Abstract
Cell based therapies are attractive approaches to promote myelin repair. Recent studies demonstrated a reduction in disease burden in mice with EAE treated with mouse mesenchymal stem cells (MSCs). Here we demonstrated human bone marrow derived MSCs (BM-hMSCs) promote functional recovery in both chronic and relapsing-remitting models of mouse EAE, traced their migration into the injured CNS and assayed their ability to modulate disease progression and the host immune response. Injected BM-hMSCs accumulated in the CNS, reduced the extent of damage and increased oligodendrocyte lineage cells in lesion areas. The increase in oligodendrocytes in lesions may reflect BM-hMSC induced changes in neural fate determination since neurospheres from treated animals gave rise to more oligodendrocytes and less astrocytes than non-treated neurospheres. Host immune responses were also influenced by BM-hMSCs. Inflammatory T-cells including interferon gamma (IFN-γ) producing Th1 cells and IL-17 producing Th17 inflammatory cells and their associated cytokines were reduced along with concomitant increases in IL-4 producing Th2 cells and anti-inflammatory cytokines. Together these data suggest the BM-hMSCs represent a viable option for therapeutic approaches.
Keywords: Mesenchymal stem cells, Migration, Repair, Differentiation, Neurons, Oligodendrocytes, Immune regulation
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the CNS characterized by extensive mononuclear cell infiltration and demyelination. MS is generally considered to be a T-cell mediated disease based on local inflammation (Sospedra and Martin 2005), response to immune modulation or immunosuppression (Perini et al. 2007; Stuve et al. 2006), and the genetic association with the major histocompatability complex (Haines et al. 1996). The best characterized model of MS is experimental allergic encephalomyelitis (EAE) (Martin 1997) induced by immunization of susceptible host animals with specific myelin proteins. In demyelinating diseases, remyelination and subsequent restoration of neuronal function can be achieved by either promoting endogenous repair mechanisms or by providing an exogenous source of myelinating cells via transplantation. Clinical trials using transplantation of myelin-forming cells and approaches that utilize either endogenous stem cells are currently appealing.
Long term functional recovery requires regulation of the pathogenic process, which may be modulated by mesenchymal stem cells (MSCs). For example, in models of demyelinating diseases such as EAE, treatment with syngeneic mouse MSCs reduces disease burden (Gerdoni et al. 2007; Kassis et al. 2008) with an accompanying down regulation of activated T-cells and the induction of T-cell anergy (Zappia et al. 2005), suggesting that immunosuppression is a characteristic of adult stem cells (Miller and Bai 2007). Under resting conditions MSCs reside in bone marrow and differentiate along lineages of mesodermal origin to form muscle, bone, cartilage, fat and tendon (Pittenger et al. 1999). Upon tissue injury or with appropriate stimuli MSCs have been proposed to be capable of differentiating into non-mesenchymal lineages including endothelial cells (Oswald et al. 2004) and neural cells (Jiang et al. 2002). Whether this reflects trans-differentiation, ectopic marker expression or cell fusion is currently unclear (Rutenberg et al. 2004). In vitro studies, however suggest that MSCs provide soluble cues that influence fate choices in neural cells (Bai et al. 2007; Rivera et al. 2006) increasing neurons and oligodendrocytes while decreasing astrocytes.
Development of MSC based therapies requires that human MSCs have similar disease regulatory characteristics to murine cells and previous studies (Aggarwal and Pittenger 2005; Zhang et al. 2005) provide strong evidence in support of this notion. Here we extend these studies and show that bone marrow derived human MSCs (BM-hMSCs) have a profound effect on disease progression in both chronic and relapsing-remitting models of MS when delivered after the onset of disease. More importantly, treatment with MSCs influenced neural cell fate in EAE host animals, promoting oligodendrogenesis and inhibiting astrogliosis as well as regulating the nature of the host immune system responses. Based on these observations we propose that MSCs represent a powerful cell based therapy for immune mediated demyelinating disease such as MS.
Materials and Methods
Isolation and culture of BM-hMSCs
The isolation and expansion of BM-hMSCs were performed as previously described (Bruder et al. 1994). Briefly, bone marrow samples were combined with 25 ml of DMEM-LG (Sigma, St. Louis, MO) supplemented with 1% (v/v) antibiotic–antimycotic solution (Life Technologies, Grand Island, NY) and 10% selected fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT). The cell suspension was centrifuged at 500g for 5 min and resuspended in DMEM-LG layered over a 63% (v/v) Percoll in Tyrode's salt solution (Sigma). Following centrifugation at 460g the top 25% of the gradient was resuspended, nucleated cells seeded at 107 cells per 75 cm2 flasks and maintained at 37°C in a 5% CO2. Large monolayer cell colonies were recovered by trypsinization and re-plated at a density of 105 cells/ml.
MOG35-55 and PLP139-151 peptides
Peptides corresponding to the sequences of mouse MOG35-55 were synthesized by the W.M. Keck Biotechnology Resource Center at Yale University. Peptides were purified by reverse-phase (C18) column HPLC using a trifluoroacetic acid/acetonitrile gradient. The sequence of the rodent MOG peptide was MEVGWYRSPFSRVVHLYRNGK. The PLP139–151 sequence used was HSLGKWLGHPDKF (Cleveland Clinic Foundation, Cleveland OH, USA).
Animal immunization, EAE induction and BM-hMSC treatment
All animal experiments were approved by Case Western Reserve University School of Medicine IACUC and were conducted in accordance with the United States Public Health Service Policy on Human Care and Use of Laboratory Animals. Six to eight week female C57BL/6 or SJL mice were used in all experiments. In some studies to facilitate detection of oligodendrocyte lineage cells, female mice from the EGFP transgenic strain on a C57BL/6J background expressing enhanced green fluorescent protein (EGFP) driven by the promoter from the myelin proteolipid protein (plp) gene (Wight et al. 2007) were used. Standard C57BL/6 and SJL mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and chronic EAE was induced in the C57BL/6 animals by subcutaneous immunization with 200μl of 200μg MOG35-55. Relapsing-remitting EAE was induced in SJL mice by subcutaneous immunization with 150μg PLP139-151. Both antigens were mixed in Complete Freund's adjuvant (CFA) with 500μg of Mycobacterium tuberculosis (Difco, Detroit, MI) and 500ng of pertussis toxin (List Biological Laboratories, Campbell, CA) was injected IP at 1and 2 days. To track hMSCs, they were labeled with either 5-chloromethylfluorescein diacetate (Cell Tracker™ red CMFDA, Invitrogen, #2925) or CFSE (Renovar, Madison, WI) and injected intravenously; 3×106 cells via the tail vein on either day 16, day 27 (C57BL/6J) or day 15 (SJL) post immunization. Animals were sacrificed at day 45 (C57BL/6J), day 70 (PLP-EGFP) and day 65 (SJL) by transcardial perfusion with 4% paraformaldehyde and cervical spinal cords were excised for analyses.
