Abstract
The transcription factor STAT1 plays a role in promoting apoptotic cell death, whereas the related STAT3 transcription factor protects cardiac myocytes from ischemia/reperfusion (I/R) injury or oxidative stress. Cytokines belonging to the IL-6 family activate the JAK-STAT3 pathway, but also activate other cytoprotective pathways such as the MAPK-ERK or the PI3-AKT pathway. It is therefore unclear whether STAT3 is the only cytoprotective mediator against oxidative stress-induced cell death. Overexpression of STAT3 in primary neonatal rat ventricular myocytes (NRVM) protects against I/R-induced cell death. Moreover, a dominant negative STAT3 adenovirus (Ad ST3-DN) enhanced apoptotic cell death (81.2 ± 6.9%) compared to control infected NRVM (46.0 ± 3.1%) following I/R. Depletion of STAT3 sensitized cells to apoptotic cell death following oxidative stress. These results provide direct evidence for the role of STAT3 as a cytoprotective transcription factor in cells exposed to oxidative stress.
Keywords: STAT1, STAT3, Apoptosis, Ischemia, Myocardial infarction, Oxidative stress
Introduction
Apoptosis has become increasingly recognized as one mechanism of cell death during ischemia/reperfusion (I/R) injury in cultured cardiac myocytes and in the isolated intact heart [1–3]. While research into the underlying molecular mechanisms of I/R remains challenging there is great potential to uncover candidate targets for novel therapeutic intervention through elucidation of the precise signalling cascades that control cell fate following cardiac damage. One of the pathways which is instrumental in determining cell fate following the oxidative stress which occurs during I/R is the JAK-signal transducers and activators of transcription (STAT) factor pathway [4].
STAT activation is initiated following ligand binding to the extracellular domain of JAK receptors and autophosphorylation of the JAK cytoplasmic domain, creating a docking site for the SH2 domain of STATs [4]. This association causes JAKs to phosphorylate STATs at distinct tyrosine residues, allowing the bound STATs to be released from the receptor and undergo homo- or heterodimersation [4]. Phosphorylated STATs are then translocated to the nucleus where they bind specific sequences in the promoters of target genes leading to transcriptional regulation. Recent studies have shown that STATs can modulate the apoptotic programme in a variety of cell types [5]. For example, STAT1 deficient cells are resistant to tumour necrosis factor α-induced apoptotic cell death [6]. STAT1 also has tumour suppressor properties and is required to modulate cell cycle checkpoint response following DNA damage [7]. STAT1 can also function as a co-activator and modulate the transcriptional activity of p53 and p73 [8,9]. We have previously reported that overexpression of STAT1 enhances apoptotic cell death in cardiac myocytes exposed to simulated I/R [10,11]. In contrast to STAT1, the related STAT3 transcription factor has oncogenic properties and is overexpressed in many cancers [12,13]. In vitro studies have described cytoprotective actions of STAT3 activity following cytokine stimulation in cardiac cells exposed to I/R [14]. Additional in vivo studies have confirmed that hearts from transgenic mice expressing a constitutively active STAT3 are protected from I/R injury [15]. STAT3 is also required for ischaemic preconditioning in the brain [16], and liver [17], as well as in the heart [18].
Many of the in vitro studies of STAT3 relied on cytokines that activated the JAK-STAT3 pathway. However, these cytokines are also known to activate other cytoprotective pathways such as the MAPK-ERK and the PI3-AKT pathways. In the present study we examined whether genetically depleting STAT3 in the absence of cytokine stimulation compromises cytoprotection.
Materials and methods
All reagents were obtained from Sigma–Aldrich unless otherwise stated. STAT3 deficient mouse embryonic fibroblasts were a kind gift from Prof. Valeria Poli, University of Turin, Italy and were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (GIBCO) [19]. The p21 reporter construct was a kind gift from Dr. W. El-Deiry, University of Pennsylvania, Pennsylvania, USA and the Ly6E and STAT3 constructs were kind gifts from James Darnell, Rockefeller University, New York.
