Abstract
Background:
Endothelins (ET-1, ET-2, ET-3) are peptides with vasoactive properties interacting with ETA and ETB receptors. ET-1 inhibits secretin-stimulated ductal secretion (hallmark of cholangiocyte growth) of cholestatic rats by interaction with ET receptors.
Aim:
The aims of the studies were to evaluate (i) the effect of ET-1 on cholangiocarcinoma growth in Mz-ChA-1 cells and nude mice and (ii) whether ET-1 regulation of cholangiocarcinoma growth is associated with changes in the expression of vascular endothelial growth factor-A (VEGF-A), VEGF-C, VEGF receptor-2 (VEGFR-2) and VEGFR-3.
Methods:
We determined the expression of ETA and ETB receptors on normal and malignant (Mz-ChA-1) cholangiocytes and human cholangiocarcinoma tissue and the effect of ET-1 on the proliferation and expression of VEGF-A, VEGF-C (regulators of tumour angiogenesis) and its receptors, VEGFR-2 and VEGFR-3, in Mz-ChA-1 cells. In vivo, Mz-ChA-1 cells were injected into the flanks of athymic mice and injections of ET-1 or saline into the tumours were performed daily. The effect of ET-1 on tumour size, cell proliferation, apoptosis, collagen quantity and the expression of VEGF-A and VEGF-C and VEGFR-2 and VEGFR-3 were measured after 73 days.
Results:
Higher expression of ETA and ETB was observed in malignant compared with normal cholangiocytes. ET-1 inhibited proliferation and VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression of Mz-ChA-1 cells. Chronic ET-1 treatment decreased tumour volume, tumour cell proliferation and VEGF-A and VEGF-C expression but increased apoptosis and collagen tissue deposition compared with controls.
Conclusions:
Modulation of VEGF-A and VEGF-C (by ET-1) may be important for managing cholangiocarcinoma growth.
Keywords: biliary cancer, cholangiopathies, neuroendocrine hormones, proliferation, vascular endothelial growth factor
Cholangiocarcinoma, the tumour originating from the biliary epithelium, accounts for about 3% of all gastrointestinal cancers and represents the second most common primary liver tumour after hepatocarcinoma (1). Biliary tumours are extremely aggressive and display a poor prognosis (1-4). The lack of therapeutic tools for such a devastating disease is due, at least in part, to the lack of knowledge regarding the mechanisms regulating cholangiocarcinoma growth (2-7). However, increasing evidence has shown that neuropeptides and neuroendocrine hormones are among those factors that are able to affect cholangiocarcinoma biology, either promoting or inhibiting its growth (1, 4, 7-11). While oestrogens and insulin-like growth factor 1 stimulate cholangiocarcinoma proliferation (12), gastrin inhibits cholangiocarcinoma growth by activation of protein kinase C-α (10). In Mz-ChA-1 cells, α2-adrenoreceptor stimulation causes upregulation of cyclic adenosine 3′,5′-mono-phosphate, which leads to inhibition of cholangiocarcinoma growth by decreased extracellular-signal-regulated kinase1/2 (ERK1/2) activity (11). A recent study has shown that leptin increased the cholangiocarcinoma growth by phosphorylation of STAT-3 and ERK1/2 (7). The two endocannabinoids, anandamide and 2-arachidonylglycerol, exert differential effects on cholangiocarcinoma growth via receptor-independent mechanisms, with anandamide inhibiting growth and 2-arachidonylglycerol stimulating cholangiocarcinoma cell growth (13).
Endothelin-1 (ET-1), a 21 amino acid vasoconstrictor peptide, regulates the pathogenesis of hypertension and heart failure (14). ET-1 exerts a multitude of effects in liver cells (15-19). ET receptors are expressed by rat hepatic stellate cells (16). ET-1 has been shown in human gallbladder epithelial cells (17) and in bile ducts of human cirrhotic liver (18). The importance of ET-1 in participating to liver homeostasis is supported by the finding that cholangiocytes synthesize hepatic ET-1 during the development of hepatopulmonary syndrome after extrahepatic bile duct obstruction (19). ET-1 inhibits secretin-induced ductal secretion (15) (a hallmark of cholangiocyte proliferation) (20, 21) in cholestatic rats by interacting with ETA receptors on cholangiocytes.
A number of angiogenic factors including vascular endothelial growth factor (VEGF) are overexpressed by human cholangiocarcinoma tissues and cell lines such as KMC-1, KMC-2, KMBC and KMG-C (22). Overexpression of VEGF has been suggested to contribute to the ‘angiogenic switch’ of the malignant phenotype in human cholangiocarcinoma (23). Increased expression of VEGF (by oestrogens) plays an important role in regulating human cholangiocarcinoma growth (24). A number of studies have shown that VEGF regulates the function of normal and hyperplastic cholangiocytes (25-27). No information exists regarding the in vitro and in vivo effects of ET-1 on (i) the growth and apoptosis of cholangiocarcinoma cells and (ii) the expression of the angiogenic factor, VEGF-A and VEGF-C, and its receptors VEGFR-2 and VEGFR-3 in cholangiocarcinoma. On the basis of these studies, we evaluated (i) the expression of ETA and ETB in the human cholangiocarcinoma cell line, Mz-ChA-1 (10, 11, 13) and the normal cell line, H69 (6), and tissue arrays containing cholangiocarcinoma and normal biopsy samples, (ii) the in vitro effects of ET-1 on the growth of H69 and Mz-ChA-1 cells, (iii) the in vivo effect of ET-1 on the growth of tumour cell lines implanted in nude mice and (iv) whether the in vitro and in vivo effects of ET-1 on MzChA-1 cell growth are associated with changes in VEGF-A and VEGF-C expression.
Materials and methods
Materials
Reagents were purchased from Sigma Chemical Company (St. Louis, MO, USA) unless otherwise specified. ET-1 was purchased from Calbiochem (La Jolla, CA, USA). The polyclonal antibodies (sheep antisera) against the rat ETA and ETB receptors were purchased from Alexis Co. (San Diego, CA, USA). Dulbecco's phosphate-buffered saline (PBS) was obtained from Celox (Hopkins, MN, USA).
