Abstract
The development of a safe and effective malaria vaccine is impeded by the complexity of the Plasmodium life cycle. A vaccine that elicits both cell-mediated and humoral immune responses might be needed for protection against this multistage parasitic infection. Apical membrane antigen 1 (AMA-1) plays a key role in erythrocytic invasion but is also expressed in sporozoites and in late stage liver schizonts, where it may provide a target of protective cell-mediated immunity (CMI). A Phase 1 trial of a vaccine consisting of recombinant AMA-1 protein and the Adjuvant system AS02A was conducted in 60 Malian adults aged 18–55 years who were randomized to receive either half-dose (25µg/0.25 ml) or full dose (50µg/0.5 ml) FMP2.1/AS02A or a control rabies vaccine. Interleukin 5 (IL-5) and interferon-γ (IFN-γ) production as evaluated by ELISpot and lymphocyte proliferation were measured after in vitro AMA-1 stimulation of peripheral blood mononuclear cells (PBMCs) collected on Days 0 and 90. Post-FMP2.1/AS02A immunization mean stimulation indices were significantly elevated as were the number of IL-5 spot forming cells (SFC)/106 PBMC, but no difference was noted in INF-γ production between the AMA-1/AS02A vaccinated group and the rabies group. These results provide evidence that complex immune responses can be induced by this vaccination strategy and add further impetus for the continuing clinical evaluation of this vaccine.
Keywords: Malaria, Vaccine, Cell-mediated immunity, Plasmodium falciparum
1. Introduction
Apical membrane antigen 1 (AMA-1) is an asexual blood-stage antigen that has been identified in all Plasmodium species examined and is a leading malaria vaccine candidate [1–3]. Although its precise biological function remains undefined, AMA-1 appears to play a role in erythrocytic invasion possibly by orienting merozoites and mediating close junctional apical contact [4,5] or direct erythrocytic binding [6]. The protein is comprised of an ectodomain in which non-overlapping groups of disulfide bonds define four separate domains (pro-domain and domains I, II, and III) [7]. Correct conformational folding is critical for the invocation of protective or inhibitory antibodies, implying that protection is directed at epitopes dependent upon correct disulfide bonding [7–9].
Antibodies against Plasmodium falciparum AMA-1 (PfAMA-1) inhibit antigen processing and merozoite invasion of erythrocytes [10] as well as P. falciparum growth in vitro [11,12]. Passive immunization of P. chabaudi-infected mice with AMA-1-specific polyclonal antibodies prevents lethal parasitemia [8]. Protective immunity against malaria has been elicited after immunization of mice and monkeys with recombinant AMA-1 [8,9,13–15]. Sero-prevalence studies have demonstrated the presence of antibodies to AMA-1 in malaria endemic areas [16,17] and in a longitudinal study of subjects parasitemic at entry, antibodies to AMA-1 were significantly associated with fewer episodes of clinical malaria [18].
While antibody-mediated immunity to AMA-1 appears to be critical for immune protection, AMA-1-specific T cell-mediated immunity (CMI) might also play a significant role in protective immunity. AMA-1 is also present on the sporozoite surface and a strong T cell response against AMA-1 may have an inhibitory effect on sporozoite invasion and exoerythrocytic stage development in the liver [19]. Just before the liver phase where CMI plays a key role in host defense, AMA-1 is translocated to the surface of the sporozoite, undergoes proteolytic cleavage and is essential to hepatocyte invasion [20,21]. T cells specific for AMA-1 may regulate antibody production as demonstrated in immunized BALB/c mice [9]. Evidence of antibody-independent protective CMI can be demonstrated by the finding that AMA-1 immunized mice and immunized B-cell-deficient mice, which are then depleted of CD4+ T cells, experience a loss of immunity unrelated to changes in antibody levels. CD4+ T cells specific for a conserved AMA-1 cryptic epitope transferred into athymic mice resulted in partial protection from parasite challenge [22]. T cell epitopes that elicit proliferative responses in Kenyan volunteers have been demonstrated in P. falciparum AMA-1 [23]. Strong T cell responses to AMA-1 were also seen in irradiated sporozoite-immunized volunteers protected against experimental malaria challenge [24]. Thus, while antibodies play a crucial role in protection post-AMA-1 immunization, antibody-independent CMI to AMA-1 contributes separately to the overall protective immune response in mice and might also do so in humans.
Immunization strategies that induce both a strong humoral response and CMI may be desirable in development of an AMA-1-based malaria vaccine. A Phase 1 trial of AMA-1/AS02A in malaria-naïve adults conducted at the Walter Reed Army Institute for Research (WRAIR) revealed strong humoral and cellular responses [3]. In the subsequent, presently described trial we measured CMI in semi-immune Malian adults after immunization with three doses of AMA-1 (FMP2.1/AS02A). Enhanced AMA-1-specific CMI was demonstrated in vaccine recipients as compared to control volunteers. These results, together with those demonstrating strong anti-AMA-1 antibody responses [25] provide evidence that multifaceted immune responses can be induced by this vaccination strategy and add further impetus for the continuing clinical evaluation of this vaccine.
