Abstract
Antibiotics are routinely used to control bacterial infection but the acquisition of acquired immunity following successful treatment has rarely been examined. We developed a model that allows visualization of acquired immunity during and following antibiotic treatment of typhoid. Pathogen-specific humoral and cellular immune responses were activated rapidly in antibiotic-treated mice but were not sustained after successful antibiotic treatment and did not confer protection to secondary infection. In marked contrast, pathogen-specific Th1 and antibody responses matured over several weeks following immunization with a live vaccine strain (LVS). The deficiency in protective immunity following antibiotic treatment could be overcome by administering flagellin during antibiotic therapy. Thus, development of protective immunity is hindered by rapid therapeutic elimination of bacteria but can be overcome by providing additional inflammatory and/or antigenic stimuli.
Keywords: T cells, Bacterial Infections, Memory, Rodent
Introduction
Live attenuated vaccines allow the transient colonization of an immunized host and induce robust humoral and cellular immune responses (1). Successful treatment of an active bacterial infection also results in transient colonization of the host, but it is unclear whether a similar protective response is induced. Induction of acquired immunity following successful antibiotic treatment is particularly important for individuals in endemic areas where re-infection is likely to occur and antibiotic therapy is widely used.
Human typhoid, caused by infection with Salmonella enterica serovar typhi (S. typhi) is a public health concern in many developing nations (2), and is also recognized as a potential bioterrorism agent in the US (3). S. typhi, does not infect other mammals (4), but several Salmonella serovars cause a typhoid-like disease in mice, sometimes referred to as murine typhoid (5). It has been demonstrated that immunization with a live vaccine strain (LVS) of Salmonella confers robust protective immunity to secondary infection in both murine and human typhoid (6, 7), and both CD4 Th1 cells and antibody are required (8, 9).
Much less is known about the induction of acquired immunity during successful treatment of bacterial infections, including typhoid. In theory, antibiotic treatment should liberate bacterial antigens and Pathogen Associated Molecular Patterns (PAMPs) from dead bacteria and therefore allow efficient activation of pathogen-specific T and B cell responses. However, antibiotic treatment of primary typhoid failed to induce significant immunity to secondary infection in a study of human volunteers (10). Other clinical studies indicate that acquired immunity after recovery from primary typhoid is insufficient to prevent re-infection. Relapse of primary infection is reported in 5–10% of typhoid patients and 1–4% can become long-term carriers of disease (11). Indeed, re-infection with a molecularly distinct strain of Salmonella has been reported in patients that have previously resolved typhoid (12). Overall, these clinical observations suggest that acquired immunity following clearance of primary typhoid may differ substantially from protective immunity induced by transient colonization with LVS-Salmonella.
We decided to examine this issue directly by generating a mouse model that allows visualization of Salmonella-specific immunity during and after resolution of typhoid with antibiotic therapy. These experiments demonstrate that antibiotic-mediated resolution of murine typhoid elicits a limited acquired immune response that is insufficient to protect against secondary infection. Although CD4 Th1 cells were efficiently activated during the early stage of antibiotic treatment, they failed to fully develop into an effective Th1 memory pool. In marked contrast, effective Th1 memory matured over a period of several weeks in mice administered LVS-Salmonella. However, the weak acquired immunity evident in antibiotic-treated mice could be markedly enhanced by administration of a TLR5 agonist during the period of antibiotic treatment.
Materials and Methods
Mouse strains
RAG-deficient SM1 TCR transgenic mice expressing the CD90.1 allele have been described (13, 14). C57BL/6 mice were purchased from NCI (Frederick, MD) and used at 6–12 weeks of age. IFN-γ-deficient, Rag-deficient, B cell-deficient (Igh-6tm1Cgn), and MHC class-II-deficient mice were purchased from The Jackson Laboratory. All mice were housed in specific pathogen-free conditions and cared for in accordance with Research Animal Resources (RAR) practices at the University of Minnesota.
Salmonella infection and antibiotic treatment
S. typhimurium strains BRD509 (AroA−AroD−) and SL1344 were grown overnight in Luria-Bertani broth without shaking and diluted in PBS after determining bacterial concentrations using a spectrophotometer. Mice were infected orally by gavage with 5×109 bacteria, immediately following administration of 100ul of a 5% NaHCO3 solution. In all infection experiments, the actual bacterial dose was confirmed by plating serial dilutions onto MacConkey’s agar plates and incubating overnight at 37°C. Mice infected with SL1344 were treated with Enrofloxacin (Baytril) at 2mg/ml in their drinking water for five weeks, beginning 2 days post-infection. Five days after antibiotic withdrawal, mice were re-challenged with 5×107 SL1344 and monitored daily for survival. When moribund, mice were euthanized by cervical dislocation as stipulated by our animal care protocol.
Bacterial colonization in vivo
Spleens and mesenteric lymph nodes from infected mice were removed and homogenized in Eagle’s Hanks Amino Acids (EHAA, Biofluids, Rockville, MD) containing 2% fetal bovine serum. Serial dilutions were plated on MacConkey’s agar plates, incubated overnight at 37°C, and bacterial counts calculated for each organ.
Adoptive transfer of SM1 T cells
Spleen and lymph nodes (cervical, axillary, brachial, inguinal, periaortic, and mesenteric) of RAG-deficient, CD90.1 congenic, SM1 TCR transgenic mice were harvested. After generating a single-cell suspension, the percentage of SM1 cells was determined using a small aliquot of this suspension and antibodies to CD4, CD90.1, and Vβ2 (eBioscience, San Diego, CA). A FACS Canto (BD Biosciences) was used to determine the percentage of CD4+ Vβ2+ SM1 cells, and the total number of SM1 cells calculated. SM1 cells were then incubated with CFSE at 37°C for 8 minutes with shaking every 2–3 minutes. Cells were washed two times in cold HBSS before adjusting the concentration and injecting 1–3×106 SM1 T cells into the lateral tail vein of recipient C57BL/6 mice.
