Abstract
Multiple sclerosis (MS) is a demyelinating autoimmune disease characterized by infiltration of T cells into the central nervous system (CNS) after compromise of the blood-brain barrier. A model used to mimic the disease in mice is experimental autoimmune encephalomyelitis (EAE). In this report, we examine the clinical and histopathological course of EAE in eNOS-deficient (eNOS−/−) mice to determine the role of nitric oxide (NO) derived from this enzyme in the disease progression. We find that eNOS−/− mice exhibit a delayed onset of EAE that correlates with delayed BBB breakdown, thus suggesting that NO production by eNOS underlies the T cell infiltration into the CNS. However, the eNOS−/− mice also eventually exhibit more severe EAE and delayed recovery, indicating that NO undertakes dual roles in MS/EAE, one proinflammatory that triggers disease onset, and the other neuroprotective that promotes recovery from disease exacerbation events.
Keywords: eNOS, nitric oxide, EAE, mice, microglia
INTRODUCTION
Multiple sclerosis (MS) is a demyelinating autoimmune disease resulting from immune attack on myelin, leading to neurodegeneration. Neurological dysfunction ranges from sensory defects to paralysis (Swanborg 1995). One MS animal model is experimental autoimmune encephalomyelitis (EAE), which mimics histopathological hallmarks of MS. During EAE, T cells, normally excluded from CNS, infiltrate through a compromised blood-brain barrier (BBB). T cells interact with microglia and macrophages (Steinman 1996) that release cytokines and chemokines and affect disease progression (Kreutzberg 1996; Raivich and Banati 2004a; Rott et al. 1994; Stevens et al. 1994) by promoting neurodegeneration or neuroprotection.
Activated microglia produce nitric oxide (NO), which can modulate demyelination and inflammation (Farias et al. 2007; Mitrovic et al. 1994). NO can aggravate CNS inflammation, but studies using NO synthase (NOS) inhibitors to treat MS produced confusing results (Kahl et al. 2003; Okuda et al. 1997; 1998). NO can have proinflammatory roles promoting cytotoxicity, and or anti-inflammatory, suppressing the immune response (Cowden et al. 1998; O’Brien et al. 1999; Okuda et al. 1997).
There are three major NOS isoforms: neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) (Stuehr et al. 1989;Dawson et al. 1991;Lamas et al. 1992). Because the NOS isoforms are expressed primarily by distinct cell types, part of the complexity of NO effects results from the location and kinetics of NO production. Neuronal NO produced by nNOS has toxic consequences in focal ischemia models, whereas endothelial NO produced by eNOS confers neuroprotection (Samdani et al. 1997). iNOS, expressed primarily by glia (Bal-Price and Brown 2001; Marques et al. 2008; Tran et al. 1997), generates the high NO output seen in pathological CNS states (Calabrese et al. 2002; Chao et al. 1992; Farias et al. 2007; Shin et al. 2000). iNOS mRNA (Koprowski et al. 1993) and protein (Cross et al. 1997; Van Dam et al. 1995) expression within the EAE animals coincide temporally and quantitatively with the severity of clinical symptoms (Dalton and Wittmer 2005; Kahl et al. 2004; Kahl et al. 2003; Zehntner et al. 2004); the other two NOS isoforms have not been evaluated.
BBB disruption is associated with several CNS diseases including MS. Acting as a vasodilator, NO modulates BBB permeability. In pathological conditions, exaggerated NO-mediated vasodilation increases BBB permeability to allow inflammatory cells to infiltrate the CNS (Hurst and Fritz 1996; Shukla et al. 1996; Thiel and Audus 2001) in stroke (Mayhan and Didion 1996) and hypertension (Mayhan 1995). We showed previously that mice deficient in individual NOS isoforms respond differently to excitotoxicity, and that BBB integrity is differentially affected by each isoform’s loss (Parathath et al. 2006; 2007). It not known which NOS isoform produces the NO that mediates BBB breakdown in EAE (Thiel and Audus 2001).
The BBB is composed primarily of endothelial cells which express eNOS (Reese and Karnovsky 1967). NO produced by endothelial cells would be ideally situated to mediate a powerful local effect on BBB integrity.
To investigate the role of eNOS in MS, we applied the myelin oligodendrocyte glycoprotein (MOG)-induced EAE model to eNOS-deficient (eNOS−/−) mice and examined disease progression. Our results indicate that eNOS deficiency affects EAE onset, severity and recovery in a complex manner: lack of eNOS protects the BBB, but enhances the severity and persistence of the autoimmune response. The late pathology was largely rescued by delivering an NO donor, suggesting that an approach to this disease might be through the judicious use of NOS inhibitors and NO agonists at different phases of the process.
MATERIALS AND METHODS
Animals
C57BL6 (wild-type, wt) and eNOS deficient (eNOS−/−) (Shesely et al. 1996) mice were bred in-house under pathogen-free conditions [Division of Laboratory Animal Resources at Stony Brook University], controlled for temperature (21°C), and maintained under a 12-hour light/dark cycle. Access to food and water was ad libitum. eNOS−/− mice have been backcrossed into the C57/BL6 background. Adult (6- to 8-week-old) female mice used in experiments were routinely genotyped to confirm the eNOS-deficiency.
MOG peptide
MOG35-55 peptide (MEVGWYRSPFSRVVHLYRNGK) was synthesized by Quality Controlled Biochemicals and purified using reverse-phase (C18) HPLC.
Induction of EAE with MOG35–55 peptide
EAE was induced (Bernard et al. 1997; Bhasin et al. 2007; Lu et al. 2002) by subcutaneous injection into the mouse flank on day 0 with 300 μg of MOG35–55 peptide emulsified in complete Freund’s adjuvant (CFA) containing 500 μg of heat-inactivated Mycobacterium tuberculosis (Difco, Detroit, MI). One week later (day 7), the mice were boosted with 300 μg of MOG35–55 peptide subcutaneously in the other flank. 500 ng Pertussis toxin (List Biologicals, Campbell, CA) in 200 μl of PBS was injected intraperitoneally on days 0 and 2.
Evaluation of EAE symptoms
After MOG immunization, mice were observed and weighed daily blindly. Symptom severity was scored on a scale of 0 to 5 with gradations of 0.5 for intermediate symptoms, as follows (Hjelmstrom et al. 1998): 0, no detectable symptoms; 1, loss of tail tone; 2, hindlimb weakness or abnormal gait; 3, complete paralysis of hindlimbs; 4, complete hindlimb paralysis with forelimb weakness or paralysis; 5, moribund or death.
