Abstract
Background
Adequate relief of IBS symptoms (IBS-AR) has been used as a primary endpoint in many randomized controlled trials of IBS and is considered by the Rome III Committee to be an acceptable primary endpoint. However, controversy exists on whether baseline severity confounds the effect of this treatment patient outcome.
Aims
In a randomized controlled treatment trial (1) to compare subjective report of IBS-AR to global assessment of improvement (IBS-GAI), change in IBS symptom severity scale (IBS-SSS) and IBS Quality of Life (IBS-QOL); (2) to explore whether initial IBS symptom severity influences the sensitivity of these outcome measures; (3) to determine whether psychological symptoms influence the sensitivity of these measures.
Methods
289 adult IBS patients were recruited to a treatment trial. Baseline IBS-SSS scores were used to classify IBS severity as mild (<150), moderate (150-300), or severe (>300). Questionnaires were completed at baseline and after 3 weeks of treatment with sham acupuncture or waitlist control.
Results
IBS baseline severity significantly affected the proportion of patients who reported IBS-AR at 3 weeks (mild, 70%; moderate, 49.7%; severe, 38.8%) (p<0.05). However, once the patients who reported IBS-AR at baseline (28.0%) were excluded from the analysis, baseline severity no longer affected the proportion of patients reporting IBS-AR. Baseline severity did not have a significant of effect patients reporting moderate or significant improvement on the IBS-GAI (mild, 30%; moderate, 25.3%; severe, 18.8%) (p=NS). Psychological symptoms had no significant correlations with responders after adjusting for baseline severity.
Conclusions
These data suggest that IBS-AR as an endpoint is confounded with initial IBS symptom severity as measured by baseline reporting of adequate relief. The confounding effects of adequate relief can be eliminated if patients who report adequate relief at screening are excluded from study participation.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by chronic or recurrent abdominal pain or discomfort, usually in the lower abdomen, which is associated with disturbed bowel function and feelings of abdominal distention and bloatedness (1). The abdominal discomfort and/or pain associated with IBS is usually relieved by defecation (2, 3). There are other symptom features that can be associated with IBS. This makes it difficult to gauge treatment response or select a primary outcome primarily by improvement of a specific symptom (4, 5).
Furthermore, in contrast to conditions like ulcerative colitis or Crohn's disease where treatment response can be gauged by reduction in mucosal inflammation, there are no biological markers in IBS, making treatment response completely subjective. Patient reported outcome measures that assess global improvement in IBS capture the range of symptoms experienced by a patient. The Food and Drug Administration (FDA) has accepted a patient reported outcome of global improvement in IBS as a primary endpoint (6). One of the most commonly used global outcome measures in IBS clinical trials asks the patient weekly if they have experienced adequate relief (AR) of their IBS symptoms or pain (i.e., “In the last seven days, have you had adequate (or satisfactory) relief of your IBS symptoms?”).
The endpoint of IBS-AR has been shown to be a clinically and statistically relevant benefit in therapeutic IBS trials with alosetron (7-9), cilansetron (10-12) and tegaserod (13-15). These studies suggest that as an endpoint IBS-AR is responsive, reproducible, and moves in the same direction as other meaningful measures (16). Furthermore, the Rome III committees identified IBS-AR as the current standard of assessment for clinical trials noting that validation of this endpoint is needed (17).
There have been some criticisms of the use of adequate or satisfactory relief as a patient reported outcome. In a 6 month study of IBS patients receiving standard medical care in an HMO setting, Whitehead et al. (18) found that at baseline the patients with the mildest severity reported the highest proportion of satisfactory relief when asked the measure once, 6 months later. These findings suggest that satisfactory relief (or by implication adequate relief) is confounded by IBS symptom severity and correlated poorly with the amount of symptom improvement. However, it may be difficult to extrapolate this finding to a clinical trial situation since it was based on data obtained from a usual care cohort of an HMO. An additional caveat to such an extrapolation is that patients not reporting symptoms were considered to have satisfactory relief. Therefore it is possible that expectation of satisfactory relief may be different in a usual care situation as compared to a true clinical trial. Finally, it is possible that a larger proportion of subjects in a usual care situation might report satisfactory or adequate relief at baseline than in a clinical trial, since the former are not specifically seeking to be enrolled in a clinical trial. Accordingly, as noted by the authors there is a pressing need to re-evaluate these data through use of a prospective clinical trial.