2. Methods
2.1. Study design and vaccine administration
The clinical study was conducted at the Bandiagara Malaria Project research clinic in Bandiagara, a rural town of 13,634 inhabitants in the Dogon country in central Mali. Malaria transmission is seasonal and heavy with children aged less than 10 years having an average of two clinical malaria episodes per transmission season [26] and severe malaria affecting 2.3% of children less than 6 years of age [27]. The malaria transmission season extends from July to December. Venous blood was obtained from semi-immune Malian adults aged 18–55 years (mean age 28 years) on enrollment into a study evaluating the safety and immunogenicity of an AMA-1 malaria vaccine and again, at defined post-vaccination intervals [25]. The recombinant E. coli-expressed protein consists of a lyophilized preparation of a major portion of ectodomain of the 3D7 clone of PfAMA-1 (FMP2.1, Lot 0917) manufactured under current Good Manufacturing Practices (cGMP) by WRAIR Pilot Bio-production facility (Forest Glen, MD, United States) [28]. The AS02A adjuvant, manufactured by GSK Biologicals (Rixensart, Belgium), is composed of an oil-in-water emulsion and two immunostimulants, 3-deacylated monophosphoryl lipid A (MPL®) and QS21 derived from Quillaja saponaria (Antigenics Inc., Lexington, MA). A comparator vaccine consisting of the RabAvert® rabies vaccine (Chiron Corporation, Emeryville, CA), a sterile preparation of killed rabies virus was used. Vaccines were administered by intramuscular injection in the left deltoid muscle.
Sixty adults were randomized in a double-blind 2:1 ratio in two staggered cohorts. Cohort 1 was randomized to either half-dose (25µg) of FMP2.1 in 0.25 ml AS02A (n = 20) or comparator vaccine (n = 10) whereas Cohort 2 was randomized to either full dose (50µg) of FMP2.1 in 0.5 ml AS02A (n = 20) or comparator vaccine (n = 10). Volunteers were vaccinated at 0, 1, and 2 months and followed for 12 months after enrollment. The first vaccination was administered in December 2004 at the end of transmission season with the second and third doses administered in January and February 2005. Day 90 of the study was during March 2005. The trial was conducted in compliance with the International Conference on Harmonization Good Clinical Practices, the Declaration of Helsinki and regulatory requirements of Mali. Study protocols were reviewed and approved by institutional review boards (IRBs) of the University of Bamako, the University of Maryland, the United States Army Surgeon General and the National Institute of Allergy and Infectious Diseases. Individual informed consent was obtained from each participant prior to screening and enrollment. Consent of illiterate participants was documented by their thumbprints and by signatures of independent witnesses. Permission to import and administer the investigational products in Mali was granted by the Republic of Mali Ministry of Health. The trial was monitored by the United States Army Medical Materiel Development Activity and the National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases.
2.2. Peripheral blood mononuclear cell (PBMC) collection
Whole blood was collected in sterile EDTA tubes on enrollment, prior to vaccination (Day 0), and at Day 90 (30 days post-vaccination dose 3). Blood was refrigerated and processed by density centrifugation within 2 h of acquisition, utilizing lymphocyte separation medium (ICN Biomedical Inc.) following standard techniques. PBMC were linear rate frozen using isopropyl alcohol containers to −70 °C at the field site before transferring to liquid nitrogen storage containers for transportation to the University of Maryland. Immunologic assays were performed at the University of Maryland. The specific assays and methodology were chosen in order to compare results to those used in malaria-naïve volunteers recruited previously at WRAIR [3]. Vaccine grade antigen for the immunologic assays was kindly donated by WRAIR.
2.3. CMI assays
2.3.1. Lymphocyte proliferation assay
150,000 cells/well were plated in triplicates in 96-well plates, incubated with media, or FMP2.1 (AMA-1) at 3µg/ml for 6 days and 3H-thymidine incorporation determined [29]. Stimulation indices (SI) were calculated by dividing the mean counts per minute (cpm) of triplicate wells containing AMA-1-specific antigen by the mean cpm observed in media controls. Values that fell outside the 95% confidence interval (CI) of replicate wells were excluded from analysis. Positive samples were defined as (1) mean of wells with AMA-1 antigen differing significantly from media control by paired t-test (one-tailed, P < 0.05); (2) SI > 2; (3) mean cpm> 1000 over that of media control.