Clinical disease scoring
Mice were monitored daily for clinical signs of EAE. Clinical scores were based on a scale of 0–5 (Zargari et al. 2007) with a score of 0 indicating no disease and 5 indicating death. A score of 1 indicates limp tail, 2 paresis or partial paralysis of the hind limbs, 3 total hind limb paralysis, and 4 hind and front limb paralysis. Data are presented as mean clinical scores for each group, with dead animals given a score of 5 on the day of death.
Histological analysis
Following fixation with 4% paraformaldehyde, 20μm sections were examined with an Olympus Provis optical microscope using Hematoxylin and Eosin (HE) for detection of inflammatory infiltrates and Luxol Fast Blue (LFB) and Toluidine Blue for myelin detection. Inflammatory activity was assayed with anti-CD45 monoclonal antibody (Millipore, Billerica, MA). Astrocytes were identified by anti-GFAP mAb (Sigma), and neurons by anti-β-tubulin III (BD Pharmingen, San Diego, CA). Oligodendrocyte precursors were identified by mAbA2B5 (BD Pharmingen) and anti-NG2 (Covance, Princeton, NJ). Binding of primary antibodies was detected with rhodamine labeled secondary antibodies (Sigma). Controls included omission of the primary antibody. Quantification was performed on 8 sections per animal and 5 animals per group. Cells were counted by investigators blinded to the status of the animal. Counts are expressed per section and normalized to the average value obtained in vehicle treated mice. Lesion load and density of myelinated axons was determined from randomly selected areas within the lesion areas from at least 3 sections taken from the same spinal cord level from 5 animals in each group.
Cytokine production and antigen-specific proliferation
Spleens were harvested and single-cell suspensions generated. In 96-well microtiter plates, 5×105 erythrocyte-free (Tris-NH4Cl-treated) splenocytes/well were incubated in culture medium with or without antigen for 48h and pulsed with 1uCi/well thymidine for the final 24h of culture. Proliferation was determined using a Top count Microplate Scintillation Counter (Packard Instruments, Meridan, CT). Results are expressed as the mean of triplicate cultures ±SEM. Supernatants were collected from parallel experiments at 72 h for cytokine analyses. Cytokine measurements were performed using the Mouse Cytokine 10-Plex system and Luminex LiquiChip analyzer.
IFN-γ and IL-4 producing -cells measured with ELISPOT assay
Characterization of IFN- γ and IL-4 producing splenocytes was performed as previously described (Burton et al. 2006; Lalvani et al. 1997). Ninety-six-well plates (Millititer, Bedford, MA, #01730) were coated with 2 μg/ml of anti-IFN- γ (eBioscience, San Diego, CA, #AN-18) or 4 μg/ml of anti-IL-4 (eBioscience, #11B11) monoclonal antibodies. Spleen cells from (1 ×106/well in triplicate) 4 mice (MOG35-55) or 3 mice (PLP139-151) per group from control and hMSC treated EAE animals at day 45 (MOG35-55) and day 60 (PLP139-151), were incubated 12-18h for INF- γ and 48h for IL-4 in the presence of 10 μg/ml MOG35-55 and 20μg/ml PLP139-151 for recall response. Wells were incubated overnight at 4°C with 50 μl of the secondary antibody (2μg/ml), Biotin conjugated anti-IFN- γ (eBioscience, #R4-A2) and anti-IL-4 (2μg/ml, eBioscience, #BVD6-24G2) mAbs. After incubation in Streptavidin-Alkaline Phosphatase at 1:2000 (DAKO, Denmark, #D0396) for 2hrs, cells were developed for 15-60 min in BCIP/NBT (KPL, Gaithersburg, MD, #50-81-78). The number of IL-4 and IFN-γ–spot-forming cells was measured using KS ELISPOT software (Carl Zeiss Vision, Hallbergmoos, Germany). Individuals were designated as responders if the numbers of spots in the presence or absence of hMSCs were significantly higher (P < 0.05) than in control wells. The frequency of myelin reactive splenocytes was calculated as follows: (spot numbers in wells with hMSCs – spot numbers in negative control wells) ÷ cell numbers per well (Bai et al. 2002).
Statistical analysis
Statistical significance was set to P < 0.05. Values are mean +/- standard deviation and the P-value and plotted either as mean +/- one standard deviation or as mean +/- two standard errors of the mean. In all plots, significance is indicated by asterisks, where one asterisk =P < 0.05, two = P < 0.01, and three = P < 0.001. Histology scores were analyzed by Mann-Whitney U test.
Results
BM-hMSCs ameliorate MOG35-55 induced chronic and PLP139-151 induced relapsing-remitting EAE
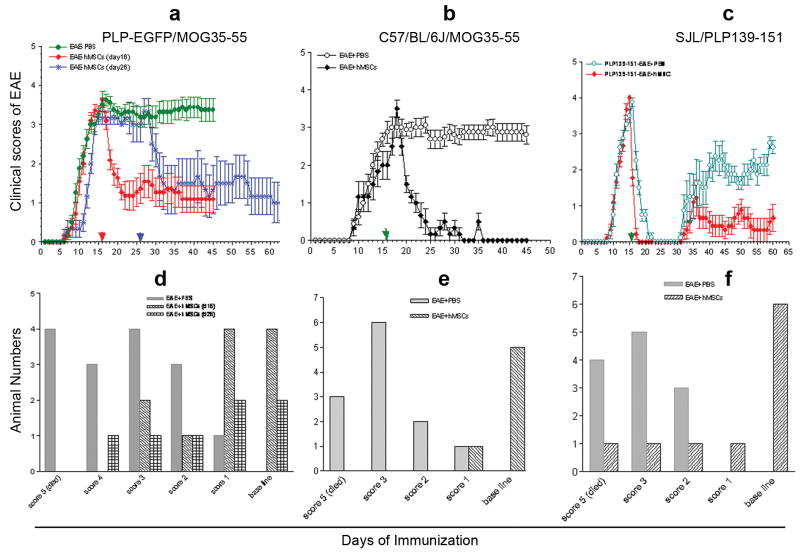
To evaluate the effects of BM-hMSCs on disease progression in EAE, cells were intravenously injected into host animals when the clinical score was significantly elevated (Fig 1 arrows). Following immunization with MOG35-55, PLP-EGFP transgenic and C57BL/6 animals developed comparable levels of disease. In both models disabilities were initially detected between days 6-9 and peaked around day 16 with an average clinical score of 3.4-4. In PLP-EGFP transgenic mice with MOG35-55 induced EAE that received BM-hMSCs on day 16 (Fig 1a red squares) or day 26 (Fig 1a blue stars), disease symptoms were rapidly reduced compared to carrier control animals (Fig 1a green circles). Four of ten animals with hind limb paralysis that received BM-hMSCs on day 16 and 2/8 on day 26 recovered normal gait. By contrast, no animal recovered normal gait in the carrier group (Fig 1d). Similar results were obtained in a cohort of non-transgenic C57BL/6 animals immunized with MOG35-55 (Fig 1b and e). These data suggest that infusion of hMSCs results in rapid and sustained improvement in chronic EAE.
