Abstract
Objective: Coarctation of the aorta has often been described as a simple form of congenital heart disease. However, rates of re-coarctation reported in the literature vary from 7% to 60%. Re-coarctation of the aorta may lead to worsening systemic hypertension, coronary artery disease and/or congestive cardiac failure. We aimed to describe the rates of re-coarctation in subjects who had undergone early coarctation repair (<2 years of age) and referred for clinically indicated or routine magnetic resonance (MR) surveillance. Methods: We retrospectively identified 50 consecutive subjects (20.2 ± 6.9 years post-repair) imaged between 2004 and 2008. Patient characteristics, rates of re-coarctation and LV/aortic dimensions were examined. Results: Forty percent of subjects had bicuspid aortic valves (BAV). There were 40 cases of end-to-end repair and 10 cases of subclavian flap repair. Re-intervention with balloon angioplasty or repeat surgery had been performed in 32% of subjects. The MRI referrals were clinically indicated in 34% and routine in 66% of patients. Re-coarctation was considered moderate or severe in 34%, mild in 34% and no re-coarctation was identified in 32% of patients. There was no significant difference in the number of cases of re-coarctation identified in the clinically indicated versus routine referrals for MR imaging (p = 0.20). There were no cases of aortic dissection or aneurysm formation identified amongst the subjects. The mean indexed left ventricular mass and ejection fraction was 72 ± 16 g/m2 and 66 ± 6%, respectively. Amongst those subjects with BAV there were larger aortic sinus (30 ± 1 mm vs 27 ± 1 mm, p = 0.03) and ascending aortic (27 ± 1 mm vs 23 ± 1 mm, p = 0.01) dimensions when compared to subjects with morphologically tricuspid aortic valves. Conclusions: We demonstrate that many years after early repair of coarctation of the aorta, MR surveillance detects significant rates of re-coarctation. These findings were independent of whether or not there was a clinical indication for imaging. Those patients with BAV disease had larger ascending aortic dimensions and may require more frequent non-invasive surveillance.
Keywords: Coarctation, Aorta, Surgery, Magnetic resonance imaging
1. Introduction
Coarctation of the aorta has often been described as a simple form of congenital heart disease by cardiologists. However, rates of re-coarctation reported in the literature vary from 7% to 60% [1–4]. Re-coarctation of the aorta may lead to worsening systemic hypertension, coronary artery disease and/or congestive cardiac failure [5], making long-term follow-up of this condition more complex than initially thought.
Repair of coarctation of the aorta in infancy has the reported benefits of increased long-term survival [6]. Despite this, late cardiovascular complications have been shown to develop even after apparently successful coarctation surgery [7–9]. Thus, recent attention has been given to the optimal long-term imaging strategy for this condition. Specifically, Therrien et al. have demonstrated that the combination of clinical examination plus magnetic resonance (MR) imaging is the most cost-effective non-invasive strategy to diagnose complications at the site of repair many years after coarctation surgery [5].
We therefore aimed to retrospectively examine findings from MR imaging for both clinically indicated and routine scans in individuals where early repair (<2 years of age) of coarctation was performed and who are now followed-up in our adult congenital heart disease service.
2. Materials and methods
2.1. Patient recruitment
Between 2004 and 2008 we retrospectively identified 50 consecutive subjects referred from our adult congenital heart disease service for MR follow-up of repaired coarctation of the aorta. In all subjects coarctation repair surgery was performed <2 years of age at our institution. Scans were regarded as either clinically indicated by the referring physician or as routine where no clinical indication was identified on physical examination or 2D-echocardiography.
Patient characteristics including; age at repair, type of repair, re-intervention procedures required, associated cardiac conditions, body mass index, heart rate, and resting blood pressure were recorded. The local research ethics committee approved the study, and all subjects gave informed consent at the time of the MR scan.
2.2. Cardiac MR imaging
MR imaging was performed using 1.5T MR scanner (Avanto, Siemens Medical Systems, Erlangen, Germany).
2.2.1. Measurement of aortic dimensions
Dimensions of the aorta were determined from MR angiography. This was performed with a T1 weighted spoiled gradient echo sequence. Imaging parameters: TR = 3.3; TE = 1.0; flip angle = 25°; matrix = 256 × 256; which was triggered visually and was acquired in a 15 s breath-hold. Measurements were systematically made at the level of the sinus of Valsalva, proximal ascending aorta, transverse aortic arch, residual coarctation site, proximal descending aorta and distal descending thoracic aorta at the level of the diaphragm. Aortic re-coarctation was defined according to the percent residual coarctation diameter/descending thoracic aorta diameter at the level of the diaphragm where, no re-coarctation >80%, mild re-coarctation 61–80%, moderate re-coarctation 41–60% and severe re-coarctation <40%.