The aims of this study of a randomized clinical trial were: (1) To compare subjective report of adequate relief (IBS-AR) to global assessment of improvement (IBS-GAI), change in IBS symptom severity scale (IBS-SSS) and IBS Quality of Life (IBS-QOL) in a randomized clinical trial (RCT); (2) To explore whether and how initial IBS symptom severity may influence the sensitivity of these outcome measures; (3) To determine whether psychological symptoms influence the sensitivity of these measures.
Methods
In this single center, single-blind, randomized, control trial, 289 IBS-patients were randomized to Waitlist or Sham Acupuncture with either a neutral or an “augmented” patient-practitioner interaction. The main study had 262 patients; a nested study that included qualitative interviews had an additional 27 patients randomized to each arm. For ethical reasons patients were subsequently re-randomized to receive sham or verum acupuncture for an additional 3 weeks. The rationale for this design was to assess the effect of placebo response and patient-practitioner interaction in IBS. The results of the main study will be reported elsewhere. For the purposes of this study patient assessments at baseline and 3 weeks were used and all subjects were combined into one group for analysis.
Subjects
Participants were recruited from advertisements in media, fliers and referrals from health professionals and were all at least 18 years old and met the Rome II criteria for IBS (19). Patients were excluded if they had such unexplained findings as weight loss >10% body weight, fever, blood in stools, family history of colon cancer, or inflammatory bowel disease. The diagnosis of IBS was based on typical symptoms (20, 21) and confirmed by a board certified gastroenterologist experienced in functional bowel disorders (AJL). Participants were allowed to continue IBS medications (e.g., fiber, anti-spasmodics, loperamide) as long as they had been on stable doses for at least 30 days prior to entering the study and agreed not to change medications or dosages during the trial. The Institutional Review Boards at the Beth Israel Deaconess Medical Center and Harvard Medical School approved the design.
Questionnaires
The questionnaires at baseline and 3 week follow-up included the following: (1) The IBS Global Assessment of Improvement Scale (IBS-GAI) (13); (2) The IBS Symptom Severity Scale (IBS-SSS) (22); (3) A binary response question, Adequate Relief (IBS-AR), assessing whether the patient had obtained adequate relief of bowel symptoms during the preceding 7 days (7, 9, 10); (4) IBS Quality of Life Scale (IBS-QOL) (23); (5) NEO Inventory, of which the Neuroticism scale of the multi-scale NEO Inventory was used to assess personality dysfunction relating to resiliency in the face of stress (24); (6) Beck Depression Inventory (BDI) which assessed symptoms of depression (25).
IBS Global Assessment of Improvement Scale (IBS-GAI)
IBS Global Improvement Scale (IBS-GAI) asks participants: “Compared to the way you felt before you entered the study, have your IBS symptoms over the past 7 days been: 1) “Substantially Worse”, 2) “Moderately Worse”, 3)“Slightly Worse”, 4) “No Change”, 5)“Slightly Improved”, 6)“Moderately Improved” or 7) “Substantially Improved” (26, 27). (26, 27)(26, 27)
IBS Symptom Severity Scale
The IBS Symptom Severity Scale (IBS-SSS) contains five questions that measure, on a 100-point VAS the severity of abdominal pain, the frequency of abdominal pain, the severity of abdominal distention, dissatisfaction with bowel habits, and interference with quality of life (22). All five components contribute to the score equally yielding a theoretical range of 0-500, with a higher score indicating worse condition. Previous studies have established that scores below 175 represent mild IBS symptoms, 175–300 represents moderate severity, and scores above 300 represent severe IBS (22). The total severity score on the IBS-SSS was treated as the gold standard measure of IBS severity. In the study by Francis et al. (22) a decrease of 50 points correlated with improvement in clinical symptoms.