Figure 1. Intravenous injection of BM-hMSCs ameliorates MOG35-55 and PLP139-151 induced EAE.
EAE induced in PLP-EGFP, C57BL/6J and SJL mice by immunization with MOG35-55 and PLP139-151 peptide (a-c). Administration of BM-hMSCs to EGFP transgenic mice at day 16 and day 26 (a), C57BL/6J mice at day 16 (b) or SJL mice at day 16 (c) resulted in improved clinical scores. The clinical scores distributed for each BM-hMSC-treated EGFP transgenic mice at day 45 (d), C57/BL/6J mice at day 45 (e), and in SJL mouse at day 60 (f). Recovery was sustained for up to 70 days the longest time point examined. Arrows indicate days of BM-hMSC injection.
The influences of BM-hMSCs were not restricted to MOG35-55 induced EAE. Female SJL animals immunized with PLP139-151 developed a relapsing-remitting disease in which clinical scores peaked during the second week after immunization and subsequently resolved and then followed with relapsing-remitting disease. Animals that received BM-hMSCs on day 16 had considerably milder disease overall (Fig. 1c) and 6/10 mice recovered completely and no animals died. By contrast, no carrier treated animals demonstrated complete recovery (Fig 1f).
To determine whether the influence of BM-hMSCs was a consequence of off-target effects of injection of human cells or a characteristic of BM-hMSCs, studies were repeated using 3.5×106 Hela cells. No amelioration in clinical progression was detected; rather there was significantly increased animal loss. In MOG EAE mice, 5/6 animals died over 45 days, likewise 6/8 SJL EAE mice died. The reduction in clinical score seen after BM-hMSC treatment is consistent with studies indicating that rodent and human MSCs modulate EAE disease progression (Aggarwal and Pittenger 2005; Karussis and Kassis 2007; Zhang et al. 2005) and suggest this is a common characteristic of MSCs.
Reduction of inflammatory cells and demyelination in MOG35-55 induced EAE mice treated by BM-hMSCs
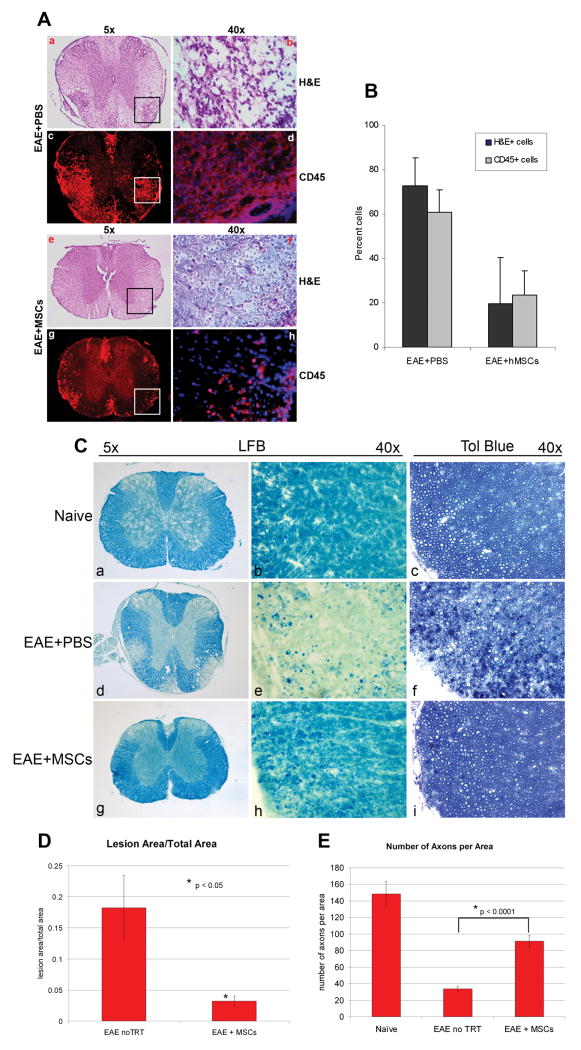
The improvement in EAE clinical scores following BM-hMSCs treatment may reflect influences on inflammatory cell infiltration into the CNS, systemic effects on the immune system and/or direct effects on CNS cells. To characterize the influence of BM-hMSCs on inflammatory cell influx, sections of spinal cord from MOG35-55 EAE animals were labeled with H&E and anti-CD45 to identify mononuclear cell and leukocyte infiltration. The numbers of infiltrating cells (Fig 2A e,f) and CD45+ expressing leukocytes (Fig 2A g,h) were significantly decreased in animals treated with BM-hMSCs compared to carrier controls (Fig 2A a-d), (Fig 2B, p<0.05), indicating that BM- hMSCs reduce inflammatory cell infiltration into the CNS during EAE.
Figure 2. Treatment with BM-hMSCs reduces cellular infiltration and tissue damage in EAE.
(A) Low (a,e,c,g) and high (b,f,g,h) power images of H&E and anti-CD45 antibody labeled sections of cervical spinal cord at day 45 post immunization. Carrier treated (d16) EAE mice induced by MOG35-55 contained prominent H&E positive infiltrating cells and large numbers of CD45+ mononuclear leukocytes in lesion areas of white matter (a-d). In animals treated with BM-hMSCs on day 16 when the clinical score was approximately 3.5 and sacrificed on day 45 the numbers of both H&E (f) and anti-CD45 (h) cells were greatly reduced. High magnification images (40X) from marked lesion areas (b,d,f,h). (B) Quantization of the relative proportion of cells in lesion areas in PBS and BM-hMSC treated animals. The number of infiltrating cells is significantly reduced in BM-hMSC treated animals (Mean +/- SD with p <0.05). (C) Myelin staining with Luxol Fast Blue (LFB) at the same time point as A and B. In cervical spinal cord sections from EAE+PBS mice with a score of 3.5 extensive myelin degeneration (d,e) is seen compared to naïve animals (a,b). Animals treated with BM-hMSCs have substantially less myelin loss that than carrier controls (g,h). Similarly, 1μm sections labeled with Toludine Blue (TB) showed increased myelin debris and greater numbers of demyelinated axons in the EAE+PBS mice (f) compared to naïve mice (c). Normalization of cytoarchitecture and reduced myelin debris is evident in lesions from mice treated with BM-hMSCs (i). (D) Comparison of lesion load between BM-MSC treated and non-treated EAE animals and (E) density of myelinated axons in naïve, BM-MSC treated and non-treated animals. Lesion load data was taken from 5 different sections of cervical spinal cord. Axonal counts were taken from lesion areas in the dorsal columns from the same cohort of animals. (N=5) P values determined by two-tailed t test.