Cholangiocarcinoma cell lines
We used (i) H69 cells that are SV40-transformed normal human cholangiocytes (a gift from Dr Gores, Mayo Clinic, Rochester, MN, USA) (6) and (ii) the human cholangiocarcinoma cell line, Mz-ChA-1, which has been widely used for evaluating the mechanisms of cholangiocarcinoma growth by us and others (5, 10, 11, 13, 28-30). Mz-ChA-1 cells from human gallbladder (10, 11, 13, 31) were obtained from Dr Fitz (University of Texas Southwestern Medical Center, Dallas, TX, USA). Mz-ChA-1 cells were cultured as described (5, 10, 11, 13, 28).
Expression of endothelin A and endothelin B receptors
We evaluated the expression of ETA and ETB receptors in H69 and Mz-ChA-1 cells by immunofluorescence and real-time polymerase chain reaction (PCR) (13, 32). For immunofluorescence, cells were seeded onto coverslips and allowed to adhere overnight. Following washes with cold 1 × PBS, coverslips were fixed with 4% paraformaldehyde (in 1 × PBS) at room temperature for 5 min. Cells were permeabilized in 1 × PBS containing 0.2% triton-X100 (PBST) and blocked in 4% bovine serum albumin (BSA in PBST) for 1 h at room temperature. Appropriate dilutions (in 1% BSA/PBST) of ETA or ETB receptor antibodies were added to the coverslips and incubated overnight at 4 °C. Cells were washed three times for 10 min in PBST and a 1:100 dilution (in 1% BSA/PBST) of a cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) was added for 2 h at room temperature. Cells were washed three times for 10 min in PBST and mounted onto microscope slides with Antifade gold containing 4,6-diamidino-2-phenylindole as a counterstain (Molecular Probes, Eugene, OR, USA). Negative controls (with pre-immune serum substituted for primary antibody) were also included. Images were taken on an Olympus IX71 fluorescence microscope with a DP70 digital camera (Olympus, Tokyo, Japan).
For real-time PCR analysis of receptor expression, RNA was extracted from Mz-ChA-1 cells using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer's instructions and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SuperArray, Frederick, MD, USA). These reactions were used as templates for the PCR assays using a SYBR Green PCR master mix and specific primers designed against human ETA and ETB genes (SuperArray) in the real-time thermal cycler Applied Biosystems Inc, Foster City, CA (ABI Prism 7900HT sequence detection system). One microlitre of the cDNA template was added to 12.5 μl of master mix, 10.5 μl of DI water and 1 μl of reverse transcriptase PCR human primers designed specifically for the messages for ETA and ETB and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), the housekeeping gene (32). A delta delta of the threshold cycle (ΔΔCT) analysis was performed using H69 as the control sample. Data are expressed as relative mRNA levels ± SEM (n = 3).
Cholangiocarcinoma tissue array analysis
Immunoreactivity for ETA and ETB was assessed in commercially available Accumax tissue arrays (Isu Abxis Co. Ltd, Seoul, Korea) by immunohistochemistry (33) using specific antibodies for ETA and ETB. These tissue arrays contain 48 well-characterized cholangiocarcinoma biopsy samples from a variety of tumour differentiation grades as well as four control liver biopsy samples. Semiquantitative analysis was performed using the following parameters. Staining intensity was assessed on a scale from 1 to 4 (1 = no staining, 4 = intense staining) and the abundance of positively stained cells was given a score from 1 to 5 (1 = no cells stained, 5 = 100% stained). The staining index was calculated by the staining intensity multiplied by the staining abundance, which gives a range from 1 to 20.
Effect of endothelin-1 on cholangiocarcinoma cell growth in vitro
The growth of Mz-ChA-1 cells was evaluated by a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt (MTS) proliferation assay (5, 13). After trypsinization, Mz-ChA-1 cells were seeded into 96-well plates (10 000 cells/well) in a final volume of 200 μl medium. In dose–response curve experiments, cells were stimulated for 48 h with 0.2% BSA (basal) or ET-1 (from 0.1 to 10 nM) before evaluating growth by MTS assays (5, 13). We also evaluated the effect of ET-1 (10 nM for 48 h) on the growth of H69 cells. In separate experiments, Mz-ChA-1 cells were stimulated for 48 h with 0.2% BSA (basal) or ET-1 (10 nM) in the absence or presence of 1 h pre-incubation with BQ-610 (a specific ETA receptor antagonist, 100 nM) (15, 34) or BQ-788 (a specific ETB receptor antagonist, 100 nM) (35). Cells were also incubated with only BQ-610 or BQ-788 (100 nM) for 48 h. Cell proliferation was assessed using a colorimetric cell proliferation assay (CellTiter 96Aqueous; Promega Corp., Madison, WI, USA) and absorbance was measured at 490 nm by a microplate spectrophotometer (Versamax; Molecular Devices, Sunnyvale, CA, USA). The proliferation index was calculated as the ratio of the absorbance of cells incubated with ET-1 compared with controls.
Effect of endothelin-1 on the expression of vascular endothelial growth factor-A, -C, vascular endothelial growth factor receptor-2 and -3 in cholangiocarcinoma cells
After trypsinization, Mz-ChA-1 cells (1 × 106) were incubated until they reached 70% confluence and were serum starved overnight. Then, cells were stimulated with (i) 0.2% BSA (basal), (ii) ET-1 (10 nM) for 6 h in the absence or presence of BQ610 and BQ788 (100 nM) or (iii) BQ610 or BQ788 (100 nM). Subsequently, we evaluated the expression of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 by real-time PCR using specific primers and the methods described above. The ΔΔCT analysis was performed and data were expressed as fold change compared with either basal or inhibitor treatment alone.