Flow cytometry
A single-cell suspension was generated from harvested mouse spleens, mesenteric lymph nodes and Peyer’s Patches, and samples incubated on ice in the dark for 30 minutes in Fc block (spent culture supernatant from the 24G2 hybridoma, 2% rat serum, 2% mouse serum, and 0.01% sodium azide) containing primary antibodies. FITC-, PE-, PE-Cy5-, or APC-conjugated antibodies specific for CD4, CD11a, CD90.1, Vβ2, TNFα, and IFNγ were purchased from eBioscience and BD Bioscience. After staining, cells were analyzed by flow cytometry using a FACS Canto and data analyzed using FlowJo software (Tree Star, San Carlos, CA).
Tracking SM1 cells in vivo
After infection with Salmonella, spleens, mesenteric lymph nodes, and Peyer’s Patches were harvested on days 3, 5, 10, and 20 into EHAA medium containing 2% fetal bovine serum. Cells were stained as described above. The percentage of SM1 cells per organ was determined, as well as the activation and expansion of SM1 cells using the cell-surface marker CD11a and CFSE dye dilution, respectively, using flow cytometry.
Salmonella-specific antibody
Mice were infected with Salmonella as described above. Each week post-infection, for five weeks, mice were bled retro-orbitally and serum was prepared by centrifugation and collection of supernatant. In addition, stool was collected, weighed, and suspended at 10% weight/volume in a fecal diluent (10mM Tris, 100mM NaCl, 1mM CaCl2, 0.05% Tween 20, 5mM sodium azide, 1ug of aprotinin/ml, 1mM benzamidine, 10ug of leupeptin/ml, 10ug of pepstatin A/ml [pH 7.4]) (15) before centrifugation to remove fecal solids. Serum and processed stool samples were stored at −20°C before direct use in antibody ELISA. High protein binding plates were coated with heat-killed S. typhimurium diluted in 0.1M NaHCO3 and incubated overnight at 4°C. After incubation in Fc block for one hour at 37°C, plates were washed twice in PBS/0.05% Tween 20. Samples were added in serial dilutions, diluted in 10% FCS/PBS, and incubated for two hours at 37°C. Plates were washed four times before biotin-conjugated antibody specific for the desired isotype was added. After incubation for one hour at 37°C, plates were washed six times. Finally, plates were incubated for one hour at 37°C in alkaline phosphatase diluted in 10% FCS/PBS. Plates were washed eight times and a substrate containing sodium phosphate, citric acid, O-phenylenediamene, and H2O2 was added. After sufficient color-change was observed, 2N H2SO4 was added to stop the reaction before plates were analyzed using a spectrophotometer.
Detection of in vivo cytokine production
Mice were infected with Salmonella as described above. Each week post-infection, for six weeks, mice were injected i.v. with bacterial antigens to activate Salmonella-specific T cells (108 heat-killed S. typhimurium). Six hours later, spleens and mesenteric lymph nodes were harvested into EHAA containing 2% fetal bovine serum and a single-cell suspension generated. Following rapid surface staining on ice, cells were fixed with formaldehyde, permeabilized using saponin (Sigma-Aldrich), and stained intracellularly using cytokine-specific antibodies.
Flagellin immunization
Mice were infected with virulent S. typhimurium and treated with antibiotics as described above. On the first day of antibiotic treatment, mice were injected i.v. with 100ug of highly purified purified Salmonella flagellin (16). Injections were repeated each week for the duration of antibiotic treatment. After rechallenge with virulent S. typhimurium, as previously described, mice were monitored daily for signs of morbidity and euthanized when moribund.
Results
Effective treatment of murine typhoid with antibiotics
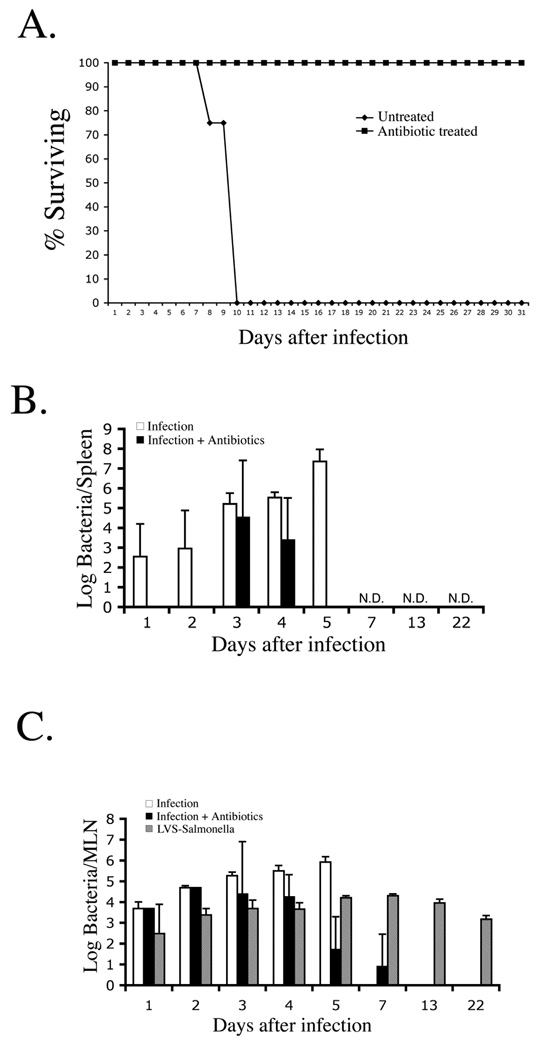
Ciprofloxacin is a fluoroquinolone antibiotic commonly used to treat human typhoid (17). We examined whether the veterinary fluoroquinolone derivative Enrofloxacin would allow resolution of fatal primary typhoid in C57BL/6 mice (18). Indeed, simply adding Enrofloxacin to the drinking water two days after oral infection allowed 100% of mice to survive a fatal infectious dose (Fig. 1A). Although Salmonella could not be detected in the spleen 72 hours after Enrofloxacin treatment (Fig. 1B, and data not shown), a much longer period of treatment was required to prevent recurrence of bacterial shedding following antibiotic withdrawal (data not shown). Similar reports of chronic bacterial shedding following apparent resolution of primary typhoid have been reported (19).
Figure 1. Enrofloxacin effectively resolves active murine typhoid.