2.3.2. ELISpot
(a) Gamma interferon-(IFN-γ)-secreting cells were quantified using a modified cytokine enzyme-linked immunospot (ELISpot) assay [30]. 50,000 and 100,000 cells/well were plated in duplicate on 96-well plates (Millipore, MAHA, Bedford, MA) coated with 5µg/ml human anti-IFN-γ (clone 2G1, Endogen, Rockford, IL) and incubated overnight with AMA-1 (1.0 and 10µg/ml). Anti-CD28/anti-CD49d monoclonal antibodies (clones 28.2 and 9F10, respectively; Pharmingen, San Diego, CA) at 1µg/ml were added as co-stimulants [31]. The negative and positive controls were Aim-V media alone and anti-CD3/anti-CD28 beads (3µl/ml, Dynal Biotech, Oslo, Norway), respectively. Bound IFN-γ was detected using biotinylated anti-IFN-γ mAb (2µg/ml, clone B133.5, Endogen) followed by a 2-h incubation. Avidin peroxidase (1:400) was added followed by 1-h incubation. A 50:1 dilution of True Blue substrate (Kirkegaard and Perry Lab, Gaithersburg, MD) was added for 15 min followed by a distilled water wash. (b) Interleukin 5 (IL-5) secreting cells were quantified using an ELISpot kit (Mabtech USA, Cincinnati, OH) [32]. Cells were plated in duplicate at 200,000 and 400,000 cells/well on 96-well plates coated with 15µg/ml human anti-IL-5 (clone TRFK5) and incubated overnight with AMA-1 (1.0 and 10µg/ml). Aim-V media and phytohemagglutinin (PHA) at 1µg/ml (Sigma Leucoagglutinin (PHA-L) lectin from Phaseolus vulgaris L-2769) were used as negative and positive controls, respectively. Bound IL-5 was detected using biotinylated anti-IL-5 mAb (2µg/ml, clone 5A10) followed by avidin peroxidase and True Blue substrate detection of spots as described above. For both ELISpot assays, cells were counted both manually and with the aid of an automatic counter (Bioreader 3000, BioSys Inc., The Colony, TX). Stringent criteria identified responders: (1) stimulant wells >8 spots per well, (2) the number of spots in stimulant wells greater than twice that in media control wells and (3) the mean number of spots in stimulant wells differing significantly from media controls by paired t-test (one-tailed, P < 0.05). Responders were defined as subjects with a detectable response to antigen at either the 100,000 cell or at both the 50,000 and 100,000 cell concentrations. Media results were subtracted from those observed in stimulant wells and expressed as net SFC/106 PBMC. CMI results were excluded if anti-CD3/anti-CD28 stimulated cells failed to exhibit strong (>800 SFC/106 PBMC or >5 SI) responses. All experiments were done in a blinded, randomized fashion using standardized reagents of identical lots.
2.4. Statistical analysis
Analyses of differences in IFN-γ and IL-5 production by ELISpot or proliferation of cells between clinical groups were performed using χ2-analysis (EpiInfo; Center for Disease Control and Prevention; 2000). A two-sided Student’s t-test was used for analysis of continuous variables with equal variance and χ2-test for categorical variables using SPSS 10.0 (2000; SPSS Corp., Chicago, IL). Spearman rank order correlation (SigmaStat 3.0) was used to analyze the correlation of CMI and antibody assays at Day 90 since the data did not appear to meet assumptions about normality, hemoscedasticity, and linearity. Statistical significance was set at a P < 0.05 without adjustment for multiple comparisons.
3. Results
3.1. Lymphocyte proliferation
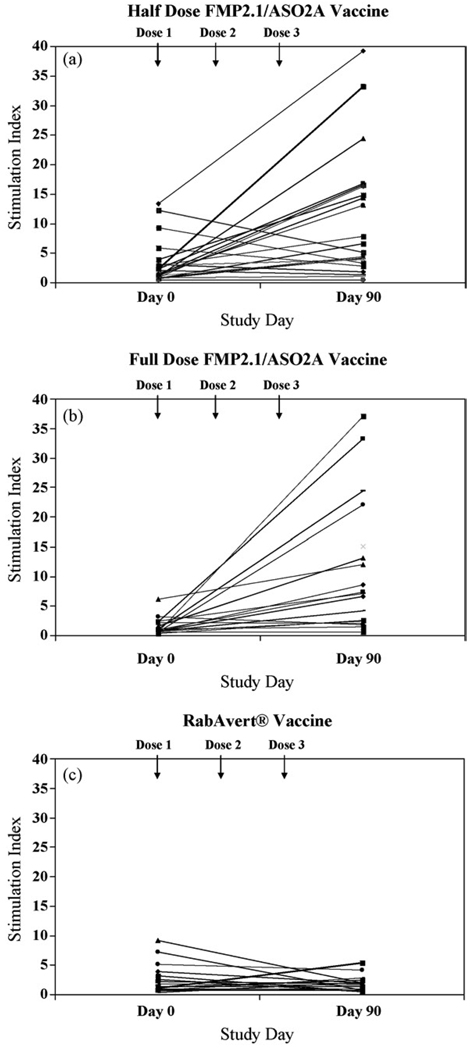
Lymphocyte proliferation assays were performed with PBMC from volunteers on Day 0 and 90. Fifty-five of 60 volunteers had PBMC from both time points (19 controls receiving RabAvert®, 18 volunteers receiving the half-dose FMP2.1/AS02A and 18 volunteers receiving the full dose FMP2.1/AS02A). Three participants had insufficient cells collected for analysis and two participants’ PBMC did not respond to the positive control stimuli. The Day 0 geometric mean SI for all participants with paired samples was 1.63 (range 0.33–13.33) (1.59 for RabAvert®, 2.38 for half-dose FMP2.1/AS02A, and 1.15 for full dose FMP2.1/AS02A; no statistical difference was noted between groups) (Table 1). The Day 90 geometric mean SI was 1.41 for RabAvert®, 5.19 for half-dose FMP2.1/AS02A, and 6.53 for full dose FMP2.1/AS02A. No differences in SI were noted between the half and full dose FMP2.1/AS02A. Intercohort analyses reveal higher FMP2.1/AS02A Day 90 vaccine responses compared to RabAvert® (25µg: 5.19 vs. 1.08, P = 0.005 and 50µg: 6.53 vs. 1.88, P = 0.004). Paired pre- and post-vaccination SI is depicted in Fig. 1. The SI was positive in 28/37 (76%) of the combined FMP2.1/AS02A vaccine recipients compared to 4/19 (21%) of RabAvert® recipients (Yates corrected χ2 < 0.0001, 95% CI [0.2–0.54]).