IBS-Adequate Relief
IBS-Adequate Relief (IBS-AR) is a dichotomous single item that asks participants “Over the past week have you had adequate relief of your IBS symptoms?” (6, 16).
IBS-Quality of Life
The IBS-Quality of Life (IBS-QOL) is a 34-item measure assessing the degree to which IBS interferes with patient quality of life. Each item is rated on a 5-point Likert scale, thus yielding a total score that has a theoretical range of 34 to 170, with higher scores indicating worse QOL (23, 28).
Definition of a Responder
For this analysis we identified those who responded to the intervention according to the four main outcomes described above. As such, four different responder definitions were compared: (1) IBS-AR, where a responder was defined as a patient who reported at the 3-week follow-up that for the last 7 days they had experienced “adequate relief” (who responded “yes”); (2) IBS-GAI, where a responder was defined as a patient who responded that, compared to 3 weeks ago, their symptoms were either “moderately improved” or “substantially improved”; (3) change in total IBS-SSS score from enrollment to 3 weeks follow-up, where a responder was defined as a patient whose overall symptom severity on the IBS-SSS changed ≥ 50 points, and (4) change in IBS-QOL from enrollment to 3 weeks follow-up, where a responder was defined as a clinically meaningful change of ≥ 10 points in this measure.
Statistical Analysis
All data were analyzed using SAS (Version 6.12). To address aim 1 (correlation among outcome measures), the nonparametric correlation coefficient, Kendall's tau-b, was computed. To address aim 2, χ2 was used to test whether the responder rates differed between patients classified as having mild, moderate and severe IBS symptoms at enrollment. For the continuous outcome variables (i.e., more than 50% reduction of IBS-SSS at week three compared with that at enrollment, change in total IBS symptom severity score from enrollment to 3 weeks follow-up, and change in IBS-QOL from enrollment to 3 weeks follow-up), analysis of variance was used to compare the IBS symptom severity groups. To address aim 3 (determine whether psychological status influenced outcome measures), a t-test was used to compare patients reporting adequate relief to no adequate relief. For other outcome measures (i.e., IBS-GAI, change in IBS-SSS, and change in IBS-QOL score), Kendall's tau-b was computed.
Results
Patient Characteristics
Between December 2003 and February 2006, 350 prospective participants were screened, and 289 were enrolled into the study. Seventy-five percent of the patients were female, and 88% were white. The mean age was 38.4 (range 18.8 – 76.4, s.d. 14.7 yr). Ninety-seven percent graduated from high school and 80% were employed. IBS subtypes were as follows: 50% IBS-Mixed, 28% IBS-Diarrhea and 23% IBS-Constipation. Most patients (94%) had IBS for more than 1 year. At baseline, IBS-SSS was used to assess symptom severity; 23 (8%) had mild, 170 (59%) had moderate, and 96 (33%) had severe symptoms. No statistically significant differences existed between these three symptom severity groups with regard to age, education, pain scores, subtype of IBS, individual gastroenterology symptoms and psychosocial status.
Correlations among Responder Definitions
At week 3, there was a significant, although modest, correlation between the 4 endpoints used in this study (Table 1).
Table 1. Correlations among outcome measures at week 3.