C57BL/6 mice were orally infected with 5×109 virulent S. typhimurium (SL1344) and some mice treated with enrofloxacin drinking water. (A) Date show percent survival of antibiotic-treated and untreated mice and is representative of three similar experiments. (B) Spleens were harvested from infected mice at various time-points after infection and bacterial loads were determined by plating organ homogenates on MacConkey’s agar. All untreated infected mice were dead by day 7. Data show mean bacterial load +/− SD for 3–5 mice per time point. (N.D. = no bacteria detected in antibiotic-treated mice).
Recovery from primary infection confers limited protective immunity to re-infection
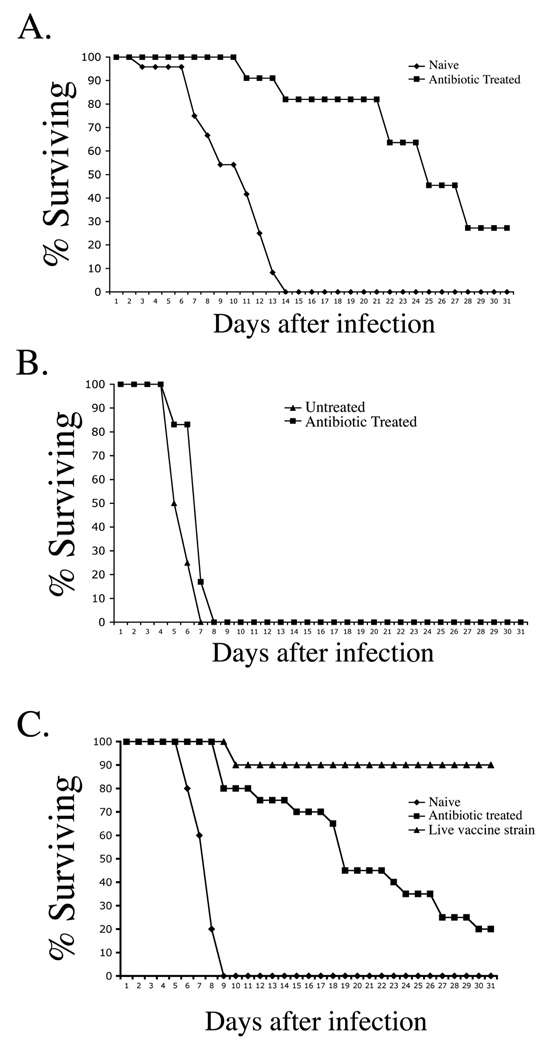
Mice that completely resolved primary infection using antibiotic treatment were re-infected to determine whether acquired immunity developed during this period of transient bacterial colonization. Indeed, naïve mice succumbed to infection with virulent Salmonella (Fig. 2A), whereas antibiotic-treated mice were protected against secondary infection, as demonstrated by increased survival time (Fig. 2A), and lower bacterial loads in the spleen and liver one week after secondary infection (data not shown). However, despite evidence of acquired immunity, almost all antibiotic-treated mice eventually succumbed to secondary typhoid (Fig. 2A), thus demonstrating that immunity following antibiotic treatment is incomplete. This weak protective immunity was absent in uninfected mice treated with antibiotics and therefore not attributable to residual antibiotics in tissues (Fig. 2B). When compared directly with effective LVS-Salmonella immunization, immunity in antibiotic-treated mice was considerably less efficient at protecting against secondary infection (Fig. 2C). Since the protective effect of LVS-Salmonella immunization correlated with a longer period of host colonization (Fig. 1C), it was of interest to determine whether antibiotic clearance of LVS-Salmonella would also result in incomplete acquired immunity. Indeed, treatment of mice immunized with LVS-Salmonella resulted in an inability of these mice to resolve secondary infection with virulent Salmonella (Fig. 3).
Figure 2. Incomplete protective immunity to typhoid in antibiotic-treated mice.
(A) C57BL/6 mice were orally infected with 5×109 virulent S. typhimurium and treated with enrofloxacin for 35 days to resolve primary infection. Five days after antibiotic withdrawal, these treated mice and a group of naïve C57BL/6 mice were orally infected with 5×107 virulent S. typhimurium. (B) Naïve C57BL/6 mice were administered enrofloxacin in drinking water for 30 days before oral infection with virulent S. typhimurium five days after antibiotic withdrawal (C) Some C57BL/6 mice were immunized orally with 1×1010 LVS-Salmonella (BRD509) while other mice were treated exactly as in A. Forty two days later, both group of mice were challenged orally with 5×107 virulent S. typhimurium. Graphs show percent survival of infected mice and are representative of 2–5 similar experiments.
Figure 3. Incomplete protective immunity in antibiotic-treated mice immunized with LVS Salmonella .
(A) C57BL/6 mice were orally infected with 5×109 LVS-Salmonella and some mice were treated with enrofloxacin for 35 days to resolve primary infection. Five days after antibiotic withdrawal, these mice and a group of naïve C57BL/6 mice were orally infected with 5×107 virulent S. typhimurium.