Table 1.
Summary of lymphocyte proliferative responses as group mean stimulation indices as measured by 3H-thymidine incorporation in PBMC from semi-immune Malian adults receiving either the three-dose series of FMP2.1/AS02A (half (25µg/0.25 ml) or full (50µg/0.5 ml) dose) or the comparator RabAvert® vaccine.
| Cohort | Vaccine (n) | Stimulation index Day 0a | Stimulation index Day 90 | P valueb(study vs. control) | P value (Day 0 vs. Day 90) |
|---|---|---|---|---|---|
| 1 | Half-dose FMP2.1/AS02A (18) | 2.38 | 5.19 | nsc | |
| RabAvert® (10) | 2.00 | 1.08 | 0.005 | ns | |
| 2 | Full dose FMP2.1/AS02A (18) | 1.15 | 6.53 | 0.002 | |
| RabAvert® (9) | 1.19 | 1.88 | 0.004 | ns | |
| Combined | FMP2.1/AS02A (36) | 1.65 | 5.84 | 0.002 | |
| RabAvert® (19) | 1.59 | 1.41 | <0.0001 | ns |
(n) = (number of volunteers).
No statistical difference of stimulation indices noted between cohorts at Day 0, pre-vaccination.
Statistical analysis performed on Day 90 FMP2.1 vaccine vs. RabAvert® comparator vaccine with level of significance defined as P < 0.05.
Not statistically significant (ns).
Fig. 1.
Lymphocyte proliferative responses in subjects immunized with FMP2.1/AS02A or RabAvert®, Paired Day 0 and 90 stimulation indices (SI) of proliferating PBMC from semi-immune Malian adults receiving either the three-dose series of FMP2.1/AS02A (a) half (25µg/0.25 ml) or (b) full (50µg/0.5 ml) doses or (c) comparator RabAvert® vaccine.
3.2. ELISpot
(a) ELISpot assays (IFN-γ and IL-5) were performed on paired PBMC from Days 0 to 90 in 56 of 60 enrolled volunteers. The mean IFN-γ secretion by PBMC in response to AMA-1 (1 and 10µg/ml) as detected by ELISpot was quantified. Media SFC were subtracted from final SFC counts and expressed as SFC/106 PBMC (Table 2). A measurable background response to AMA-1 stimuli was noted at both the 1 and 10µg/ml concentrations for all groups (105–182 mean SFC/106 PBMC) with 55% of PBMC collected from volunteers before vaccination (Day 0) producing IFN-γ. A significant increase in detectable IFN-γ production was noted from Days 0 to 90 for both the FMP2.1/AS02A and the RabAvert® groups. However, there were no differences between the mean SFC/106 PBMC from the vaccine group compared to the control vaccine group. Similarly, there were no differences in the amounts of IFN-γ secreted by PBMC from volunteers who received the full and half-doses of FMP2.1/AS02A. (b) The mean IL-5 secretion by PBMC in response to AMA-1 (1 and 10µg/ml) as detected by ELISpot was also quantified (Table 2). Little detectable IL-5 secretion was measured before vaccination (1.5–3.3 SFC/106 PBMC). Significant increases in IL-5 production were observed at Day 90 for both the half and full dose FMP2.1/AS02A groups compared to Day 0 results and the RabAvert® group. The half-dose FMP2.1/AS02A group had the highest cytokine secretion measured although this was not significantly different than the full dose FMP2.1/AS02A group (1µg/ml: 90.5 vs. 38.6 SFC/106 PBMC, P=0.13, 10µg/ml: 121.5 vs. 69.4 SFC/106 PBMC, P = 0.19). A small but significant increase in IL-5 secretion was also noted between Days 0 and 90 RabAvert® results after stimulation of PBMC with 10µg/ml of AMA-1 (3.3 vs. 42.4 SFC/106 PBMC, P = 0.01). This was also observed between Days 0 and 90 in the RabAvert® group in cultures stimulated with 1.0µg/ml AMA-1 but was statistically insignificant.
Table 2.