Time-controlled Drug Delivery
Alzet miniosmotic pumps (Durect, Cupertino, CA) were used for time-controlled drug delivery. 14-day pumps (infusion rate 0.25 μl/hr, 100 μl volume) were filled with PBS or 1mM NO donor 2,2′-(Hydroxynitroshydrazino)is-ethanamine [NOC-18 (Calbiochem; San Diego, CA)] and incubated at 37°C overnight.
One day before MOG-immunization, adult wild-type and eNOS−/− female mice (6–10 weeks old) were deeply anesthetized and pumps were implanted subcutaneously in the back of the mice. The pumps were replaced on day 15 and day 29.
Eriochrome Cyanine stain
Eriochrome cyanine staining was used to visualize myelin. Spinal cord sections were air-dried for 1 hour at room temperature and then at 37°C in a dry incubator. After incubation with acetone for 5 minutes, the slides were air-dried and stained in eriochrome cyanine solution (0.2% eriochrome cyanine RS (Sigma), 0.5% H2SO4 (Sigma), 10% iron alum (Sigma) in distilled water) for 30 minutes, differentiated in 5% iron album (Sigma) for 10 minutes, and placed in borax-ferricyanide solution (1% borax (Sigma), 1.25% potassium ferricyanide (Sigma), in distilled water) for 5 minutes. The slides were then dehydrated through graded ethanol solutions and coverslipped using Permount (Fisher Scientific, NJ, USA).
FluoroMyelin stain
FluoroMyelin fluorescent myelin stain was used to visualize myelin. Slides were hydrated in PBS or PBS-T for 20 minutes, incubated in staining solution for 20 minutes at room temperature, washed 3 times with PBS, and then mounted with Fluoromount-G (Southern Biotech, AL, USA).
To co-image Iba1, after the wash step the slides were blocked with 5% goat serum and processed with anti-Iba1 antibodies.
Immunofluorescence
Mice were transcardiacally perfused using 4% paraformaldehyde/PBS and the spinal cords removed and post-fixed in 4% paraformaldehyde/PBS for 1 hour at room temperature followed by 30% sucrose dehydration at 4°C overnight. The spinal cords were embedded in Tissue-Tek (Miles, Elkhart, IN) optimal cutting temperature compound, frozen on dry ice, and stored at −80°C until use. Cross sections (20 lm) were cut on a cryostat (Leica, Nussloch, Germany) at −20°C.
Spinal cord sections were blocked in serum [5% serum in PBS-T (0.5% TritionX-100 in PBS)], and then incubated overnight at 4°C in rabbit anti-mouse Iba1 (Wako, Japan) at a 1:500 to detect resting and activated microglia/macrophages. Sections were then incubated with fluorescence-conjugated (FITC or Texas Red) goat anti-rabbit secondary antibody for 1 hour at room temperature, washed with PBS, and mounted using Fluoromount-G (Southern Biotech, AL, USA).
We used occludin immunostaining as an indicator of BBB integrity (Bangsow et al. 2008; Date et al. 2006; Parathath et al. 2006; 2006): spinal cord sections (20μm) were fixed in 95% ethanol and then acetone. After blocking in serum, sections were incubated with 3μg/mL mouse anti-occludin (Zymed; San Francisco, CA) overnight at 4°C. FITC-goat anti-mouse secondary antibody was added for 1hr at room temperature. Sections were washed in PBS and mounted using Fluoromount-G. The stained sections were photographed using a SPOT RT camera with a Nikon Eclipse E600 microscope.
Western blotting
The spinal cords were isolated and homogenized in 0.25% Triton X-100 in PBS. Debris was removed by centrifugation, and the supernatant protein concentration measured using the BioRad Bradford detergent-compatible (DC) assay. Protein (20 mg) was separated by 15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Membranes were blocked using 5% nonfat dry milk in PBS containing 0.05% Tween 20, and then incubated with rabbit anti-mouse Iba1 antibody (1:1000) and rabbit anti-mouse actin antibody (1:2500) overnight at 4°C. Horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immunoresearch) was used and detected using the LumiGLO Chemiluminescent Substrate System (KPL).
BBB Breakdown
BBB breakdown was assessed by the disruption of occludin immunostaining and Evans Blue diffusion, as described before (Parathath et al. 2006; Yepes et al. 2003). eNOS−/− and wild-type mice were injected intravenously with 2% Evans blue (Sigma). After 24 hours, the mice were sacrificed by transcardiac perfusion with saline, and the spinal cords removed, weighed, and homogenized in 0.5% TritonX-100, and centrifuged at 21,000×g. The amount of Evans blue in the supernatant was quantified at 620nm, subtracted from background, and divided by the tissue wet weight. Values are presented as percent of total signal and represent average values for at least three mice per group.
Measurement of nitrite levels
Nitrite levels measurement was based on the reaction of nitrates/nitrites with 2,3-diaminonaphthalene (DAN) under acidic conditions, which results in 2,3-naphthyltriazole formation. In brief, 100 μl of sample or standard (NaNO2) were loaded in each well of a black 96-well plate with a clear bottom. 20 μl DAN (0.05 mg/ml in 0.62 M HCl) was added to each well. The reaction proceeded at RT for 20 min and terminated by the addition of 100 μl 0.28 M NaOH. After 10 min incubation at RT, fluorescence was measured on a Titertek Fluoroscan II fluorescence plate reader using an excitation 355 nm and an emission 460 nm filter pair.
Statistics
Statistical analysis was performed using one-way ANOVA followed by a Bonferroni-Dunn test for multiple comparisons within a group, or a two-tailed t-test for comparisons between groups as indicated by the figure legends; p < 0.05 was considered significant and is marked by an (*) or (#); p < 0.01 and p < 0.001 were considered very significant and are marked by two (**) or (##); and three (***) or (###), respectively. Results are represented as average with error bars indicating the standard error of the mean. n refers to the number of animals used for each genotype or condition.