Acquired immunity following antibiotic therapy is mediated by CD4 T cells and antibody
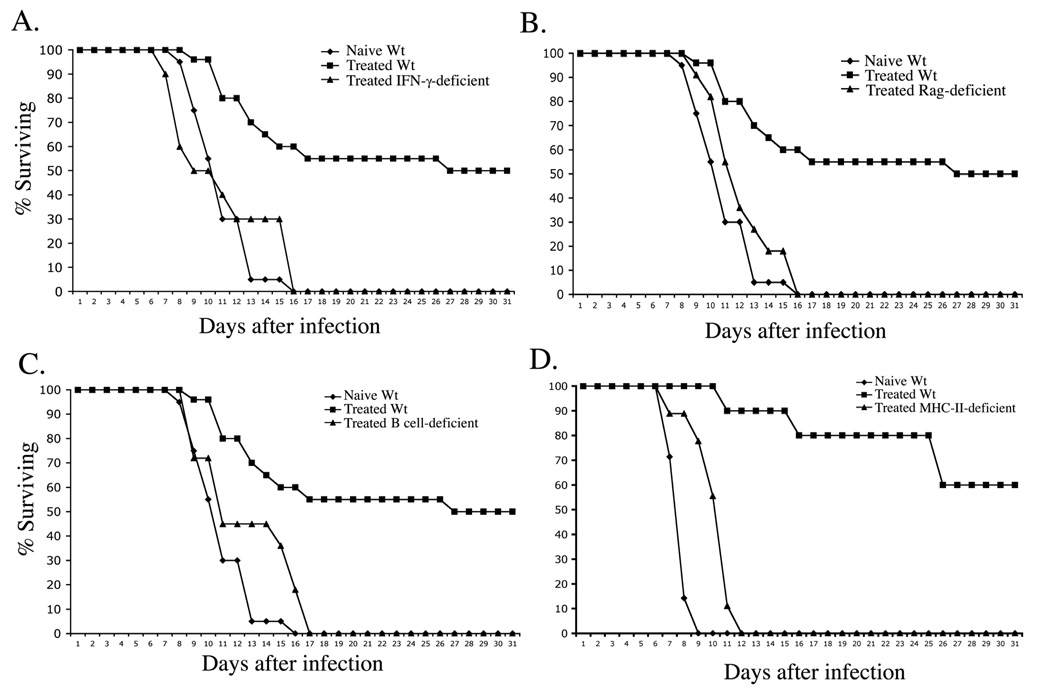
CD4 Th1 cells and antibody are required for protective immunity conferred by LVS-Salmonella immunization (8, 20, 21). We examined the basis of weak acquired immunity in antibiotic-treated mice by resolving primary infection in several gene-deficient strains and then examining secondary immunity to typhoid. As expected, naïve wild-type (Wt) mice succumbed rapidly to typhoid whereas antibiotic-treated Wt mice survived longer (Fig. 4). Interestingly, antibiotic-treated IFN-γ-deficient mice (Fig. 4A), Rag-2-deficient mice (Fig. 4B), B cell-deficient mice (Fig. 4C), or mice lacking CD4 T cells (Fig. 4D) displayed no evidence of acquired immunity to secondary infection, demonstrating that both Th1 cells and antibody are required for limited acquired immunity following antibiotic treatment of primary typhoid.
Figure 4. Acquired immunity after antibiotic therapy of typhoid requires class-II restricted T cells and antibody.
C57BL/6 (Wt) and, (A) IFN-γ-deficient, (B) Rag-deficient, (C) B cell deficient, or (D) MHC class-II deficient mice, were infected orally with 5×109 virulent S. typhimurium and treated with antibiotics in drinking water for 35 days. Antibiotic-treated and naïve Wt mice were re-infected orally with 5×107 virulent S. typhimurium. Graphs show the percent survival of infected mice and are representative of 2–3 similar experiments.
Early activation of pathogen-specific T cells in antibiotic-treated mice
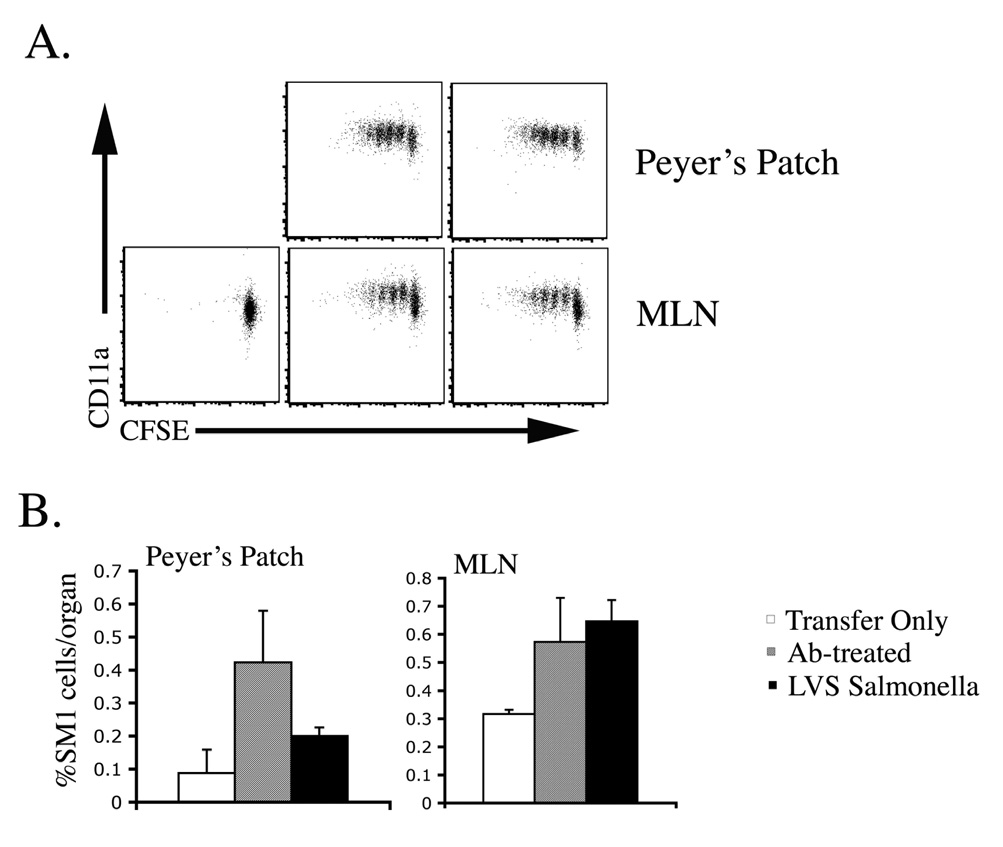
The failure of antibiotic-treated mice to resist secondary infection suggested a deficiency in the initial activation or development of Salmonella-specific CD4 T cells. We used Salmonella-specific SM1 T cells to compare the initial activation of Salmonella-specific CD4 T cells after infection and antibiotic treatment versus immunization with LVS-Salmonella. SM1 cells were activated to increase surface expression of CD11a, underwent several rounds of cell division, and accumulated in the Peyer’s patch and mesenteric lymph nodes (MLN) of mice administered LVS-Salmonella or treated with antibiotics to resolve primary typhoid (Fig. 5). Therefore, the initial activation of Salmonella-specific CD4 T cells was similar after oral immunization with LVS-Salmonella or antibiotic treatment of typhoid.
