Abstract
Background
Seizures are a common presenting feature in children with cerebral malaria (CM) and neurologic deficits have been described in survivors of CM. However few prospective studies have described the frequency of seizure activity and neurologic deficits in survivors of CM over time.
Methods
Eighty-two children aged 3 to 12 years who survived an episode of CM were followed up and monitored for seizure activity and neurologic deficits at discharge, 3, 6 and 24 months. Seventy six children with uncomplicated malaria (UM) and 105 healthy community controls (CC) age 3 to 12 years were recruited as comparison groups and the frequency of seizures in the 6 to 24 month follow-up period was compared in the 3 groups.
Results
Cumulative incidence of seizures increased over time in children with CM, with a total of 2 of 76 children (2.6%) reporting seizures at 3 months, 3 of 74 children (4.1%) at 6 months and 11 of 68 children (16.2%) at 24 months (Chi square for trend = 9.36, P=0.002). In contrast, neurologic deficits almost completely resolved over time, occurring in 19 of 76 children with CM (25%) at discharge, 2 of 74 children (2.7%) at 6 months, and 1 of 68 (1.5%) children at 24 months.
Conclusions
During the 24 months following a CM episode, neurologic deficits resolve but the cumulative incidence of seizures increases in children with CM. Neurologic impairment after an episode of CM may not be limited to the neurologic deficits seen at discharge.
Keywords: Seizures, cerebral malaria, neurological deficits, survivors, children
Running head title: Seizure activity in survivors of cerebral malaria
Introduction
Cerebral malaria (CM) is the most severe neurological complication of malaria. Even with appropriate antimalarial treatment, an estimated 19.2% of patients die of the disease and 10–17% of survivors have some form of neurologic deficit1. While some of the neurological deficits (like ataxia) are transient lasting not more than six months, others (like hemiplegia) are persistent and lead to permanent impairments while others like epilepsy appear much later in life for some survivors 2.
Seizures are a common complication of CM, occurring in more than 80 % of children at the time of presentation and in more than 50% of the children during hospitalization 3, 4. Seizures associated with CM tend to be more complex than simple febrile seizures associated with fevers5. They also tend to be prolonged and recur several times during the illness, they may be subtle and only seen on EEG, and may be focal or generalised5. Observational studies have noted that CM patients who have prolonged or multiple seizures (subtle or unsubtle) have worse outcomes4, 6 and other studies have shown that repeated seizures may lead to development of epilepsy later in life7–9. Few studies have however examined the frequency of seizures in CM survivors.
Retrospective studies suggest an association between increased frequency of seizures and CM but recall bias and other flaws may affect retrospective studies. Secondly the timing of when seizures start to occur after CM is not defined, though retrospective studies suggest both early10 and late onset11.
Several studies have described various neurological deficits in CM patients discharged from hospitals7–9, 12, 13. Most of these studies however did not follow CM survivors beyond six months after discharge; the long-term impact of CM therefore remains poorly described.
The present study was designed to prospectively assess frequency of seizures and neurologic deficits in children with CM and their development or resolution over a 24 month period of follow-up. We also compared seizure frequency in CM over the 6 to 24 month follow-up period to a similar group of children with uncomplicated malaria and to a group of otherwise healthy children from the same community.
Methods and Materials
Study site
The study was done at Mulago Hospital which is the national referral hospital of Uganda and the teaching hospital of Makerere of University Medical School. It also serves as the district hospital for Kampala and the surrounding areas. Children with CM were recruited from the paediatric acute care unit (ACU), the paediatric emergency unit at Mulago that admits children (up to 12 years) with medical conditions for management and observation before being transferred to the main paediatric wards or discharged and followed up as outpatients.