Neonatal rat ventricular cardiac myocyte culture. Neonatal rat ventricular cardiac myocytes (NRVM) were isolated from the hearts of 1- to 3-day-old Sprague Dawley rats as previously described [10].
Determination of cardiac myocyte viability and assesment of cell death by flow cytometry. Measurement of cell death by Trypan Blue was carried out as previously described [10]. Annexin V and 7AAD fluorescence was measured using a Becton Dickinson flow cytometer. Cell death is given as the combined percentage of cells positive for Annexin V (apoptotic cells) and 7AAD (necrotic cells).
Terminal dUTP nick end labelling (TUNEL). Labelling of 3′-hydroxyl ends of DNA fragments was performed using terminal deoxynucleotidyl transferase (TdT) and rhodamine conjugated nucleotides (Roche, Hertfordshire, UK). Cells were grown on 1% gelatin coated coverslips and transfected for 48 h with 0.25 μg pcDNA3 or STAT3 per well and co-transfected with 0.5 μg GFP. Following I/R injury, cells were washed in PBS and fixed for 10 min in 4% paraformaldehyde. The TUNEL assay was performed as previously described [10].
Luciferase reporter assays. Cells were seeded in 24 well plates and transfected with 0.5 μg luciferase reporter construct and 50 ng CMV-renilla per well. After 24 h, cells were lysed in passive lysis buffer (Promega) and luciferase activity was measured using the dual luciferase reporter system according to the manufacturer’s instructions. Relative luciferase activity was calculated as luciferase activity/renilla activity and normalised to controls.
Adenoviral infections. The dominant negative STAT3 and GFP adenoviruses were kind gifts from Prof. Brian Foxwell, Imperial College London, UK and the STAT3C adenovirus was a kind gift from Prof. Michitaka Ozaki, Okayama University, Japan. Recombinant viruses were propagated using human embryonic kidney 293 cells and purified through a cesium chloride gradient and PD-10 column (GE Healthcare). Viral titre was determined by plaque assay in 293 cells. Viral transduction was carried out by incubating cells at the indicated multiplicity of infection (MOI) in serum free medium, after 2 h cells were washed and fresh media added.
Quantitative real-time PCR (qPCR). One micro gram of RNA extracted from cardiac myocytes and cDNA was prepared using Superscript II (Invitrogen, Paisley, UK) and random hexamers (Promega, UK). qPCR was carried out using Platinum SYBR Green (Invitrogen, Paisley, UK) on the DNA Engine Opticon system (MJ Research, Waltham, MA). HPRT, Actin and GAPDH were used together as normalising genes and for each experiment both target and normalising gene PCR efficiency was firstly determined to ensure normalizing genes were acceptable. To test primer efficiency, qPCR was carried out on a 2-fold dilution series from a pooled set of cDNA and the threshold Ct value was plotted against the log cDNA dilution. Efficiency was then calculated using the equation m = (−1/log E), where m is the slope of the line and E is the efficiency and primer pairs were used only if the PCR efficiency of the normalising and control genes were found to be within 10% of each other. Expression changes were claculated using the 2−ΔΔCt method and expressed as fold change over control [20]. Specific primers were designed with the aid of CloneWorks and the Ensembl database and are shown below.
| Primer | Primer sequence |
|---|---|
| Socs3 forward | 5’-TGGTCACCCACAGCAAGTTT-3’ |
| Socs3 reverse | 5’-ACCAGCTTGAGTACACAGTC-3’ |
| c-Fos forward | 5’-GCCTTTCCTACTACCATTCC-3’ |
| c-Fos reverse | 5’-CCGTTTCTCTTCCTCTTCAG-3’ |
| Hprt forward | 5’-CTCATGGACTGATTATGGACAGGAC-3’ |
| Hprt reverse | 5’-GCAGGTCAGCAAAGAACTTATAGCC-3’ |
| Actin forward | 5’-AGATGACCCAGATCATGTTTGAG-3’ |
| Actin reverse | 5’-AGGTCCAGACGCAGGATG-3’ |
| Gapdh forward | 5’-GTGTGAACGGATTTGGCCG-3’ |
| Gapdh reverse | 5’-CCAGTAGACTCCACGACATA-3’ |
Western blot. Antibodies against the phosphorylated pSTAT3Y705 and pSTAT3S727 were purchased from Cell Signaling Technology, MA, USA, pSTAT1Y701 was from Zymed, CA, USA, pSTAT1S727 was from Upstate, Cambridge, UK, caspase-9 was from Santa Cruz Biotechnology, CA, USA and GAPDH was from Chemicon, CA, USA. Horseradish peroxidase-conjugated secondary antibodies were from Dako (Glostrup, Denmark).