RESULTS
Altered progression of EAE in eNOS−/− mice
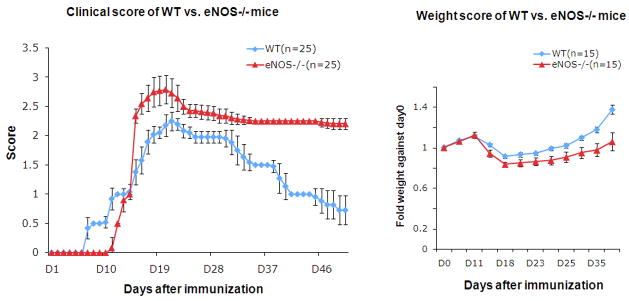
To evaluate the role of eNOS in EAE, the clinical course of MOG-induced EAE was assessed in wild-type (wt) and eNOS−/−mice (Fig. 1A). Wt mice exhibited disease signs on average at day 7 (7.2±0.4) after immunization, in contrast to the eNOS−/− mice which showed a significant delayed disease onset (day 11.9±0.3). However, the eNOS−/− mice exhibited a dramatic exacerbation on about day 15 (score 2.36±0.12). Symptoms worsened faster than the wt mice which had a more gradual onset of disease severity (score 1.36±0.23 on day 15). Disease severity also peaked at different levels for the eNOS−/− mice and wt mice. In wild-type mice, the peak of disease severity was around days 20 – 22, with a maximum score of 2.25±0.14. In contrast, the disease peak in eNOS−/− mice started on day 17 and persisted until day 22 with a maximum score of 2.75±0.25. All of the eNOS−/− mice exhibited paralysis of one or both hindlimbs, which was not the case for the wt mice. Following the peak of disease, wild-type mice showed a progressive recovery; by day 50, the clinical score observed for wt mice was 0.85±0.23 (loss of tail tone) and they exhibited no other motor dysfunction. The eNOS−/− mice exhibited sustained symptoms with a slow and limited recovery (score 2.18±0.11 on day 50), and their neurological and motor dysfunction was persistent.
Figure 1. eNOS−/− mice exhibit delayed EAE onset, exaggerated disease severity and limited recovery.
A. Clinical course of EAE. Wild-type (WT) and eNOS−/− mice were injected with MOG35–55 peptide in CFA and pertussis toxin to induce EAE. Disease severity was scored on a clinical scale from 0 to 5. B. Weight score of WT and eNOS−/− mice during EAE. A two-tailed t test was performed to analyze the weight score differences between WT and eNOS−/− mice for each timepoint (*** p<0.001; **p<0.01). (Experiment was repeated at least three times. Cumulative data are provided; total number of animals tested n=25/genotype).
An alternate approach to characterize EAE severity is to weigh the mice daily, since mice become sick and lose weight as a consequence of the disease. Wt mice exhibited weight loss as disease progressed peaking between days 18 to 22 (Fig. 1B). The mice then re-gained weight as the recovery began. The eNOS−/− mice similarly showed significant weight loss with biggest drop between days 18 to 22. However, the eNOS−/− mice only slowly re-gained weight during the recovery period, paralleling the slow symptomatic recovery seen in Fig. 1A.
Taken together, the delayed onset of disease and prolonged recovery period suggested that eNOS-generated NO plays an early damaging role and a late protective role in MOG-induced EAE.
eNOS−/− mice exhibit delayed BBB breakdown
The delay in EAE onset in eNOS−/− mice indicates that the NO produced by eNOS contributes to the induction of disease. Since eNOS is primarily expressed by endothelial cells and astrocytes (Lin et al. 2007), cells that are the main constituents of the BBB, and T cell infiltration through a compromised BBB is a requisite early event in the disease, this raised the possibility that the role of the NO generated might be to affect BBB permeability.
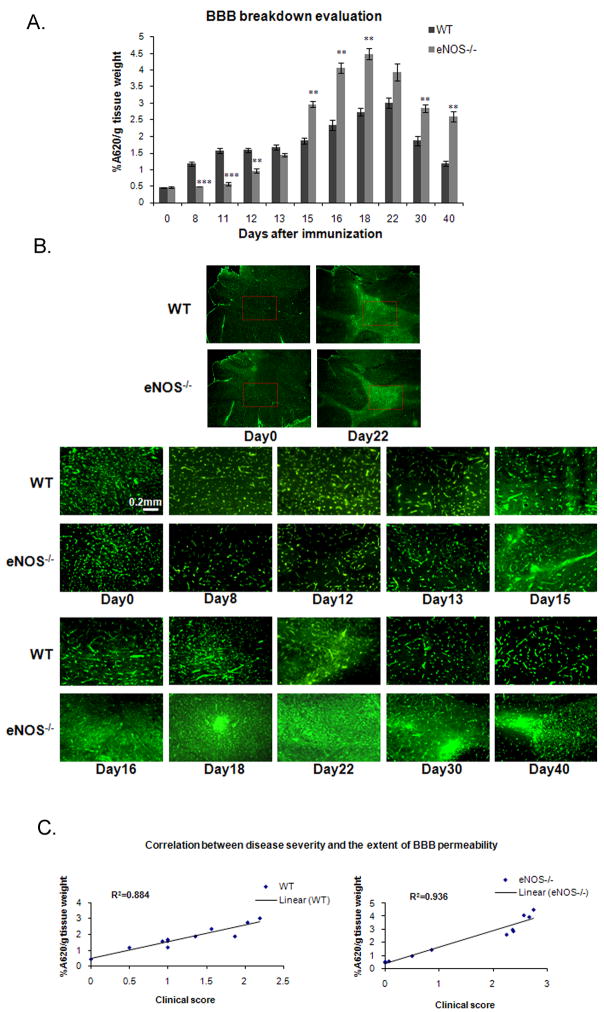
We evaluated BBB integrity by Evans Blue dye diffusion in the spinal cord at different time points after MOG immunization; the presence of Evans Blue after perfusion indicates breakdown of the BBB. On day 0, WT and eNOS−/− mice displayed very low levels of Evans Blue in the spinal cord (0.46±0.01 and 0.46±0.02, respectively), indicative of BBB integrity (Fig. 2A). Increased levels of Evans Blue were detected on day 8 in WT mice (1.18±0.06), but no increase was observed for the eNOS−/− mice until day 12 (0.96±0.06), which correlated with the delayed disease onset observed in Fig. 1. The extent of BBB breakdown increased progressively in WT mice with the maximal level seen on day 22 (3.01±0.16). In contrast, eNOS−/− mice showed a dramatic increase on day 15 (2.97±0.09) that went beyond that seen in the WT mice and that peaked on day 18 (4.5±0.18). Recovery of BBB integrity was observed for both genotypes; however, the eNOS−/− mice lagged substantially behind the WT mice.