Study design
A prospective cohort study design was undertaken to monitor children who had survived CM and compare them to a similar group of children with uncomplicated malaria (UM) and healthy community controls (CC). Written informed consent was obtained from the parents or guardians of study participants. Ethical approval for the study was granted by the Institutional Review Boards for Human Studies at Makerere University Faculty of Medicine, University Hospitals of Cleveland, Case Western Reserve University, Indiana Wesleyan University, University of Minnesota and the Uganda National Council for Science and Technology.
Study population
In this study we followed up a cohort of children enrolled in an earlier study describing the short term (six months) neurological and cognitive sequelae of cerebral malaria. Details of enrolment and follow up have been described elsewhere14 but briefly; children aged 3 to 12 years were enrolled if they were admitted to the Acute Care Unit (ACU) at Mulago Hospital and met the WHO criteria for CM: coma (Blantyre coma scale d”2 or Glasgow coma scale d”8), P. falciparum on blood smear, and no other cause for coma). Children with uncomplicated malaria (UM) and community children (CC) aged 3 to 12 years without evidence of acute illness were also recruited. Children with UM were recruited from the ACU and the Makerere University-University of California San Francisco (MU-UCSF) clinic presenting with malaria without other complications. The CC children were recruited from the families or neighbourhoods of the CM and UM children. The total numbers of children included in the analysis were the numbers available from the original study14.
Measurements and study instruments
Children who met inclusion criteria and whose parents provided consent were taken over by the study physicians who provided appropriate care and monitored the children throughout the hospitalisation and the follow up period. Upon recruitment all CM children had complete clinical history and physical examination performed by the study physicians. Data was extracted on history of present illness, including a description of associated seizures before and during the illness. Neurologic features monitored during the hospitalisation included level of consciousness, posture, motor function, deep tendon reflexes and abnormal movements. A detailed neurologic examination using a standardised questionnaire was performed by the study neurologist on day six of hospitalisation or the day before discharge to determine gross neurologic impairments. Vision was assessed by perception of a light source or moving objects and hearing was assessed by the child's responses to a source of sound.
Children with UM were assessed to rule out any features of severe malaria like impaired consciousness, seizures, severe pallor and CC children were assessed to rule out any obvious illnesses. Nutrition was assessed by comparing weight for age to standardised references using z-scores (Epi Info 6; Centers for Disease Control and Prevention, Atlanta, GA)15. Socioeconomic status was assessed using a scoring instrument incorporating a checklist of material possessions, quality of home environment and home structure, living density, food resources, cooking and bathroom facilities, and access to electricity and clean water as previously described16.
Follow up
The children were asked to return for follow-up assessments at 3, 6 and 24 months after discharge for the CM group or after recruitment for the UM and CC groups. At each follow up visit, the assessments consisted of medical history, physical and a detailed neurological assessment in the CM group. Seizure occurrence and frequency in the UM and CC children was recorded at 24 months only and covered the period between the 6 month and the 24 month visit. The lack of questioning about seizures for the first 6 months of follow-up in children with UM and CC was an inadvertent omission.
Parents were also asked for the description of seizures. Patients who reported seizures were referred to the paediatric neurology clinic for management. All children who were found to be ill during evaluations at the follow up visits were sent for appropriate treatment and re-evaluated after a week or upon recovery from the illness.
Data management
Data was entered into databases using FileMaker Pro 7 and analysed using SPSS 11.0 after editing and cleaning. Chi square and Student's t-test were used to compare frequencies and mean values of continuous variables, respectively. Chi square and Analysis of Variance (ANOVA) were used to determine whether the CM group had more occurrences of seizures, mortality and number of seizures per episode than the UM and CC groups.