Statistical analysis. Data are expressed as means ± SEM, all experiments were repeated in triplicate. Statistical analysis was carried out using a Student’s t-test, one-way ANOVA with Dunnet’s post or two-way ANOVA with Bonferroni post test, p-values of less than 0.05 were considered significant.
Results
Overexpression of wild type STAT3 protects against cell death in cardiac myocytes exposed to I/R whereas, a STAT3Y705F mutant enhances I/R-induced cell death
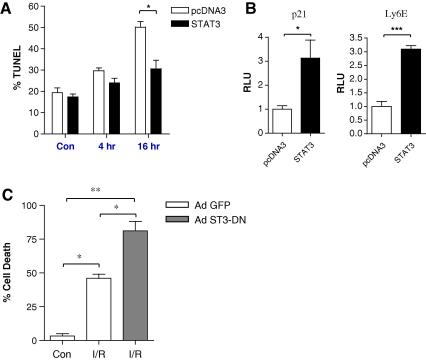
To determine whether STAT3 has a direct role in modulating the levels of cell death, we carried out transient transfection experiments in primary neonatal rat ventricular myocytes (NRVM) exposed to I/R injury. As shown in Fig. 1A transient expression of wild type STAT3 significantly reduced the levels of apoptotic cell death from 50 ± 5% to 31 ± 7% in NRVM exposed to I/R. This suggests that STAT3 may indeed function as a cytoprotective factor in cells subjected to oxidative stress. In order to show and confirm that transfection and overexpression of STAT3 allows it to mediate its transcriptional activity we also carried out reporter luciferase assays using two well characterised STAT3 target promoters, p21 and Ly6E [21,22]. In both cases transient expression of STAT3 induced a 3-fold increase of p21 and Ly6E promoter activity in NRVM (Fig. 1B), thus confirming that STAT3 is transcriptionally active when overexpressed in NRVM.
Fig. 1.
STAT3 protects cardiac myocytes from I/R injury. (A) Cardiac myocytes were transfected with 0.5 μg GFP and 0.25 μg STAT3-pcDNA3 or pcDNA3 empty vector for 48 h and subjected to I/R injury for the indicated times. Cell death was measured using TUNEL, a minimum of 200 GFP positive cells were counted per experiment. Experiments were repeated in triplicate, and statistical analysis was carried out using a 2-way ANOVA with a Bonferroni post test, ∗p < 0.05. (B) STAT3 is transcriptionally active in neonatal rat ventricular myocytes; 0.5 μg of the p21 or Ly6E promoter construct and 50 ng CMV-renilla were transfected into cardiac myocytes with 0.5 μg STAT3 or pcDNA3. Luciferase activity was measured after 48 h and expressed as relative luciferase units (RLU) normalised to 1. ∗p < 0.05, ∗∗∗p < 0.001, Student’s t-test, n = 3, experiment repeated in triplicate. (C) Dominant negative STAT3 increases I/R-induced cell death in cardiac myocytes. Cardiac myocytes were transduced for 48 h with control Ad GFP or Ad STAT3-DN at MOI = 100. Cells were then subjected to 4 h ischaemia and 16 h reperfusion and cell death was measured using annexin V and 7AAD staining, ∗p < 0.05, ∗∗p < 0.01, one way ANOVA with Bonferroni post test, n = 3 per group, average of two experiments.
