with UV-inactivated TMEV (Fig. 4B), suggesting that TLR2-mediated signaling is virus replication-dependent, similar to TLR3-mediated signaling (So et al. 2006). In contrast, viral infection in TLR2-expressing HEK293 cells failed to activate IRF3 or IRF7, unlike TLR3-expressing cells (Fig. 4C). These data indicate that TMEV infection directly activates NF-κB via TLR2, but not IRFs associated with the type I IFN production (Honda and Taniguchi 2006).
Fig. 4. TMEV infection induces TLR2-dependent NF-κB activation, but not IRF3 or IRF7.
Parental HEK 293 and HEK 293 cells stably expressing TLR2 or TLR3 were transfected with luciferase reporter gene containing NF-κB-binding sequence (A and C), or with GAL4-IRF7 and UAS(GAL)-reporter plasmid (B), in conjunction with pRL-TK luciferase gene. After 24 h, cells were treated with TMEV, UV-inactivated TMEV, LTA (10 μg/ml) or poly (I-C) (50 μg/ml) for another 24 h. The luciferase activity was determined by fold induction in stimulated cells, compared to the basal level in media-treated cells. The data represent the means of three independent experiments. *, p<0.05 and **, p<0.01.
TLR2 contributes to the overall activation of NF-κB after TMEV infection in astrocytes
To determine the contribution of TLR2 to the overall level of TMEV-induced NF-κB activation, NF-κB activation levels in WT and TLR2-/- astrocytes were assessed following TMEV infection using EMSA (Fig. 5). The NF-κB activation in response to poly (I-C) or TNF-α in TLR2-/- astrocytes was not affected, contrast to no activation by TLR2 ligand, LTA (Fig 4A). Interestingly, the TMEV-induced NF-κB binding activity was significantly lower in TLR2-/- astrocytes compared to that in WT cells (Fig. 5B). Similar to the observation with TLR2-expressing HEK 293 cells, the TMEV-induced IRF3 binding activity was not affected by TLR2-deficiency. These results strongly suggest that TLR2-mediated NF-κB activation significantly contributes to the overall level of NF-κB activation induced in astrocytes after TMEV infection. Taken together, these results demonstrated that TLR2 is required for the maximal NF-κB activation, leading to the subsequent cellular activation and cytokine production in TMEV-infected astrocytes.
Fig. 5. TLR2 facilitates a maximal NF-κB activation during TMEV infection in astrocytes.
(A) Primary astrocytes from WT or TLR2-/- mice were treated with LTA (5 μg/ml), poly I-C (pIC, 50 μg/ml), or TNF-α (5 ng/ml) for 1 h. (B) Some cells were infected with TMEV (10 MOI) for various time periods. Nuclear extracts from these cells were incubated with oligonucleotide containing the consensus NF-κB binding sequence or ISRE sequence. Some nuclear extracts were incubated with anti-p65 or anti-IRF3 antibody before incubation with radio-labeled probe. These mixed samples were subjected to electrophoretic mobility shift assay (EMSA).
IL-6 production is associated with TLR3-dependent upregulation of TLR2 expression
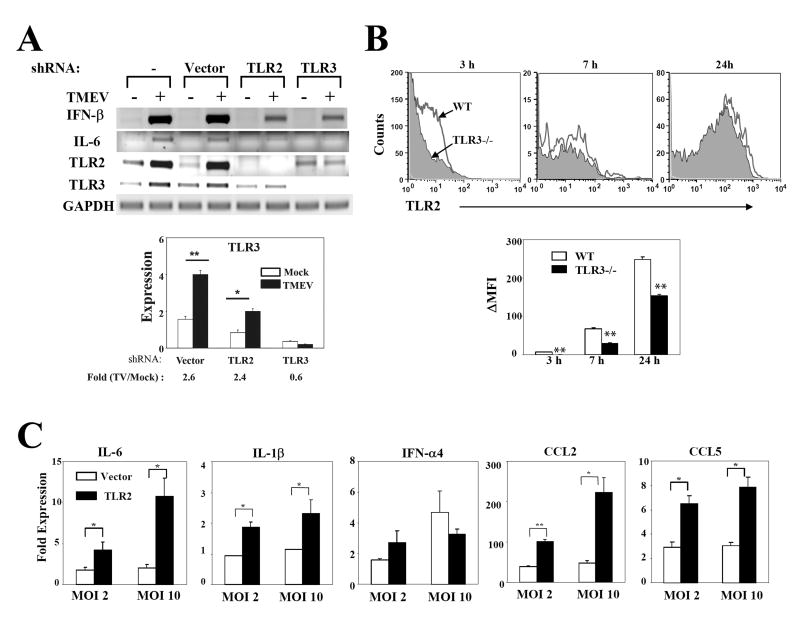
Low-levels of TLR2, TLR4, TLR5 and TLR9 messages are constitutively expressed in resting astrocytes and microglia, and their expression in these cells is upregulated after exposure to TLR ligands or TMEV (Bowman et al. 2003; Olson and Miller 2004). To examine whether TLR3 expression is critical for TMEV-induced TLR2 expression in astrocytes, we established 3 different stable C8D1A astrocyte lines after transfecting with the empty vector, vector containing TLR2-shRNA or TLR3-shRNA. We used this astrocyte line to establish astrocytes expressing the shRNAs, because of the difficulties in