To determine whether BM-hMSCs reduced tissue damage in EAE animals, sections of cervical spinal cord from naïve, carrier, and BM-hMSCs treated EAE animals were labeled with Luxol fast blue (LFB) and Toluidine blue (TB). Multiple areas of demyelination were detected in carrier and BM-hMSC treated EAE animals but not naïve animals (Fig 2C). The extent of the chronic demyelinated regions was considerably reduced in the BM-hMSC treated animals (Fig 2Cg,h) compared to carrier controls (Fig 2Cd,e) and they contained greater numbers of myelinated axons, fewer demyelinated or partially demyelinated axons, and reduced myelin debris (Fig 2C, D and E) compared to carrier treated EAE animals (Fig 2C, D and E) suggesting that BM-hMSCs either promote repair or provide neuroprotection in EAE. The protective effects of BM-hMSCs were not limited to the cervical spinal cord, rather in EAE substantial demyelination was seen in lumbar spinal cord and in the brain. In all regions, lesion load appeared to be reduced in animals treated with BM-hMSCs while normal myelination was seen in naïve animals (Fig. 2C).
BM-hMSCs are found in regions of demyelination in EAE animals
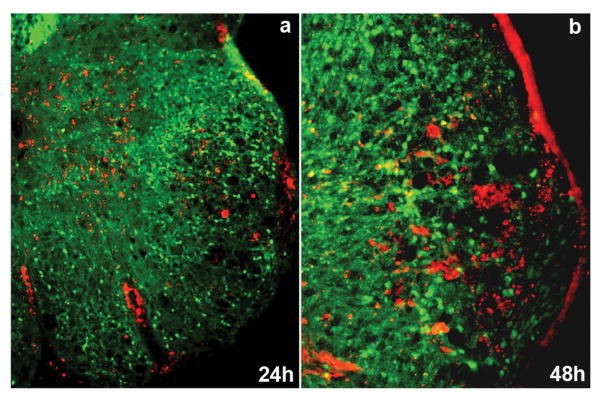
To assess whether BM-hMSCs might act locally in EAE to reduce the inflammatory response and normalize histology their distribution was assayed. Labeled BM-hMSCs were intravenously injected into both models of EAE. Within 24 hrs labeled BM-hMSCs were observed in the CNS of host animals. In the spinal cord BM-hMSCs were detected in demyelinated areas many of which were closely associated with blood vessels (Fig. 3a, b). The injected cells persisted in the CNS and could be detected 45 days after injection although in reduced numbers (data not shown). Very few injected cells were detectable in naïve spinal cords any time after injection.
Figure 3. BM-hMSCs accumulate in demyelinating lesions in EAE induced by MOG35-55.
BM-hMSCs labeled with CMFDA (red) were found in lesion areas of PLP-EGFP transgenic EAE mice at 24h (a) and 48h (b) post-injection.
Treatment with BM-hMSCs enhances oligodendrogenesis and reduces astrogliosis in MOG35-55 induced EAE animals
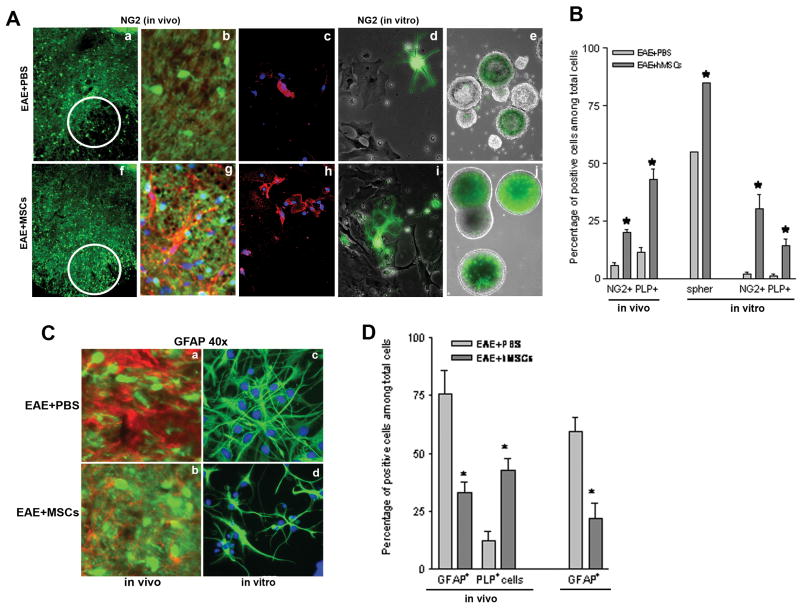
The neural cell composition of EAE lesions was altered in the presence of BM-hMSCs. In EAE animals, lesion areas in cervical spinal cord contained reduced proportions of NG2+ and PLP+ cells compared to naïve tissue and this loss was significantly reversed in animals that received BM-hMSCs. The proportion of NG2+ cells in lesions increased from approximately 5% in EAE animals (Fig 4Aa,b) to 20% (p<0.05) in BM-hMSC treated animals (Fig 4Af,g) compared to approximately 22% in naïve animals (not shown). Likewise the number of PLP+ oligodendrocytes increased from approximately 10% to 44% (p<0.05) (Fig 4B) closer to naïve values of 70% suggesting that BM-hMSCs are either protective of or selectively recruit the oligodendrocyte lineage (Bai et al. 2007). To assess whether BM-hMSCs promoted oligodendrogenesis in EAE animals, neurospheres were generated from BM-hMSC treated and carrier-treated EAE PLP-transgenic animals and grown in control or BM-hMSC conditioned media. While neurospheres from both sets of animals contained EGFP+ cells, the proportion of strongly positive spheres (green) was significantly higher (p<0.05) in the BM-hMSC treated animals (16/20 total spheres) compared to carrier controls (11/20 total spheres) (Fig. 4Ae,j). The increased number of EGFP+ cells in BM-hMSCs treated neurospheres was maintained in vitro. A higher proportion of NG2+ cells (30%) (Fig 4Ac,h,B) and oligodendrocytes (14% cells) (Fig 4Ad,i,B) were found in BM-hMSC treated cultures compared to controls (2% NG2+ cells and 1% oligodendrocytes) (p<0.05), suggesting that BM-hMSCs enhance endogenous repair in part by stimulating oligodendrogenesis.
Figure 4. Treatment with BM-hMSCs increases oligodendrogenesis and decreases astrogliosis in EAE animals.