2.2.2. Aortic cine and flow imaging
Cine images of the entire thoracic aorta were acquired using retrospectively gated steady-state free precession (SSFP) MR imaging. Image parameters: TR = 2.4 ms; TE = 1.2 ms; flip angle = 78°; slice thickness = 6–8 mm; matrix = 144 × 192; field of view = 300–380 mm and temporal resolution = 25 phases; acquired during a single breath-hold. Specific attention was given to the region of the proximal descending aorta where previous aortic surgery was performed. Aortic flow data were acquired using a flow-sensitive gradient-echo sequence (TR, 27 ms; TE, 3.2 ms; flip angle, 30°; slice thickness, 5 mm; and matrix, 192 × 256) during free breathing. Imaging planes were located in the proximal ascending aorta [10]. Through-plane flow data (30 phases per cardiac cycle) were acquired by use of retrospective cardiac gating. Arterial blood flow was calculated from phase contrast images by use of a semiautomatic vessel edge-detection algorithm (Argus; Siemens Medical Systems, Erlangen, Germany) with operator correction. Aortic valve regurgitation fraction (RF %) was calculated as percent backward aortic flow/total aortic flow.
2.2.3. Assessment of ventricular volumes, function and mass using cine MR imaging
SSFP cine MR images of the heart were acquired in the vertical long-axis, four-chamber view and the short-axis view covering the entirety of both ventricles (9–12 slices). Image parameters used were as described above. Assessment of left ventricular volumes was performed by manual segmentation of short-axis cine images with endocardial outline at end-diastole and end-systole (Argus; Siemens Medical Systems, Erlangen, Germany). End-diastolic and end-systolic volumes were calculated by use of Simpson's rule, and from these volumes the ejection fraction was calculated. Left ventricular mass was determined in end-diastole via manual segmentation of the endo- and epi-cardium from short-axis images. This value was indexed to body surface area.
2.3. Statistical analysis
All data are presented as mean ± SD or median and range. Statistical comparison of parametric data was performed with a two-tailed unpaired Student's t-test. The relationship between dichotomous variables was tested with Fisher's exact test. A value of p < 0.05 was considered statistically significant. Statistical testing and data analysis were performed with GraphPad Prism Version 4.0 (San Diego, CA).
3. Results
3.1. Patient characteristics (Table 1)
Table 1.
Patient characteristics.
| n = 50 | |
|---|---|
| Mean age (years) | 22.0 ± 6.5 |
| Median age at repair (days) | 4.0 (range 1.0–630) |
| Mean years since repair | 20.2 ± 6.9 |
| Gender | 62% male, 38% female |
| Body mass index (kg/m2) | 24.5 ± 4.9 |
| Heart rate (beats/min) | 74 ± 11 |
| Blood pressure (mmHg) | 127/70 ± 15/9 |
| Type of repair | 80% end-to-end 20% subclavian patch repair |
| Re-interventions post-initial surgery | 1st re-intervention performed in 36% |
| 2nd re-intervention performed in 6% | |
| Associated conditions at the time of surgery | BAV (n = 20), VSD (n = 8), sub-Ao stenosis (n = 5), AR (n = 3), MVD (n = 1) |
Characteristics for all 50 patients are detailed with data presented as mean ± SD or median and range where stated. BAV: bicuspid aortic valve disease, VSD: ventricular septal defect, sub-Ao: sub aortic, AR: aortic regurgitation, MVD: mitral valve disease.
The mean age of patients in the study and years from repair of coarctation was 22.0 ± 6.5 years and 20.2 ± 6.9 years, respectively. The median age at repair was 4.0 days (range 1–630 days) with 62% of repair surgery being performed by 1 month of age. End-to-end repair was used in 80% (40/50) of patients and subclavian flap surgery was used in the remainder. Thirty-two percent (16/50) of patients required a re-intervention procedure after the initial surgical repair (12 cases treated with percutaneous balloon procedures and four cases treated with surgery). Three patients required an additional procedure subsequent to their first re-intervention. Patients had normal resting haemodynamics and body mass index (Table 1).