generating primary astrocytes uniformly expressing shRNAs. TLR2 mRNA expression appears to be dependent on the presence of TLR3, as TLR2 mRNA was not induced in TLR3-shRNA-expressing cells which show an undetectable TLR3 mRNA level (Fig. 6A). In contrast, no such induction of TLR3 mRNA by TLR2–mediated signals was detected. In fact, the quantitative real-time PCR results (Fig. 6A, lower panel) indicated that upregulation of TLR3 mRNA is similar between WT and TLR2-knockdown cells (2.6 fold vs. 2.4, respectively). Since TMEV infection induces a strong activation of NF-κB via TLR3 (So et al. 2006), the TLR3-mediated NF-κB activation may result in the upregulation of TLR2 expression. This possibility is supported by a recent report indicating that the TNF-α-induced TLR2 expression in microglia is mediated by activation of the NF-κB pathway (Syed et al. 2007). More importantly, the TMEV-induced expression of IL-6 gene was significantly impaired in both TLR2 and TLR3 knocked-down astrocyte lines (Fig. 6A). Similar results were obtained with primary astrocytes transfected with the shRNAs (not shown). These results suggest that TLR3-dependent upregulation of TLR2 expression modifies the cytokine profile of virus-infected astrocytes.
Fig. 6. IL-6 and CCL5 gene activation by TMEV in astrocytes is dependent on the TLR3-mediated upregulated TLR2 expression.
(A) Three different stable C8D1A murine astrocyte lines were generated by transfection with siRNA vector containing insert specific for TLR2, TLR3 or none. At 5 h after TMEV infection, the expression levels of genes were determined using RT-PCR. TLR3 expression levels were assessed by real-time PCR. Fold induction of TLR3 was calculated based on differences in the expression between mock- and TMEV-infected samples. (B) Surface TLR2 expression levels on primary astrocytes from WT or TLR3-/- mice infected with TMEV for 3, 7 or 24 h were measured by flow cytometry. ΔMFI (MFI of infected cells – MFI of uninfected cells) was shown as the mean ±SD of two independent experiments. (C) TLR3-/- astrocytes were transfected with 5 μg of mouse TLR2 plasmid or vector alone. Forty-eight hours later, the cells were infected with TMEV (2 or 10 MOI) for 6 h. Their relative cytokine gene expression levels were determined using real-time PCR. Data are presented as the fold-induction in mRNA expression compared with uninfected cells. **, p<0.01 and *, p<0.05 by Student's t test.
To examine whether the upregulation of TLR3-dependent TLR2 mRNA expression in astrocytes is associated with an increase in TLR2 level on the cell surface, primary astrocytes from WT or TLR3-/- mice were infected with TMEV for 3, 7, and 24 h, and then the TLR2 levels were compared using flow cytometry (Fig. 6B). TLR3-/- astrocytes failed to induce early expression (3 h post-infection) of TLR2, and the TLR2 level was gradually increased to the WT level at 24 h. These results suggest that a TLR3-mediated signaling up-regulates TLR2 expression most drastically at the early stage of viral infection. Furthermore, TLR3-/- astrocytes which over-express TLR2 following transfection with a TLR2 expression plasmid significantly increased the activation levels of IL-6, IL-1β, CCL2 and CCL5 genes, but not IFN-α4 (Fig. 6C), confirming that the production level of these cytokines is TLR2-dependent. Taken together, these results indicate that TMEV-induced, TLR3-dependent signals mediate the TLR2 upregulation, leading to the production of various proinflammatory cytokines, which are not vigorously induced by the TLR3-mediated signaling alone.