To further clarify the role of STAT3 in NRVM following I/R injury, we utilized a mutant STAT3 adenovirus in which the tyrosine (Y705) was mutated to a phenylalanine (F705). This mutant has been shown to act as a dominant negative (ST3-DN) [23]. Importantly, this approach using an adenoviral STAT3Y705F allows us to transduce > 90% of NRVM and as indicated in Fig. 1C, ST3-DN enhanced significantly the effects of I/R-induced death to 81.2 ± 6.9% compared with control (GFP) infected cells 46.0 ± 3.1%. This data provides further evidence that a fully transcriptionally active STAT3 that requiring the 705 tyrosine phosphorylation site is necessary for its cytoprotective properties.
Deletion of STAT3 sensitizes cells to cell death
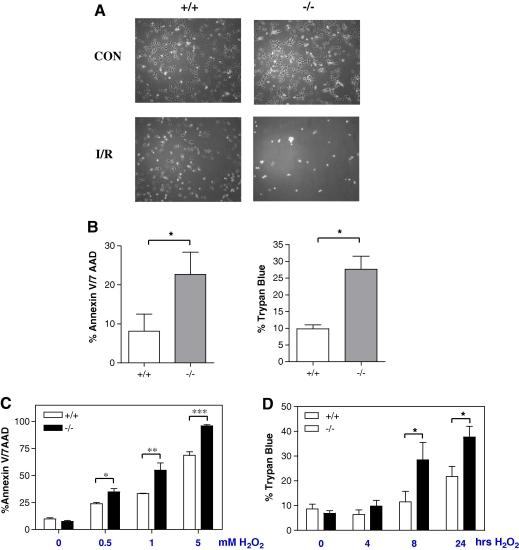
While overexpression using a variety of methods is useful to examine the effects of factors on cell fate, it may lead to spurious results due to the non-physiological levels produced using such protocols. Since STAT3 deficient mice are not viable [24], we therefore, utilized STAT3 deficient mouse embryonic fibroblasts (MEF) to further examine the role of STAT3 in MEF cells exposed to I/R injury. Interestingly, as indicated in Fig. 2A, STAT3 deficient MEF cells were very sensitive to I/R injury compared to wild type MEF cells with very few STAT3 deficient MEFs (ST3−/−) remaining attached to the culture dishes following 4 h of simulated ischaemia and 16 h of reperfusion (Fig. 2A lower panel). We therefore, reduced the simulated ischaemic period from 4 h to 2 h and the simulated reperfusion period from 16 h to 1 h and once again as shown in Fig. 2B, there was significantly enhanced cell death in the ST3−/− MEF compared to wild type MEF ST3+/+ cells. Since MEF cells are not usually exposed physiologically to I/R we instead subjected MEF cells to H2O2 as a model of oxidative stress. Once again, ST3−/− cells were significantly more sensitive to cell death than ST3+/+ MEF cells in a dose and time dependent manner (Fig. 2C and D). These data are further evidence that STAT3 deficiency renders cells more susceptible to oxidative stress.
Fig. 2.
STAT3−/− MEFs are highly sensitive to H/R damage. (A) STAT3+/+ and STAT3−/− MEFs were subjected to 4 h ischaemia and 16 h reperfusion and visualised under light microscopy. (B) MEFs were subjected to 2 h ischaemia followed by 1 h reperfusion and cell death was quantified by annexin V/7AAD staining (left) and trypan blue uptake (right) ∗p < 0.05, Student’s t-test, n = 3 per group, average of three experiments (C) STAT3+/+ and STAT3−/− MEFs were treated with indicated concentrations of H2O2 for 24 h and cell death was assessed by annexin V/7AAD staining. (D) STAT3+/+ and STAT3−/− MEFs were treated with 0.5 mM H2O2 for the indicated times and cell death was measured by trypan blue uptake. For C and D, statistical analysis was carried out using a 2-way ANOVA with Bonferroni post test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n = 3 per group, experiment repeated in triplicate.