| IBS-GAI | >50% Reduction IBS-SSS | Change in IBS-SSS | Change in IBS-QOL | |
|---|---|---|---|---|
| Adequate relief | 0.42 | 0.39 | 0.35 | 0.22 |
| IBS-GAI | 0.41 | 0.39 | 0.15* | |
| >50% Reduction IBS-SSS | 0.85 | 0.19 | ||
| Change in IBS-SSS | 0.19 |
All correlations (Kendall's tau-b) in table were significant at p<0.001 (except *p<0.01)
Effects of Baseline Severity on Response to Treatment
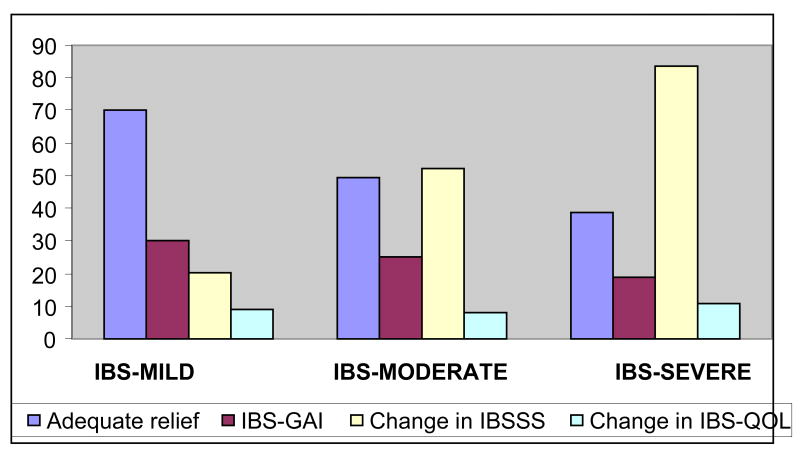
Responder rates differed between patients classified as having mild, moderate and severe IBS symptoms at baseline. Table 2 demonstrates the effects of IBS symptom severity at baseline and the response to outcome measures at 3 week follow-up. Patients with mild IBS symptoms were significantly more likely to be a responder for IBS-AR (70%) than were patients with moderate (49.7%) or severe (38.8%) symptom severity. Similar results were seen when a responder was defined in terms of IBS-GAI: patients with moderate and severe IBS symptoms at baseline were less likely to report that they were moderately or substantially better (moderate (25.3)% and severe (18.8%)) compared to mild IBS symptoms (30%). In contrast, change in IBS-SSS at week 3 was least in patients with mild IBS (20.3%) and greatest in patients with severe IBS (83.7%). Change in IBS-QOL did not correlate with IBS severity. These results are graphically depicted in Figure 1.
Table 2. Effects of IBS-SSS on the magnitude of symptom reduction at week 3.
| Baseline | IBS-AR | IBS-GAI | >50% I Reduction BS-SSS | Change in IBS-SSS | Change in IBS-QOL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity | Responder | Responder | |||||||||
| Level | N | Rate (%)* | CI | Rate | CI | Mean | CI | Mean† | CI | Mean | CI |
| Mild | 23 | 70.0 | 49.4, 90.6 | 30.0 | 9.4, 50.6 | 12.55 | -4.12, 29.23 | 20.25 | -5.18, 45.68 | 8.95 | 5.17, 12.74 |
| Moderate | 170 | 49.7 | 41.6, 57.7 | 25.3 | 18.3, 32.3 | 21.61 | 17.01, 26.22 | 52.28 | 41.07, 63.49 | 7.93 | 5.52, 10.34 |
| Severe | 96 | 38.8 | 28.0, 49.5 | 18.8 | 10.1, 27.4 | 22.99 | 17.64, 28.33 | 83.73 | 63.82, 103.63 | 10.66 | 6.64, 14.67 |
| Total | 289 | 47.8 | 41.6, 54.0 | 23.6 | 18.3, 28.9 | 21.34 | 17.88, 24.80 | 59.94 | 50.27, 69.61 | 8.89 | 6.94, 10.85 |
Difference between groups was significant at p<0.05
p<0.001, and all others at p>0.05
Figure 1. Proportion of IBS responders according the severity of symptoms.
Proportion reporting adequate relief and IBS-GAI is lowest in patients with severe at baseline, but the magnitude of symptom improvement is greatest in patients with severe IBS. No correlation was found in improvement IBS-QOL and severity of symptoms.
Comparison of Adequate Relief Responder and non-Responders
Patients who were responders to IBS-AR at week 3 when compared to non-responders clearly had better health status and milder symptoms at that point as evidenced by the greater improvement in IBS-SSS subscales for pain, bowel habit, interference with daily activities and greater improvement in the total score of the IBS-SSS at week 3 as compared to patients who were not IBS-AR responders. Therefore, adequate relief was seen to correlate well with improvements in symptoms and other clinical parameters thus providing concurrent validity for this responder definition (Table 3).