TLR2 and TLR3-induced soluble factors also induce TLR2-dependent cytokines
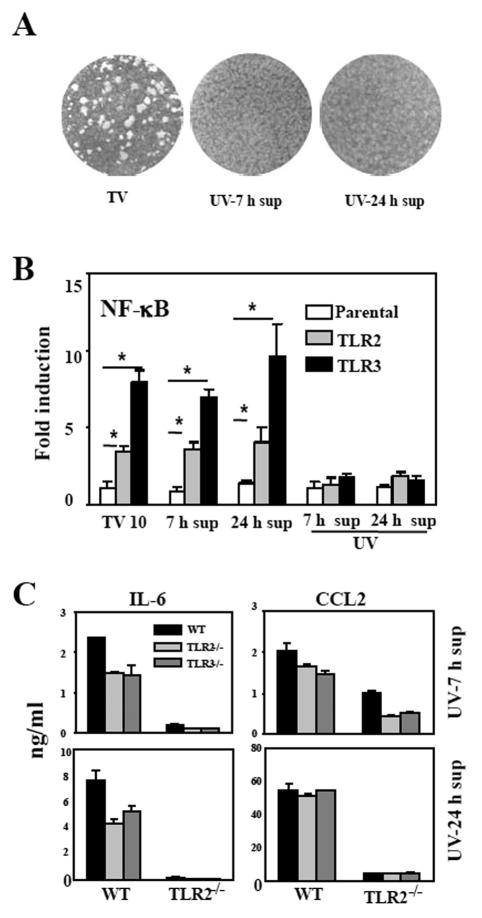
To examine the possibility that TLR3-induced soluble factor(s) may function as a ligand for TLR2 following TMEV infection, supernatants from WT, TLR2-/-, or TLR3-/- astrocytes incubated for 7 or 17 h (between 7-24 h) after viral infection were irradiated with UV to inactivate the infectious virus (Fig. 7). The viral inactivation was verified by plaque assays on BHK cells (Fig. 7A) and NF-κB activation reporter gene assays using HEK293 cell lines that stably express TLR2 or TLR3 (Fig. 7B). The UV-treatment completely abrogated the ability of supernatants to form plaques and to induce TLR-dependent NF-κB activation. Aliquots of the supernatants were added to fresh WT and TLR2-/- astrocytes and then their levels of cytokine gene activation were compared. Interestingly, the infectious virus-free supernatant from TMEV-infected WT astrocytes strongly induced IL-6, IL-1β and CCL2 mRNA expression, whereas those from TLR2-/- and TLR3-/- astrocytes were unable to fully activate these cytokine genes (Fig. 7C). In addition, these supernatants failed to induce the cytokine genes in TLR2-/- astrocytes (Fig. 7C), indicating that the induction of cytokine genes by the supernatants is strictly dependent on the presence of TLR2. These results suggest that TLR2 or TLR3-induced soluble factors are able to mediate TLR2-dependent cytokine production in mouse astrocytes.
Fig. 7. Factors produced by TMEV-infected astrocytes via TLR2/3 promote TLR2-dependent IL-6 production.
The supernatants were harvested from wild-type (WT-TV), TLR2-/- (TLR2-/-TV), or TLR3-/- (TLR3-/-TV) astrocytes at 7 h post-infection (7 h sup), and then cells were washed, re-cultured with fresh media for another 17 h to collect additional supernatants (24 h sup). (A) The lack of infectious virus in UV-irradiated (1 h) culture supernatants of wild-type astrocytes was verified by plaque assay on BHK cells and (B) NF-κB activation in HEK293 cells expressing TLR2 or TLR3 as described in Fig 4. (C) UV-inactivated supernatants were added to WT or TLR2-/- astrocytes. After 3 h, cells were washed and re-cultured with fresh media for another 21 h. The supernatants were then assessed for IL-6 and CCL2 levels using ELISA. The results represent the mean of three independent experiments. *p<0.05 by Student's t test.