Effect of endothelin-1 on the growth of cholangiocarcinoma cells implanted in nude mice
Male 8-week-old BALB/c nude (nu/nu) mice were purchased from Taconic Farms, Germantown, NY, USA. The mice were kept in a temperature-controlled environment (20–22 °C) with 12-h light–dark cycles and free access to water and mouse chow. All animal experiments were performed in accordance with a protocol approved by the Scott and White Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). Mz-ChA-1 cells (3 × 106 viable cells) were suspended in 0.5 ml of extracellular matrix gel and injected subcutaneously in the left back flank of these animals. This cell line was chosen for its ability to form solid tumours when injected into nude mice. Mice were divided into two groups: (i) the first (n = 3, control) received 0.9% NaCl injections (150 μl) into the implanted tumour and (ii) the second (n = 3) received ET-1 injections (100 ng/kg body weight in 150 μl of 0.9% NaCl) (36) into the tumour. The same operator performed the injections every day starting from ‘day 0’, when the tumours were implanted, for 73 days. The size of the tumours was assessed by a tri-axial measurement, twice a week. Tumour parameters (length, width, height) were measured by an electronic calliper and the volume was determined using the following equation: tumour volume (mm3) = 0.5 × [length (mm) × width (mm) × height (mm)]. The measurements started from the third week, when the tumour mass was well established. A third operator, in a coded and blinded fashion, evaluated morphometric parameters. Latency of tumour growth was determined as the time needed for the tumour to increase to 150% of the initial volume. At the end of the observation period of 73 days, mice were anaesthetized with sodium pentobarbital (50 mg/kg body weight, IP) and sacrificed according to the institutional guidelines. Heart, liver and kidney were isolated from the two groups of animals, fixed in formalin, embedded in paraffin, processed for histopathology and stained with haematoxylin and eosin (H&E) for the detection of tissue damage.
Morphological analysis of tumour tissues
A blinded operator dissected tumour tissues from each group of mice. Neoplastic tissues were fixed in formalin, embedded in paraffin, processed for histopathology and sections (4 μm) were stained with H&E for routine examination or with Masson's trichrome for collagen deposit evaluation and examined by light microscopy. Tumour sections (n = 4, from each group of animals) were stained for cytokeratin-7 (CK-7) and proliferating cellular nuclear antigen (PCNA) as described (37). Following staining, sections were counterstained with haematoxylin and examined with a microscope (Olympus BX 40; Olympus Optical Co. Ltd, Tokyo, Japan). Data were expressed respectively as percentage of total area occupied by CK-7-positive tumour cells and number of PCNA-positive malignant cholangiocytes per 100 malignant cells counted in seven different tumour fields.
To evaluate the protein expression of VEGF-A and VEGF-C, tumour sections (n = 4) from each group of animals were incubated overnight at 4 °C with antibodies specific for VEGFA (1:400) or VEGF-C (1:50). Sections were rinsed with 1 × PBS for 5 min, incubated for 10 min at room temperature with a secondary biotinylated antibody (Dako LSAB2, code K0675; Dako, Milan, Italy), followed by DAKO ABC (Dako LSAB2, code K0675) and developed with 3,3′-diaminobenzidine. Sections were analysed in a coded fashion by an Olympus BX 40 light microscope (Olympus) with a Videocam (Spot Insight Diagnostic Instrument Inc., Sterling Heights, MI, USA) and processed with an Image Analysis System (Delta Sistemi, Rome, Italy). For all the immunoreactions, negative controls (the primary antibody was replaced with pre-immune serum) were included. Data were expressed as the percent of VEGF-A- and VEGF-C-positive malignant cholangiocytes counted in seven different tumour fields. In tumour samples from ET-1- or vehicle-treated mice, for the detection of apoptosis on single cells, the terminal deoxynucleotide transferase end labelling (TUNEL) method (ApopTag; Chemicon Int., Temecula, CA. USA) was used according to the manufacturer's instructions. In each tumour section (n = 4), the number of TUNEL-positive tumour cells was assessed in six slides for each group. Positive cells were counted in six non-overlapping fields (magnification × 20) for each slide. Negative controls, obtained by incubating the tumour sections with normal pre-immune serum from the same species and then with the secondary antibody, were used to confirm the specificity of the staining.
Statistical analysis
All data are expressed as mean ± SEM. Differences between groups were analysed by the Student unpaired t-test when two groups were analysed and analysis of variance (ANOVA) when more than two groups were analysed. A P-value < 0.05 was used to indicate statistical significance.
Results
Expression of endothelin receptors
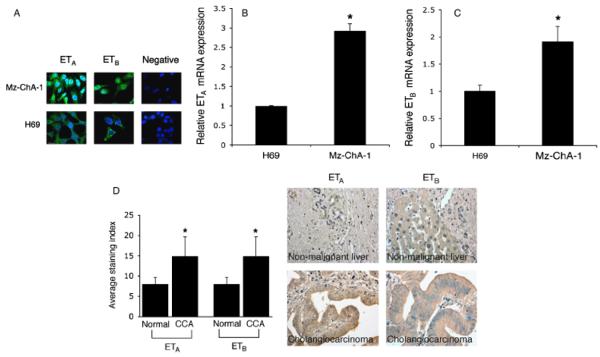
Immunofluorescence showed a positive reaction for ETA and ETB receptors in H69 and Mz-ChA-1 cells (Fig. 1A). By real-time PCR, ETA and ETB receptor mRNA levels were significantly higher in Mz-ChA-1 cells compared with H69 cells (Fig. 1B and C). Array analysis on human cholangiocarcinoma tissues confirmed that ETA and ETB receptors are expressed more in these neoplasias with respect to normal tissues (Fig. 1D).
Fig. 1.
(A) Immunofluorescence for endothelin receptor A (ETA) and ETB in H69 cells and the human cholangiocarcinoma cell line, Mz-ChA-1. A positive reaction (green) for ETA and ETB receptors is evident in all the cell lines. Nuclei were counterstained with DAPI (blue). Negative controls (with pre-immune serum substituted for primary antibody) were also included. Scale bar = 10 μm. (B and C) Real-time polymerase chain reaction analysis for ETA and ETB receptors in total RNA from H69 and Mz-ChA-1 cells. Data are mean ± SEM of three experiments. #P < 0.05 vs. H69 cells. (D) Evaluation of protein expression for ETA and ETB in tissue arrays containing 48 well-characterized cholangiocarcinoma biopsy samples from a variety of tumour differentiation grades as well as four control liver biopsy samples. Immunoreactivity showed a higher expression of ETA and ETB in human cholangiocarcinoma compared with normal tissue. Data are mean ± SEM of four experiments. *P < 0.05 vs. normal liver.