Table 3. Comparison of Responders to Non-responders on IBS-AR.
| No Adequate Relief (n=130) |
Adequate Relief (n=119) |
|||
|---|---|---|---|---|
| Mean | CI | Mean | CI | |
| IBS-SSS at 3 weeks FU (0-300 scale) | 251.8 | 237.6, 266.1 | 168.5 | 155.0, 182.0 |
| Pain rating (0-100) | 32.3 | 27.8, 36.8 | 19.7 | 15.9, 23.4 |
| Pain days in last 10 (0-10) | 5.0 | 4.4, 5.6 | 3.4 | 2.9, 3.8 |
| Distention rating (0-100) | 36.6 | 31.3, 41.8 | 21.4 | 17.3, 25.4 |
| Dissatisfaction w/Bowel Habits (0-100) | 73.5 | 70.2, 76.7 | 50.7 | 47.2, 54.3 |
| Interfere w/Daily activities (0-100) | 59.0 | 55.6, 62.3 | 43.3 | 39.6, 47.0 |
| Change in IBS-SSS | 28.0 | 16.3, 39.7 | 94.2 | 80.6, 107.7 |
| IBS-QOL at week 3 (0-100)* | 78.4 | 74.2, 82.5 | 71.7 | 67.4, 76.0 |
| Change in IBS-QOL | 4.9 | 2.8, 7.1 | 13.7 | 10.5, 17.0 |
| IBS-SSS at baseline (0-500 scale)† | 279.8 | 267.8, 291.8 | 262.6 | 249.7, 275.6 |
| IBS-QOL at baseline (0-100 scale) † | 83.3 | 79.1, 87.5 | 85.2 | 80.4, 90.1 |
Comparison significant at P>0.05
at P<0.05, and other comparisons significant at P<0.001.
Effect of Baseline Reports of Relief
At the baseline visit participants were asked whether they had experienced adequate relief of symptoms over the past week, in this case prior to entry into the study. As seen in Table 4, IBS-AR at baseline was answered ‘yes’ by 39% of patients with mild symptom severity, 36.5% with moderate symptom severity and only 10.4% of patients with severe symptom severity.
Table 4. Report of Outcome Measure at Baseline.
| IBS-AR* | IBS-QOL* | ||||
|---|---|---|---|---|---|
| IBS Severity (IBS-SSS 0-500) |
N | (0-100 scale) | |||
| % | CI | Mean | CI | ||
| Mild (mean 157.2; CI 153.1 -161.2) | 23 | 39.1 | 19.2, 59.0 | 67.1 | 58.0, 76.2 |
| Moderate (mean 241.0: CI 235.7 -246.4) | 170 | 36.5 | 29.2, 43.8 | 80.4 | 76.9, 84.0 |
| Severe (mean 356.5; CI 348.2 – 364.8) | 96 | 10.4 | 4.3, 16.5 | 94.5 | 89.0, 99.9 |
| Total | 289 | 28.0 | 22.8, 33.2 | 84.0 | 81.0, 87.0 |
Difference between three severity levels at P<0.001
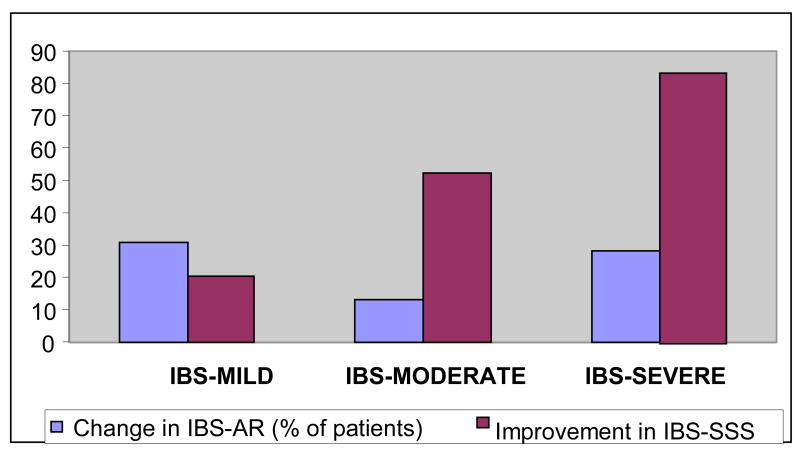
When patients who reported AR at baseline were removed, the discordance between adequate relief being associated with less change in IBS-SSS as previously shown in Figure 1 was no longer present. Thus severity of IBS no longer related inversely to the proportion with AR at 3 weeks (Figure 2). In contrast change in IBS-SSS improved more significantly in patients severe IBS symptoms (p=0.035).