Figure 5. Rapid activation of Salmonella-specific CD4 T cells following antibiotic-cure or LVS-Salmonella immunization.
C57BL/6 mice were adoptively transferred with 2×106 CFSE-stained CD90.1+ SM1 T cells before oral infection with 5×109 LVS-Salmonella (BRD509) or oral infection with 5×109 virulent S. typhimurium (SL1344) followed by antibiotic treatment 2 days later. Three days after infection Peyer’s Patches and mesenteric lymph nodes (MLN) were harvested and SM1 T cells identified by flow cytometry staining for CD4 and CD90.1 (A) CD11a surface staining and CFSE dye dilution, and (B) the percentage of SM1 T cells from antibiotic-treated and LVS-Salmonella immunized mice.
Lack of sustained effector Th1 and antibody response following antibiotic treatment
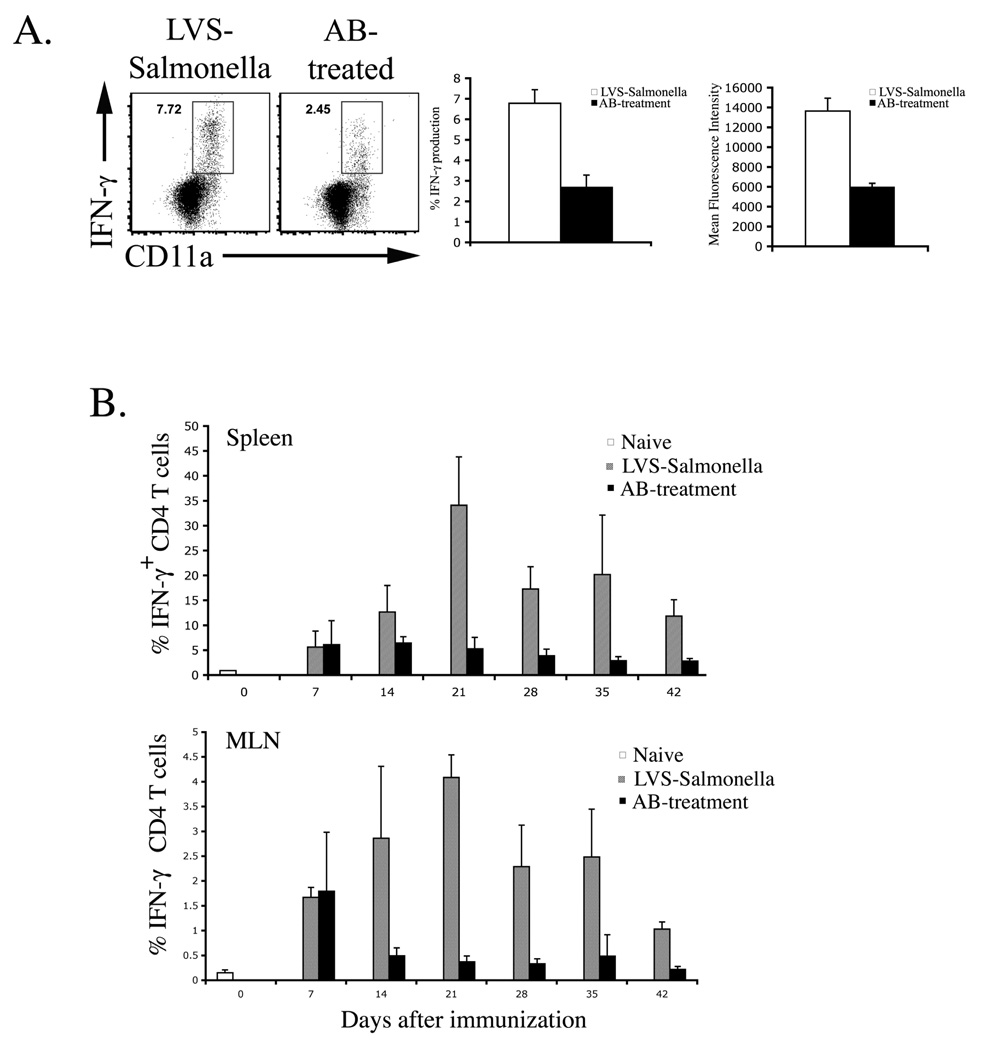
Next we examined the effector Th1 response at the time of secondary challenge. In mice immunized with LVS-Salmonella, a large proportion of spleen and mesenteric lymph node CD11aHi CD4 T cells produced IFN-γ in response to re-stimulation (Fig. 6A). In contrast, a lower percentage of Th1 cells was detected in antibiotic-treated mice (Fig. 6A). Furthermore, Th1 cells in antibiotic-treated mice produced less IFN-γ on a per cell basis than Th1 cells recovered from LVS-Salmonella immunized mice (Fig. 6A). Therefore, weak protective immunity in antibiotic treated mice correlates with a deficiency in the number and activity of effector/memory Th1 cells.
Figure 6. Deficient maturation of Th1 responses in antibiotic-treated mice.
C57BL/6 mice were immunized with LVS-Salmonella or orally infected with virulent S. typhimurium and treated with antibiotics for 35 days. (A) Forty-two days after immunization or infection, CD4 Th1 cell responses in the spleen were examined by re-stimulation with Salmonella lysate and direct ex-vivo detection of intracellular cytokine production. FACS plots show IFN-γ production by gated CD4 T cells in response to six hours of stimulation with Salmonella lysate. Bar graphs show (middle) mean percentage of IFN-γ+ CD4 T cells +/− SD, and (right) mean-fluorescence intensity +/− SD of IFN-γ producing cells from 4 mice per group. Data are representative of three similar experiments. (B) At weekly time points, CD4 Th1 responses were examined exactly as described in A. Bar graphs show mean percentage of IFN-γ+ CD4 T cells +/− SD in the spleen and MLN of 3–5 mice per group and are representative of 2 similar experiments.