Discussion
Viral infection induces various TLR-mediated innate responses, which subsequently play a pivotal protective or pathogenic role in conjunction with virus-specific adaptive immune responses (Boehme and Compton 2004; Mogensen and Paludan 2005). TMEV infection also triggers innate inflammatory responses in various cell types through TLR3-mediated signaling (So et al. 2006). In this study, we have further investigated the potential involvement of other TLRs and the downstream innate cytokine responses. Our study indicates that TMEV activates the innate immunity through a TLR2-dependent pathway as well, similar to that mediated by TLR3 (Figs. 2 and 3). The induction of proinflammatory cytokines, such as IL-6, IL-1β, CCL2 and CCL5, but not type I IFNs, was significantly decreased after TMEV infection in TLR2-deficient primary astrocytes (Fig. 3), suggesting that TLR2 is also associated with the production of these cytokines. This differential stimulation of cytokine production seems to reflect the differences in TMEV-induced NF-κB and IRF activation mediated via TLR2 and TLR3 (Fig. 4). Interestingly, the activation of TLR2-dependent cytokine genes correlates with the TLR3-mediated upregulation of TLR2 expression (Fig. 7A). These results indicate that TMEV-induced TLR3-mediated signals enhance TLR2 expression, leading to the production of a set of proinflammatory cytokines, which may not be strongly induced by TLR3-engagement alone.
Recent studies suggested that Coxsackievirus B4 (CVB4), another member of the Picornavirus family as same as TMEV, triggers cytokine production through a TLR4-dependent pathway in human pancreatic cells (Triantafilou and Triantafilou 2004). CVB3 and CVB4, as well as TMEV, are also potent inducers for the expression of proinflammatory cytokine genes in human astrocytes (Kwon et al. 2004). To examine the possibility that these similarities between CVB and TMEV may reflect utilization of the same set of TLRs for their cytokine gene activation, we assessed the effects of TLR4-deficiency on TMEV-induced cytokine gene expression. Surprisingly, there was no difference between TMEV-infected WT and TLR4-/- astrocytes in the expression of IL-6 and type 1 IFNs (Fig. 3B). Therefore, it is conceivable that utilization of TLRs by an identical virus is different dependent on the cell types. Recently it was reported that intracellular helicase family members, retinoic acid-inducible gene-I (RIG-I) and MDA-5, also play an important role particularly in the production of type I IFNs (Gitlin et al. 2006; Kato et al. 2006; Yoneyama et al. 2004). MDA-5 is critical for the innate immunity selectively to picornavirus infection, including TMEV infection (Kato et al. 2006). However, it is not yet known whether the MDA-5-dependent activation is important for TMEV-induced cytokine responses in primary glial cells. Nevertheless, our results here demonstrate that TLR2 and TLR3, but not TLR4, are involved in the TMEV-induced proinflammatory cytokine gene expression in mouse astrocytes.
TLR3 plays a critical role in TMEV-induced cytokine gene expression in primary mouse astrocytes (So et al. 2006). In addition, treatment of astrocytes with TLR3 ligand induces the expression of other TLRs, including TLR2 in human and mouse astrocytes (Carpentier et al. 2005; Jack et al. 2005). Our current study directly demonstrates that TMEV infection rapidly upregulates the expression of TLR2 on mouse astrocytes in a TLR3-dependent manner (Fig. 6). It is interesting to note that primary astrocytes express a low level of TLR2 and TLR3-/- astrocytes display even lower level of TLR2, resulting in compromised cytokine gene activation by a TLR2 ligand, LTA (Fig. 2C). Therefore, upregulation of TLR2 is likely required to effectively activate the cell population with LTA. The low level of TLR2 expression on unstimulated astrocytes (Figs. 2 & 6) agrees with the previous reports indicating rare- to-undetectable levels of TLR2 on primary astrocytes (Carpentier et al. 2005; Jack et al. 2005). Interestingly, other investigators recently observed that Pam3CysK4, another TLR2 ligand, activates the intracellular expression of CCL2 equally well in primary TLR3-/- and WT astrocytes (Zhou et al. 2008). However, their experimental system differs from ours in many aspects. The assessment of intracellular expression based on flow cytometry measures the proportion of cells producing CCL2 rather than the level of chemokine, while our ELISA data measure the protein levels in the supernatants. In addition, Pam3CysK4 activates TLR2 via TLR1/TLR2 heteromers, but LTA recognizes TLR2 via TLR2/TLR6 heteromers, leading to different stimulation signals for cytokine gene activation as previously suggested (Henneke et al. 2005). Nevertheless, the upregulation of TLR2 expression may not be unique to TLR3-mediated activation since treatments with other TLR ligands also elevate TLR2 expression.