In vitro effect of endothelin-1 on cholangiocarcinoma cell growth
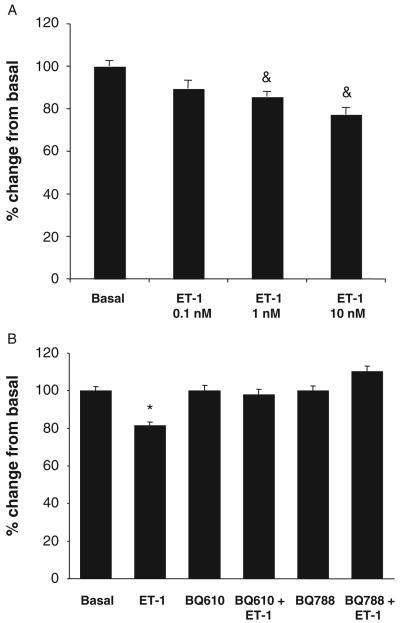
In vitro studies have shown that ET-1 (at 1 and 10 nM for 48 h) significantly decreased the proliferation of Mz-ChA-1 cells (Fig. 2A). The rationale for using Mz-ChA-1 cells to begin to evaluate the mechanisms by which ET-1 inhibits both In vitro and in vivo is based on the fact that these cells are able to form tumours when injected into nude mice (5, 10). The inhibitory effect of ET-1 (10 nM for 48 h) on Mz-ChA-1 growth was blocked by both BQ-610 and BQ-788 (Fig. 2B), suggesting that ET-1 exerts its inhibitory effects on cholangiocarcinoma growth by interaction with both ETA and ETB receptors. ET-1 did not affect the growth of the normal human cholangiocytes, H69 (not shown).
Fig. 2.
(A) Effect of endothelin-1 (ET-1), by MTS assays, on the growth of the cholangiocarcinoma cell line, Mz-ChA-1. ET-1 (at 0.1–10 nM for 48 h) inhibits the growth of Mz-ChA-1 cells. (B) ET-1-induced decrease of Mz-ChA-1 cell growth at 48 h is blocked by pre-incubation with both BQ610 and BQ788. Data are mean ± SEM of three experiments. &P < 0.05 vs. corresponding basal value; *P < 0.02 vs. other values.
Effect of endothelin-1 on the expression of vascular endothelial growth factor-A, -C, vascular endothelial growth factor receptor-2 and -3 in Mz-ChA-1 cells
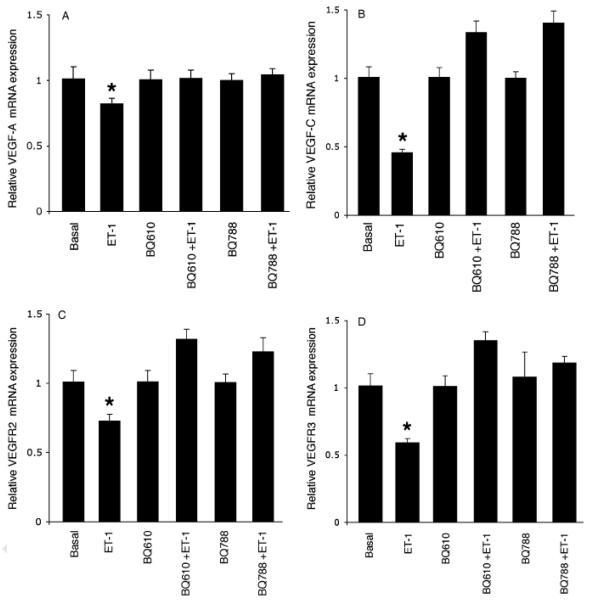
By real-time PCR, treatment with ET-1 (10 nM) induced a significant decrease in the message expression of VEGF-A and VEGF-C (measured as ratio to GAPDH mRNA) (Fig. 3A and B). Furthermore, ET-1 inhibited the expression of the message for VEGFR-2 (to which VEGF-A binds) (26) and VEGFR-3 (to which VEGF-C binds) (26) (Fig. 3C and D). Consistent with the concept that both ETA and ETB receptors regulate the effects of ET-1 on cholangiocarcinoma growth, ET-1 inhibition of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression was blocked by BQ610 (an ETA receptor inhibitor) (15) and BQ788 (an ETB receptor inhibitor) (15) (Fig. 3A–D).
Fig. 3.
Effect of endothelin-1 (ET-1) on the message expression (by real-time polymerase chain reaction) of vascular endothelial growth factor-A (VEGF-A), VEGF-C, VEGF receptor-2 (VEGFR-2) and VEGFR-3 expression in Mz-ChA-1 cells. (A and B) ET-1 (10 nM) decreased the mRNA expression of VEGF-A and VEGF-C of Mz-ChA-1 cells, a decrease that was blocked by pretreatment with BQ-610 and BQ-788. (C and D) ET-1 showed an inhibitory effect on the expression of the message for VEGFR-2 and VEGFR-3, a decrease that was blocked by pretreatment with BQ-610 and BQ-788. Data are mean ± SEM of three experiments. *P < 0.05 vs. corresponding basal values or treatment with inhibitors alone.
Effect of endothelin-1 on the growth of tumours implanted in nude mice
At 73 days (the time when mice were sacrificed), a significant difference in tumour size was found in ET-1-treated mice compared with mice injected with vehicle only (431.47 ± 55.45mm3, ET-1-treated mice vs. 904.08 ± 194mm3, vehicle-treated mice; P < 0.02) (Fig. 4A and B). No significant difference in body weight was detected between the two groups of animals following 73 days of treatment (Table 1). There was a significant decrease in the tumour latency of control mice (12.3 ± 4.1 days) vs. mice treated with ET-1 (31.25 ± 8.6 days) (P < 0.02) (Table 1).
Fig. 4.
(A and B) Malignant xenograft growth was evaluated in mice treated with daily injections of endothelin-1 (ET-1) or NaCl (vehicle) into the tumours. Nude mice were subcutaneously injected with Mz-ChA-1 cells (3 × 106 viable cells) suspended in 0.5 ml extracellular matrix. Mice were injected every day with NaCl or ET-1 inside the implanted tumours, for 73 days. (A) At 73 days, a significant difference in tumour size (arrows) was macroscopically evident in ET-1-treated mice compared with mice injected with vehicle. (B) ET-1 effect on cholangiocarcinoma xenograft growth. At 73 days, the cholangiocarcinoma xenograft volume of mice periodically injected with ET-1 was 431.47 ± 55.45 vs. 904.08 ± 194mm3 for the control mice. Tumour size is expressed in (mm3) ± SEM. *P < 0.05 vs. corresponding basal value.