Figure 2. Delayed, but ultimately, more extensive BBB breakdown in eNOS−/− mice.
A. WT and eNOS−/− mice were injected with Evans Blue at different time points after MOG immunization. 24 hours later, the mice were perfused with PFA and the spinal cords removed, weighed, and homogenized. The extent of BBB breakdown was assessed as the amount of Evans Blue (quantified using A620 nm) present into the spinal cord normalized to the tissue wet weight. Values are presented as percent of the total amount of Evans Blue Dye and represent the average of at least three mice per experimental group in three separate experiments. A two-tailed t test analyzed the BBB breakdown difference between WT and eNOS−/− mice for each timepoint (***, p<0.001; **, p<0.01). B. BBB breakdown was assessed in the cerebellum of animals by occludin immunofluorescence. Two representative low magnification figures (day 0 and day 22) show area of interest (red box). C. Correlation between BBB permeability and disease severity.
Breakdown of the BBB was also investigated by immunofluorescence using an anti-occludin antibody, since occludin localizes exclusively to endothelial tight junctions (Hirase et al. 1997). In cerebella of untreated wt and eNOS−/− mice on day 0, occludin showed strong and typical staining of the endothelial layer at contact points between cells (Fig. 2B). By day 8, WT mice began to exhibit diffuse occludin staining, indicating significant BBB breakdown. This pattern persisted, peaking on day 22 and then resolving by day 30. In contrast, the eNOS−/− mice showed a normal-looking staining pattern until day 15, at which point a marked and much more diffuse occludin staining was observed that progressed until day 22, suggesting almost complete breakdown of the BBB. The diffuse staining pattern improved subsequently but still remained incompletely resolved at day 40.
The results from these experiments suggested that the considerable difference in the clinical course of EAE between eNOS−/− and wild-type mice correlates with the differences in extent of BBB breakdown. To determine whether there was a general correlation between BBB permeability and disease severity, the quantification of the levels of Evans Blue in the spinal cord from different time points were plotted against the clinical score on that day (Fig. 2C). A close linear correlation was evident (r2 = 0.884 for wild-type, and r2 = 0.936 for eNOS−/− mice).
Altered demyelination pattern during disease progression in eNOS−/− mice
Inflammation and demyelination are two well-defined characteristics of EAE. Eriochrome cyanine (EC) staining was used to detect myelin profiles in mouse spinal cord. On day 0, wild-type and eNOS−/− mice showed compact myelin staining in the white matter of spinal cord (Fig. 3A). In contrast, severe demyelination was detected in eNOS−/− mice on day 15, characterized by a large area devoid of EC staining (asterisks), whereas only minimal demyelination was observed in WT mice. By day 22, WT mice showed large areas lacking EC staining, and eNOS−/− mice exhibited almost 50% demyelination of the white matter. Recovery (remyelination) was apparent by day 30 and complete by day 40 for the WT mice. In contrast, although partial remyelination was seen in the eNOS−/− mice, large areas of demyelination still remained at days 30 and 40.
Figure 3. Increased demyelination in eNOS−/− mice during EAE.
A. Frozen cross-sections of spinal cords isolated from WT and eNOS−/− mice at different time points during the EAE course were stained with Eriochrome cyanine (EC). Sites of demyelination are indicated by asterisks. B, C. Use of FluoroMyelin to image myelin quantitatively. (Quantifications were performed on five sections per mouse for three different mice in three separate EAE experiments; results are presented as an average with error bars indicating the standard error of the mean. A two-tailed t test was performed to analyze the significance of the difference of FluoroMyelin staining intensity between WT and eNOS−/− mice at each timepoint (*** p<0.001; **p<0.01).
Demyelination was also evaluated by FluoroMyelin, a fluorescent myelin stain, for which similar results were obtained (Fig. 3B). Quantification of the fluorescence intensities (Fig. 3C) revealed that the extent of demyelination was significantly different from day 15 through the end of the experiment at day 40.
eNOS−/− mice exhibit increased microglial infiltration and activation
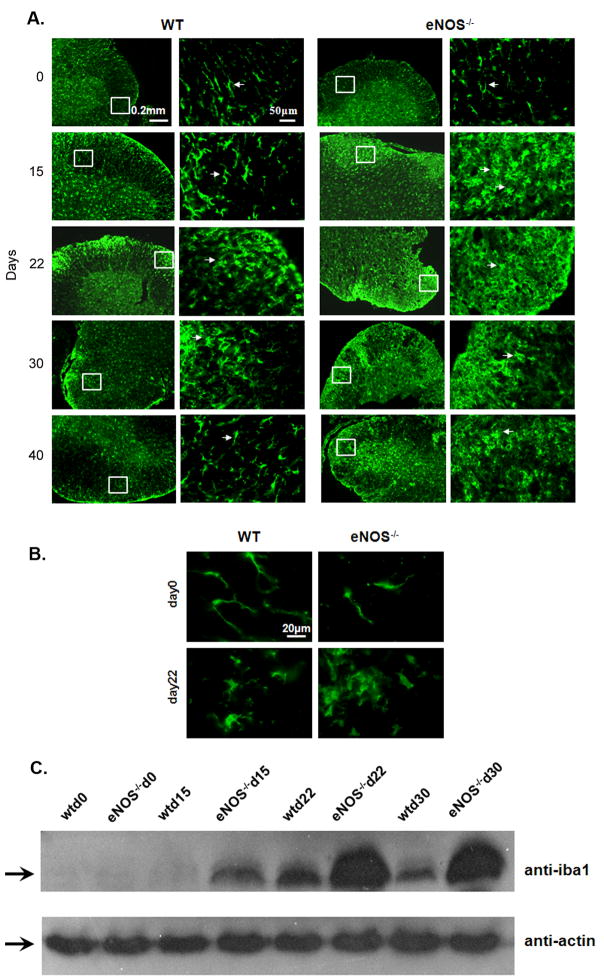
During EAE, infiltrating T cells attack the endogenous myelin. Activated microglia are recruited to the demyelinated area and through their phagocytotic activity remove cellular debris (Benveniste 1997; Diemel 1998). We evaluated the levels of microglia/macrophage activation at different time-points (Day 15, 22, 30, and 40) during EAE, using the Iba1 antibody which detects resting microglia/macrophages and becomes upregulated during activation (Fig. 4A, B). Iba1 staining in WT and eNOS−/− mice on day 0 revealed resting microglia/macrophages, defined by ramified morphology with long thin cell bodies (arrows). On day 15 in WT mice, the microglia/macrophages observed still appeared to be largely in the resting state, whereas in eNOS−/− mice, dramatic numbers of activated cells were visible in the white matter of spinal cord, defined by an ameboid shape and extensive branching. This accumulation of activated microglia/macrophages continued in eNOS−/− mice through day 40. WT mice, on the other hand, where characterized by activated microglia/macrophage between days 22 – 30, but a return to the resting state and a decrease in the total number of Iba1+ cells was apparent by day 40.