Activated STAT3 is induced following I/R
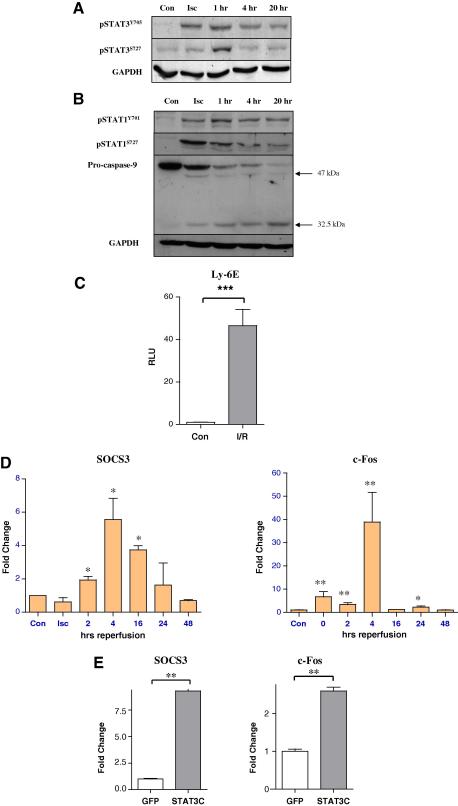
The above data demonstrates that exogenous levels of STAT3 are cytoprotective in cardiac myocytes exposed to I/R injury. However, it is unclear whether endogenous STAT3, phospho-pSTAT3Y705 or pSTAT3S727 is activated in NRVM during I/R. As shown in Fig. 3A, pSTAT3Y705 levels increased following 4 h of ischaemia peaked at 1 h of reperfusion. The levels of pSTAT3Y705 remained elevated even after 20 h of reperfusion compared to control resting cells. The levels of pSTAT3S727 also increased following 4 h of ischaemia and again peaked at the 1 h reperfusion time point, but unlike pSTAT3Y705, pSTAT3S727 returned to control levels thereafter. As previously reported in the isolated intact heart [10], STAT1 phosphorylation of pSTAT1Y701 was also enhanced during ischaemia and remained elevated 20 h after reperfusion in NRVM (Fig. 3B) and this was also associated with increased apoptotic activity as assessed by the processing and cleavage of procaspase-9. In contrast, pSTAT1S727 was increased during ischaemia, but decreased over time following reperfusion (Fig. 3B).
Fig. 3.
Activation of STAT3 following I/R injury. NRVMs were subjected to 4 h ischaemia or ischaemia plus the indicated times of reperfusion and lysates were subjected to Western blotting using antibodies for (A) tyrosine and serine phosphorylated STAT3 or (B) tyrosine and serine phosphorylated STAT1 and caspase-9. GAPDH was used as a loading control. (C) STAT3 dependent luciferase reporter activity is enhanced by I/R injury. 1 μg of Ly6E luciferase reporter and 0.1 μg CMV-renilla were transfected into NRVMs in 6-well plates for 24 h. Cells were then subjected to 4 h ischaemia and 24 h reperfusion (I/R) and luciferase activity was measured ∗∗∗p < 0.001, n = 3, experiment repeated in duplicate. (D) STAT3 dependent gene expression is increased by I/R injury. NRVMs were subjected to a time course of I/R injury and the levels of SOCS3 and c-Fos were measured by qPCR and normalised to control (con) levels. Isc = Ischaemia alone without reperfusion. Statistical analysis was carried out using a one-way ANOVA with Dunnett’s post test, n = 3 samples, experiments repeated in triplicate, ∗p < 0.05, ∗∗p < 0.01 compared to con. (E) NRMVs were transduced with STAT3C adenovirus at MOI = 100. After 24 h, the expression of SOCS3 and c-fos and was examined by qPCR. ∗∗p < 0.01, Student’s t-test, n = 3 per group, repeated in duplicate.