(A) Low numbers of PLP- EGFP+ oligodendrocyte (green) and NG2+ oligodendrocyte precursor cells (red) are present in EAE lesions of carrier treated animals sacrificed at day 45 (a,b). Similarly, cell cultures from lesioned spinal cords (c,d) or neurospheres derived at day 45 from the SVZ of PLP-EGFP EAE mice treated with PBS showed low numbers of oligodendrocyte lineage cells. By contrast, in animals treated with BM-HMSCs on day 16 the numbers of PLP+ cells and NG2+ cells are increased in lesion area, (f,g) in vitro (h,i) and in neurospheres (B) Quantification of relative cell numbers shown in A. The proportion of NG2 cells increases from 5% to 20% in vivo and 2% to 30% in vitro after BM-hMSC treatment. Likewise the proportion of EGFP-PLP+ cells increased from 10% to 57% in vivo and 1% to 14% in vitro after BM-hMSC treatment. The proportion of strongly PLP-EGFP+ neurospheres was significantly increased from 50% to 80% after treatment with BM-hMSCs. (C) Astrogliosis and oligodendrogenesis characterized by increases in GFAP expression (red) and decreases in EGP-PLP expression (green) are prominent in EAE lesions (a) in mice treated with PBS (b) Parallel lesions from animals treated with BM-hMSCs. Note the reduced number of astrocytes (GFAP+) and enhanced oligodendrocytes (PLP-EGFP+ cells). Consistent with reduced astrogliosis in BM-hMSC treated lesions, the proportion of GFAP+ cells in neurosphere cultures from the SVZ of EAE mice treated with BM-hMSCs on day 16 was significantly reduced (d) compared to EAE mice treated with PBS (c). (D) Quantification of the relative cell numbers shown in C. The proportion of GFAP+ cells decreased from 73% to 37% in vivo and 52% to 14% in vitro after BM-hMSC treatment while the number of PLP-EGFP+ cells increased from 10% to 54%. (*) = Significance p<0.05.
Lesions from cervical spinal cords in carrier treated EAE animals showed increased GFAP expression (approx. 73% GFAP+ cells) (Fig 4Ca,D) concomitant with a reduction in oligodendrocytes (approx. 10% PLP+ cells) (Fig 4Ca,D) while in lesions of BM-hMSC treated animals GFAP expression was less pronounced (approx. 37% GFAP+ cells) (Fig 4Cb,D) and the proportion of oligodendrocytes was increased (approx. 44% PLP+ cells) (Fig 4Cb,D). The decreased GFAP expression seen in the treated EAE animals may reflect either decreased numbers of astrocytes, reduced astrocyte hypertrophy or both. To address this question, NSCs derived from control and BM-hMSC treated EAE adult subventricular zones (SVZ) were cultured in the presence of growth factors and allowed to differentiate for three days. Cultures derived from BM-hMSCs treated EAE animals contained significantly fewer GFAP+ astrocytes (approx 14% of total cells) (Fig. 4Cd,D) than did non-BM-hMSC treated EAE animals (approx 52% GFAP+ cells) (Fig 4Cc,D) (Fig 4B p< 0.05) suggesting that. BM-hMSCs modulate the CNS responses to injury in part through suppression of astrogliosis.
Treatment with BM-hMSCs reduces myelin-specific memory splenocytes in EAE
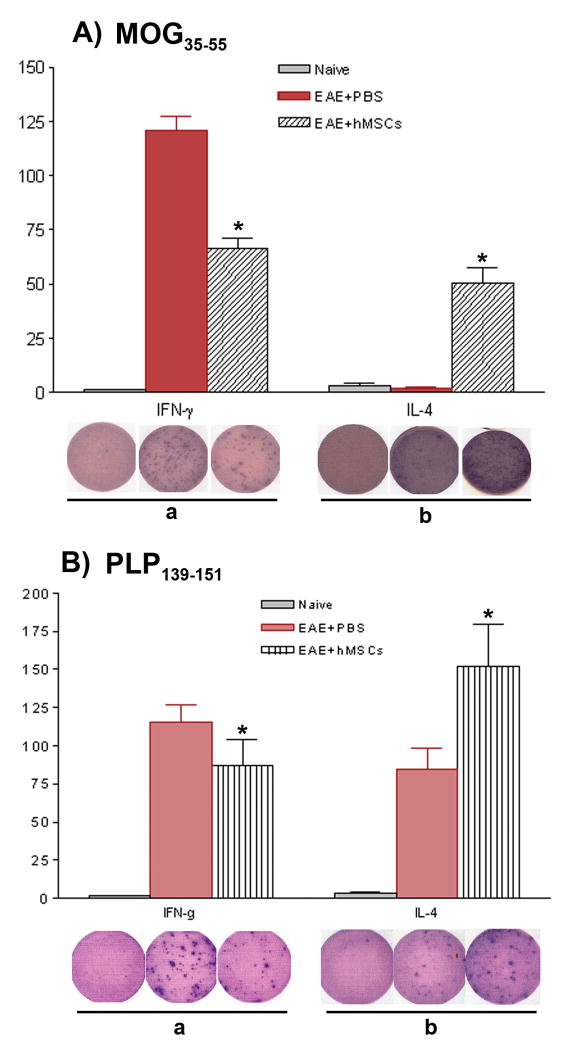
To determine whether BM-hMSCs altered myelin-specific memory cells in EAE, in vitro antigen specific IFN-γ and IL-4 producing T cell ELISPOT assays were performed. Spleen derived cells were stimulated with recall antigen (MOG35-55 10μg/ml, or PLP139-151 20μg/ml). In MOG induced EAE, the relative frequency of IFN-γ producing cells, assayed after 24 hrs, was reduced from approximately1/1,500 to approximately 1/8,300 in BM-hMSCs treated animals (Fig. 5Aa) (p<0.05). By contrast, the relative frequency of IL-4 producing cells at 48hrs was increased from 1/11,900 in EAE to 1/6,600 (Fig 5Ab) (p<0.01). Similar trends were seen in the PLP139-151 EAE model. The frequency of IFN-γ producing PLP139-151 specific cell responses was lower in BM-hMSCs treated EAE animals, from 1/8,650 in carrier EAE to 1/11,547 in BM-hMSCs-treated animals (Fig 5Ba) (p<0.05), while the frequencies of IL-4 producing cells increased from 1/11,800 to 1/6,600 (Fig 5Bb) (p<0.05). No specific responses were detected in naïve mice (Fig. 5A,B). These observations suggest that BM-hMSCs reduce inflammatory myelin-specific TH1 cells and increase inflammatory-inhibiting TH2 cells in EAE.
Figure 5. BM-hMSC treated EAE mice display reduced frequencies of myelin peptide-specific TH1 but increased frequencies of myelin peptide-specific TH2 cells.
106 splenocytes from naïve, PBS-treated or BM-hMSC-treated PLP-EGFP transgenic mice at day 45 post-immunization with MOG35-55-induced EAE (A) or SJL mice at day 60 post-immunization with PLP139-151-induced EAE (B) were stimulated with myelin peptide, then IFN-γ and IL-4 producing T cell ELISOPTS were performed. BM-hMSC-treated mice from both strains displayed decreased frequencies of TH1, but higher frequencies of myelin peptide-specific TH2 compared to PBS-treated controls. The wells shown are representatives from triplicates. Counts represent mean standard deviation from one of three experiments with similar results. (*) = significant difference of p< 0.05.