Summary of ELISpot responses as measured by SFC/106 peripheral blood mononuclear cells (PBMC) after stimulation with apical membrane antigen 1 (AMA-1) at 1.0 or 10.0µg/ml concentrations in semi-immune Malian adults receiving either the three-dose series of FMP2.1/AS02A (half (25µg/0.25 ml) or full (50µg/0.5 ml) dose) or the comparator RabAvert® vaccine.
| Vaccine (n) | Cytokine | AMA-1 concentration (µg) |
Spot forming cells/106 cells Day 0a |
Spot forming cells/106 cells Day 90 |
P valueb(study vs. control) |
P value (Day 0 vs. Day 90) |
|---|---|---|---|---|---|---|
| Half-dose FMP2.1/AS02A (19) | IFN-γ | 1 | 126 | 222 | ns | nsc |
| 10 | 138 | 313 | ns | 0.01 | ||
| Full dose FMP2.1/AS02A (18) | IFN-γ | 1 | 131 | 243 | ns | 0.01 |
| 10 | 182 | 378 | ns | 0.01 | ||
| Combined FMP2.1/AS02A (37) | IFN-γ | 1 | 129 | 233 | ns | 0.01 |
| 10 | 161 | 347 | ns | 0.02 | ||
| RabAvert® (19) | IFN-γ | 1 | 105 | 244 | – | 0.02 |
| 10 | 163 | 347 | – | <0.001 | ||
| Half-dose FMP2.1/AS02A (19) | IL-5 | 1 | 1.5 | 90.5 | 0.06 | 0.01 |
| 10 | 2.3 | 121.5 | 0.049 | 0.004 | ||
| Full dose FMP2.1/AS02A (18) | IL-5 | 1 | 1.6 | 38.6 | ns | 0.006 |
| 10 | 2.0 | 69.4 | ns | <0.001 | ||
| Combined FMP2.1 (37) | IL-5 | 1 | 1.5 | 63.1 | 0.08 | <0.001 |
| 10 | 2.1 | 94 | 0.029 | <0.001 | ||
| RabAvert® (19) | IL-5 | 1 | 2.1 | 25.1 | – | ns |
| 10 | 3.3 | 42.4 | – | 0.01 |
(n) = (number of volunteers).
SFC results represent mean number of spots measured per 106 PBMC plated. No statistical differences in SFC were noted between cohorts at Day 0, pre-vaccination.
Statistical analysis performed on Day 90 FMP2.1 vaccine vs. RabAvert® comparator vaccine with level of significance defined as P < 0.05. Italicized results are reported when close to the level of significance.
Not statistically significant (ns).
3.3. Correlation of immunologic responses
Anti-FMP2.1 antibody responses were determined by enzyme-linked immunosorbent assay (ELISA) at WRAIR and previously reported [25]. We studied the correlation of immunological responses by analyzing the results of the CMI assays against the antibody response and also between concomitant CMI assays. Correlations were noted between the lymphocyte proliferative and anti-FMP2.1 antibody responses [correlation coefficient (ρ) = 0.543, P < 0.001] and between IL-5 secretion as measured by ELISpot assay and anti-FMP2.1 antibody responses (ρ = 0.383, P = 0.003). A correlation was also observed between the results of the IL-5 ELISpot assay and the lymphocyte proliferative responses (ρ = 0.526, P < 0.001). No correlation was observed between IFN-γ secretion as measured by ELISpot and all other immunological measurements.
4. Discussion
The FMP2.1/AS02A malaria vaccine appears to induce detectable cellular as well as humoral responses in malaria semi-immune adults. Measurable T cell-mediated immune responses (IL-5 production and lymphocyte proliferative responses) were detected in semi-immune malaria-exposed adults who received the AMA-1 vaccine. A balanced Th1/T2 cytokine response has been described in preclinical monkey trials after vaccination with AS02A adjuvanted to P. falciparum MSP142 antigen as compared to other adjuvants (AS01B, AS05, and AS08) formulated to MSP142 which invoked an enhanced Th1 response [33]. Likewise, both IFN-γ and IL-5 production has been described in preclinical studies with RTS,S/AS02A [34], and Phase I studies in malaria-naïve U.S. volunteers vaccinated with FMP2.1/AS02A [3]. Enhanced IL-5 production was noted in the half-dose FMP2.1/AS02A group as compared to the full dose FMP2.1/AS02A group. While the significance of this is unclear, the paradoxical findings of higher CMI responses in response to lower doses of adjuvanted protein, was also observed in U.S. malaria-naïve volunteers receiving AMA-1/AS02A [3] as well as volunteers receiving AMA-1/AS01B, Liver stage antigen 1 (LSA-1)/AS02A and LSA-1/AS01B (M. Spring, J. Cummings and D.G. Heppner, personal communication). In contrast to the results seen in the U.S.-based trial, no differences in IFN-γ production were noted between recipients of FMP2.1/AS02A and the comparator rabies vaccine [3].