Figure 2. Change in Adequate Relief and improvement in IBSSS*.
* Baseline to 3 weeks - p=0.035
The 4 possible combinations of IBS-AR responses were also compared at baseline and week 3 with change in IBS-SSS (Table 5). Patients who did not have AR at baseline but reported AR at week 3 had the greatest change in IBS-SSS (104.44) relative to those who did not report AR at baseline or at week 3 (32.5; p>0.0001) or others who reported adequate relief at baseline whether or not they had adequate relief at 3 weeks. This data confirms the importance of the assessment of adequate relief at baseline in order to eliminate them from the treatment trial. When patients with baseline adequate relief were eliminated from the analysis, baseline symptom severity had no effect on endpoint adequate relief at week 3 (results not shown).
Table 5. Change in IBS-AR at baseline and week 3.
| IBS-AR Response at Baseline and week 3 | ||||
|---|---|---|---|---|
| No-Yes N=70 |
Yes-Yes N=48 |
Yes-No N=22 |
No-No N=108 |
|
|
Change in IBS-SSS (mean ± S.D.) |
85.3 ± 38 | 60.1 ± 37 | 35.9 ± 32 | 19.7 ± 16 |
Difference between groups was significant at P<0.0001
Effects of Psychological Symptoms on the Responder Rate
There were no significant differences between the responders in NEO and Beck questionnaires from baseline to 3 weeks. Psychological symptoms did not correlate with responder status (Table 6).
Table 6. NEO and BECK correlation with responders: coefficient of correlation.
| IBS-AR | IBS-GAI | Change in IBS-SSS | Change in IBS-QOL | |
|---|---|---|---|---|
| Neuroticism | 0.6659 | 0.7813 | 0.9687 | |
| Extraversion | 0.2993 | 0.4223 | 0.1025 | |
| Beck Anxiety | 0.9532 | 0.5895 | 0.8187 | 0.6603 |
| Beck Depression | 0.7804 | 0.8286 | 0.4055 | 0.1924 |
Proportion reporting adequate relief and IBS-GAI is lowest in patients with severe at baseline, but the magnitude of symptom improvement is greatest in patients with severe IBS. No correlation was found in improvement IBS-QOL and severity of symptoms.
Discussion
Assessment of improvement in IBS clinical trials continues to evolve. Patient reported assessment of adequate relief of IBS symptoms has been used as a primary endpoint in a number of treatment trials and has been shown to be responsive to change, reproducible and correlate with bowel symptoms. However, a recent study by Whitehead and colleagues (18) performed in a usual care HMO found that patients with milder symptoms at baseline were more likely to report satisfactory relief than patients with moderate or severe symptoms. This finding might limit the value of global patient reported outcomes since baseline severity appears to confound their validity as gauges of treatment response. Whitehead et al. recommended that similar data be obtained via prospective assessment in a treatment trial. This seemed logical since the expectation for improvement in symptoms might be greater for patients entering a treatment trial compared to patients in a usual care situation. Furthermore, it is possible that patients seen in a usual care setting might report satisfactory relief at baseline since they are not a seeking to meet severity criteria for enrollment in a treatment trial.