Results
Baseline characteristics
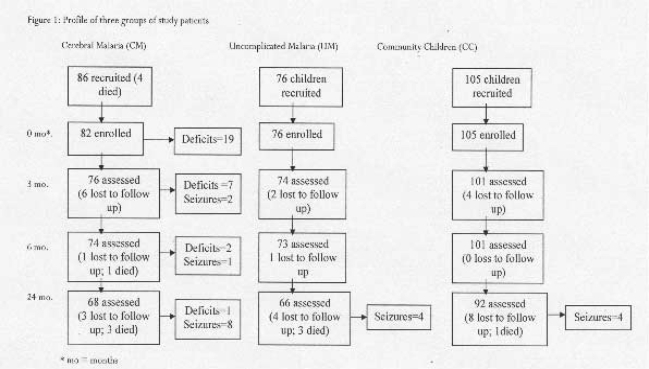
Eighty-six children with CM were enrolled, of whom 4 died during admission, and the 82 survivors followed up over 24 months. Seventy-six children with UM and 105 healthy CC were also recruited and followed up over 24 months. Children in the CM group were significantly younger and were most likely male when compared to the UM and CC groups, mean age 6.0 years vs 7.2 years (p = 0.002) and 7.6 years (p<0.001); percent male 59.3% vs 42.7% and 47.6% respectively. There was no significant difference in the nutritional status or socioeconomic scores among the three groups of children (Table 1).
Table 1.
Baseline characteristics of the three groups of study participants
| Cerebral Malaria (CM)N=86 |
Uncomplicated Malaria (UM)N=75 |
Community Children (CC)N=105 |
P value* (CM vs UM) |
P value* (CM vs CC) |
|
| Age in years (SD)± | 5.99 (2.29) | 7.21 (2.64) | 7.55 (2.22) | 0.002 | <0.001 |
| Number of males (%) | 51 (59.3) | 32 (42.7) | 50 (47.6) | 0.51 | 0.11 |
| Mother's level of education (SD) | 6.24 (2.07) | 6.41 (2.29) | 6.21 (2.11) | 0.66 | 0.92 |
| Nutrition (weight for age z-score) | −1.15 (1.45) | −1.07 (1.00) | −1.04 (1.15) | 0.71 | 0.60 |
| Socioeconomic status score (SD) | 9.86 (2.76) | 10.73 (3.79) | 10.14 (2.77) | 0.18 | 0.57 |
± SD = Standard deviation
* = Frequencies were compared using the ?Frequencies were compared using the malaria on c
Study follow-up and mortality over 24 months. Overall 86% (226/262, excluding 4 CM children who died during admission) of the children recruited for the study completed the 24 months follow up, eight children died and 28 could not be traced during the follow up period. Of the children who survived the CM episode, 83% (68/82) completed the 24 months follow up; four children died and 10 were lost to follow up. In the UM group, 84% (66/76) completed follow up; three died and six were lost to follow up and 88% (92/105) of the CC group completed follow up; one died and 12 were lost to follow up. (See study profile figure 1)
Figure 1.
Profile of three groups of study patients
A higher frequency of children with CM died (4/72, 5.6%), compared to UM, (3/69, 4.3%) and CC (1/93, 1.1%) died over the 24 months follow up period, though this difference did not achieve statistical significance (P= 0.17 for CM vs CC). The cause of death in all these cases was reported as deaths following short febrile illnesses.
Seizure activity
In the CM group the cumulative number of children reporting seizures increased over time from 2 of 76 children at three months, and 3 of 74 at six months to 11 of 68 children by 24 months follow up (Chi square for trend = 9.36, P=0.002, Table 2). Frequency of seizures between the 6 and 24 month period was recorded in children with CM, UM and CC. Children with CM had a higher frequency of seizures (8/68, 11.8%) as compared to children with UM (4/66, 6.1%, OR= 2.07 (95% CI 0.53–8.68, P= 0.25) or CC (4/92, 4.4%, OR= 2.93 (95% CI 0.75–12.21, P= 0.08), but the difference was not statistically significant. Similarly, the frequency of epilepsy, defined as two or more unprovoked seizure, was not significantly higher in children with CM (3/68, 4.4%) than children with UM (2/66, 3.0%, OR= 1.48 (95% CI 0.2–13.1, P= 0.51) or CC (1/92, 1.1%, OR= 4.2 (95% CI 0.4–107.1, P=0.21).