To confirm that endogenous STAT3 activity was enhanced following I/R, we carried out our Ly6E STAT3 responsive reporter assay and showed that there was a significant increase (40-fold) in Ly6E promoter activity in NRVM cells exposed to I/R (Fig. 3C). Furthermore we also demonstrate that two well known STAT3 responsive genes SOCS3 [25] and c-Fos [26] were also upregulated in NRVM cells exposed to I/R as assessed by qPCR (Fig. 3D). As a positive control we also transduced NRVM with a constitutively active STAT3 adenovirus (Ad-STAT3C) [27] and showed that enhanced levels of STAT3 activity was also associated with upregulation of SOCS3 and c-Fos mRNAs (Fig. 3E). Taken together, these data strongly suggests that endogenous STAT3 does indeed become activated and induces a transcriptional programme that may modulate the levels of I/R-cell death in cardiac cells.
Discussion
Myocardial infarction or I/R injury results from occlusion of blood flow in the left ventricle via the coronary arteries, leading to cardiac damage, cell death and eventually loss of cardiac output. The molecular mechanism that mediates myocardial damage following I/R are not fully understood. STAT transcription factors have been reported to play a prominent role in cell fate following various stresses including I/R [5,10,11]. Our present in vitro data demonstrates that STAT3 plays a cytoprotective role in cardiac cells exposed to I/R injury and oxidative stress and that depleting STAT3 in the absence of cytokine stimulation compromises cytoprotection.
Recently we showed that the levels of phosphorylated STAT1 and STAT3 are significantly modulated in the infarcted area in the in vivo rat myocardium exposed to I/R injury [28]. Thus, the free radical scavenger tempol reduced STAT1 and STAT3 phosphorylation and reduced the infarct size following I/R [28], suggesting that activation of STAT1 and STAT3 within the infarct area may contribute to cardiac remodelling. Also free radical production following I/R injury may be a trigger for distinct kinases that are responsible for STAT1 or SATAT3 phosphorylation.
STAT1 overexpression has been shown to induce apoptosis in cardiac myocytes after I/R injury in vitro, [11]. Our previous in vivo studies also showed both STAT1 and STAT3 phosphorylation is enhanced but with different time kinetics [28]. Although it was observed that there was less overall activated STAT3 in the infarcted heart, it may still counteract the effects of activated STAT1 and reduce the degree of damage. This was further highlighted following IFN-γ treatment, a potent activator of STAT1, which exacerbated cardiac damage as evidenced by increased infarct size [28]. Moreover, IFN-γ-induced STAT1 activation during reperfusion and significantly reduced the cardioprotective effect of tempol, indicating that this antioxidant’s anti-apoptotic effect is likely to be mediated partly through reduction in activated STAT1 [28]. Since we previously showed that the cardioprotective affects of epigallocatechin-3-gallate (EGCG), a natural antioxidant found in green tea, were partly mediated by inhibition of STAT1 activity [29], this highlights the role of STAT1 as a general molecular target for antioxidants in the ischemic myocardium.
Although total STAT3 deletion is embryonic lethal, these mice had developmental heart defects also suggesting an important role for STAT3 in cardiac development [24]. Generation of cardiac-specific STAT3 knock outs further highlighted the important role of STAT3 in the heart. These mice suffer from decreased left ventricular capillary density and showed symptoms of heart failure [25,26]. Furthermore these mice were more susceptible to I/R-induced cardiac damage and had larger infarct sizes and increased myocyte apoptotic cell death [30,31].
Granulocyte-colony stimulating factor (G-CSF) has been reported to confer cardioprotection, reduced infarct size and inhibited apoptotic cell death. These effects of G-CSF were abolished by in mice expressing an inactive STAT3 in cardiac myocytes, suggesting that the cardioptotective effects are mediated via G-CSF-JAK-STAT3 pathway [32]. Thus, these data are further evidence that STAT3 has cytoprotective properties and in particular highlight a role for STAT3 in cardioprotection.
Acknowledgment
S.B is a Ph.D. student funded by the British Heart Foundation, London, UK.