In regards to associated lesions, 40% (20/50) of patients had bicuspid aortic valves, 16% (8/50) of patients had a ventricular septal defect repaired at the time of coarctation surgery and 10% (5/50) of patients had sub-aortic stenosis resection performed. There were three patients with significant aortic valve regurgitation (regurgitation fraction >10%) and one patient with mitral valve disease.
Scans were clinically indicated in 34% (17/50) of patients. Specifically, new-onset hypertension or hypertension refractory to therapy was the reason for referral in 14 cases. In a further three cases 2D echocardiography findings of increased peak velocity across the aortic arch led to the referral for MR imaging. The remaining 66% (33/50) of cases were regarded as routine scans, where there were no abnormal clinical/echo findings identified.
3.2. Cardiac MR imaging (Table 2, Figs. 1 and 2)
Table 2.
Aortic dimensions and left ventricular parameters.
| n = 50 | Aortic dimensions (mm) |
|---|---|
| Sinus of Valsalva | 28 ± 5 |
| Proximal ascending aorta | 25 ± 5 |
| Transverse aortic arch | 16 ± 4 |
| Region site of previous CoA surgery | 13 ± 4 |
| Proximal descending aorta | 19 ± 4 |
| Distal descending thoracic aorta (diaphragm level) | 17 ± 3 |
| LV parameters | |
|---|---|
| Indexed EDV (ml/m2) | 79 ± 15 |
| Indexed ESV (ml/m2) | 27 ± 10 |
| Indexed SV (ml/m2) | 52 ± 8 |
| Cardiac index (l/min/m2) | 4.0 ± 0.6 |
| Ejection fraction (%) | 66 ± 6 |
| Indexed mass (g/m2) | 72 ± 16 |
Aortic dimensions for the entire thoracic aorta and indexed parameters for left ventricular volumes, function and mass are detailed for the patients (n = 50). Data are presented as mean ± SD. CoA: coarctation, LV: left ventricle, EDV: end-diastolic volume, ESV: end-systolic volume, SV: stroke volume.
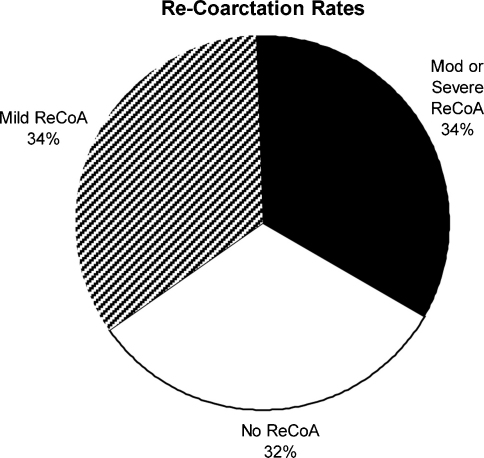
Fig. 1.
Rates of re-coarctation. Pie graph showing the proportion of patients with no, mild or moderate/severe re-coarctation identified in follow-up MR imaging. Re-CoA: re-coarctation.
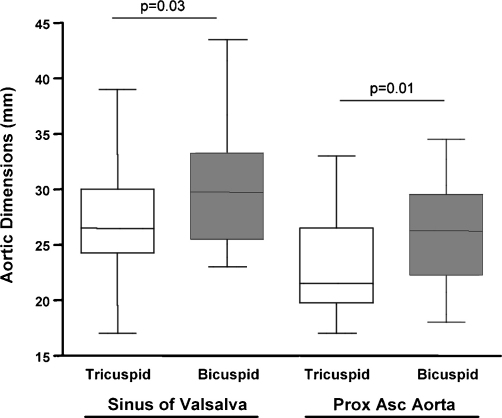
Fig. 2.
Aortic dimensions-tricuspid versus bicuspid aortic valves. Mean aortic dimensions for patients with morphologically tricuspid (n = 30) and bicuspid (n = 20) aortic valves are shown. Measurements were made at the level of the sinus of Valsalva and proximal ascending aorta. The results are expressed as mean ± SD, p < 0.05 was considered statistically significant.
Re-coarctation of the aorta was identified in 68% (34/50) of patients. Specifically, mild re-coarctation was found in 34% (17/50) of patients and moderate or severe re-coarctation was found in 34% (17/50) of patients (Fig. 1). Eighty-two percent of patients in whom re-coarctation was identified had an end-to-end repair performed initially. There were no cases of aortic dissection or aneurysm formation identified amongst the cohort. There was no statistically significant difference in the number of cases of re-coarctation identified in either the clinically indicated or routine referrals for MR imaging (p = 0.20).