Similar to the study by Whitehead and colleagues (18) we found that patients with milder IBS symptoms at baseline were more likely to report adequate relief during a treatment trial. It should be noted that other studies have not found such an association (29, 30). However, we also found that a significant proportion of patients reported adequate relief during their baseline assessment. When those patients were removed from the analysis there was no longer an inverse relationship of adequate relief with baseline severity. Because of this, we believe that patients who report adequate relief at baseline should not be considered eligible for a treatment trial. Removing patients with adequate relief at baseline addresses the issues raised by Whitehead and colleagues (18) and strengthens the validity of adequate relief as a primary patient reported outcome in IBS clinical trials.
Our study highlights the fact that patient reported adequate relief of symptoms is a multidimensional outcome that is influenced by a number of factors, including coping mechanisms and psychological status, which may not directly related to symptom severity. Thus patients may even have severe symptoms, yet the perception of adequate relief may be influenced in the positive direction by modifying factors including coping and adjustment to chronic illness. Notably, baseline IBS-QOL mirrored the severity of the symptoms as measured by IBS-SSS, highlighting the importance of quality of life in patient assessments of global severity.
This study has several limitations. First, this treatment trial was designed to assess the effects of acupuncture and practitioner-patient relationship on IBS and did not employ a pharmacological agent, which may have influenced patient expectations. Second, the study cohort consisted of relatively small number of patients reporting mild symptoms. Future research evaluating study endpoints should include a greater number of these patients. Third, our study was only 3 weeks in duration and we assessed adequate relief only at baseline and at 3 weeks. However, this observation must be balanced by our trial data which shows clinically meaningful responses. Therefore, future studies assessing adequate relief as an endpoint should include additional assessment points and should be of longer duration (i.e., weekly assessments over a 12 week intervention).
In conclusion, our study demonstrates that as an endpoint in an IBS treatment trial adequate relief is confounded by baseline symptom severity. However, when patients who report adequate relief at baseline are removed from analysis baseline severity no longer confounds response to adequate relief. Based on the results of our study we recommend that if adequate relief is going to be used as a primary endpoint that patients be tested for this at baseline and if they report adequate relief they should not enter into the study.
Acknowledgments
This research was made possible by NIH Grant Numbers 1R01 AT01414-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the National Institutes of Digestive, Diabetes and Kidney Disease (NIDDK), Grant Number 1R21 AT002860-01 from NCCAM and the Office of Behavioral and Social Science Research (OBSSR), and Grant Numbers 1 R21 AT002564 and 1K24 AT004095 from NCCAM. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Maria Passos was supported by a grant from National Council for Scientific and Technological Development (CNPq) from Brazil.
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Lembo A, Ameen VZ, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clin Gastroenterol Hepatol. 2005;3:717–25. doi: 10.1016/s1542-3565(05)00157-6. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580–9. doi: 10.1053/j.gastro.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ. Irritable bowel syndrome. Intern Med J. 2006;36:724–8. doi: 10.1111/j.1445-5994.2006.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tack J, Fried M, Houghton LA, et al. Systematic review: the efficacy of treatments for irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:183–205. doi: 10.1111/j.1365-2036.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 6.Mangel AW, Hahn BA, Heath AT, et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76–81. doi: 10.1177/030006059802600203. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M, Mayer EA, Drossman DA, et al. Improvement in pain and bowel function in female irritable bowel patients with alosetron, a 5-HT3 receptor antagonist. Aliment Pharmacol Ther. 1999;13:1149–59. doi: 10.1046/j.1365-2036.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Northcutt AR, Kong S, et al. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035–40. doi: 10.1016/S0140-6736(00)02033-X. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri M, Chey WY, Mayer EA, et al. A randomized controlled clinical trial of the serotonin type 3 receptor antagonist alosetron in women with diarrhea-predominant irritable bowel syndrome. Arch Intern Med. 2001;161:1733–40. doi: 10.1001/archinte.161.14.1733. [DOI] [PubMed] [Google Scholar]