Table 2.
Frequency of seizure episodes in children with cerebral malaria over 24-month follow-up period.
| Follow-up period | Number (%) of children with new seizure episodes during the follow-up interval |
Cumulative number (%) of children with seizure activity |
| 3 months (N= 76) | 2 (2.6) | 2 (2.6) |
| 6 months (N=74) | 1 (1.4) | 3 (4.1) |
| 24 months (N=68) | 8 (11.8) | 11 (16.2) a |
P=0.003, ?2 test for trend.
In children with CM, the majority of the seizures were generalised tonic clonic seizures (6 of 9 episodes, 66.7%). Five of the 9 seizure episodes (55.6%) were associated with a febrile illness and 4 of 9 children (44.4%) reported more than two seizure episodes.
There was no association between seizures at admission and risk of seizures during the 24-months follow up (P=0.68). Likewise numerous clinical and laboratory factors were assessed but none correlated with risk of seizure during the follow up period (data not shown).
Neurologic deficits
Neurologic examinations were done on the CM group only. At discharge 25.0 % (19/76) of children were identified to have gross neurologic impairments, but this reduced to 9.2% (7/76) at 3 months , 2.7% (2/74) at 6 months, and all but one had resolved at 24 months (1/68, 1.5%) (Table 3). Cortical spinal signs, that is hyperreflexia with increased muscle tone, were the commonest deficits described at discharge, accounting for 74% (14/19) of all gross neurologic impairments (Table 3). The others were: spastic quadriplegia with vision and hearing impairments (1), ataxia (1), lack of coordination (2) and attentions deficit with inability to follow instructions (1) (table 3).
Table 3.
Frequency of neurologic deficits after cerebral malaria over a 2-year follow-up period
| Follow-up period | Number with neurologic deficits(% of total) |
Number with cortical spinal signs (% of those with neurological deficits)a |
Number with other neurologic deficits (% of those with neurological deficits)b |
| Discharge (N=76)c | 19 (25.0) | 14 (73.7) | 5 (26.3) |
| 3 months (N= 76) | 7 (9.2) | 4 (57.1) | 3 (42.9) |
| 6 months (N=74) | 2 (2.7) | 1 (50) | 1 (50) |
| 24 months (N=68) | 1 (1.5) | 0 (0) | 1 (100) |
Cortical spinal signs = Hypereflexia and hypertonia
Includes: spastic quadriplegia, vision and hearing impairments, ataxia, lack of coordination and attention deficit with inability to follow instructions.
Neurologic examinations missing or incomplete on 6 of the 82 children discharged after episode of cerebral malaria.
Discussion
It has increasingly been recognised that children exposed to CM experience a variety of neurological impairments. This two-year prospective follow up of children shows that, while gross neurologic deficits tend to resolve over time in survivors of CM, cumulative prevalence of seizures increases over time.
Seizure activity has been well described during an acute episode of CM. In children admitted with a diagnosis of CM, about 80%17, 18 of the children present with seizures compared to 69% 5 of children admitted with primary diagnosis of malaria. In addition over 60% of CM children continue to experience seizures following admission19, however the prevalence of seizure activity in CM survivors over time has not been described previously. In this prospective study, we found that between six and 24 months follow up, the prevalence of seizures was higher in the CM survivors (11.8%) than in children with UM (6.1%) and CC (4.3%) respectively, and this difference approached statistical significance in comparison to CC (P=0.08). The higher prevalence rates of seizures in CM survivors found in this study was similar to those found in other studies. For example, in a retrospective study in Kenyan children aged between six and nine years, Carter et al 20 found that children with a history of CM had an increased prevalence of epileptiform seizures of 9.2% compared to community children unexposed to malaria with 2.2%. However, the present study is the first to describe the cumulative seizure risk over time, and document that the cumulative incidence of seizures in children who survive CM increases over time. The findings suggest that CM may be associated with neuronal damage that predisposes children to seizures, and that the effects of this damage are often not seen until more than 6 months after the CM episode.