Protective immunity to malaria has been shown to correlate with detectable cellular immune responses. B and T cell responses have been measured in response to defined AMA-1 epitopes in volunteers naturally exposed to malaria [17,23]. Moreover, proliferative responses to parasitized RBC (pRBC) in U.S. volunteers immunized with attenuated falciparum sporozoites correlated with protection against subsequent sporozoite challenge [24]. We detected measurable increases in cellular immunologic responses to AMA-1 after vaccination in an adult Malian population. This is encouraging in that responses to vaccination are detectable against a high background of acquired immunity. Differences in immune responses between vaccine and control groups may be more pronounced in the target population of children who have less acquired immunity. The paucity of long-lived T cell memory responses that develop in response to malaria antigens even in the setting of repetitive antigen exposure has long been noted. The immune response to blood stage malaria antigens appears to evolve over years and acquisition of immunity may continue into adulthood [17]. While T helper cells may facilitate AMA-1-specific antibody production [9], other effector T cell functions may contribute to host immunity independently of antibody. Immunization with recombinant P. chabaudi AMA-1 in mice stimulates immune protection against homologous P. chabaudi infection. This appears to be partially mediated by CD4+ cells in an antibody-independent fashion but in synergism with anti-AMA-1 antibodies [22]. In this model, IFN-γ was not detected in response to vaccination and challenge. This parallels our findings showing no difference in IFN-γ production as measured by ELISpot between vaccine groups at Day 90 but contrasts with the findings in malaria-naïve adults in which potent IFN-γ production was measured in response to vaccination [3]. Of note, in the latter study vaccination also stimulated dose dependent IL-5 production in malaria-naïve adults. In areas of ongoing malaria transmission, IFN-γ production to this vaccine as measured by ELISpot may not serve as a relevant measure of CMI acquisition. Whether a Th2-biased CMI, as evidenced by IL-5 production, correlates with enhanced protective efficacy will be assessed in greater detail in upcoming Phase 2 vaccine trials.
Vaccinations occurred between December 2004 and February 2005 and Day 90 serum was collected in March 2005 at the height of the dry season when malaria transmission is virtually undetectable. Samples were processed in random order, with representative Day 0 and 90 samples included in each experiment using identical reagents and lots of fetal calf serum for cell cryopreservation and for processing of immunologic assays. Negligible IL-5 was detected pre-vaccination (Day 0). In contrast, detectable IFN-γ was noted at Day 0 in both vaccine recipients and RabAvert® controls. This observation is likely the result of CMI acquired by ongoing exposure to parasites during the transmission season. Similarly, 20/56 (35.7%) volunteers had detectable lymphocyte proliferative responses at Day 0 as well as antibody responses with significant vaccine-induced boosting peaking by Day 74 [25].Modest increases in IFN-γ production were detected after stimulation of post-vaccination PBMC (collected during the dry season) with AMA-1 in all groups, including the rabies comparator. The mechanism(s) for these increases in the absence of ongoing malaria transmission are unclear. It is possible that during the malaria transmission season, persistent sub-clinical infection, which is common in semi-immune adults, results in continuous exposure to high levels of malaria antigens leading to down-regulation of IFN-γ responses to these antigens and to decreased inflammatory responses. Similarly, it is known that inducible T regulatory cells may influence the immunologic response to malaria infection during transmission season [35]. These phenomena might not occur during the dry season when exposure to malaria antigens is greatly diminished. Blood smears were not done on asymptomatic individuals so we cannot investigate these theories. Potential explanations for the absence of a measurable vaccine-induced increase in IFN-γ production as detected by ELISpot may have been the failure of the vaccine to induce persistent cellular response in this population. Additionally, we sampled PBMC 28-day post-dose three in this study whereas in the previous study in malaria naïve adults, we sampled PBMC at 14 days post-dose three and did find significant increases in IFN-γ by ELISpot. Other potential factors include a technical difference in the assay whereby PBMC were plated at 50,000 and 100,000 per well from the Malian adult study (to reduce background) compared to 200,000 per well PBMC from the malaria naïve adults.
Both the lymphocyte proliferation and the IL-5 ELISpot assays demonstrated a clear increase in CMI correlating with vaccination. A mild but significant increase in IL-5 production in the control group receiving rabies vaccine at Day 90 was noted in response to antigenic stimulation by AMA-1 (10µg/ml). This increase was modest in comparison to the vaccine recipients and was not observed in cultures stimulated with 1µg/ml AMA-1. The results suggest that Th2 cytokine responses may be a better indicator of this vaccine’s “take” than Th1 responses represented by IFN-γ although more research needs to be performed including measurement of other Th2 cytokine responses to validate this contention and to assess the impact of the adjuvant in this response. Furthermore, the fact that close correlations were observed between antibody response, lymphocyte proliferation responses and IL-5 production argues strongly for this vaccine construct being able to elicit a wide array of host immune responses. An ongoing Phase 2 trial of this vaccine in children will assess associations between these immune responses and protection against disease and measure the impact of parasite diversity on CMI and antibody responses.
We have demonstrated a CMI response in relation to vaccination with FMP2.1/AS02A in malaria-experienced Malian adults. Our studies demonstrate AMA-1-specific lymphocyte proliferation and IL-5 production in response to vaccination and a close correlation of these CMI responses to antibody production. Whether these CMI responses are mainly supporting antibody production or represent other important mechanisms of CMI effector immunity (e.g., expansion of T memory subsets, production of other cytokines not evaluated in the present study, etc.) remains to be determined. Nevertheless, the finding that a multifaceted immune response is elicited after a three-dose vaccination series with AMA-1 is encouraging for future development of this protein construct alone or as part of a vaccine comprised of multiple malaria antigens. If protective efficacy is demonstrated in the Phase 2 pediatric trial of the FMP2.1/AS02A vaccine that is presently underway in Mali the cellular immunity associated with this protection will be studied.