Treatment with BM-hMSCs alters the cytokine profiles and reduces proliferation of myelin-specific spleen cells
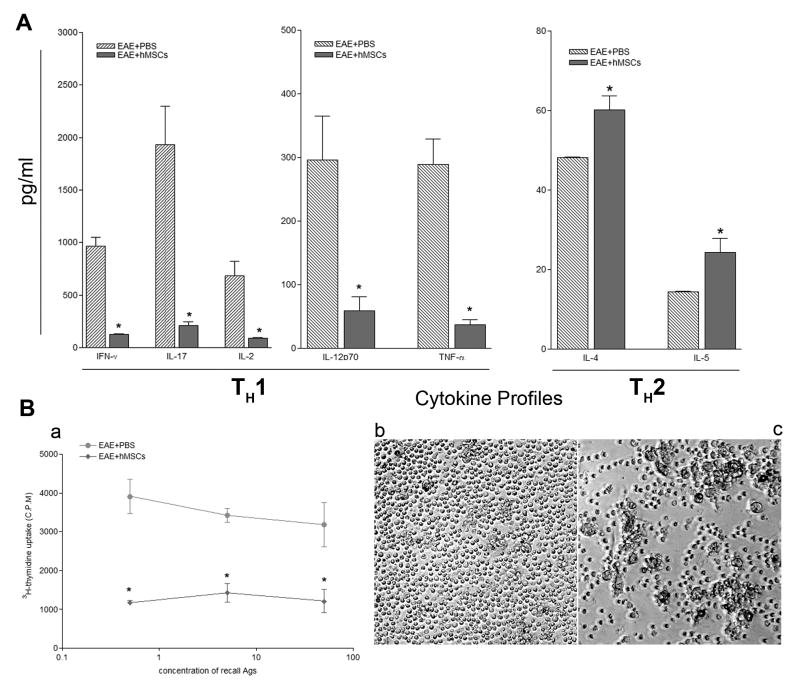
Stimulation of spleen cells from EAE animals induced with MOG35-55 enhanced the secretion of a spectrum of cytokines including IFN-γ, IL-17, IL-2, IL-12p70, TNF-α, IL-4 and IL-5. To define the effects of BM-hMSCs on this cytokine production, cells were harvested 16 days after MOG35-55 immunization and cultured in the absence or presence of MOG35-55 (0μg/ml, 0.5μg/ml, 5μg/ml and100μg/ml) and assayed for cytokine production by a Liqui-Chip system. All MOG35-55 concentrations provoked a spleen cell response and were compared at 5μg/ml. The average levels of Th1/Th17 inflammatory cytokines (n=3) (IFN- γ: 965.6pg/ml, IL-17: 1934.5pg/ml, IL-2: 684.5pg/ml, IL-12p70: 295.7pg/ml, TNF-α: 288.8pg/ml) in EAE mice were significantly reduced in BM-hMSC-treated EAE animals (IFN-γ: 127.9pg/ml, IL-17: 208.1pg/ml, IL-2: 88.7pg/ml, IL-12p70: 68.7pg/ml, TNF-α: 37.2pg/ml) (Fig 6A) (p<0.05). By contrast the levels of anti-inflammatory TH2 cytokines including IL-4 and IL-5 were significantly increased in the BM-hMSC-treated group (IL-4: 60.1pg/ml, IL-5: 24.3pg/ml) compared to carrier only group (IL-4: 48.1pg/ml, IL-5: 14.4pg/ml) (Fig 6A) (p<0.05). These values represent significant changes in cytokine production. In BM-MSC treated mice, cytokine production decreased 9.3 fold for IL-17 (TH17), 7.8 fold for TNF-α, 7.7 fold for IL-2, 7.5 fold for IFN- γ (TH1), and 4.3 fold in IL-12p70, while TH2 cytokine production increased 1.7 fold in IL-5 and 1.3 fold in IL-4. These data suggest that one of the most down-regulated cytokines is IL-17, a molecule that has recently been strongly implicated in mediating the progression of disease in EAE.
Figure 6. Differential expression of TH1, TH17 and TH2 cytokine profiles in peripheral lymph organs and suppression of MOG35-55-specific T-cell proliferative responses in BM-hMSC-treated mice with EAE induced by MOG35-55.
Cells were isolated from both PBS and BM-hMSC-treated mice at day 16 after immunization and cultured in the presence of MOG35-55 peptide. (A) Responses from the BM-hMSC-treated animals displayed significantly decreased levels of pro-inflammatory cytokines (IFN-γ, IL-17, IL-2, IL-12p70, TNF-α) compared to those from PBS-treated controls. In contrast, the expression of anti-inflammatory cytokines (IL-4, IL-5) was significantly increased in the BM-hMSC-treated group compared to the controls (B). Values are means ± SEM of triplicate cultures. Data represent three separate experiments using spleen cells pooled from three different donors. (*) = significant difference of p< 0.05. (C) Splenic T-cells from control and BM-hMSC-treated EAE mice were cultured with different concentrations of MOG35-55 (0.5 μg/ml, 5 μg/ml, 50 μg/ml) and 3H-thymidine incorporation assessed three days later (a). T cells, at 5ug/ml from BM-hMSC treated mice, showed fewer proliferating colonies (b) than T cells from control mice in response to 5 μg/ml of MOG35-55 (c). Proliferative responses are expressed as mean CPM of at least 3 independent experiments. (*) = significant difference of p< 0.05.
When spleen cells from MOG35-55 induced EAE animals were challenged in vitro with MOG35-55, they demonstrated a robust proliferative response. By contrast, cells derived from animals treated with BM-hMSCs showed little or no proliferative response on challenge with antigen (Fig 6Ba). Furthermore, colony forming assays demonstrated significantly higher numbers of colony forming cells in EAE (Fig. 6Bb) than in BM-hMSC-treated animals (Fig. 6Bc), suggesting that the BM-hMSCs induce a state of myelin antigen-specific T-cell unresponsiveness.