Figure 4. Increased microglia/macrophage infiltration and activation in eNOS−/− mice.
A. Frozen cross-sections of spinal cords from WT and eNOS−/− mice at different time points in EAE were stained with anti-Iba1. Iba1 is expressed on resting and activated microglia. Arrows indicate resting microglia, characterized by long, thin cell bodies. Arrowheads indicate activated microglia that are characterized by thicker, rounded cell bodies and multi-branched cellular protrusions. (Experiment has been repeated three times. In total, five mice were assessed for immunostaining per timepoint per genotype; representative data are provided.) B. Higher magnification (boxed areas in panel A) shows morphology of resting state (day 0) and activated state (day 22) microglia/macrophages. C. Protein extracts from WT and eNOS−/− mice spinal cords were collected at different timepoints in EAE and analyzed using western blotting to quantify levels of Iba1. Equal loading of protein was confirmed by anti-actin blotting. (Western blot was repeated three times on mouse spinal cord samples from three separate EAE experiments).
Spinal cord extracts were analyzed at different time points during EAE by western blotting to determine Iba1 expression as a quantitative measure of the extent of microglia/macrophage recruitment and activation (Fig. 4C). In both WT and eNOS−/− spinal cord extracts, the levels of Iba1 increased as disease progressed. eNOS−/− spinal cords showed early upregulation of Iba1 starting on day 15, whereas no increase was observed in WT spinal cords. By day 22, increased Iba1 was seen for both genotypes, but the levels of expression were higher in the eNOS−/− spinal cords. They remained elevated at day 30, whereas the levels of expression in WT spinal cords had begun to decrease. These results correlate with the extent of microglia/macrophage activation described in panels A and B, and with the clinical symptoms described earlier.
Early stages of EAE are characterized by extensive axonal damage that results in recruitment and activation of microglia which phagocytose myelin debris and hasten the progression of myelin loss (Bauer et al. 1994; Matsumoto et al. 1992; Raivich and Banati 2004b). To visualize the microglia during the course of demyelination, we stained spinal cords with FluoroMyelin (for myelin) and Iba1 (for microglia/macrophages). Our results show that activated microglia/macrophage accumulated mostly in the demyelinated areas (Suppl. Fig 1), which supported the model that microglia/macrophage are recruited into areas that are undergoing/will undergo demyelination.
Providing nitric oxide using an NO donor does not affect the onset and severity of disease, but is beneficial for recovery in eNOS−/− mice
As shown above, eNOS−/− mice have a delayed but then worsened clinical course, pattern of demyelination and extent of microglial infiltration compared to WT mice. To determine whether it is the lack of eNOS gene or the lack of NO produced during disease that caused these differences, we provided NO using the NO donor 2,2′-(Hydroxynitroshydrazino)is-ethanamine (NOC-18) by delivery through mini-osmotic pumps into the eNOS−/− mice. 14-day pumps (rate of infusion 0.25 μl/hr, 100 μl total volume) filled with PBS or 1mM NOC-18 in PBS were implanted in the back of the mice on day 1 after MOG immunization, and replaced with fresh pumps on days 15 and 30. As shown in Fig. 5A, the NOC-18-infused eNOS−/− mice showed delayed disease onset with symptoms starting around day 12, similar to mice infused just with PBS. Rapid exacerbation of disease symptoms started on day 15 with maximal severity score of 2.67±0.24 on day 18. However, in contrast to the limited recovery seen in eNOS−/− mice, the mice infused with NOC-18 recovered faster, with the clinical score on days 30–40 indistinguishable from that of WT mice, and EC staining (Fig. 5B) that showed a remyelination pattern like that of WT animals. The extent of microglia infiltration and activation also changed during the recovery period in eNOS−/− mice infused with NOC-18: fewer activated microglia were recruited on day 30 to the spinal cord (Fig. 5C). On day 40, most Iba1+ microglia were in a resting state, correlating with the better remyelination at this timepoint.
Figure 5. Therapeutic administration of an NO donor is beneficial in the recovery phase for eNOS−/− mice.
A. Clinical course and weight score in eNOS−/− mice infused with NOC-18. (Experiment has been repeated three times. Cumulative data are provided; total number of animals tested: n=19/WT, n=19/eNOS−/−, n=21/eNOS−/− infused with NOC-18.) B. Eriochrome cyanine (EC) staining in spinal cords of eNOS−/− mice supplemented with NOC-18. C. Anti-Iba1 staining in eNOS−/− mice supplemented with NOC-18. (At least four mice were assessed each timepoint per genotype for both EC staining and Iba-1 staining; representative data are provided.) D. BBB breakdown was assessed by occludin immunofluorescence in eNOS−/− mice supplemented with NOC-18. E. Nitrite production during EAE. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni-Dunn test for multiple comparisons within each timepoint. *p < 0.05 indicates significant difference in nitrite production, **p < 0.01 and ***p < 0.001 indicates very significant difference in nitrite production. Within each genotype or treatment (i.e. WT mice, eNOS−/− mice and eNOS−/− mice infused with NOC-18), one-way ANOVA was conducted and followed by a Bonferroni-Dunn test for multiple comparisons for all timepoints versus control (day 0). #p < 0.05 indicates significant difference in nitrite production, ##p < 0.01 and ###p < 0.001 indicates very significant difference in nitrite production.
BBB breakdown in NOC-18-infused eNOS−/− mice was investigated using occludin immunofluorescence (Fig. 5D). More diffused occludin staining was observed in the animals starting on day 15, progressed to day 22, and resolved by day 30. The early BBB breakdown in these mice is consistent with the rapid exacerbation of disease symptoms, which resembled that of eNOS−/− mice. However, these mice showed an almost complete BBB restoration during the recovery period, comparable to the wt mice, suggesting that the NO provided by NOC-18 plays a beneficial role.