The dimensions of the entire thoracic aorta and functional data and dimensions for the left ventricle are summarised in Table 2. Twenty-eight percent (14/50) of patients had post-stenotic dilatation of the proximal descending aortic arch. The mean left ventricular ejection fraction and indexed mass were normal for the cohort, being 66 ± 6% and 72 ± 16 g/m2, respectively. When subjects in whom there was evidence of re-coarctation were compared with no re-coarctation, left ventricular ejection fraction and indexed mass were similar for both (67 ± 1% vs 66 ± 2%, p = 0.59 and 71 ± 3 g/m2 vs 74 ± 6 g/m2, p = 0.82, respectively).
When patients with morphologically tricuspid aortic valves (n = 30) were compared with bicuspid aortic valves (n = 20), there was no difference in the number of cases reported to have re-coarctation (p = 0.14). However, patients with morphologically bicuspid aortic valves had larger aortic dimensions at the level of the sinus of Valsalva (30 ± 1 mm vs 27 ± 1 mm, p = 0.03) and proximal ascending aorta (27 ± 1 mm vs 23 ± 1 mm, p = 0.01) than patients with tricuspid aortic valves (Fig. 2). Aortic dimensions for the transverse arch and the descending thoracic aorta were similar between the groups.
4. Discussion
We demonstrate that many years after infantile repair of coarctation of the aorta, MR surveillance detects significant rates of re-coarctation. Specifically, we identified at least mild re-coarctation in over 2/3 of the cohort referred for follow-up scanning. These findings were independent of whether a clinical indication for imaging was identified. Additionally, those patients with bicuspid aortic valves had larger ascending aortic dimensions when compared to patients with morphologically tricuspid aortic valves.
The rationale for early repair of coarctation of the aorta in infancy has been the reported benefits of increased long-term survival [6,11]. Despite this, late cardiovascular complications have been shown to develop even after apparently successful coarctation surgery [7–9]. Re-coarctation of the aorta may lead to worsening systemic hypertension, coronary artery disease and/or congestive cardiac failure [5]. Our data suggest significant rates of re-coarctation amongst patients in whom early operative repair of aortic coarctation was performed two decades prior.
Therrien et al. have demonstrated that the combination of clinical examination plus MR imaging is the most cost-effective strategy to diagnose complications at the site of repair many years after coarctation surgery [5]. Although the sensitivity of echocardiography is reasonably high for detection of re-coarctation, this is tempered by lower image resolution (particularly in adults) and technical difficulties in obtaining a representative Doppler gradient in the presence of collateral vessel flow. The issue that remains unresolved and warrants further prospective investigation is the optimal timing of serial imaging in this group.
We identified larger ascending aortic dimensions in patients with repaired coarctation of the aorta and morphologically bicuspid aortic valves which is consistent with previous descriptions [12]. It is recognised that bicuspid aortic valve disease is often associated with connective tissue disorders [13–17], which may lead to aortic dilatation even in the absence of haemodynamically significant aortic valve pathology. This leaves the patient at risk of aneurysm formation, aortic dissection and sudden death. Importantly amongst our cohort we were unable to identify aneurysm formation or dissection in either the region of coarctation repair or the ascending portion of the aorta.
Although there is a paucity of data pertaining to the risk of aortic dissection in subjects with bicuspid aortic valve disease, it is generally believed that increasing risk is directly proportional to increasing aortic dimensions [18]. Hence current ACC/AHA guidelines recommend that patients with bicuspid aortic valves and dilated aortic root dimensions (>40 mm) should undergo routine yearly serial evaluation [18]. It can be argued that subjects with previously repaired coarctation and bicuspid aortic valve disease present a ‘high risk’ group amongst these subjects and in whom more frequent screening protocols may be warranted.
The retrospective nature of this study and relatively small numbers presents a limitation to our findings. Serial prospective data detailing the rate of progression of re-coarctation and ascending aortic dimensions would have been ideal in order to more completely understand optimal timing for screening protocols. This is especially the case in those patients who have mild re-coarctation. The rate of progression and clinical significance of this group can only be accurately determined prospectively. The patients in our study were referred from our adult congenital heart disease service and although the majority of scans were deemed routine there may well still be a selection bias which explains the relatively high rate of re-coarctation identified.