Discussion
Multiple sclerosis is characterized by areas of demyelination and inflammation throughout the brain and spinal cord and is modeled in animals by EAE. Here we show that IV injection of BM-hMSCs resulted in a dramatic reduction in disease progression in 2 models of EAE. Intravenous infusion of BM-hMSCs into animals with MOG 35-55 EAE resulted in a rapid improvement in clinical score and reduced lesion burden concomitant with reduced inflammatory cell infiltration into the CNS. These data are consistent with earlier studies demonstrating a reduction in EAE disease in animals treated with murine and human stromal cells (Zhang et al. 2005). The BM-hMSC induced improvement was accompanied by changes in neural cell responses with increased oligodendrocytes and decreased astrocytes in lesion areas as well as changes in spleen-cell responses. In active EAE the predominant response is mediated through Th1 proinflammatory cells and expression of their associated cytokines. In animals that received BM-hMSCs there was a significant reduction in pro-inflammatory cytokines including IL-17, IFN-γ, IL-2, IL-12p70, and TNF-α and a significant increase in anti-inflammatory cytokines including IL-4 and IL-5. Recent studies have described an important role for Th-17 T-cells in the pathogenesis of EAE (Harrington et al. 2005) and treatment with BM-MSCs significantly down regulates IL-17 levels and results in a reduction in astrogliosis. Previous studies suggest that astrocytes can stimulate IL-17 production (Miljkovic et al. 2007) although whether the decrease in IL-17 expression is directly linked to changes in astrogliosis in the current model is unknown. Indeed, the precise role of astrocytes in response to CNS injury and inflammation is not clear and it has been proposed that they possess anti-inflammatory properties (Xiao et al. 1998). In the setting of EAE, however, astrocytes appear unlikely to reduce spinal cord inflammation but rather they form a gliotic response that was reduced by BM-hMSC treatment. The therapeutic benefits of MSCs are not limited to the nervous system (Uccellii et al. 2008). In other inflammatory diseases, treatment with MSCs has been shown to reduce disease burden and enhance repair (Bacigalupo 2004). For example, in heart disease MSCs appear to be more beneficial for cardiac repair (Ohnishi et al. 2007) than other stem cells and this may reflect their ability to influence IL-17 expression (Antonysamy et al. 1999; Mangan et al. 2006) as well as modulation of TH1 cell function. It is unlikely that all BM-hMSC influences on the immune system in EAE are dependent on IL-17 modulation. The observed suppression of T-cell proliferation may reflect either a direct effect of MSCs on division arrest in T cells (Glennie et al. 2005) and/or a reflection of decreasing MHC class II (Tse et al. 2003) as well as CD80/CD86 expression on dendritic cells (DCs) (Jiang et al. 2005). Regardless of the precise mechanisms, consistent with previous observations (Dazzi and Horwood 2007; Gerdoni et al. 2007; Krampera et al. 2007; Nauta and Fibbe 2007), these data demonstrate that BM-hMSCs altered the immunological responses in two distinct models of EAE.
The substantial functional and histological recovery seen in the CNS of EAE animals treated with BM-hMSCs suggests they may act locally to promote repair through several distinct mechanisms. First, BM-hMSCs that infiltrate lesion areas may differentiate directly into neural cells. Early studies suggested that MSCs injected into the developing lateral ventricles differentiated into astrocytes and more recently into other neural cell types (Deng et al. 2001; Woodbury et al. 2000). This seems unlikely to account for the histological changes seen in the current study, however, since when labeled MSCs were injected into naïve or EAE animals no evidence of them adopting a neural fate was detected based on their expression of neuronal (TuJ1) or glial (GFAP, CC1) antigens (data not shown). Furthermore, even when grown in highly neuralizing conditions in vitro, the proportion of “neuralized” hMSC progeny remained relatively small and their functional properties not well known (Alexanian 2007; Bai et al. 2007). One likely explanation for the appearance of GFAP+ MSCs in other models of neural damage is cell fusion (Terada et al. 2002; Weimann et al. 2003). Indeed, intravenously injected bone marrow-derived cells are known to fuse with hepatocytes in liver, Purkinje neurons in the cerebellum and cardiac muscle in the heart. The differentiation of MSCs is however critically dependent on environmental signals and it may be that a small proportion differentiate into neural cells in the setting of a demyelinating lesion.
The capacity of BM-hMSCs to alter the endogenous neural responses to demyelination is likely to have a major influence on functional recovery. Analyses of BM-hMSCs suggest they produce a variety of neurotrophic factors (Crigler et al. 2006; Pisati et al. 2007) that have neuroprotective (Ankeny et al. 2004) functions. These include BDNF that regulates oligodendrogenesis in the CNS (Du et al. 2003). Indeed, treatment with several neurotrophins has bee suggested to reduce disease burden in EAE possibly by enhancing oligodendrocyte survival (Linker et al. 2002). Thus, transplanted neural or mesenchymal stem cells may exert trophic effects on the host CNS that enhances cell survival (Caplan and Dennis 2006) and endogenous remyelination (Aharonowiz et al. 2008; Munoz et al. 2005; Pluchino et al. 2003). A characteristic of BM-hMSCs likely to enhance their ability to influence disease progression in EAE is their localization to areas of damage. This localization facilitates close contact with inflamed tissue and enhances suppression of inflammation and reduction of demyelination and axonal injury. In addition, the suppression of the immune responses by BM-hMSCs, a potentially universal property of stem cells (Fandrich et al. 2002; Miller and Bai 2007), prevents loss of injected cells. This may be of major importance in the application of transplantation therapy for immune-mediated diseases such as MS since it would protect the transplanted cells from immune attack (Aggarwal and Pittenger 2005). Although recent studies suggest that in certain circumstances MSCs are immunogenic and can stimulate graft rejection (Eliopoulos et al. 2005; Nauta et al. 2006) the long term retention of hMSCs in our animal model makes this unlikely in the setting of EAE.
The rapid and sustained functional recovery seen in animals with EAE after treatment with BM-hMSCs suggests these cells alter several aspects of disease progression. We propose that BM-hMSCs suppress T-lymphocyte activities thereby exerting an immunoregulatory capacity (Di Nicola et al. 2002; Gerdoni et al. 2007; Nauta and Fibbe 2007). Though the mechanisms mediating such effects are still only partially understood, it is likely they involve both cell-to-cell contact and soluble factors. Further, endogenous neural stem or progenitor cells are activated by BM-hMSCs (Munoz et al. 2005). Neural stem cells exist in the developing and adult mammalian nervous system including humans. They are capable of undergoing expansion and differentiation into neurons, astrocytes, and oligodendrocytes in vitro (Reynolds and Weiss 1992) and after transplantation in vivo (Svendsen et al. 1997). Although their restricted locations in the brain may limit their clinical effectiveness, stimulation by BM-hMSCs may enhance their response and facilitate endogenous CNS repair. In the current studies it is likely that the recovery of myelination is a reflection of suppression of the autoimmune responses in combination with induced proliferation or enhanced differentiation of endogenous progenitor cells. Consistent with this hypothesis, an increase in the density of NG2+ cells and oligodendrocytes were seen in BM-hMSC-treated animals presumably reflecting the release of multiple bioactive factors by BM-hMSCs (Caplan and Dennis 2006) and earlier studies have suggested that bone marrow stromal cells can promote neurogenesis in the hippocampus (Munoz et al. 2005). Whether the functional improvement seen in EAE reflects remyelination or neuroprotection derived from OPCs is currently unclear.
In conclusion, our data indicate that BM-hMSCs migrate to demyelinating lesions of the inflamed CNS from the bloodstream and inhibit myelin-specific memory T cells that drive chronic and relapsing-remitting clinical disease in EAE. We demonstrate that BM-hMSCs regulate the balance of T lymphocytes between TH1/TH17 to TH2 and modify the cytokines released during EAE. As human MSCs have already been used for the treatment of acute graft-versus-host disease, the demonstration of an involvement of BM-hMSCs in controlling the pathogenesis of chronic and relapsing-remitting EAE offer a new perspective for the treatment of demyelinating diseases such as MS and other neurodegenerative diseases like Alzheimer's disease and Parkinson's disease.