- 10.Miner PBJ, Pruitt RE, Carter F. Cilansetron is efficacious in treating diarrheal symptoms in irritable bowel syndrome with diarrhea predominance (IBS-D) over 3 months. Gastroenterology. 2004;128:A29. [Google Scholar]
- 11.Bradette M, Moennikes H, Carter F, et al. Cilansetron in irritable bowel syndrome with diarrhoea predominance (IBS-D): efficacy and safety in a 6 month global study. Gastroenterology. 2004;126:A42. [Google Scholar]
- 12.Coremans G, Clouse RE, Carter F, et al. Cilansetron, a novel 5-HT3 antagonist demonstrated efficacy in males with irritable bowel syndrome with diarrhoea-predominance (IBS-D) Gastroenterology. 2004;126:A643. [Google Scholar]
- 13.Kellow J, Lee OY, Chang FY, et al. An Asia-Pacific, double blind, placebo controlled, randomised study to evaluate the efficacy, safety, and tolerability of tegaserod in patients with irritable bowel syndrome. Gut. 2003;52:671–6. doi: 10.1136/gut.52.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyhlin H, Bang C, Elsborg L, et al. A double-blind, placebo-controlled, randomized study to evaluate the efficacy, safety and tolerability of tegaserod in patients with irritable bowel syndrome. Scand J Gastroenterol. 2004;39:119–26. doi: 10.1080/00365520310006748. [DOI] [PubMed] [Google Scholar]
- 15.Tack J, Muller-Lissner S, Bytzer P, et al. A randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipation. Gut. 2005;54:1707–13. doi: 10.1136/gut.2005.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangel AW, Fehnel S. Adequate relief in IBS treatment trials: corrections to errors stated by Whitehead et al. Am J Gastroenterol. 2006;101:2884–5. 2885–7. doi: 10.1111/j.1572-0241.2006.00867_1.x. author reply. [DOI] [PubMed] [Google Scholar]
- 17.Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–51. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Palsson OS, Levy RL, et al. Reports of “satisfactory relief” by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol. 2006;101:1057–65. doi: 10.1111/j.1572-0241.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WG. Irritable bowel syndrome: a management strategy. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:453–60. doi: 10.1053/bega.1999.0039. [DOI] [PubMed] [Google Scholar]
- 20.Hammer J, Eslick GD, Howell SC, et al. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666–72. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanner SJ, Depew WT, Paterson WG, et al. Predictive value of the Rome criteria for diagnosing the irritable bowel syndrome. Am J Gastroenterol. 1999;94:2912–7. doi: 10.1111/j.1572-0241.1999.01437.x. [DOI] [PubMed] [Google Scholar]
- 22.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 23.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–11. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 24.Costa PJ, McCrae RR. Psychological Assessment Resources. Odessa, FL: Psychological Assessment Resources; 1985. The NEO personality inventory manual. [Google Scholar]
- 25.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 26.Lembo T, Wright RA, Bagby B, et al. Alosetron controls bowel urgency and provides global symptom improvement in women with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2662–70. doi: 10.1111/j.1572-0241.2001.04128.x. [DOI] [PubMed] [Google Scholar]
- 27.Gordon S, Ameen V, Bagby B, et al. Validation of irritable bowel syndrome Global Improvement Scale: an integrated symptom end point for assessing treatment efficacy. Dig Dis Sci. 2003;48:1317–23. doi: 10.1023/a:1024159226274. [DOI] [PubMed] [Google Scholar]
- 28.Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999–1007. doi: 10.1111/j.1572-0241.2000.01941.x. [DOI] [PubMed] [Google Scholar]
- 29.Ameen V, Heath AT, McSorley D, et al. Global measure of Adequate relief predicts clinically important difference in pain and is independent of baseline pain severity in Irritable bowel syndrome. Gastroenterology. 2007;132:A140. [Google Scholar]
- 30.Spiegel BM, Chang L, Kanwal F. Is asking “How are you doing” enough to capture health related quality of life (HRQOL) and overall severity in irritable bowel syndrome (IBS)? Gastroenterology. 2007;132:A140. [Google Scholar]