Table 1.
Effect of endothelin-1 treatment on cholangiocarcinoma xenograft growth and nude mice parameters after 73 days of treatment
| Parameter | Vehicle | ET-1 |
|---|---|---|
| Body weight (g) | 26 ± 0.47 | 23.5± 0.75NS |
| Tumour latency (days) | 12.3 ± 4.1 | 31.25± 8.6* |
| Tumour size (mm3) | 904.08 ± 194 | 431.47 ± 55.45* |
Data are mean ± SEM of four mice.
P < 0.02 vs. the corresponding value of vehicle-treated mice.
ET-1, endothelin-1; NS, not significant; SEM, standard error of mean.
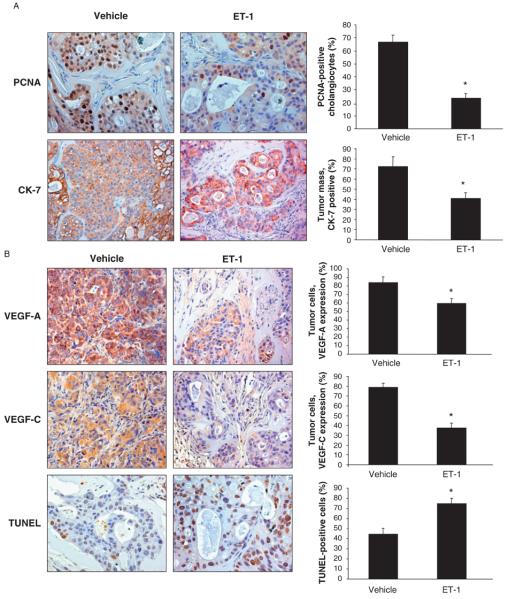
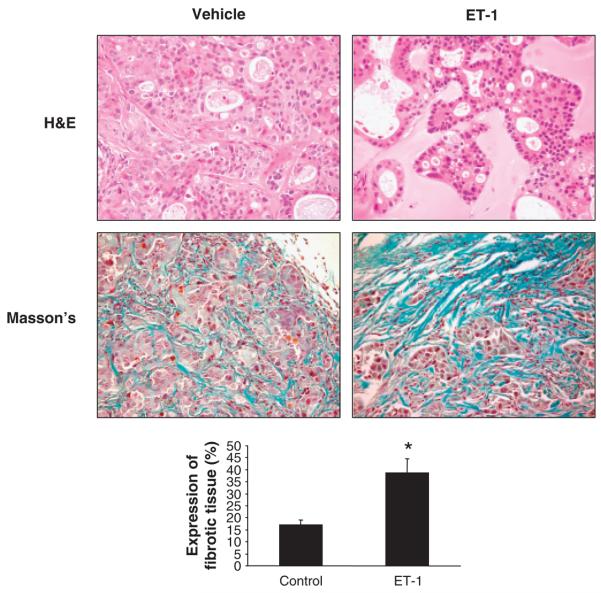
Immunohistochemistry for PCNA shows a decrease in the proliferative activity of the tumour cells from ET-1-treated mice compared with tumour cells from vehicle-treated mice (Fig. 5A). By immunohistochemistry, a decrease of area occupied by CK-7-positive malignant cholangiocytes in tumour mass of ET-1-treated vs. vehicle-treated animals is shown (Fig. 5A). Immunohistochemistry for VEGF-A and VEGF-C (Fig. 5B), trophic angiogenic factors involved in tumorigenesis and highly expressed in neoplastic tissue (5, 38), shows a decrease of both VEGF-A and VEGF-C in tumours obtained from ET-1-treated mice compared with vehicle-treated mice (Fig. 5B). TUNEL staining analysis (Fig. 5B) showed an increase in apoptosis in tumour cells of ET-1-treated mice compared with vehicle-treated mice. H&E and Masson's trichrome staining (Fig. 6) showed a more expressed fibrotic tissue in ET-1-treated mice compared with vehicle-treated mice (Fig. 6). No organ damage was found by histological analysis in the two groups of animals (not shown).
Fig. 5.
(A) Evaluation of proliferation [by staining for proliferating cellular nuclear antigen (PCNA)] in liver sections from tumour specimens from nude mice treated with endothelin-1 (ET-1) or vehicle. ET-1 decreased proliferation and increased apoptosis of cholangiocarcinoma cells in an in vivo mouse model. Data are mean ± SEM of three experiments. *P < 0.05 vs. PCNA-positive malignant cholangiocytes from vehicle-treated nude mice. Original magnification × 20. (B) Measurement of area occupied by cytokeratin-7 (CK-7)-positive malignant cholangiocytes from mice treated with ET-1 or vehicle. ET-1 administration significantly reduced the area occupied by CK-7-positive malignant cholangiocytes compared with mice treated with control solution. Data are mean ± SEM of three experiments. *P < 0.02 vs. CK-7-positive cells of tumour mass from vehicle-treated nude mice. Original magnification × 20. (B) Expression of vascular endothelial growth factor-A (VEGF-A) and VEGF-C by immunohistochemistry in tumours from ET-1- or vehicle-treated mice. Both VEGF-A and VEGF-C expression were reduced in malignant cells from ET-1- vs. vehicle-treated mice. Data are mean ± SEM of three experiments. *P < 0.05 vs. VEGF-A- and VEGF-C-positive tumour cells from vehicle-treated nude mice. Original magnification × 20. (B) Evaluation of apoptosis by terminal deoxynucleotide transferase end labelling (TUNEL) staining in tumours from ET-1- or vehicle-treated mice. An increase in the number of TUNEL-positive cholangiocytes demonstrated an increase in apoptosis in ET-1-treated tumours. Data are mean ± SEM of three experiments. *P < 0.02 vs. TUNEL-positive tumour cells from vehicle-treated nude mice. Negative controls (with pre-immune serum substituted for primary antibody) were also included. Original magnification × 20.
Fig. 6.