The most common type of seizure reported in the three groups was generalised tonic clonic seizures, which were mostly associated with episodes of febrile illnesses. Focal seizures were also reported in the CM and UM groups but none in the CC groups. There was therefore no consistent pattern in any group which is generally consistent with the description of seizures in malaria endemic areas; the type of seizures associated with CM are usually undistinguishable from those reported by children with otherwise uncomplicated malaria. The seizures could be typically febrile seizures brought on by fever5, 20 or could be complex seizures brought on by P. falciparum malaria per se5.
In the CM group there was no relationship between the seizure activity during the episode of CM and the occurrence of seizure activity during the follow up period. This suggests that the pathophysiology of seizures during the acute illness of cerebral malaria might be different from seizures experienced during the follow up period. We were also unable to demonstrate any clinical factors during the acute episode of CM that was associated with later development of seizures in survivors.
In the CM group, seizure activity seems to mature with time. At the three months follow up only two survivors reported history of seizure activity, the number increased to three at 6 months and by 24 months 11 children reported history of seizures (P=0.003, ?2 test for trend). Assessing epilepsy, which has been defined as two or more episodes of unprovoked seizures (e.g., not associated with fever) 20, the numbers were small and although epilepsy was more frequent in children with CM (4.4%) than children with UM (3.0%) or CC (1.1%), the differences were not statistically significant. In a cohort of Malian children aged six months to 14 years who were followed up a mean of 2.5 to 3.0 years after an episode of CM or non-cerebral malaria, the incidence of epilepsy after CM was higher than subjects who had suffered non-cerebral malaria21. Further evidence is provided by a case-control study from the same area10, in which children who had an episode of CM had an adjusted 3.9-fold increased risk of epilepsy as compared to those who had never had CM. In the present study, similar trends were seen, but with the smaller numbers assessed and a follow up period of only 24 months, none of the trends achieved statistical significance. It is possible that significant differences in epilepsy frequency might be seen over a longer period of follow-up between children with CM and community children.
The aetiology of seizures and subsequent development of epilepsy in CM survivors remains unclear. It might be that those who are genetically prone to seizures are also prone to epilepsy and to cerebral malaria22. However the pattern of increased cumulative incidence of seizures in the time after the CM episode suggests that injury suffered during CM might be the initiating event in a cascade of events that leads to development of epilepsy.
In contrast to seizures, which continue to present over time in survivors, neurological deficits documented on physical exam at discharge seem to resolve with time. Twenty-five percent of the children had one or more neurological impairments identified at discharge, but these gradually resolved over time, such that only one out of 68 children (1.5%) had a neurological impairment after 24 months. The prevalence of neurological deficits has been previously reported to range from 8% to 28%9, 12, 13, 23, 24. In this study cortical spinal signs (hyperreflexia with increased muscle tone) were the most common impairments found at discharge, accounting for three quarters of all neurologic impairments. A few studies25–27 have previously reported increased muscle tone and reflexes as impairments but not as frequently as in this study. The very comprehensive neurological exam conducted in the present study may have enabled us to identify subtle muscle tone and reflex problems. The one child with persistent neurologic deficits at 24 months had quadriplegia, speech and hearing impairments.
With the exception of one child, the children in this study did not have persistent gross neurologic impairments unlike in a recent Kenyan study where persistent gross motor deficits were found in 9.8% of CM survivors 28. The differences may be explained in part by the younger age of the children in the Kenyan cohort as compared to the cohort in the present study (mean age 30 versus 72 months). Its been reported that children who have brain injury early in childhood during the period of brain development and organisation are likely to suffer more severe and lasting neurological damage29, 30 Further evidence is that in the subset of children 5 to 12 years of age in whom we tested cognition, neurological impairment at 6 months was 0% 10 but studying the entire cohort, including children age 3–4 years, the rate is slightly higher at 2.7%.