Recent reports have suggested that CD4 T cells require sustained antigen presentation for maximal proliferation and development of effector function (22, 23). We therefore examined the maturation of effector/memory Th1 responses over several weeks in mice immunized with LVS-Salmonella and mice resolving primary typhoid with antibiotic therapy. There was no difference in CD4 IFN-γ production between antibiotic-treated and LVS-Salmonella mice at 1 week (Fig. 6B), in broad agreement with the detection of efficient early activation of SM1 cells in antibiotic-treated mice (Fig. 5), and suggest that initial priming conditions are similar in both models. However, at later time points, Th1 cell responses increased dramatically in LVS-immunized mice but actually declined in mice administered antibiotics (Fig. 6B). Together, these data demonstrate a remarkable difference in the development of Th1 cells in LVS-vaccinated and antibiotic-treated mice.
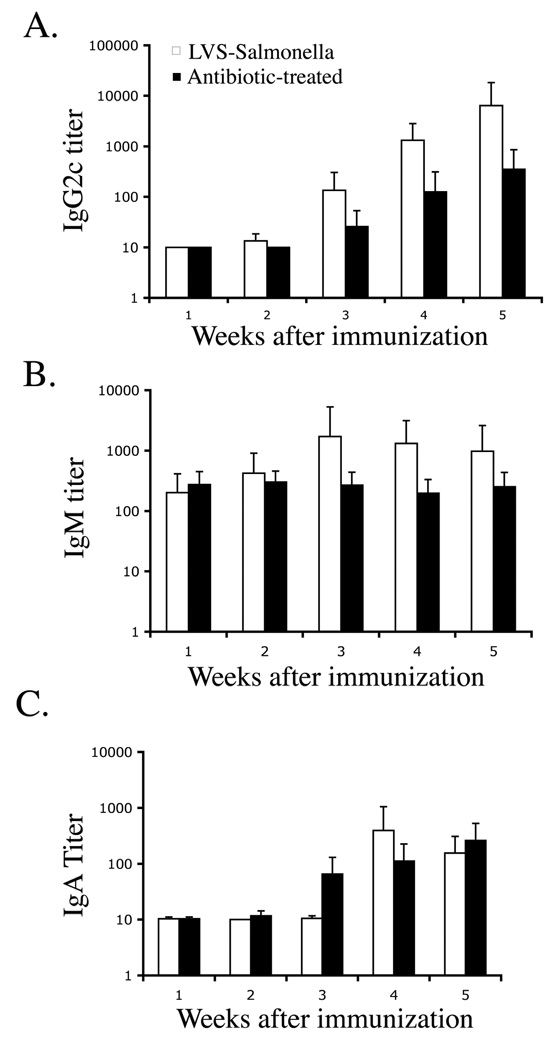
A similar deficiency was noted in the Salmonella-specific antibody response. Rising titers of Salmonella-specific IgG2c were detected in the serum of LVS-Salmonella immunized mice (Fig. 7A). A smaller increase in Salmonella-specific IgM (Fig. 7B) and fecal IgA (Fig. 7C) titers was detected, but there was no increase in IgG1 (data not shown). In antibiotic-treated mice, lower titers of Salmonella-specific IgG2c were detected and a significant IgM response was absent (Fig. 7A, B). However, Salmonella-specific fecal IgA responses developed normally in antibiotic treated mice (Fig. 7C), perhaps because intestinal IgA class switching can occur in the absence of CD4 T cell help (24). Together, these data demonstrate that resolution of primary typhoid with antibiotic therapy causes a deficiency in the maturation of Salmonella-specific CD4 Th1 and serum antibody responses.
Figure 7. Deficient development of Salmonella-specific serum antibody responses in antibiotic-treated mice.
C57BL/6 mice were immunized with LVS-Salmonella or orally infected with virulent S. typhimurium and treated with antibiotics for 35 days. (A–B) Blood was collected at weekly time points and Salmonella-specific antibody responses examined using isotype-specific antibody ELISAs (C) Stool was collected, weighed, suspended in a fecal diluent, and the presence of Salmonella-specific IgA determined using an antibody ELISA.
Recovery of protective immunity by co-administration of flagellin
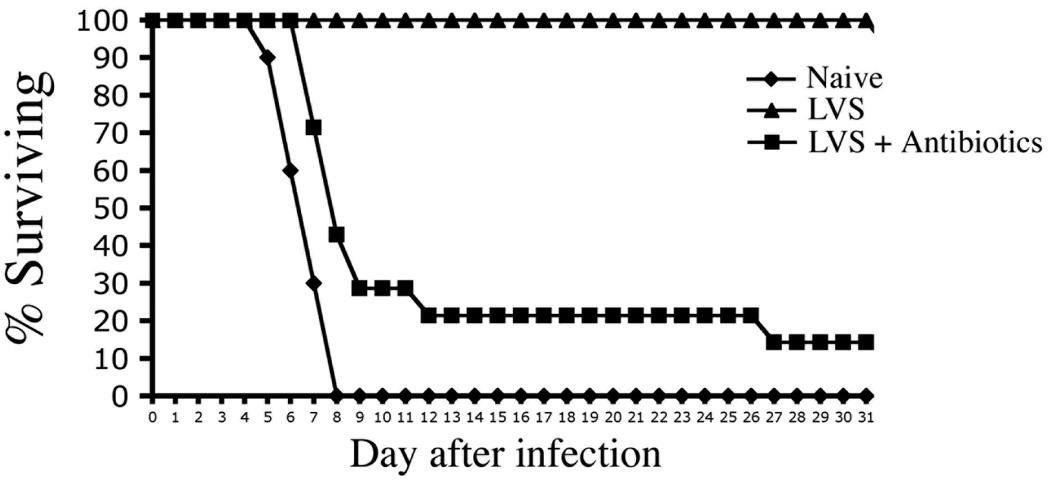
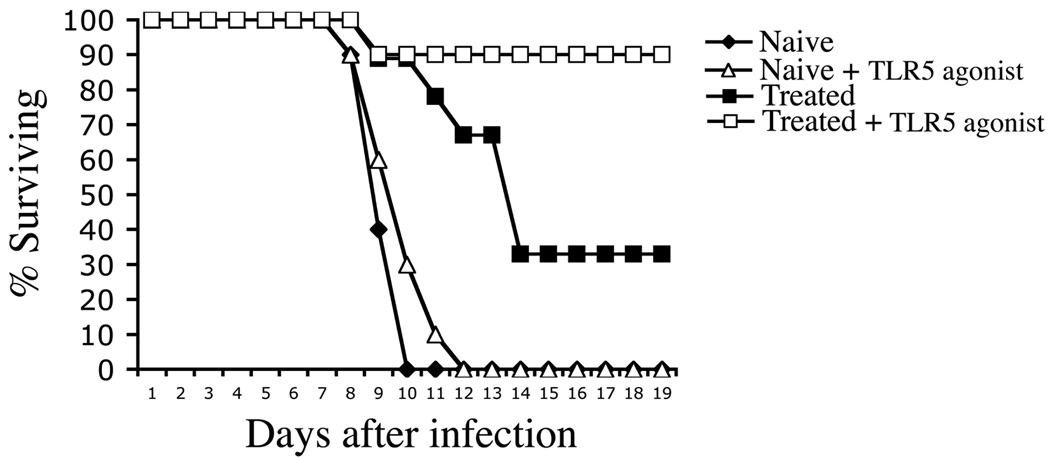
Incomplete development of Th1 and antibody responses following antibiotic treatment might be due to very rapid elimination of bacterial antigen and PAMPs, causing CD4 T cell to mature in the absence of sustained inflammation. Therefore, we examined whether acquired immunity could be enhanced by administration of the TLR5 agonist, flagellin, which has been shown to function as an effective adjuvant and is also an immunodominant antigen recognized by CD4 T cells (25). As expected, naïve wild-type (Wt) mice succumbed rapidly to infection but mice that had previously resolved typhoid using antibiotics survived longer (Fig. 8). Weekly administration of bacterial flagellin during the period of antibiotic treatment allowed mice that resolve primary infection to resist re-infection with typhoid (Fig. 8). This protective effect was not simply due to the immunizing effect of flagellin itself as flagellin administration failed to induce significant protective immunity in uninfected mice (Fig. 8). Thus, administration of a simple TLR5 agonist and antigen during the period of antibiotic therapy allowed recovery of robust protective immunity to typhoid.
Figure 8. Treatment with flagellin enhances protective immunity in antibiotic-treated mice.
C57BL/6 mice were orally infected with virulent S. typhimurium and treated with antibiotic drinking water for 35 days. Groups of infected and naïve mice were injected i.v. with 100 µg purified flagellin at weekly intervals during antibiotic treatment. Five days after antibiotic treatment was halted, naïve and antibiotic-treated mice were infected orally with virulent S. typhimurium. Data shows the percent survival of infected mice and is representative of 2 similar experiments.
Discussion
Immunization with live attenuated organisms can induce cellular and humoral immunity responses and effective protection against numerous vial and bacterial pathogens. One unique aspect of these live vaccines is that they involve a short period of host colonization with a weakened microbe. Much less is known about the development acquired immunity following antibiotic treatment of an infection with virulent bacteria, despite the fact that this is a common occurrence in endemic areas.