The rapid TLR3-dependent upregulation of TLR2 expression upon TMEV infection may play an important role in inflammatory responses in the CNS of virus-infected mice. Viral infection appears to induce proinflammatory cytokine genes such as IL-6 and IL-1β in CNS cells after upregulating TLR2 expression and consequent utilization of the TLR2-dependent signal pathway. These cytokines may establish the local environment favoring for amplification of less protective and more pathogenic populations of T cells, like Th17 (Bettelli et al. 2007). The TLR2-mediated cytokine gene expression after TMEV-infection is associated with NF-κB activation (Figs. 4 and 5). This is consistent with our previous studies that NF-κB activation is essential for the activation of innate immune responses to TMEV in primary astrocytes (Palma and Kim 2004; Palma et al. 2003). These results suggest that viral replication intermediates, TMEV-induced proteins, and/or apoptotic cellular products following viral infection may be important for the TLR2-mediated activation.
TMEV-infected astrocytes produce many different cytokines in a TLR3-dependent manner, most likely triggered by dsRNA TMEV replication intermediates (Fig. 2). In addition, an infectious virus-free supernatant of TMEV-infected astrocytes is also capable of inducing these cytokines (Fig. 7), suggesting the involvement of other soluble factors produced following viral infection. However, the nature of soluble factor(s) triggering TLR2-mediated signaling is not yet clear. It was previously shown that host cell heat-shock proteins induce the production of proinflammatory cytokines such as IL-6 and NO via TLR2 (Asea et al. 2002). In addition, soluble CD14 is an endogenous ligand for TLR2 of human astrocytes, leading to the production of IL-6 (Bsibsi et al. 2007). Thus, it is conceivable that any of these and/or additional unknown factors may lead to the activation of TLR2-dependent cytokine genes in astrocytes. Such soluble factors may critically affect the type and function of anti-viral immune responses. Nevertheless, our current studies indicate that a TLR (TLR2) is secondarily involved in inducing proinflammatory cytokines in consequence of the primary TLR (TLR3)-mediated signals induced by viral infection. Such an indirect activation pathway easily overlooked previously may critically affect the inflammatory process in virus-induced chronic immune-mediated demyelinating disease.
Footnotes
Supported by RO1 NS28752 and RO1 NS33008 from the NIH, USPHS.
References
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–93. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, et al. Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci. 2006;26:12826–37. doi: 10.1523/JNEUROSCI.4937-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH. Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect Immun. 2008;76:250–62. doi: 10.1128/IAI.00949-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme KW, Compton T. Innate sensing of viruses by toll-like receptors. J Virol. 2004;78:7867–73. doi: 10.1128/JVI.78.15.7867-7873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Bajramovic JJ, Van Duijvenvoorden E, Persoon C, Ravid R, Van Noort JM, Vogt MH. Identification of soluble CD14 as an endogenous agonist for Toll-like receptor 2 on human astrocytes by genome-scale functional screening of glial cell derived proteins. Glia. 2007;55:473–82. doi: 10.1002/glia.20473. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–52. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Kim BS, Miller SD, Melvold RW. Theiler's murine encephalomyelitis virus-induced demyelination: a model for human multiple sclerosis. Methods. 1996;10:453–461. doi: 10.1006/meth.1996.0123. [DOI] [PubMed] [Google Scholar]
- Dal Canto MC, Melvold RW, Kim BS, Miller SD. Two models of multiple sclerosis: experimental allergic encephalomyelitis (EAE) and Theiler's murine encephalomyelitis virus infection. A pathological and immunological comparison. Microsc Res Tech. 1995;32:215–29. doi: 10.1002/jemt.1070320305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr Alzheimer Res. 2007;4:185–9. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA. 2006;103:8459–64. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneke P, Morath S, Uematsu S, Weichert S, Pfitzenmaier M, Takeuchi O, Muller A, Poyart C, Akira S, Berner R, et al. Role of lipoteichoic acid in the phagocyte response to group B streptococcus. J Immunol. 2005;174:6449–55. doi: 10.4049/jimmunol.174.10.6449. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–58. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–30. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Kang MH, So EY, Park H, Kim BS. Replication of Theiler's virus requires NF-kappaB-activation: higher viral replication and spreading in astrocytes from susceptible mice. Glia. 2008;56:942–53. doi: 10.1002/glia.20668. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–83. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Kinsner A, Boveri M, Hareng L, Brown GC, Coecke S, Hartung T, Bal-Price A. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide. J Neurochem. 2006;99:596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci USA. 2004a;101:1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones EA, Sandor F, Ortiz Y, Bowen GN, Counter SL, Wang TC, Finberg RW. Use of murine embryonic fibroblasts to define Toll-like receptor activation and specificity. J Endotoxin Res. 2004b;10:419–424. doi: 10.1179/096805104225006516. [DOI] [PubMed] [Google Scholar]
- Kwon D, Fuller AC, Palma JP, Choi IH, Kim BS. Induction of chemokines in human astrocytes by picornavirus infection requires activation of both AP-1 and NF-kappa B. Glia. 2004;45:287–296. doi: 10.1002/glia.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Means TK, Golenbock DT, Fenton MJ. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–32. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- Mines M, Ding Y, Fan GH. The many roles of chemokine receptors in neurodegenerative disorders: emerging new therapeutical strategies. Curr Med Chem. 2007;14:2456–70. doi: 10.2174/092986707782023686. [DOI] [PubMed] [Google Scholar]
- Mogensen TH, Paludan SR. Reading the viral signature by Toll-like receptors and other pattern recognition receptors. J Mol Med. 2005;83:180–92. doi: 10.1007/s00109-004-0620-6. [DOI] [PubMed] [Google Scholar]
- Morath S, Geyer A, Spreitzer I, Hermann C, Hartung T. Structural decomposition and heterogeneity of commercial lipoteichoic acid preparations. Infect Immun. 2002;70:938–44. doi: 10.1128/iai.70.2.938-944.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–53. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kim BS. The scope and activation mechanisms of chemokine gene expression in primary astrocytes following infection with Theiler's virus. J Neuroimmunol. 2004;149:121–129. doi: 10.1016/j.jneuroim.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Palma JP, Kwon D, Clipstone NA, Kim BS. Infection with Theiler's murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-κB activation: potential role for the initiation of demyelinating disease. J Virol. 2003;77:6322–6331. doi: 10.1128/JVI.77.11.6322-6331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebenko-Moll NM, Liu L, Cardona A, Ransohoff RM. Chemokines, mononuclear cells and the nervous system: heaven (or hell) is in the details. Curr Opin Immunol. 2006;18:683–9. doi: 10.1016/j.coi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Rubio N, Capa L. Differential IL-1 synthesis by astrocytes from Theiler's murine encephalomyelitis virus-susceptible and -resistant strains of mice. Cell Immunol. 1993;149:237–47. doi: 10.1006/cimm.1993.1151. [DOI] [PubMed] [Google Scholar]
- Skias DD, Kim DK, Reder AT, Antel JP, Lancki DW, Fitch FW. Susceptibility of astrocytes to class I MHC antigen-specific cytotoxicity. J Immunol. 1987;138:3254–3258. [PubMed] [Google Scholar]
- So EY, Kang MH, Kim BS. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia. 2006;53:858–67. doi: 10.1002/glia.20346. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Syed MM, Phulwani NK, Kielian T. Tumor necrosis factor-alpha (TNF-alpha) regulates Toll-like receptor 2 (TLR2) expression in microglia. J Neurochem. 2007;103:1461–71. doi: 10.1111/j.1471-4159.2007.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435–45. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M. Coxsackievirus B4-induced cytokine production in pancreatic cells is mediated through toll-like receptor 4. J Virol. 2004;78:11313–11320. doi: 10.1128/JVI.78.20.11313-11320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami K, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol. 2002;169:2026–33. doi: 10.4049/jimmunol.169.4.2026. [DOI] [PubMed] [Google Scholar]
- Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R. Inflammation in Parkinson's diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des. 2007;13:1925–8. doi: 10.2174/138161207780858429. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–19. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–9. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Halle A, Kurt-Jones EA, Cerny AM, Porpiglia E, Rogers M, Golenbock DT, Finberg RW. Lymphocytic Choriomeningitis Virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J Neuroimmunol. 2008;194:70–82. doi: 10.1016/j.jneuroim.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-β. Blood. 2007;109:619–25. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]