Study weaknesses included the lack of information about seizures in children with UM and CC for the first 6 months, which may have prevented the detection of statistically significant differences in cumulative incidence of seizure activity in these groups as compared to children with CM. In addition, with the fairly low incidence of epilepsy over the 2- year follow-up period in the children with CM, the study sample size lacked sufficient power to detect a difference in the frequency of epilepsy between CM and CC.
In conclusion, in this study we demonstrate that neurologic impairment after an episode of CM is not limited to the neurologic deficits seen at discharge. While physical neurologic deficits in children who survive CM largely resolve over time, the cumulative incidence of seizures in CM survivors increases over time. Additional prospective studies are required to determine the complete long-term neurologic impact of CM in children.
Acknowledgements
We are grateful to the families that participated in this study and our project staff especially Esther Ssebyala, Mary Simensen, Joseph Otim, Jean Pierre Ndayizeyi, Drs Christine Mugasha, Moorine Sekadde and Judy Orikiriza who participated in recruiting, assessing and following up these children and data management.
This research was supported by a grant from the National Institutes of Health Fogarty International Center (R21 TW-006794) to Dr John and a Swedish International Development Agency (SIDA) Makerere University Medical School/Mulago Hospital Program for the Development of Medical Research grant to Dr. Opoka.
References
- 1.Murphy SC, Breman JG. Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. The American journal of tropical medicine and hygiene. 2001 Jan–Feb;64(1–2 Suppl):57–67. doi: 10.4269/ajtmh.2001.64.57. [DOI] [PubMed] [Google Scholar]
- 2.Mung'Ala-Odera V, Snow RW, Newton CR. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-saharan Africa. The American journal of tropical medicine and hygiene. 2004 Aug;71(2 Suppl):64–70. [PubMed] [Google Scholar]
- 3.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, et al. Indicators of life-threatening malaria in African children. The New England journal of medicine. 1995 May 25;332(21):1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. The Quarterly journal of medicine. 1989 May;71(265):441–459. [PubMed] [Google Scholar]
- 5.Waruiru CM, Newton CR, Forster D, New L, Winstanley P, Mwangi I, et al. Epileptic seizures and malaria in Kenyan children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996 Mar–Apr;90(2):152–155. doi: 10.1016/s0035-9203(96)90120-0. [DOI] [PubMed] [Google Scholar]
- 6.Jaffar S, Van Hensbroek MB, Palmer A, Schneider G, Greenwood B. Predictors of a fatal outcome following childhood cerebral malaria. The American journal of tropical medicine and hygiene. 1997 Jul;57(1):20–24. doi: 10.4269/ajtmh.1997.57.20. [DOI] [PubMed] [Google Scholar]
- 7.Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990 Oct 27;336(8722):1039–1043. doi: 10.1016/0140-6736(90)92498-7. [DOI] [PubMed] [Google Scholar]
- 8.Bondi FS. The incidence and outcome of neurological abnormalities in childhood cerebral malaria: a long-term follow-up of 62 survivors. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992 Jan–Feb;86(1):17–19. doi: 10.1016/0035-9203(92)90420-h. [DOI] [PubMed] [Google Scholar]
- 9.van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. The Journal of pediatrics. 1997 Jul;131(1 Pt 1):125–129. doi: 10.1016/s0022-3476(97)70135-5. [DOI] [PubMed] [Google Scholar]
- 10.Ngoungou EB, Koko J, Druet-Cabanac M, Assengone-Zeh-Nguema Y, Launay MN, Engohang E, et al. Cerebral malaria and sequelar epilepsy: first matched case-control study in Gabon. Epilepsia. 2006 Dec;47(12):2147–2153. doi: 10.1111/j.1528-1167.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- 11.Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung'ala-Odera V, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005 Jan;10(1):3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 12.Idro R, Karamagi C, Tumwine J. Immediate outcome and prognostic factors for cerebral malaria among children admitted to Mulago Hospital, Uganda. Annals of tropical paediatrics. 2004 Mar;24(1):17–24. doi: 10.1179/027249304225013240. [DOI] [PubMed] [Google Scholar]