Unlike several other antibiotics (Griffin et al, unpublished), we found that Enrofloxacin is highly effective at clearing primary typhoid in the mouse model. Indeed, other investigators have recently used Enrofloxacin to resolve Salmonella infection in mice (26). Although Enrofloxacin rapidly eliminated bacteria from the spleen and liver, mice recommenced the shedding live bacteria in fecal pellets if treatment was withdrawn before 30 days of treatment. Under these circumstances spleens and livers were re-colonized by rapidly growing bacteria and all mice succumbed to recurrent infection (data not shown). The anatomical site where persistent Salmonella survive during treatment is not yet clear, but this issue is under investigation in our laboratory. If mice were treated with Enrofloxacin in drinking water for at least 30 days, no recurrent infection developed, no bacteria were shed in fecal pellets, and these mice survived in our animal facility for over 6 months. Prolonged Enrofloxacin treatment is therefore effective at resolving murine typhoid in highly susceptible mice.
Mice that fully resolved primary infection with antibiotic treatment displayed evidence of acquired immunity to re-infection in that they survived longer and had lower bacterial burdens. This protective immunity required production of IFN-γ and the presence of class-II-restricted T cells indicating an important protective role for Th1 cells, similar to the protective immunity induced by LVS-Salmonella (27–30). Our experiments also demonstrate a prominent role for B cells in resistance following antibiotic treatment, consistent with previous studies of immunity mediated by live attenuated Salmonella (31–33). However, it should be noted that B cell deficient mice have also been reposted to have a deficiency in the development of Salmonella-specific Th1 cells (32, 34), complicating interpretation of this particular experiment. The presence of specific antibody may simply reduce the initial challenge dose before intracellular infection takes place or prevent cell-to-cell transmission after macrophage apoptosis. However, a role for B cells and/or antibody in the enhancement of antigen presentation to CD4 T cells by dendritic cells has also been suggested (32, 34, 35).
Surprisingly, the adaptive immune response induced following antibiotic therapy was considerably less robust than LVS-Salmonella immunization and was insufficient to protect against re-infection. The weak response was not due to an intrinsic difference in virulent versus LVS strains since antibiotic treatment of LVS-immunization severely hindered the development of protective immunity. Failure to develop robust acquired immunity after antibiotic treatment is consistent with clinical reports suggesting weak protective immunity in typhoid endemic areas and the lack of protective immunity in studies using human volunteers (10–12). It is unlikely that incomplete immunity was due to a lack of antigen, since at the time of antibiotic administration bacterial loads are very high in the spleen and liver of infected mice. Furthermore, Salmonella-specific CD4 T cells were effectively activated and initially developed effector Th1 responses that were similar to mice immunized with LVS-Salmonella. These data suggest that the initial priming of bacterial-specific responses occurs normally in antibiotic treated-mice.
In marked contrast, we detected a deficiency in Th1 and antibody responses at later time points after antibiotic treatment. This immune deficiency correlated with the inability of these mice to resist secondary infection. We propose that the rapid elimination of bacterial antigen and PAMPs under antibiotic therapy disrupts the normal development of Th1 immunity that is detected in LVS-Salmonella immunized mice. Indeed, it has previously been noted that the full proliferative capacity and effector development of CD4 T cells requires prolonged antigen stimulation (22, 23). However, unlike these previous reports, our experiments have been completed in an infectious disease model that requires CD4 T cells for protective immunity. Together, our data indicate that an effective therapy that rapidly eliminates bacteria from the host may actually be detrimental to the subsequent acquisition of CD4-mediated protective immunity to secondary infection. If this is the case, it represents an important challenge to antibiotic therapy in endemic areas since successful treatment may therefore leave the individual susceptible to re-infection.