In conclusion, coarctation of the aorta has often been described as a simple form of congenital heart disease. Rates of re-coarctation in adult subjects who were repaired early in life suggest that this is in fact a complex condition, which requires intensive long-term surveillance in order to avert serious morbidity and/or mortality. In our study, there was a significant rate of re-coarctation identified in scans performed on a routine basis i.e. in which there was no obvious clinical indication. More frequent screening protocols may also be warranted in those patients who have bicuspid aortic valve disease in addition to previously repaired coarctation of the aorta.
Acknowledgments
Wendy Norman and Jeff Critchley provided radiography support for the cardiac MR imaging. Steven Kimberley provided administrative support for the recruitment of patients into the protocol.
Footnotes
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14–17, 2008.
Sources of Funding: RP is funded by the Neil Hamilton Fairley NHMRC/NHF of Australia Postdoctoral Fellowship, AMT is funded by the Higher Education Funding Council for England (HEFCE) and British Heart Foundation (BHF).
Appendix A. Conference discussion
Dr G. Sarris (Athens, Greece): The stated aim of this study was to assess the rate, the late re-coarctation rate, in patients who fulfilled two criteria: they had coarctation repair early in life, less than 2 years of age, and who were referred for MRI evaluation.
The conclusions of the study were that MRI detects significant rates of re-coarctation late after repair and that this finding was independent of whether there was a clinical indication for stenting, the implication perhaps being that MRI could be used as a routine screening method for possible re-coarctation.
It is my feeling that your conclusion ought to be qualified by the statement that the 30% re-coarctation rate detected is not 30% incidence of re-coarctation In the population of all patients following repair, but in a selected patient sample who are already referred for evaluation and of whom one half had a clinical indication for scanning; therefore, it seems that the percentage of 30% represents an overestimate of the probability of re-coarctation long-term since the denominator is not precisely known.
Further, while it is not surprising that up to one third of this patient population were actually found by MRI to have re-coarctation (and, in fact, indeed, one third of these patients had had a prior intervention, which was a balloon plasty), it is somewhat surprising that an equal percentage of patients without any clinical indication were also found to have significant re-coarctation. My question is whether data was collected, recorded and analysed regarding the blood pressure difference between arm and leg in these two groups of patients and whether this measurement is comparable in the two groups since it seems difficult to explain why this one third of patients with more than 50% stenosis would have no detectable blood pressure difference on clinical examination. Therefore, could you comment on whether an MRI would be indicated as a routine surveillance method even in patients who have no blood pressure difference between arm and leg following coarctation repair?
Dr Puranik: In the data that I presented, the blood pressure difference between arm and leg was not something that I had access to, it was only the resting blood pressure from the clinical examination that we report.
We do acknowledge the possibility that there exists a selection bias amongst our referrals and therefore we may be presenting an over-estimate of the true rate of re-coarctation.
The fundamental point still remains though, that even in an experienced centre clinicians relying on clinical examination and echocardiography may still not be able to determine if patients repaired early in life have significant re-coarctation.
This being the case, we recommend that routine follow-up every 3 to 5 years of patients who have a morphologically tricuspid aortic valve would be a reasonable, and perhaps with patients who had a bicuspid valve probably more frequently, such as every 2 years.
Dr V. Hraska (Sankt Augustin, Germany): It's amazing how many patients were detected with re-coarctation. Based on your data, approximately 30% patients were detected with a severe re-coarctation. What was the destiny of these patients? What happened with these patients? Did you take your surgeons seriously? In other words, were these patients reoperated based on your findings?
Dr Puranik: I don’t have the complete dataset and it was outside the scope of this retrospective paper. However, I know that all of the patients who were reported as severe coarctation went on to have a procedure. Those who were deemed as moderate re-coarctation varied between being watched for another 12-month period and being operated on.