Photomicrographs of endothelin-1 (ET-1)- or vehicle-treated tumour sections stained with haematoxylin and eosin (H&E) and Masson's trichrome. Cholangiocarcinoma cells forming several acinar or tubular structures are shown in these images (H&E). An amorphous matrix localized between groups of cancer cells was observed in all the tumours. Masson's trichrome analysis showed fibrotic septa (blue) surrounding cholangiocarcinoma nodules. In ET-1-treated tumours, a wide area of fibrotic tissue is evident. Histological analysis demonstrated increased necrosis and damage and a more prominent fibrotic tissue in the ET-1-treated tumours with respect to controls. Original magnification × 80. Data are mean ± SEM of three experiments. *P < 0.05 vs. vehicle-treated tumour.
Discussion
We have shown that (i) normal and malignant cholangiocyte cell lines and normal and cholangiocarcinoma human tissue of biopsy samples express ETA and ETB receptors, (ii) ETA and ETB expression is higher in cholangiocarcinoma cells compared with normal cholangiocytes and (iii) ET-1 decreases the growth of the cholangiocarcinoma cell line, Mz-ChA-1 cells. Furthermore, chronic treatment of the Mz-ChA-1 cells, subcutaneously implanted in a nude mouse model, with ET-1 inhibits tumour growth in vivo. ET-1 In vitro and in vivo inhibition of Mz-ChA-1 cell growth was associated with decreased expression of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 in Mz-ChA-1 cells and in sections from tumours. The data suggest that modulation of the expression of VEGF and VEGF receptors (by ET-1) may be important for managing cholangiocarcinoma growth.
Endothelin-1, by interacting with its own receptors, regulates the growth and metastasis of several types of tumours by modulating mitogenesis, angiogenesis, invasion and cell meta-static diffusion (39). Specifically, ET-1 enhances the growth and progression of prostatic, ovarian, renal, pulmonary, colorectal, breast, bladder, endometrial carcinomas, brain tumours, melanoma and bone metastases (39). These findings suggest the use of ET-receptor antagonists for the treatment of several types of cancers. Recently, it has been shown that DSL6A pancreatic cancer cells express ET receptors and that the ET-1 receptor antagonist, bosentan, inhibits the proliferation of pancreatic cancer cells (40). A study has demonstrated a beneficial effect of an ETA receptor antagonist, in combination with paclitaxel and carboplatin, as first-line therapy for advanced non-small cell lung cancer (41). Contrary to these findings (39-42), our study demonstrated an inhibitory effect of ET-1 on cholangiocarcinoma growth by interaction with both ETA and ETB receptors. The inhibitory effect of ET-1 on cholangiocarcinoma growth resembles a similar one that is observed for gastrin on hyperplastic and neoplastic cholangiocyte growth (10, 20). Gastrin exerts trophic effects on parietal and enterochromaffin-like cells in the gastric mucosa as well as on several gastrointestinal and non-gastrointestinal tumour cell lines (43-46) but does not promote the growth of rat liver and pancreas (47, 48). Furthermore, a study has shown that transfected cholecystokinin receptors mediate growth inhibition of human pancreatic cancer cell lines (49). Moreover, we have shown that gastrin inhibits secretin-stimulated ductal secretion (15) (a functional marker of cholangiocyte proliferation) (50-54) and both hyperplastic and neoplastic cholangiocyte growth (10, 20, 50). Consistent with the concept that ET-1 has inhibitory properties on cholangiocyte growth, we have shown that ET-1 exerts a similar effect seen by gastrin by inhibiting secretin-induced ductal secretion (15).
In our study, ET-1 inhibition of Mz-ChA-1 proliferation was associated with decreased expression of VEGF-A and VEGF-C, two important regulators of hyperplastic and neoplastic cholangiocyte growth (5, 24-26, 55). Cholangiocarcinoma cells stimulate the development of a rich vascular network, which functions to sustain the metabolic needs and to ensure an adequate support of oxygen and nutrients to the malignant cells (55). In intrahepatic cholangiocarcinoma, high levels of VEGF are required to maintain tumour vascularization and to stimulate malignant cholangiocyte proliferation (55). We propose that the inhibition of cholangiocarcinoma growth by ET-1 is also due to a decreased synthesis or release of VEGF-A and VEGF-C, which (in addition to regulating cholangiocyte functions) (25, 26) are potent inductors of vascularization, development and growth of several cancers (5, 55), such as cholangiocarcinoma (5, 24). Indeed, VEGF has been shown to stimulate cholangiocyte proliferation by an autocrine mechanism (26) and to prevent cholangiocyte damage by interruption of the blood flow of the hepatic artery (25) that is the main blood supply of the biliary epithelium (27, 56). These findings, demonstrating that increased VEGF expression sustains cholangiocyte proliferation (26) and that reduced cholangiocyte VEGF expression induces a decrease in cell growth and an increase in apoptosis (25), support the concept that VEGF is an important player in ET-1 inhibition of cholangiocarcinoma growth. We also speculate that the inhibitory effect on cholangiocarcinoma growth by ET-1 may be due to the vasoconstrictive properties of this peptide, which is able to modify the tumour blood flow (57, 58).
The inhibitory effect of ET-1 on tumour growth was accompanied by increased apoptosis of cholangiocytes and development of abundant fibrosis. The presence of rich connective tissue into the mass could be explained by the fact that ET-1 represents a key mediator of fibrogenesis and that it is responsible for the differentiation of fibroblasts towards a myofibroblast phenotype (59). Moreover, the fibrogenic mechanism could be activated in response to apoptotic and necrotic processes, more expressed in ET-1-treated tumours with respect to controls (60). In conclusion, our findings raise the possibility of a therapeutic approach for the treatment of biliary tumours by the modulation of the ET axis.
Acknowledgements
We thank Dr Andreea Trache and Anna Webb of the Texas A&M University Health Science Center Microscopy Imaging Center for assistance with confocal microscopy.