References
- 1.Scarabelli T.M., Stephanou A., Pasini E., Comini L., Raddino R., Knight R.A., Latchman D.S. Different signalling pathways induce apoptosis in endothelial cells and cardiac myocytes during ischaemia/reperfusion injury. Circ. Res. 2002;90:745–748. doi: 10.1161/01.res.0000015224.07870.9a. [DOI] [PubMed] [Google Scholar]
- 2.Stephanou A., Brar B.K., Scarabelli T.M., Knight R.A., Latchman D.S. Distinct initiator caspases are required for the induction of apoptosis in cardiac myocytes during ischaemia versus reperfusion injury. Cell Death Differ. 2001;8:434–435. doi: 10.1038/sj.cdd.4400846. [DOI] [PubMed] [Google Scholar]
- 3.Yaoita H., Ogawa K., Maehara K., Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998;97:276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- 4.Levy D.E., Darnell J.E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 5.Stephanou A., Latchman D.S. Opposing actions of STAT1 and STAT-3. Growth Factor. 2005;23:177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Commane M., Flickinger T.W., Horvath C.M., Stark G.R. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 7.Townsend P.A., McComick J.M., Barry S.B., Lawrence K.M., Knight R.A., Hubank M., Chen P.-L., Latchman D.S., Stephanou A. STAT-1 facilitates the ATM activated checkpoint pathway following DNA damage. J. Cell Sci. 2005;118:1629–1639. doi: 10.1242/jcs.01728. [DOI] [PubMed] [Google Scholar]
- 8.Townsend P.A., Davidson S., Scarabelli T.M., Knight R.A., Latchman D.S., Stephanou A. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J. Biol. Chem. 2004;297:5811–5819. doi: 10.1074/jbc.M302637200. [DOI] [PubMed] [Google Scholar]
- 9.Soond S.M., Carroll C., Townsend P.A., Sayan E., Melino G., Behrmann I., Knight R.A., Latchman D.S., Stephanou A. STAT1 regulates p73-mediated Bax expression. FEBS Lett. 2007;581:1217–1226. doi: 10.1016/j.febslet.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Stephanou A., Brar B.K., Knight R.A., Marber M.S., Yellon D., Latchman D.S. Ischaemia-induced STAT-1 expression and activation plays a critical role in cardiac myocyte apoptosis. J. Biol. Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 11.Stephanou A., Brar B.K., Scarabelli T.M., Nakanishi Y., Matsumura M., Knight R.A., Latchman D.S. Induction of apoptosis and Fas receptor/Fas ligand expression by ischaemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J. Biol. Chem. 2001;276:28340–28347. doi: 10.1074/jbc.M101177200. [DOI] [PubMed] [Google Scholar]
- 12.Turkson J., Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 13.Buettner R., Mora L.B., Jove R. STAT Activated signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 14.Negoro S., Kunisada K., Fujio Y., Funamoto M., Darville M.I., Eizirik D.L., Osugi T., Izumi M., Oshima Y., Nakaoka Y., Hirota H., Kishimoto T., Yamauchi-Takihara K. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation. 2001;104:979–981. doi: 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- 15.Osugi T., Oshima Y., Fujio Y., Funamoto M., Yamashita A., Negoro S., Kunisada K., Izumi M., Nakaoka Y., Hirota H., Okabe M., Yamauchi-Takihara K., Kawase I., Kishimoto T. Cardiac-specific activation of signal transducer, activator of transcription 3 promotes vascular formation in the heart. J. Biol. Chem. 2002;277:6676–6681. doi: 10.1074/jbc.M108246200. [DOI] [PubMed] [Google Scholar]