Acknowledgements
Vaccine production and laboratory assays were supported by the United States Agency for International Development, Washington, DC and by the Military Infectious Diseases Research Program, Fort Detrick, MD. The authors would like to thank the laboratory of Urszula Krzych for help in developing laboratory assay methodology, and Ms. Lisa Ware, Project Manager at WRAIR for her support. We would expressly like to acknowledge the population of Bandiagara who so graciously consented to participate in this trial and for whom we strive to develop a malaria vaccine.
References
- 1.Saul A, Lawrence G, Allworth A, Elliott S, Anderson K, Rzepczyk C, et al. A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant. Vaccine. 2005 April 27;23(23):3076–3083. doi: 10.1016/j.vaccine.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, et al. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun. 2005 June;73(6):3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polhemus ME, Magill AJ, Cummings JF, Kester KE, Ockenhouse CF, Lanar DE, et al. Phase I dose escalation safety and immunogenicity trial of Plasmodium falciparum apical membrane protein (AMA-1) FMP2.1, adjuvanted with AS02A, in malaria-naive adults at the Walter Reed Army Institute of Research. Vaccine. 2007 March 26;(25):4203–4212. doi: 10.1016/j.vaccine.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GH, Thomas AW, Margos G, Dluzewski AR, Bannister LH. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect Immun. 2004 January;72(1):154–158. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters AP, Thomas AW, Deans JA, Mitchell GH, Hudson DE, Miller LH, et al. A merozoite receptor protein from Plasmodium knowlesi is highly conserved and distributed throughout Plasmodium. J Biol Chem. 1990 October 15;265(29):17974–17979. [PubMed] [Google Scholar]
- 6.Kato K, Mayer DC, Singh S, Reid M, Miller LH. Domain III of Plasmodium falciparum apical membrane antigen 1 binds to the erythrocyte membrane protein Kx. Proc Natl Acad Sci USA. 2005 April 12;102(15):5552–5557. doi: 10.1073/pnas.0501594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodder AN, Crewther PE, Matthew ML, Reid GE, Moritz RL, Simpson RJ, et al. The disulfide bond structure of Plasmodium apical membrane antigen-1. J Biol Chem. 1996 November 15;271(46):29446–29452. doi: 10.1074/jbc.271.46.29446. [DOI] [PubMed] [Google Scholar]
- 8.Anders RF, Crewther PE, Edwards S, Margetts M, Matthew ML, Pollock B, et al. Immunisation with recombinant AMA-1 protects mice against infection with Plasmodium chabaudi. Vaccine. 1998 January;16(2–3):240–247. doi: 10.1016/s0264-410x(97)88331-4. [DOI] [PubMed] [Google Scholar]
- 9.Amante FH, Crewther PE, Anders RF, Good MF. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J Immunol. 1997 December 1;159(11):5535–5544. [PubMed] [Google Scholar]
- 10.Dutta S, Haynes JD, Moch JK, Barbosa A, Lanar DE. Invasion-inhibitory antibodies inhibit proteolytic processing of apical membrane antigen 1 of Plasmodium falciparum merozoites. Proc Natl Acad Sci USA. 2003 October 14;100(21):12295–122300. doi: 10.1073/pnas.2032858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodder AN, Crewther PE, Anders RF. Specificity of the protective antibody response to apical membrane antigen 1. Infect Immun. 2001 May;69(5):3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocken CH, Withers-Martinez C, Dubbeld MA, van der WA, Hackett F, Valderrama A, et al. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect Immun. 2002 August;70(8):4471–4476. doi: 10.1128/IAI.70.8.4471-4476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stowers AW, Kennedy MC, Keegan BP, Saul A, Long CA, Miller LH. Vaccination of monkeys with recombinant Plasmodium falciparum apical membrane antigen 1 confers protection against blood-stage malaria. Infect Immun. 2002 December;70(12):6961–6967. doi: 10.1128/IAI.70.12.6961-6967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins WE, Pye D, Crewther PE, Vandenberg KL, Galland GG, Sulzer AJ, et al. Protective immunity induced in squirrel monkeys with recombinant apical membrane antigen-1 of Plasmodium fragile. Am J Trop Med Hyg. 1994 December;51(6):711–719. doi: 10.4269/ajtmh.1994.51.711. [DOI] [PubMed] [Google Scholar]
- 15.Deans JA, Knight AM, Jean WC, Waters AP, Cohen S, Mitchell GH. Vaccination trials in rhesus monkeys with a minor, invariant, Plasmodium knowlesi 66 kDa merozoite antigen. Parasite Immunol. 1988 September;10(5):535–552. doi: 10.1111/j.1365-3024.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomas AW, Trape JF, Rogier C, Goncalves A, Rosario VE, Narum DL. High prevalence of natural antibodies against Plasmodium falciparum 83-kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am J Trop Med Hyg. 1994 December;51(6):730–740. doi: 10.4269/ajtmh.1994.51.730. [DOI] [PubMed] [Google Scholar]