This work was supported partly by a grant award from Scott & White to Heather Francis, by an NIH KO1 grant award (DK078532) to Dr DeMorrow, by the Dr Nicholas C. High-tower Centennial Chair of Gastroenterology from Scott & White, the VA Research Scholar Award, a VA Merit Award and the NIH grants DK58411, DK062975 and DK76898 to Dr Alpini, by a MIUR grant 2003060137_004 to the Department of Gastroenterology, Università Politecnica delle Marche, by a MIUR grant PRIN # 2007HPT7BA_003 to Dr Alvaro and by University funds to Dr Onori and PRIN 2007 and Federate Athenaeum funds from the University of Rome ‘La Sapienza’ to Prof. Gaudio.
Abbreviations
- BSA
bovine serum albumin
- CK-7
cytokeratin-7
- ΔΔCT
delta delta analysis of the threshold cycle
- DAPI
4,6-diamidino-2-phenylindole
- ERK1/2
extracellular-signal-regulated kinase 1/2
- ET-1
endothelin-1
- ETA
endothelin receptor A
- ETB
endothelin receptor B
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- H&E
haematoxylin and eosin
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium, inner salt
- PCNA
proliferating cellular nuclear antigen
- PCR
polymerase chain reaction
- PKC
protein kinase C
- TUNEL
terminal deoxynucleotide transferase end labelling
- VEGF
vascular endothelial growth factor
References
- 1.Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37:961–9. doi: 10.1053/jhep.2003.50200. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Gores GJ. Tumor-specific marker genes for intrahepatic cholangiocarcinoma: utility and mechanistic insight. J Hepatol. 2008;49:160–2. doi: 10.1016/j.jhep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–50. doi: 10.1016/j.cld.2007.11.003. ix. [DOI] [PubMed] [Google Scholar]
- 4.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 5.Fava G, Marucci L, Glaser S, et al. Gamma-aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–46. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 6.Werneburg NW, Yoon JH, Higuchi H, Gores GJ. Bile acids activate EGF receptor via a TGF-alpha-dependent mechanism in human cholangiocyte cell lines. Am J Physiol Gastrointest Liver Physiol. 2003;285:G31–6. doi: 10.1152/ajpgi.00536.2002. [DOI] [PubMed] [Google Scholar]
- 7.Fava G, Alpini G, Rychlicki C, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–61. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan CK, Podila PV, Taylor JE, et al. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908–16. doi: 10.1016/0016-5085(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 9.Marzioni M, Fava G, Benedetti A. Nervous and neuroendocrine regulation of the pathophysiology of cholestasis and of biliary carcinogenesis. World J Gastroenterol. 2006;12:3471–80. doi: 10.3748/wjg.v12.i22.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–91. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 11.Kanno N, Lesage G, Phinizy JL, et al. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology. 2002;35:1329–40. doi: 10.1053/jhep.2002.33330. [DOI] [PubMed] [Google Scholar]
- 12.Alvaro D, Mancino MG, Onori P, et al. Estrogens and the pathophysiology of the biliary tree. World J Gastroenterol. 2006;12:3537–45. doi: 10.3748/wjg.v12.i22.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demorrow S, Glaser S, Francis H, et al. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 14.Tostes RC, Fortes ZB, Callera GE, et al. Endothelin, sex and hypertension. Clin Sci. 2008;114:85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 15.Caligiuri A, Glaser S, Rodgers RE, et al. Endothelin-1 inhibits secretin-stimulated ductal secretion by interacting with ETA receptors on large cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1998;275:G835–46. doi: 10.1152/ajpgi.1998.275.4.G835. [DOI] [PubMed] [Google Scholar]
- 16.Housset C, Carayon A, Housset B, et al. Endothelin-1 secretion by human gallbladder epithelial cells in primary culture. Lab Invest. 1993;69:750–5. [PubMed] [Google Scholar]
- 17.Iwasaki S, Homma T, Matsuda Y, Kon V. Endothelin receptor subtype B mediates autoinduction of endothelin-1 in rat mesangial cells. J Biol Chem. 1995;270:6997–7003. doi: 10.1074/jbc.270.12.6997. [DOI] [PubMed] [Google Scholar]
- 18.Pinzani M, Milani S, de Franco R, et al. Endothelin 1 is over-expressed in human cirrhotic liver and exerts multiple effects on activated hepatic stellate cells. Gastroenterology. 1996;110:534–48. doi: 10.1053/gast.1996.v110.pm8566602. [DOI] [PubMed] [Google Scholar]
- 19.Luo B, Tang L, Wang Z, et al. Cholangiocyte endothelin 1 and transforming growth factor beta1 production in rat experimental hepatopulmonary syndrome. Gastroenterology. 2005;129:682–95. doi: 10.1016/j.gastro.2005.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 21.Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–37. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Ogasawara S, Yano H, Higaki K, et al. Expression of angiogenic factors, basic fibroblast growth factor and vascular endothelial growth factor, in human biliary tract carcinoma cell lines. Hepatol Res. 2001;20:97–113. doi: 10.1016/s1386-6346(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 23.Benckert C, Jonas S, Cramer T, et al. Transforming growth factor beta 1 stimulates vascular endothelial growth factor gene transcription in human cholangiocellular carcinoma cells. Cancer Res. 2003;63:1083–92. [PubMed] [Google Scholar]
- 24.Mancino A, Mancino MG, Glaser S, et al. Estrogens stimulate the proliferation of human cholangiocarcinoma by inducing the expression and secretion of vascular endothelial growth factor. Dig Liver Dis. 2008;41(2):156–63. doi: 10.1016/j.dld.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaudio E, Barbaro B, Alvaro D, et al. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol. 2006;291:G307–17. doi: 10.1152/ajpgi.00507.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–82. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–24. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 28.Alpini G, Kanno N, Phinizy JL, et al. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G973–82. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- 29.Isomoto H, Mott JL, Kobayashi S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–96. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietersen AM, Rutjes SA, van Tongeren J, et al. The tumor-selective viral protein apoptin effectively kills human biliary tract cancer cells. J Mol Med. 2004;82:56–63. doi: 10.1007/s00109-003-0486-z. [DOI] [PubMed] [Google Scholar]