- 16.Kim E.J., Raval A.P., Perez-Pinzon M.A. Preconditioning mediated by sublethal oxygen–glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J. Cereb. Blood Flow Metab. 2008;28:1329–1340. doi: 10.1038/jcbfm.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iñiguez M., Berasain C., Martinez-Ansó E., Bustos M., Fortes P., Pennica D., Avila M.A., Prieto J. Cardiotrophin-1 defends the liver against ischemia–reperfusion injury and mediates the protective effect of ischemic preconditioning. J. Exp. Med. 2006;203:2809–2815. doi: 10.1084/jem.20061421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith R.M., Suleman N., Lacerda L., Opie L.H., Akira S., Chien K.R., Sack M.N. Genetic depletion of cardiac myocyte STAT-3 abolishes classical preconditioning. Cardiovasc. Res. 2004;63:611–616. doi: 10.1016/j.cardiores.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Costa-Pereira A.P., Tininini S., Strobl B., Alonzi T., Schlaak J.F., Is’harc H., Gesualdo I., Newman S.J., Kerr I.M., Poli V. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc. Natl. Acad. Sci. USA. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Bellido T., O’Brien C.A., Roberson P.K., Manolagas S.C. Transcriptional activation of the P21(WAF1, CIP1, SDI1) gene by interleukin-6 type cytokines. A prerequisite for their pro-differentiating and anti-apoptotic effects on human osteoblastic cells. J. Biol. Chem. 1998;273:21137–21144. doi: 10.1074/jbc.273.33.21137. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J.J., Zhao Y., Chait B.T., Lathem W.W., Ritzi M., Knippers R., Darnell J.E., Jr. Ser727-dependent recruitment of MCM5 by Stat1alpha in IFN-gamma-induced transcriptional activation. EMBO J. 1998;17:6963–6971. doi: 10.1093/emboj/17.23.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams L., Bradley L., Smith A., Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J. Immunol. 2004;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K., Noguchi K., Shi W., Tanaka T., Matsumoto M., Yoshida N., Kishimoto T., Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasukawa H., Hoshijima M., Gu Y., Nakamura T., Pradervand S., Hanada T., Hanakawa T., Yoshimura A., Ross J., Jr., Chien K.R. Suppressor of cytokine signaling-3 is a biomechanical stress-inducible gene that suppresses gp130-mediated cardiac myocyte hypertrophy and survival pathways. J. Clin. Invest. 2001;108:1459–1467. doi: 10.1172/JCI13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang E., Lerner L., Besser D., Darnell J.E., Jr. Independent and cooperative activation of chromosomal c-fos promoter by STAT3. J. Biol. Chem. 2003;278:15794–15799. doi: 10.1074/jbc.M213073200. [DOI] [PubMed] [Google Scholar]
- 27.Haga S., Terui K., Zhang H.Q., Enosawa S., Ogawa W., Inoue H., Okuyama T., Takeda K., Akira S., Ogino T., Irani K., Ozaki M. STAT3 protects against Fas-induced liver injury by redox-dependent and –independent mechanisms. J. Clin. Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick J.M., Barry S.P., Sivarajah A., Stefanutti G., Townsend P.A., Lawrence K.M., Eaton S., Knight R.A., Thiemermann C., Latchman D.S., Stephanou A. Free radical scavenging inhibits STAT phosphorylation following in vivo ischemia/reperfusion injury. FASEB J. 2006;20:2115–2117. doi: 10.1096/fj.06-6188fje. [DOI] [PubMed] [Google Scholar]
- 29.Townsend P.A., Scarabelli T.M., Pasini E., Gitti G., Menegazzi M., Suzuki H., Knight R.A., Latchman D.S., Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–1623. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 30.Jacoby J.J., Kalinowski A., Liu M.G., Zhang S.S., Gao Q., Chai G.X., Ji L., Iwamoto Y., Li E., Schneider M., Russell K.S., Fu X.Y. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc. Natl. Acad. Sci. USA. 2003;100:12929–12934. doi: 10.1073/pnas.2134694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilfiker-Kleiner D., Hilfiker A., Fuchs M., Kaminski K., Schaefer A., Schieffer B., Hillmer A., Schmiedl A., Ding Z., Podewski E., Poli V., Schneider M.D., Schulz R., Park J.K., Wollert K.C., Drexler H. Circ. Res. 2004;95:187–195. doi: 10.1161/01.RES.0000134921.50377.61. [DOI] [PubMed] [Google Scholar]
- 32.Harada M., Qin Y., Takano H., Minamino T., Zou Y., Toko H., Ohtsuka M., Matsuura K., Sano M., Nishi J., Iwanaga K., Akazawa H., Kunieda T., Zhu W., Hasegawa H., Kunisada K., Nagai T., Nakaya H., Yamauchi-Takihara K., Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat. Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]