To determine whether NOC-18 was effective in providing NO during the 14-day infusion period, we measured nitrite production in the spinal cords during EAE. Nitrite production was measured at different time points in WT mice, eNOS−/− mice and eNOS−/− mice infused with NOC-18, and we found that the NOC-18 only partially restored the nitrite levels towards those seen in WT mice (Fig. 5E). Interesting, the overall level of nitrites decreased in correlation with the severity of EAE symptoms in all mice examined.
These experiments suggested that providing even modest amounts of NO are beneficial during the recovery phase for eNOS−/− mice, and that higher levels of NOC-18 delivery might succeed in reversing the altered onset and disease progression during the early stages of EAE.
DISCUSSION
We report here that eNOS−/− mice show delayed BBB breakdown accompanied by (1) altered EAE progression characterized by delayed onset, increased severity and limited recovery of neurological and motor dysfunction; (2) early severe demyelination on day 15 which persists through day 40; and (3) an altered pattern of microglia infiltration and activation that occurs early and is similarly persistent. These results suggest that eNOS-produced NO triggers BBB breakdown during induction of EAE, but has a protective role subsequently by promoting innate immunosuppression during EAE symptom progression and recovery.
NO secreted by iNOS plays a crucial role in EAE. iNOS activity increases during EAE in correlation with the clinical course, and the iNOS-produced-NO is thought to mediate protection, since EAE symptoms are exacerbated in iNOS−/− mice (Fenyk-Melody et al. 1998). Mechanistically, it may function to increase T-helper 1 responses (Kahl et al. 2003) or eliminate inflammatory cells from the CNS by promoting their apoptosis (Okuda et al. 1997). In eNOS−/−mice, microglia recruitment and activation in the spinal cord was persistent in comparison to WT mice, indicating a similar role, directly or indirectly, for eNOS. The immunosuppressive effects of NO might be functioning through any of several mechanisms including inhibition of T cell proliferation or down-regulation of adhesion molecules, both of which would inhibit further recruitment of inflammatory cells.
It has been reported that NO can disturb BBB permeability, although the precise mechanism is complex and poorly understood. With their cell bodies wrapping around the capillaries and the ends sealed by tight junctions, endothelial cells can regulate BBB integrity. eNOS expression is up-regulated in endothelia during EAE (Zhao et al. 1996). In kainate hippocampal excitotoxicity, eNOS-derived NO mediates BBB breakdown (Parathath et al. 2006), and we report a similar finding here in EAE. It is important to consider that the absence of NO can affect BBB permeability through changes in venous pressure. eNOS−/− mice are hypertensive and excessive venous hypertension can stretch vein walls to separate the tight junctions formed by endothelial cells and disrupt BBB (Hawkins and Davis 2005; Talbert 2008). Although this process could affect the later events of our EAE experimental results (chronic disease signs), it can’t explain the delayed BBB opening that we observe. The major Th1 cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-1-β (IL-1β), activate endothelia and modulate the BBB by inducing the expression of endothelial cell adhesion molecules (Dore-Duffy et al. 1993; Losy et al. 1999).
These eNOS findings shed light on results obtained by several NOS/iNOS inhibitor treatment studies for EAE (Okuda et al. 1998; Zhao et al. 1996): in some cases the inhibitors ameliorated disease, whereas in others more severe symptoms were provoked, possibly because the treatments were delivered or were effective at different times in the disease course. Aminoguanidine (AG), a selective inhibitor for iNOS, reportedly has different effects on EAE in the induction and progression phase. AG administration during the induction phase showed partial preventive effects, while administration during the progression phase resulted in more severe disease and higher mortality (Okuda et al. 1998). These studies, along with our study, suggest that the timing or route of drug administration is an important factor.
Early studies reported that total NOS activity is increased in MS patients CSF (Calabrese et al. 2002), but more recently total NOS activity in the spinal cord was reported to remain unchanged or decrease during EAE (Kahl et al. 2003), and calcium-dependent NOS (eNOS and nNOS) activity in the brain were decreased concomitantly with iNOS upregulation (Teixeira et al. 2002). The different outcomes reported might be explained by the use of different models for EAE induction, or the different tissue examined (brain, spinal cord or CSF). However, since NOS activity represents the combined activity of all three NOS isoforms, it is conceivable that each isoform could play a different role in EAE. Here we find that nitrite levels in the spinal cord are about 20% lower in eNOS−/− mice than in WT mice, and the nitrite levels decrease in parallel for both strains during EAE progression and then rise again during recovery (Fig. 5C). We also observe benefit in eNOS−/− mice recovery when nitrite levels are partially restored through administration of the NO donor NOC-18 (Fig. 5A, 5B). Although not tested, this raises the possibility that administration of NOC-18 to elevate the falling nitrite levels in WT animals during the course of EAE could have a beneficial outcome.
It is intriguing that NO appears to play aggravating and protective roles in EAE in different contexts. Although both could be mediated directly by NO, it is possible that the negative outcomes triggered by NO production could ensue from its conversion to the toxic metabolite peroxynitrite (ONOO−) through reaction with superoxide. ONOO− modifies proteins through the formation of nitrotyrosine adducts and, when present at sufficiently high levels, induces excitotoxicity, DNA damage and apoptosis (Brown and Bal-Price 2003; Kroncke et al. 2001). Contrasting roles for NO and ONOO− have been reported. In vitro, peroxynitrite induces lipid peroxidation whereas NO inhibits it (Hooper et al. 2000), and human adult CNS-derived oligodendrocytes are relatively resistant to NO-mediated damage but highly susceptible to peroxynitrite-mediated injury (Beckman et al. 1994). We hypothesize that NO may be a protective molecule in the recovery phase of EAE, whereas its toxic metabolite peroxynitrite triggers BBB breakdown. Future studies will focus on the effects of peroxynitrite in EAE.
Finally, in our attempt to complement eNOS deficiency by administering a synthetic NO donor (NOC-18), we noted that no effect was observed in the phase where eNOS-generated NO is harmful, i.e. BBB breakdown, since no change in the delay of EAE was observed, but beneficial outcomes were obtained in the recovery phase (Fig. 5A). It is possible that NO provision only partially restored the deficiency triggered by eNOS absence (Fig. 5C), and that lower levels of nitrite are required for immunosuppression than for BBB breakdown. Alternately, it could indicate that diffuse provision of nitrate suffices to confer immunosuppression in this setting, whereas BBB breakdown requires local production by the endothelial cells that can not be mimicked by diffuse exogenous provision. Regardless of the mechanism, this outcome is potentially useful and suggests that a combination based on an eNOS inhibitor and NOC-18 supplementation could simultaneously delay or prevent new MS episodes by preventing BBB breakdown while enhancing immunosuppression/recovery.