Acknowledgments
The authors thank Rae Wang for help with technical aspects of the manuscript and Anne DeChant for help with manuscript preparation. The support of the Myelin Repair Foundation during the course of these studies is gratefully acknowledged. We also thank the National Center for Regenerative Medicine and the Center for Stem Cells and Regenerative Medicine and NIH NS-36674 and NS-30800 for support.
References
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE. 2008;3(9):e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexanian AR. Epigenetic modifiers promote efficient generation of neural-like cells from bone marrow-derived mesenchymal cells grown in neural environment. J Cell Biochem. 2007;100(2):362–71. doi: 10.1002/jcb.21029. [DOI] [PubMed] [Google Scholar]
- Ankeny DP, McTigue DM, Jakeman LB. Bone marrow tranplants provide tissue protectionadn directional guidance for axons after contusive spinal cord injuries in rats. Exp Neurology. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in alloimmunity: a novel IL-17 antagonist promotes heart graft survival. Transplant Proc. 1999;31(1-2):93. doi: 10.1016/s0041-1345(98)01453-5. [DOI] [PubMed] [Google Scholar]
- Bacigalupo A. Mesenchymal stem cells and haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2004;17(3):387–99. doi: 10.1016/j.beha.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Bai L, Caplan A, Lennon D, Miller RH. Mesenchymal stem cell signals regulate neural stem cell fate. Neurocehmistry Research. 2007;32:353–362. doi: 10.1007/s11064-006-9212-x. [DOI] [PubMed] [Google Scholar]
- Bai L, Koopmann J, Fiola C, Fournier P, Schirrmacher V. Dendritic cells pulsed with viral oncolysates potently stimulate autologous T cells from cancer patients. Int J Oncol. 2002;21(4):685–94. doi: 10.3892/ijo.21.4.685. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56(3):283–94. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CT, Gotch F, Imami N. Rapid qualitative and quantitative analysis of T-cell responses in HIV-1-infected individuals receiving successful HAART and HIV-1 sero-negative controls: concomitant assessment of perforin, IFN-gamma and IL-4 secretion. J Immunol Methods. 2006;308(1-2):216–30. doi: 10.1016/j.jim.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Caplan A, Dennis JE. Mesenchymal stem cell as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198(1):54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. 2007;19(6):650–5. doi: 10.1097/CCO.0b013e3282f0e116. [DOI] [PubMed] [Google Scholar]
- Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001;282(1):148–52. doi: 10.1006/bbrc.2001.4570. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CD. Regionally specific effects of BDNF on oligodendrocytes. Developmental Neuroscience. 2003;25:116–126. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class 1 and class11- mismatched recipient mice. Blood. 2005;106:4057–65. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- Fandrich F, Dresske B, Bader M, Schulze M. Embryonic stem cells share immune-privileged features relevant for tolerance induction. J Mol Med. 2002;80(6):343–50. doi: 10.1007/s00109-002-0342-6. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61(3):219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, Rimmler JB, Haynes CS, Roses AD, et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat Genet. 1996;13(4):469–71. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Karussis D, Kassis I. Use of stem cells for the treatment of multiple sclerosis. Expert Rev Neurother. 2007;7(9):1189–201. doi: 10.1586/14737175.7.9.1189. [DOI] [PubMed] [Google Scholar]
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, Slavin S, Abramsky O, Karussis D. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65(6):753–61. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, Andreini A, Mosna F, Bonetti B, Rebellato E, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16(5):797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186(6):859–65. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker RA, Maurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8(6):620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin R. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis and their application for new therapeutic strategies. J Neural Transm Suppl. 1997;49:53–67. doi: 10.1007/978-3-7091-6844-8_6. [DOI] [PubMed] [Google Scholar]
- Miljkovic D, Momcilovic M, Stojanovic I, Stosic-Grujicic S, Ramic Z, Mostarica-Stojkovic M. Astrocytes stimulate interleukin-17 and interferon-gamma production in vitro. J Neurosci Res. 2007;85(16):3598–606. doi: 10.1002/jnr.21453. [DOI] [PubMed] [Google Scholar]
- Miller RH, Bai L. Cellular approaches for stimulating CNS remyelination. Regen Med. 2007;2(5):817–29. doi: 10.2217/17460751.2.5.817. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171–6. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–20. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S, Ohgushi H, Kitamura S, Nagaya N. Mesenchymal stem cells for the treatment of heart failure. Int J Hematol. 2007;86(1):17–21. doi: 10.1532/IJH97.07041. [DOI] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- Perini P, Calabrese M, Rinaldi L, Gallo P. The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert Opin Drug Saf. 2007;6(2):183–90. doi: 10.1517/14740338.6.2.183. [DOI] [PubMed] [Google Scholar]
- Pisati F, Bossolasco P, Meregalli M, Cova L, Belicchi M, Gavina M, Marchesi C, Calzarossa C, Soligo D, Lambertenghi-Deliliers G, et al. Induction of neurotrophin expression via human adult mesenchymal stem cells: implication for cell therapy in neurodegenerative diseases. Cell Transplant. 2007;16(1):41–55. doi: 10.3727/000000007783464443. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rivera FJ, Couillard-Despres S, Pedre X, Ploetz S, Caioni M, Lois C, Bogdahn U, Aigner L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells. 2006;24(10):2209–19. doi: 10.1634/stemcells.2005-0614. [DOI] [PubMed] [Google Scholar]
- Rutenberg MS, Hamazaki T, Singh AM, Terada N. Stem cell plasticity, beyond alchemy. Int J Hematol. 2004;79(1):15–21. doi: 10.1007/BF02983528. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Stuve O, Youssef S, Weber MS, Nessler S, von Budingen HC, Hemmer B, Prod'homme T, Sobel RA, Steinman L, Zamvil SS. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J Clin Invest. 2006;116(4):1037–44. doi: 10.1172/JCI25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen CN, Caldwell MA, Shen J, ter Borg MG, Rosser AE, Tyers P, Karmiol S, Dunnett SB. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148(1):135–46. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416(6880):542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5(11):959–66. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Wight PA, Duchala CS, Shick HE, Gudz TI, Macklin WB. Expression of a myelin proteolipid protein (Plp)-lacZ transgene is reduced in both the CNS and PNS of Plp(jp) mice. Neurochem Res. 2007;32(2):343–51. doi: 10.1007/s11064-006-9202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61(4):364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Xiao BG, Diab A, Zhu J, van der Meide P, Link H. Astrocytes induce hyporesponses of myelin basic protein-reactive T and B cell function. J Neuroimmunol. 1998;89(1-2):113–21. doi: 10.1016/s0165-5728(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106(5):1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zargari M, Allameh A, Sanati MH, Tiraihi T, Lavasani S, Emadyan O. Relationship between the clinical scoring and demyelination in central nervous system with total antioxidant capacity of plasma during experimental autoimmune encephalomyelitis development in mice. Neurosci Lett. 2007;412(1):24–8. doi: 10.1016/j.neulet.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195(1):16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]