- 17.Udhayakumar V, Kariuki S, Kolczack M, Girma M, Roberts JM, Oloo AJ, et al. Longitudinal study of natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in a holoendemic region of malaria in western Kenya: Asembo Bay Cohort Project VIII. Am J Trop Med Hyg. 2001 August;65(2):100–107. doi: 10.4269/ajtmh.2001.65.100. [DOI] [PubMed] [Google Scholar]
- 18.Polley SD, Mwangi T, Kocken CH, Thomas AW, Dutta S, Lanar DE, et al. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine. 2004 December 16;23(5):718–728. doi: 10.1016/j.vaccine.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Howell SA, Well I, Fleck SL, Kettleborough C, Collins CR, Blackman MJ. A single malaria merozoite serine protease mediates shedding of multiple surface proteins by juxtamembrane cleavage. J Biol Chem. 2003 June 27;278(26):23890–23898. doi: 10.1074/jbc.M302160200. [DOI] [PubMed] [Google Scholar]
- 20.Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, et al. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004 March 5;279(10):9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 21.Florens L, Washburn MP, Raine JD, Anthony RM, Grainger M, Haynes JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002 October 3;419(6906):520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Hodder AN, Yan H, Crewther PE, Anders RF, Good MF. CD4+ T cells acting independently of antibody contribute to protective immunity to Plasmodium chabaudi infection after apical membrane antigen 1 immunization. J Immunol. 2000 July 1;165(1):389–396. doi: 10.4049/jimmunol.165.1.389. [DOI] [PubMed] [Google Scholar]
- 23.Lal AA, Hughes MA, Oliveira DA, Nelson C, Bloland PB, Oloo AJ, et al. Identification of T-cell determinants in natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in an adult population exposed to malaria. Infect Immun. 1996 March;64(3):1054–1059. doi: 10.1128/iai.64.3.1054-1059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krzych U, Lyon JA, Jareed T, Schneider I, Hollingdale MR, Gordon DM, et al. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J Immunol. 1995 October 15;155(8):4072–4077. [PubMed] [Google Scholar]
- 25.Thera MA, Doumbo OK, Coulibaly D, Diallo DA, Kone AK, Guindo AB, et al. Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial. PLoS ONE. 2008;3(1):e1465. doi: 10.1371/journal.pone.0001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coulibaly D, Diallo DA, Thera MA, Dicko A, Guindo AB, Kone AK, et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg. 2002 December;67(6):604–610. doi: 10.4269/ajtmh.2002.67.604. [DOI] [PubMed] [Google Scholar]
- 27.Lyke KE, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, et al. Incidence of severe Plasmodium falciparum malaria as a primary endpoint for vaccine efficacy trials in Bandiagara, Mali. Vaccine. 2004 August 13;22(23–24):3169–3174. doi: 10.1016/j.vaccine.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 28.Dutta S, Lalitha PV, Ware LA, Barbosa A, Moch JK, Vassell MA, et al. Purification, characterization, and immunogenicity of the refolded ectodomain of the Plasmodium falciparum apical membrane antigen 1 expressed in Escherichia coli. Infect Immun. 2002 June;70(6):3101–3110. doi: 10.1128/IAI.70.6.3101-3110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, et al. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun. 2000 March;68(3):1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain Ty21a typhoid vaccine. J Immunol. 2002 August 15;169(4):2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 31.Waldrop SL, Davis KA, Maino VC, Picker LJ. Normal human CD4+ memory T cells display broad heterogeneity in their activation threshold for cytokine synthesis. J Immunol. 1998 November 15;161(10):5284–5295. [PubMed] [Google Scholar]
- 32.Jakobson E, Masjedi K, Ahlborg N, Lundeberg L, Karlberg AT, Scheynius A. Cytokine production in nickel-sensitized individuals analysed with enzyme-linked immunospot assay: possible implication for diagnosis. Br J Dermatol. 2002 September;147(3):442–449. doi: 10.1046/j.1365-2133.2002.04850.x. [DOI] [PubMed] [Google Scholar]
- 33.Pichyangkul S, Gettayacamin M, Miller RS, Lyon JA, Angov E, Tongtawe P, et al. Pre-clinical evaluation of the malaria vaccine candidate P. falciparum MSP1(42) formulated with novel adjuvants or with alum. Vaccine. 2004 September 28;22(29–30):3831–3840. doi: 10.1016/j.vaccine.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Stewart VA, McGrath SM, Walsh DS, Davis S, Hess AS, Ware LA, et al. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS S/AS02A. Vaccine. 2006 October 30;24(42–43):6483–6492. doi: 10.1016/j.vaccine.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 35.Torcia MG, Santarlasci V, Cosmi L, Clemente A, Maggi L, Mangano VD, et al. Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 2008 January 15;105(2):646–651. doi: 10.1073/pnas.0709969105. [DOI] [PMC free article] [PubMed] [Google Scholar]