References
- 1.Behl P.R., Sante P., Blesovsky A. Isolated coarctation of the aorta: surgical treatment and late results. Eighteen years’ experience. J Cardiovasc Surg (Torino) 1988;29:509–517. [PubMed] [Google Scholar]
- 2.Hesslein P.S., McNamara D.G., Morriss M.J., Hallman G.L., Cooley D.A. Comparison of resection versus patch aortoplasty for repair of coarctation in infants and children. Circulation. 1981;64:164–168. doi: 10.1161/01.cir.64.1.164. [DOI] [PubMed] [Google Scholar]
- 3.Waldman J.D., Lamberti J.J., Goodman A.H., Mathewson J.W., Kirkpatrick S.E., George L., Turner S.W., Pappelbaum S.J. Coarctation in the first year of life. Patterns of postoperative effect. J Thorac Cardiovasc Surg. 1983;86:9–17. [PubMed] [Google Scholar]
- 4.Williams W.G., Shindo G., Trusler G.A., Dische M.R., Olley P.M. Results of repair of coarctation of the aorta during infancy. J Thorac Cardiovasc Surg. 1980;79:603–608. [PubMed] [Google Scholar]
- 5.Therrien J., Thorne S.A., Wright A., Kilner P.J., Somerville J. Repaired coarctation: a “cost-effective” approach to identify complications in adults. J Am Coll Cardiol. 2000;35:997–1002. doi: 10.1016/s0735-1097(99)00653-1. [DOI] [PubMed] [Google Scholar]
- 6.Brickner M.E., Hillis L.D., Lange R.A. Congenital heart disease in adults. Second of two parts. N Engl J Med. 2000;342:334–342. doi: 10.1056/NEJM200002033420507. [DOI] [PubMed] [Google Scholar]
- 7.Ala-Kulju K., Jarvinen A., Maamies T., Mattila S., Merikallio E. Late aneurysms after patch aortoplasty for coarctation of the aorta in adults. Thorac Cardiovasc Surg. 1983;31:301–306. doi: 10.1055/s-2007-1022001. [DOI] [PubMed] [Google Scholar]
- 8.Hehrlein F.W., Mulch J., Rautenburg H.W., Schlepper M., Scheld H.H. Incidence and pathogenesis of late aneurysms after patch graft aortoplasty for coarctation. J Thorac Cardiovasc Surg. 1986;92:226–230. [PubMed] [Google Scholar]
- 9.Rostad H., Abdelnoor M., Sorland S., Tjonneland S. Coarctation of the aorta, early and late results of various surgical techniques. J Cardiovasc Surg (Torino) 1989;30:885–890. [PubMed] [Google Scholar]
- 10.Taylor A.M., Bogaert J. In: Imaging planes. Clinical cardiac MRI. Bogaert J., Dymarkowski S., Taylor A.M., editors. Springer-Verlag; Heidelberg, Germany: 2005. pp. 85–98. [Google Scholar]
- 11.Campbell M. Natural history of coarctation of the aorta. Br Heart J. 1970;32:633–640. doi: 10.1136/hrt.32.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quenot J.P., Boichot C., Petit A., Falcon-Eicher S., d’Athis P., Bonnet C., Wolf J.E., Louis P., Brunotte F. Usefulness of MRI in the follow-up of patients with repaired aortic coarctation and bicuspid aortic valve. Int J Cardiol. 2005;103:312–316. doi: 10.1016/j.ijcard.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Braverman A.C., Guven H., Beardslee M.A., Makan M., Kates A.M., Moon M.R. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Hahn R.T., Roman M.J., Mogtader A.H., Devereux R.B. Association of aortic dilation with regurgitant, stenotic and functionally normal bicuspid aortic valves. J Am Coll Cardiol. 1992;19:283–288. doi: 10.1016/0735-1097(92)90479-7. [DOI] [PubMed] [Google Scholar]
- 15.Keane M.G., Wiegers S.E., Plappert T., Pochettino A., Bavaria J.E., Sutton M.G. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102:III35–III39. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 16.Nataatmadja M., West M., West J., Summers K., Walker P., Nagata M., Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl. 1):II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 17.Nistri S., Sorbo M.D., Marin M., Palisi M., Scognamiglio R., Thiene G. Aortic root dilatation in young men with normally functioning bicuspid aortic valves. Heart. 1999;82:19–22. doi: 10.1136/hrt.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonow R.O., Carabello B.A., Kanu C., de Leon A.C., Jr., Faxon D.P., Freed M.D., Gaasch W.H., Lytle B.W., Nishimura R.A., O’Gara P.T., O’Rourke R.A., Otto C.M., Shah P.M., Shanewise J.S., Smith S.C., Jr., Jacobs A.K., Adams C.D., Anderson J.L., Antman E.M., Faxon D.P., Fuster V., Halperin J.L., Hiratzka L.F., Hunt S.A., Lytle B.W., Nishimura R., Page R.L., Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]