Supplementary Material
Supplemental Figure 1: Recruitment of microglia/macrophages into regions of demyelination. Double staining with FluoroMyelin and anti-Iba1 to image regions of demyelination and microglia/macrophage localization. A. WT day 15 section, low and high magnification. B. WT day 22 section. C. eNOS−/− day 22 section. The white boxes indicate the regions that have been magnified in the higher magnification images.
Acknowledgments
We thank Drs. Howard Crawford, Holly Colognato, Michael Frohman and members of the Tsirka lab for critical editing and discussions. This work was supported by an AHA-established Investigator award, NIH grant 5R01NS04216804, and NMSS pilot project award to SET.
References
- Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21(17):6480–91. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsow T, Baumann E, Bangsow C, Jaeger M, Pelzer B, Gruhn P, Wolf S, von Melchner H, Stanimirovic D. The epithelial membrane protein 1 is a novel tight junction protein of the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28(6):1249–1260. doi: 10.1038/jcbfm.2008.19. [DOI] [PubMed] [Google Scholar]
- Bauer J, Sminia T, Wouterlood FG, Dijkstra CD. Phagocytic activity of macrophages and microglial cells during the course of acute and chronic relapsing experimental autoimmune encephalomyelitis. J Neurosci Res. 1994;38(4):365–75. doi: 10.1002/jnr.490380402. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–40. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- Benveniste E. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- Bernard CC, Johns TG, Slavin A, Ichikawa M, Ewing C, Liu J, Bettadapura J. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J Mol Med. 1997;75(2):77–88. doi: 10.1007/s001090050092. [DOI] [PubMed] [Google Scholar]
- Bhasin M, Wu M, Tsirka S. Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol. 2007;8:10. doi: 10.1186/1471-2172-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate and mitochondria. Mol Neurobio. 2003;27:325–355. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Bella R, Foresti R, Bates TE, Giuffrida Stella AM, Pennisi G. Nitric oxide synthase is present in the cerebrospinal fluid of patients with active multiple sclerosis and is associated with increases in cerebrospinal fluid protein nitrotyrosine and S-nitrosothiols and with changes in glutathione levels. J Neurosci Res. 2002;70(4):580–7. doi: 10.1002/jnr.10408. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149(8):2736–41. [PubMed] [Google Scholar]
- Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88(1–2):1–8. doi: 10.1016/s0165-5728(98)00040-x. [DOI] [PubMed] [Google Scholar]
- Cross AH, Manning PT, Stern MK, Misko TP. Evidence for the production of peroxynitrite in inflammatory CNS demyelination. J Neuroimmunol. 1997;80(1–2):121–30. doi: 10.1016/s0165-5728(97)00145-8. [DOI] [PubMed] [Google Scholar]
- Dalton D, Wittmer S. Nitric-oxide-dependent and independent mechanisms of protection from CNS inflammation during Th1-mediated autoimmunity: evidence from EAE in iNOS KO mice. J Neuroimmunol. 2005;160(1–2):110–121. doi: 10.1016/j.jneuroim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Date I, Takagi N, Takagi K, Tanonaka K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood-brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci Lett. 2006;407(2):141–145. doi: 10.1016/j.neulet.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Diemel LT, Copelman CA, Cuzner ML. Macrophages in CNS remyelination: friend or foe? Neurochemical Research. 1998;23:341–347. doi: 10.1023/a:1022405516630. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Washington R, Dragovic L. Expression of endothelial cell activation antigens in microvessels from patients with multiple sclerosis. Adv Exp Med Biol. 1993;331:243–8. doi: 10.1007/978-1-4615-2920-0_38. [DOI] [PubMed] [Google Scholar]
- Farias AS, de la Hoz C, Castro FR, Oliveira EC, Ribeiro dos Reis JR, Silva JS, Langone F, Santos LM. Nitric oxide and TNFalpha effects in experimental autoimmune encephalomyelitis demyelination. Neuroimmunomodulation. 2007;14(1):32–8. doi: 10.1159/000107286. [DOI] [PubMed] [Google Scholar]
- Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160(6):2940–6. [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hirase T, Staddon J, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110(14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161(9):4480–3. [PubMed] [Google Scholar]
- Hooper DC, Scott GS, Zborek A, Mikheeva T, Kean RB, Koprowski H, Spitsin SV. Uric acid, a peroxynitrite scavenger, inhibits CNS inflammation, blood-CNS barrier permeability changes, and tissue damage in a mouse model of multiple sclerosis. Faseb J. 2000;14(5):691–8. doi: 10.1096/fasebj.14.5.691. [DOI] [PubMed] [Google Scholar]
- Hurst RD, Fritz IB. Nitric oxide-induced perturbations in a cell culture model of the blood-brain barrier. J Cell Physiol. 1996;167(1):89–94. doi: 10.1002/(SICI)1097-4652(199604)167:1<89::AID-JCP10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kahl K, Schmidt H, Jung S, Sherman P, Toyka K, Zielasek J. Experimental autoimmune encephalomyelitis in mice with a targeted deletion of the inducible nitric oxide synthase gene: increased T-helper 1 response. Neurosci Lett. 2004;358(1):58–62. doi: 10.1016/j.neulet.2003.12.095. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Zielasek J, Uttenthal LO, Rodrigo J, Toyka KV, Schmidt HH. Protective role of the cytokine-inducible isoform of nitric oxide synthase induction and nitrosative stress in experimental autoimmune encephalomyelitis of the DA rat. J Neurosci Res. 2003;73(2):198–205. doi: 10.1002/jnr.10649. [DOI] [PubMed] [Google Scholar]
- Koprowski H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci U S A. 1993;90(7):3024–7. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kroncke K-D, Fehsel K, Suschek C, Kolb-Bachofen V. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. International Immunopharmacology. 2001;1(8):1407–1420. doi: 10.1016/s1567-5769(01)00087-x. [DOI] [PubMed] [Google Scholar]