- 13.Meremikwu MM, Asindi AA, Ezedinachi E. The pattern of neurological sequelae of childhood cerebral malaria among survivors in Calabar, Nigeria. The Central African journal of medicine. 1997 Aug;43(8):231–234. [PubMed] [Google Scholar]
- 14.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. The Journal of infectious diseases. 2006 Sep 15;194(6):837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 15.EPI INFO 6. Atlanta, GA: Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 16.Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007 Feb;119(2):e360–e366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacology & therapeutics. 1998 Jul;79(1):1–53. doi: 10.1016/s0163-7258(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 18.Olumese PE, Gbadegesin RA, Adeyemo AA, Brown B, Walker A. Neurological features of cerebral malaria in Nigerian children. Annals of tropical paediatrics. 1999 Dec;19(4):321–325. doi: 10.1080/02724939992149. [DOI] [PubMed] [Google Scholar]
- 19.Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. Qjm. 1996 Aug;89(8):591–597. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- 20.Carter JA, Neville BG, White S, Ross AJ, Otieno G, Mturi N, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004 Aug;45(8):978–981. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- 21.Ngoungou EB, Dulac O, Poudiougou B, Druet-Cabanac M, Dicko A, Mamadou Traore A, et al. Epilepsy as a consequence of cerebral malaria in area in which malaria is endemic in Mali, West Africa. Epilepsia. 2006 May;47(5):873–879. doi: 10.1111/j.1528-1167.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 22.Versteeg AC, Carter JA, Dzombo J, Neville BG, Newton CR. Seizure disorders among relatives of Kenyan children with severe falciparum malaria. Trop Med Int Health. 2003 Jan;8(1):12–16. doi: 10.1046/j.1365-3156.2003.00965.x. [DOI] [PubMed] [Google Scholar]
- 23.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet neurology. 2005 Dec;4(12):827–840. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 24.Crawley J, Waruiru C, Mithwani S, Mwangi I, Watkins W, Ouma D, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet. 2000 Feb 26;355(9205):701–706. doi: 10.1016/S0140-6736(99)07148-2. [DOI] [PubMed] [Google Scholar]
- 25.Neequaye J, Ofori-Adjei E, Ofori-Adjei D, Renner L. Comparative trial of oral versus intramuscular chloroquine in children with cerebral malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991 Nov–Dec;85(6):718–722. doi: 10.1016/0035-9203(91)90425-x. [DOI] [PubMed] [Google Scholar]
- 26.Carme B, Bouquety JC, Plassart H. Mortality and sequelae due to cerebral malaria in African children in Brazzaville, Congo. The American journal of tropical medicine and hygiene. 1993 Feb;48(2):216–221. doi: 10.4269/ajtmh.1993.48.216. [DOI] [PubMed] [Google Scholar]
- 27.Murphy S, English M, Waruiru C, Mwangi I, Amukoye E, Crawley J, et al. An open randomized trial of artemether versus quinine in the treatment of cerebral malaria in African children. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996 May–Jun;90(3):298–301. doi: 10.1016/s0035-9203(96)90260-6. [DOI] [PubMed] [Google Scholar]
- 28.Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Archives of disease in childhood. 2006 Feb;91(2):142–148. doi: 10.1136/adc.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holding PA, Snow RW. Impact of Plasmodium falciparum malaria on performance and learning: review of the evidence. The American journal of tropical medicine and hygiene. 2001 Jan–Feb;64(1–2 Suppl):68–75. doi: 10.4269/ajtmh.2001.64.68. [DOI] [PubMed] [Google Scholar]
- 30.Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002 Oct;23(5):353–364. doi: 10.1097/00004703-200210000-00010. [DOI] [PubMed] [Google Scholar]