- 31.Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–96. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 32.Demorrow S, Francis H, Gaudio E, et al. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–19. doi: 10.1152/ajpgi.00304.2007. [DOI] [PubMed] [Google Scholar]
- 33.Alpini G, Invernizzi P, Gaudio G, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68(22):9184–93. doi: 10.1158/0008-5472.CAN-08-2133. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seki H, Elder MG, Sullivan MH. Endothelin-1 regulates human decidual cells through both A- and B-type receptors. Mol Cell Endocrinol. 1995;114:111–6. doi: 10.1016/0303-7207(95)03647-p. [DOI] [PubMed] [Google Scholar]
- 35.Janosi T, Petak F, Fontao F, et al. Differential roles of Eta and Etb receptors and vasoactive intestinal peptide (Vip) in regulation of the airways and the pulmonary vasculature in isolated rat lung. Exp Physiol. 2008;93(11):1210–9. doi: 10.1113/expphysiol.2008.042481. [DOI] [PubMed] [Google Scholar]
- 36.Gulati A, Srimal RC. Endothelin antagonizes the hypotension and potentiates the hypertension induced by clonidine. Eur J Pharmacol. 1993;230:293–300. doi: 10.1016/0014-2999(93)90564-x. [DOI] [PubMed] [Google Scholar]
- 37.Lesage G, Glaser S, Ueno Y, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–90. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 38.Veeravagu A, Hsu AR, Cai W, et al. Vascular endothelial growth factor and vascular endothelial growth factor receptor inhibitors as anti-angiogenic agents in cancer therapy. Recent Pat Anticancer Drug Discovery. 2007;2:59–71. doi: 10.2174/157489207779561426. [DOI] [PubMed] [Google Scholar]
- 39.Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–51. doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Fitzner B, Brock P, Holzhuter SA, et al. Synergistic growth inhibitory effects of the dual endothelin-1 receptor antagonist Bosentan on pancreatic stellate and cancer cells. Dig Dis Sci. 2008;54(2):309–20. doi: 10.1007/s10620-008-0366-z. [DOI] [PubMed] [Google Scholar]
- 41.Chiappori AA, Haura E, Rodriguez FA, et al. Phase I/II study of atrasentan, an endothelin A receptor antagonist, in combination with paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer. Clin Cancer Res. 2008;14:1464–9. doi: 10.1158/1078-0432.CCR-07-1508. [DOI] [PubMed] [Google Scholar]
- 42.Hoosein MM, Dashwood MR, Dawas K, et al. Altered endothelin receptor subtypes in colorectal cancer. Eur J Gastroenterol Hepatol. 2007;19:775–82. doi: 10.1097/MEG.0b013e3282c563de. [DOI] [PubMed] [Google Scholar]
- 43.Watson SA, Steele RJ. Gastrin antagonists in the treatment of gastric cancer. Anticancer Drugs. 1993;4:599–604. doi: 10.1097/00001813-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Yassin RR, Clearfield HR, Little KM. Gastrin's trophic effect in the colon: identification of a signaling pathway mediated by protein kinase C. Peptides. 1993;14:1119–24. doi: 10.1016/0196-9781(93)90164-c. [DOI] [PubMed] [Google Scholar]
- 45.Quintero E, Ohning GV, del Rivero M, et al. Gastrin mediates the increase in gastric cell growth in uremic rats. Am J Physiol Gastrointest Liver Physiol. 1995;268:G586–91. doi: 10.1152/ajpgi.1995.268.4.G586. [DOI] [PubMed] [Google Scholar]
- 46.Waldum HL, Brenna E, Kleveland PM, Sandvik AK. Gastrin – physiological and pathophysiological role: clinical consequences. Dig Dis. 1995;13:25–38. doi: 10.1159/000171484. [DOI] [PubMed] [Google Scholar]
- 47.Chen D, Nylander AG, Norlen P, Hakanson R. Gastrin does not stimulate growth of the rat pancreas. Scand J Gastroenterol. 1996;31:404–10. doi: 10.3109/00365529609006418. [DOI] [PubMed] [Google Scholar]
- 48.Chen D, Ding XQ, Rehfeld JF, Hakanson R. Endogenous gastrin and cholecystokinin do not promote growth of rat liver. Scan J Gastroenterol. 1994;29:688–92. doi: 10.3109/00365529409092495. [DOI] [PubMed] [Google Scholar]
- 49.Detjen K, Fenrich MC, Logsdon CD. Transfected cholecystokinin receptors mediate growth inhibition on human pancreatic cancer cell lines. Gastroenterology. 1997;112:952–9. doi: 10.1053/gast.1997.v112.pm9041258. [DOI] [PubMed] [Google Scholar]
- 50.Glaser S, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G1061–70. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- 51.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–78. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–97. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 53.Lesage G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–9. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 54.Lesage G, Glaser S, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–44. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 55.Shinkaruk S, Bayle M, Lain G, Deleris G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr Med Chem Anticancer Agents. 2003;3:95–117. doi: 10.2174/1568011033353452. [DOI] [PubMed] [Google Scholar]
- 56.Tavoloni N, Schaffner F. The intrahepatic biliary epithelium in the guinea pig: is hepatic artery blood flow essential in maintaining its function and structure? Hepatology. 1985;55:666–72. doi: 10.1002/hep.1840050424. [DOI] [PubMed] [Google Scholar]
- 57.Bell KM, Prise VE, Shaffi KM, Chaplin DJ, Tozer GM. A comparative study of tumour blood flow modification in two rat tumour systems using endothelin-1 and angiotensin II: influence of tumour size on angiotensin II response. Int J Cancer. 1996;67:730–8. doi: 10.1002/(SICI)1097-0215(19960904)67:5<730::AID-IJC23>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 58.Cemazar M, Wilson I, Prise VE, et al. The endothelin B (ETB) receptor agonist IRL 1620 is highly vasoconstrictive in two syngeneic rat tumour lines: potential for selective tumour blood flow modification. Br J Cancer. 2005;93:98–106. doi: 10.1038/sj.bjc.6602672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krieg T, Abraham D, Lafyatis R. Fibrosis in connective tissue disease: the role of the myofibroblast and fibroblast–epithelial cell interactions. Arthritis Res Ther. 2007;9(Suppl 2):S4. doi: 10.1186/ar2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laplante P, Raymond MA, Gagnon G, et al. Novel fibrogenic pathways are activated in response to endothelial apoptosis: implications in the pathophysiology of systemic sclerosis. J Immunol. 2005;174:5740–9. doi: 10.4049/jimmunol.174.9.5740. [DOI] [PubMed] [Google Scholar]