- Lin L, Taktakishvili O, Talman W. Identification and localization of cell types that express endothelial and neuronal nitric oxide synthase in the rat nucleus tractus solitarii. Brain Res. 2007;1171:42–51. doi: 10.1016/j.brainres.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losy J, Niezgoda A, Wender M. Increased serum levels of soluble PECAM-1 in multiple sclerosis patients with brain gadolinium-enhancing lesions. J Neuroimmunol. 1999;99(2):169–72. doi: 10.1016/s0165-5728(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Lu W, Bhasin M, Tsirka S. Involvement of tissue plasminogen activator in both onset and effector phases of experimental allergic encephalomyelitis. J Neurosci. 2002;22(24):10781–10789. doi: 10.1523/JNEUROSCI.22-24-10781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CP, Cheeran MC, Palmquist JM, Hu S, Lokensgard JR. Microglia are the major cellular source of inducible nitric oxide synthase during experimental herpes encephalitis. J Neurovirol. 2008;14(3):229–38. doi: 10.1080/13550280802093927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Ohmori K, Fujiwara M. Microglial and astroglial reactions to inflammatory lesions of experimental autoimmune encephalomyelitis in the rat central nervous system. J Neuroimmunol. 1992;37(1–2):23–33. doi: 10.1016/0165-5728(92)90152-b. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Role of nitric oxide in disruption of the blood-brain barrier during acute hypertension. Brain Res. 1995;686(1):99–103. doi: 10.1016/0006-8993(95)00460-8. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Didion SP. Glutamate-induced disruption of the blood-brain barrier in rats. Role of nitric oxide. Stroke. 1996;27(5):965–9. doi: 10.1161/01.str.27.5.965. discussion 970. [DOI] [PubMed] [Google Scholar]
- Mitrovic B, Ignarro LJ, Montestruque S, Smoll A, Merrill JE. Nitric oxide as a potential pathological mechanism in demyelination: its differential effects on primary glial cells in vitro. Neuroscience. 1994;61(3):575–85. doi: 10.1016/0306-4522(94)90435-9. [DOI] [PubMed] [Google Scholar]
- O’Brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163(12):6841–7. [PubMed] [Google Scholar]
- Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Nitric oxide via an inducible isoform of nitric oxide synthase is a possible factor to eliminate inflammatory cells from the central nervous system of mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73(1–2):107–16. doi: 10.1016/s0165-5728(96)00194-4. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Aminoguanidine, a selective inhibitor of the inducible nitric oxide synthase, has different effects on experimental allergic encephalomyelitis in the induction and progression phase. J Neuroimmunol. 1998;81(1–2):201–10. doi: 10.1016/s0165-5728(97)00180-x. [DOI] [PubMed] [Google Scholar]
- Parathath S, Gravanis I, Tsirka S. Nitric oxide synthase isoforms undertake unique roles during excitotoxicity. Stroke. 2007;38(6):1938–45. doi: 10.1161/STROKEAHA.106.478826. [DOI] [PubMed] [Google Scholar]
- Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J Cell Sci. 2006;119(Pt 2):339–49. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004a;46(3):261–81. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Research Reviews. 2004b;46(3):261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. Eur J Immunol. 1994;24(6):1434–40. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28(6):1283–8. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93(23):13176–81. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin T, Kim S, Moon C, Wie M, Kim H. Aminoguanidine-induced amelioration of autoimmune encephalomyelitis is mediated by reduced expression of inducible nitric oxide synthase in the spinal cord. Immunol Invest. 2000;29(3):233–41. doi: 10.3109/08820130009060864. [DOI] [PubMed] [Google Scholar]
- Shukla A, Dikshit M, Srimal RC. Nitric oxide-dependent blood-brain barrier permeability alteration in the rat brain. Experientia. 1996;52(2):136–40. doi: 10.1007/BF01923358. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85(3):299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Stevens DB, Gould KE, Swanborg RH. Transforming growth factor-beta 1 inhibits tumor necrosis factor-alpha/lymphotoxin production and adoptive transfer of disease by effector cells of autoimmune encephalomyelitis. J Neuroimmunol. 1994;51(1):77–83. doi: 10.1016/0165-5728(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin Immunol Immunopathol. 1995;77(1):4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- Talbert DG. Raised venous pressure as a factor in multiple sclerosis. Med Hypotheses. 2008;70(6):1112–7. doi: 10.1016/j.mehy.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Teixeira SA, Castro GM, Papes F, Martins ML, Rogerio F, Langone F, Santos LM, Arruda P, de Nucci G, Muscara MN. Expression and activity of nitric oxide synthase isoforms in rat brain during the development of experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 2002;99(1):17–25. doi: 10.1016/s0169-328x(01)00341-2. [DOI] [PubMed] [Google Scholar]
- Thiel VE, Audus KL. Nitric oxide and blood-brain barrier integrity. Antioxid Redox Signal. 2001;3(2):273–8. doi: 10.1089/152308601300185223. [DOI] [PubMed] [Google Scholar]
- Tran EH, Hardin-Pouzet H, Verge G, Owens T. Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;74(1–2):121–9. doi: 10.1016/s0165-5728(96)00215-9. [DOI] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Man AHWK, Marquette C, Tilders FJ, Berkenbosch F. Appearance of inducible nitric oxide synthase in the rat central nervous system after rabies virus infection and during experimental allergic encephalomyelitis but not after peripheral administration of endotoxin. J Neurosci Res. 1995;40(2):251–60. doi: 10.1002/jnr.490400214. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland D, Lawrence D. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112(10):1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehntner S, Bourbonniere L, Hassan-Zahraee M, Tran E, Owens T. Bone marrow-derived versus parenchymal sources of inducible nitric oxide synthase in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;150(1–2):70–79. doi: 10.1016/j.jneuroim.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Zhao W, Tilton RG, Corbett JA, McDaniel ML, Misko TP, Williamson JR, Cross AH, Hickey WF. Experimental allergic encephalomyelitis in the rat is inhibited by aminoguanidine, an inhibitor of nitric oxide synthase. J Neuroimmunol. 1996;64(2):123–33. doi: 10.1016/0165-5728(95)00158-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Recruitment of microglia/macrophages into regions of demyelination. Double staining with FluoroMyelin and anti-Iba1 to image regions of demyelination and microglia/macrophage localization. A. WT day 15 section, low and high magnification. B. WT day 22 section. C. eNOS−/− day 22 section. The white boxes indicate the regions that have been magnified in the higher magnification images.