There are two aspects of bacterial persistence that might be required for the full development of CD4 responses. First, antigen persistence may allow pathogen-specific T cells to gradually acquire full effector function through sequential restimulation of responding clones. Second, the presence of prolonged inflammatory stimuli could provide non-TCR mediated maturation signals that allow Th1 development. Our data show that intervention with flagellin, which unusually happens to be an antigenic target of Salmonella-specific T cells and a TLR agonist, can rescue the development of effective protective immunity in antibiotic-treated mice. We are currently examining whether intervention with other adjuvants or Salmonella antigens would also have a similar effect. Addition of adjuvant delivery or therapeutic vaccination to antibiotic therapy may therefore be an important requirement for optimal development of CD4 Th1 responses and protection from secondary challenge. Therefore, these studies may aid the design of attenuated vaccines or the development of a novel therapy against bacterial infection.
Footnotes
This work was supported by grants from the National Institutes of Health AI055743 and AI073672.
References
- 1.Snowden MF. Emerging and reemerging diseases: a historical perspective. Immunol Rev. 2008;225:9–26. doi: 10.1111/j.1600-065X.2008.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhutta AZ. Current concepts in the diagnosis and treatment of typhoid fever. Bmj. 2006;333:78–82. doi: 10.1136/bmj.333.7558.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobel J, Khan AS, Swerdlow DL. Threat of a biological terrorist attack on the US food supply: the CDC perspective. Lancet. 2002;359:874–880. doi: 10.1016/S0140-6736(02)07947-3. [DOI] [PubMed] [Google Scholar]
- 4.Pier GB, Grout M, Zaidi T, Meluleni G, Mueschenborn SS, Banting G, Ratcliff R, Evans MJ, Colledge WH. Salmonella typhi uses CFTR to enter intestinal epithelial cells. Nature. 1998;393:79–82. doi: 10.1038/30006. [DOI] [PubMed] [Google Scholar]
- 5.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. Animal models of salmonella infections: enteritis versus typhoid fever. Micrtobes Infect. 2001;3:1335–1344. doi: 10.1016/s1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 6.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 7.Pasetti MF, Levine MM, Sztein MB. Animal models paving the way for clinical trials of attenuated Salmonella enterica serovar Typhi live oral vaccines and live vectors. Vaccine. 2003;21:401–418. doi: 10.1016/s0264-410x(02)00472-3. [DOI] [PubMed] [Google Scholar]
- 8.VanCott JL, Chatfield SN, Roberts M, Hone DM, Hohmann EL, Pascual DW, Yamamoto M, Kiyono H, McGhee JR. Regulation of host immune responses by modification of Salmonella virulence genes. Nat. Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 9.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 10.Dupont HL, Hornick RB, Snyder MJ, Dawkins AT, Heiner GG, Woodward TE. Studies of immunity in typhoid fever. Protection induced by killed oral antigens or by primary infection. Bull World Health Organ. 1971;44:667–672. [PMC free article] [PubMed] [Google Scholar]
- 11.Levine MM, Black RE, Lanata C. Precise estimation of the numbers of chronic carriers of Salmonella typhi in Santiago, Chile, an endemic area. J Infect Dis. 1982;146:724–726. doi: 10.1093/infdis/146.6.724. [DOI] [PubMed] [Google Scholar]
- 12.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. N Engl J Med. 2002;347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 13.McSorley SJ, Asch S, Costalonga M, Rieinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173:4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 15.O’Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar-Gonzalez RM, Srinivasan A, Griffin A, Muralimohan G, Ertelt JM, Ravindran R, Vella AT, McSorley SJ. Salmonella flagellin induces bystander activation of splenic dendritic cells and hinders bacterial replication in vivo. J Immunol. 2007;179:6169–6175. doi: 10.4049/jimmunol.179.9.6169. [DOI] [PubMed] [Google Scholar]
- 17.Crum FN. Current trends in typhoid Fever. Curr Gastroenterol Rep. 2003;5:279–286. doi: 10.1007/s11894-003-0064-0. [DOI] [PubMed] [Google Scholar]
- 18.Schroder J. Enrofloxacin: a new antimicrobial agent. J S Afr Vet Assoc. 1989;60:122–124. [PubMed] [Google Scholar]
- 19.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J. Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 21.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Bajenoff M, Wurtz O, Guerder S. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4+ T cells. J. Immunol. 2002;168:1723–1729. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 23.Obst RH, Santen M, van Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gewirtz TA. Flag in the crossroads: flagellin modulates innate and adaptive immunity. Curr Opin Gastroenterol. 2006;22:8–12. doi: 10.1097/01.mog.0000194791.59337.28. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, MacLennan IC. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–6207. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 27.Nauciel C. Role of CD4+ T cells and T-independent mechanisms in aquired resistance to Salmonella typhimurium infection. J. Immunol. 1990;145:1265–1269. [PubMed] [Google Scholar]
- 28.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Role of T cells, TNF alpha and IFN gamma in recall of immunity to oral challenge with virulent salmonellae in mice vaccinated with live attenuated aro- Salmonella vaccines. Microb Pathog. 1992;13:477–491. doi: 10.1016/0882-4010(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 29.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastroeni P, Harrison JA, Chabalgoity JA, Hormaeche CE. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(−/−) (B-cell-deficient) mice fail to mount solid aquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect. Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 34.Ugrinovic S, Menager N, Goh N, Mastroeni P. Characterization and development of T-Cell immune responses in B-cell-deficient (Igh-6(−/−)) mice with Salmonella enterica serovar Typhimurium infection. Infect Immun. 2003;71:6808–6819. doi: 10.1128/IAI.71.12.6808-6819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobar JA, Gonzalez PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fcgamma receptors on dendritic cells. J Immunol. 2004;173:4058–4065. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]